Abstract
Purpose
The vascular disrupting agent fosbretabulin tromethamine selectively targets pre-existing tumor vasculature, which causes vascular shutdown and leads to cancer cell death and necrosis. Antiangiogenesis agents such as bevacizumab, a humanized antivascular endothelial growth factor monoclonal antibody, might prevent revascularization during and after treatment with a vascular disrupting agent.
Patients and Methods
Patients with recurrent or persistent epithelial ovarian, tubal, or peritoneal carcinoma, measurable or detectable disease, and three or fewer prior regimens were randomly assigned to bevacizumab (15 mg/kg intravenously once every 3 weeks) or the combination of bevacizumab (15 mg/kg) plus fosbretabulin (60 mg/m2) intravenously once every 3 weeks until disease progression or toxicity. Randomization was stratified by disease status (measurable v nonmeasurable), prior bevacizumab, and platinum-free interval. The primary end point was progression-free survival (PFS). The study was designed with 80% power for a one-sided alternative at a 10% level of significance to detect a reduction in the hazard by 37.5%.
Results
The study enrolled 107 patients. Median PFS was 4.8 months for bevacizumab and 7.3 months for bevacizumab plus fosbretabulin (hazard ratio, 0.69; 90% two-sided CI, 0.47 to 1.00; one-sided P = .05). The proportion responding (overall response rate) to bevacizumab was 28.2% among 39 patients with measurable disease and 35.7% among 42 patients treated with the combination. The relative probability of responding was 1.27 (90% CI, 0.74 to 2.17; one-sided P = .24). Adverse events greater than grade 3 were more common in the combination regimen than in bevacizumab only for hypertension (35% v 20%). There was one grade 3 thromboembolic event in the combination arm and one intestinal fistula in the bevacizumab only arm.
Conclusion
On the basis of the PFS, overall response rate, and tolerability of these two antivascular therapies, further evaluation is warranted for this chemotherapy-free regimen. Fosbretabulin in combination with bevacizumab increases the risk of hypertension.
INTRODUCTION
After eight positive randomized phase III trials, angiogenesis is an undisputed therapeutic target in epithelial ovarian cancer.1 Even as a single agent, bevacizumab, a humanized monoclonal antibody against vascular endothelial growth factor (VEGF), has clinically significant activity in both platinum-resistant and platinum-sensitive recurrent ovarian cancer.2 On the basis of its mechanism of action and favorable tolerability profile, doublet or even triplet chemotherapy combinations containing bevacizumab have improved progression-free survival (PFS) compared with chemotherapy alone with a suggestion of possible improvement in overall survival (OS) in certain populations.1 The optimal agents to combine with bevacizumab are not known. Targeted therapy doublets that include bevacizumab or other antivascular agents might be preferred to combinations containing traditional cytotoxic compounds because such combinations might be better tolerated and even more effective.
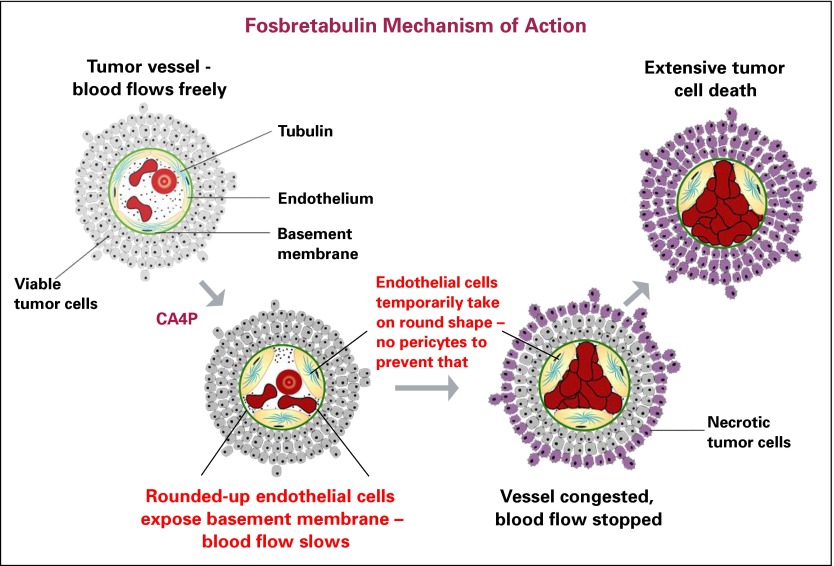
Vascular disrupting agents (VDAs) are ideal candidates to combine with antiangiogenic agents such as bevacizumab. In contrast to antiangiogenesis agents that target VEGF and angiopoetins, VDAs target existing tumor vascularity rather than preventing neovascularization.3 Tumor vessels can be selectively targeted by VDAs because the newly formed endothelial cells associated with cancer progression lack smooth muscle and pericyte coverage; thus, they rely more on intracellular tubulin to maintain their flat tube-like shape in vessel walls. VDAs that inhibit cancer-associated endothelial cell tubulin cause the affected endothelial cells to round up, thereby obstructing tumor-associated blood vessel lumens.4 This causes vessel collapse and obstruction (Fig 1).5 Thus, nontumor-associated blood vessels are relatively resistant to VDAs not only because of increased amounts of endothelial cell smooth muscle but also because of increased endothelial pericyte coverage, which allows them to maintain their shape when exposed to VDAs.
Fig 1.
Fosbretabulin mechanism of action. CA4P, combretastatin A4 mono tris phosphate. Reprinted with permission.5
Interestingly, cells on the periphery of solid tumors are also relatively insensitive to VDA-induced vascular shutdown. This resistant peripheral rim of tumor cells with ongoing neovascularization contributes to tumor regeneration, metastasis, and ongoing progression after VDA exposure. Conceptually, combining VDAs with antiangiogenesis compounds such as bevacizumab might overcome this regrowth phenomenon.6
Combretastatin A4 is a VDA originally isolated from the African bush willow (Combretum caffrum). Fosbretabulin is a water-soluble prodrug of cis-combretastatin A4 otherwise known as combretastatin A4 mono tris phosphate (abbreviated in the literature as CA4P). Fosbretabulin is a small molecule that acts as a potent and reversible tubulin depolymerizing agent. Preclinical models have shown that fosbretabulin results in massive acute vascular collapse as early as 2 hours after administration with recovery as soon as 24 hours, providing further rationale for combining it with bevacizumab.7 In a phase I study of the combination of fosbretabulin and bevacizumab, the dose-limiting toxicity seemed to be hypertension with the maximum tolerated dose of fosbretabulin being 63 mg/m2 once every other week. Importantly, this study showed dynamic contrast-enhanced diffusion-weighted magnetic resonance imaging evidence of profound vascular changes associated with fosbretabulin administration that were sustained only after bevacizumab treatment.8
The objective of this study was to assess PFS in a randomized phase II study of single-agent bevacizumab versus the combination of bevacizumab and fosbretabulin among women with recurrent epithelial ovarian cancer.
PATIENTS AND METHODS
This was an open-label prospective randomized phase II trial of single-agent bevacizumab compared with bevacizumab plus fosbretabulin (Gynecologic Oncology Group protocol 186-I; ClinicalTrials.gov. Identifier: NCT01305213). Eligible patients included women with measurable (per RECIST 1.1) or detectable persistent or recurrent epithelial ovarian, fallopian tube, or primary peritoneal carcinoma with documented disease progression.9 Detectable disease required at least one of the following conditions: cancer antigen (CA)-125 at least 2× upper limit of normal (ULN), ascites and/or pleural effusion attributed to tumor, or solid and/or cystic abnormalities on radiographic imaging that did not meet RECIST 1.1 definitions for target lesions. Patients must have had one prior platinum-based chemotherapeutic regimen for management of primary disease and were allowed to have received two additional cytotoxic regimens for management of recurrent or persistent cancer, with no more than one nonplatinum, nontaxane regimen. Patients were also allowed to have received noncytotoxic (biologic and/or targeted agents such as bevacizumab) therapy as part of their primary treatment regimen but were not allowed to have received any noncytotoxic therapy for management of recurrent or persistent disease. For the purposes of this study, poly (ADP-ribose) polymerase inhibitors were considered cytotoxic and were allowed before enrollment. Patients with either platinum-sensitive (platinum-free interval [PFI] > 182 days) or platinum-resistant (PFI ≤ 182 days) disease were eligible.
Patients age 18 years or older with a Gynecologic Oncology Group performance status of 0 or 1 were eligible. Patients with a performance status of 2 were eligible if they had received only one prior regimen. Adequate bone marrow function (platelet count ≥ 100,000/μL, absolute neutrophil count ≥ 1,500/μL, and hemoglobin > 9 g/dL), renal function (serum creatinine ≤ 1.5× institutional ULN), hepatic function (bilirubin ≤ 1.5× ULN, AST ≤ 3× ULN, and alkaline phosphatase ≤ 2.5× ULN), and electrolytes (potassium, magnesium, and calcium within institutional normal ranges) were required before random assignment. Normal blood coagulation parameters (prothrombin time international normalized ratio ≤ 1.5× ULN or an in-range international normalized ratio if a patient was receiving a stable dose of therapeutic warfarin, and a partial thromboplastin time ≤ 1.5× ULN) and normal urine protein excretion (≤ 1+ or a 24-hour urine protein < 1.0 g/day) were also required for patient enrollment. Patients with a serious nonhealing wound (including a history of abdominal fistula or GI perforation), ulcers, bone fracture, or an intra-abdominal abscess within 90 days before the first date of study treatment were ineligible. Patients with active bleeding or pathologic conditions that carried a high risk of bleeding or tumor involving major vessels were also ineligible. Those with uncontrolled hypertension, myocardial infarction, or unstable angina within 6 months before registration or a corrected QT interval (QTc) greater than 470 milliseconds were not allowed to enter the study.
All patients signed approved informed consent and authorization forms permitting release of personal health information. Patients of childbearing potential had a negative serum pregnancy test before entering the study and were practicing an effective form of contraception. Those with prior VDA treatment, other invasive malignancies, nonhealing wounds, active bleeding, brain metastases, or clinically significant cardiovascular disease were ineligible.
Drug Administration and Supportive Care
Bevacizumab was administered at 15 mg/kg as a continuous intravenous (IV) infusion once every 3 weeks. Anaphylaxis precautions were observed, and the initial dose was delivered over 90 minutes, the second infusion was administered over 60 minutes and, if the 60-minute infusion was well tolerated, all subsequent infusions were administered over 30 minutes. Among those randomly assigned to fosbretabulin, 60 mg/m2 was administered IV over 10 minutes on day 1 of each cycle after bevacizumab. This differed from the phase I schedule8 to provide a more practical and convenient regimen. Treatment was continued every 21 days (one cycle) until disease progression or until adverse effects (AEs) prohibited further therapy. The 14-day regimen in the phase I study8 of the combination was modified to once every 3 weeks to encourage symmetry in the once-every-6-weeks assessment of tumor progression.
If a bevacizumab-related infusion reaction occurred, future cycles were administered with an H1 blocker, an H2 blocker, and dexamethasone as premedications. Before fosbretabulin was administered, all patients received oral or IV dexamethasone (8 mg) and oral acetaminophen (650 mg) 1 hour before infusion. Infusion reactions that occurred despite premedications required discontinuation of the agent.
The treatment of chronic hypertension was encouraged and was administered at the discretion of the investigator. On the day of treatment, patients with systolic blood pressure (BP) readings between 120 and 130 mmHg or diastolic readings between 80 and 90 mmHg were administered amlodipine 5 mg or diltiazem 30 mg orally 2 hours before fosbretabulin (this was before the bevacizumab infusion). Amlodipine 10 mg or diltiazem 60 mg was administered orally 2 hours before fosbretabulin for patients with systolic BP readings higher than 130 mmHg or diastolic readings higher than 90 mmHg before infusion on the day of treatment and those who had previously required treatment of transient hypertension after fosbretabulin during previous cycles . Nausea, vomiting, diarrhea, and tumor pain were treated at the discretion of the investigator. Medications known to cause QTc prolongation were prohibited.
Dose Modifications
Dose reductions were not allowed for bevacizumab. Growth factors were not allowed, and subsequent cycles of therapy were administered if the absolute neutrophil count was ≥ 1,500/μL and the platelet count was ≥ 100,000/μL. Patients who failed to recover adequate counts within a 2-week delay were removed from the study. Bevacizumab was also discontinued for grade ≥ 2 (Common Terminology Criteria for Adverse Events v410) arterial thromboembolic events, left ventricular dysfunction, or hemorrhage (intracranial or pulmonary) and any grade ≥ 3 AE, including venous thromboembolic events, hemorrhage (nonintracranial or pulmonary), and wound complications. Any grade of posterior reversible encephalopathy syndrome, intestinal perforation and/or fistula, or other grade 4 AE required discontinuation of bevacizumab. If bevacizumab was discontinued secondary to AEs, the fosbretabulin was also stopped.
One dose level reduction of fosbretabulin to 50 mg/m2 was required if the QTc was greater than 480 milliseconds for grade ≥ 2 hypertension or hypotension during the 4 hours after fosbretabulin infusion and for grade ≥ 3 central neurologic symptoms, hematologic toxicity, or neuropathy. For the latter grade 3 toxicities, retreatment required a reduction in toxicity to grade ≤ 1. A second dose level reduction to 40 mg/m2 was allowed. No more than two fosbretabulin dose reductions were permitted. There were no dose escalations for fosbretabulin.
Study End Points
Tumor measurements using (computed tomography or magnetic resonance imaging) were made once during every other 3-week cycle according to RECIST 1.19 for the first 6 months and then every 3 months thereafter until disease progression. Progression (for those with measurable disease) was defined as at least a 20% increase in the sum of the diameters of target lesions, taking as a reference the smallest sum on study (this included the baseline sum if that was the smallest on study). In addition to the relative increase of 20%, the sum had to include an absolute increase of at least 5 mm. Other criteria sufficient for declaring progression included the following: a global deterioration in health status attributable to the disease, which required a change in therapy without objective evidence of progression, new lesions, or unequivocal progression of existing nontarget lesions. Patients who progressed within 6 weeks were deemed to have progressive disease (PD). Partial response was defined as at least a 30% decrease in the sum of the diameters of target lesions, taking as a reference the baseline sum of the diameters. Complete response was defined as the disappearance of all target and nontarget lesions and no evidence of new lesions. Stable disease was any condition not meeting the above criteria.
For those with detectable but nonmeasurable disease, assessment was based on CA-125, effusions (ie, ascites), and/or evaluation of indeterminate solid or cystic abnormalities. CA-125 responses, regardless of measurable status, were assessed with Rustin criteria.11 Initial values taken within 2 weeks of starting therapy had to be 2× ULN to be considered evaluable. At least a 50% reduction in CA-125 levels from the baseline value that was maintained for at least 28 days represented a partial response. A full response was defined as normalization of CA-125 levels (ie, less than ULN) maintained for at least 28 days (Appendix Tables A1-A3, online only). The date of progression by CA-125 level was determined by values greater than 2× maximum (ULN, nadir) that was confirmed at least 8 days later. If the date of progression was within 8 weeks, the patient had PD. If patients had evaluations more than 8 weeks from study entry without PD, then their response was at least stable disease. Patients were evaluated by using best overall response while receiving study therapy.
Statistical Design
The primary objective of this study was to assess the activity of the combination regimen relative to that of the control bevacizumab through the hazard ratio (HR) of disease progression or death (PFS end point) as a superiority study. The study was powered to detect a one-sided alternative hypothesis of a reduction in the hazard rate by 37.5% (HR < 0.63) by using the Cox proportional hazards model, which was considered clinically important to detect.12 The target enrollment was 110 patients (55 per arm) with 84 events to achieve 80% power with a 10% level of significance when conducted with an interim analysis. Secondary objectives included assessments of the overall proportion responding (overall response rate), OS, and toxicity. Funding was denied for translational end points. An interim futility analysis was performed after 44 PFS events (taking events from both treatment arms), which was capable of stopping the study early with 50% probability if the combination regimen did not improve the hazard of progression.13 The final primary analysis used a method given by Jennison and Turnbull.14 The study was positive if the standardized value of the HR, Z2, was less than –1.25. In addition, two interim safety evaluations were performed after 25 and 53 eligible patients received therapy on either treatment arm for at least 4 months. For treatment randomization, patients were stratified according to their disease status (measurable v detectable), prior use of bevacizumab therapy (no use v prior use), and most recent PFI (> 365 days, > 182 days and ≤ 365 days, ≤ 182 days). The analysis stratified patients by measurable disease, prior bevacizumab use, and PFI (> 365 v ≤ 365 days).
RESULTS
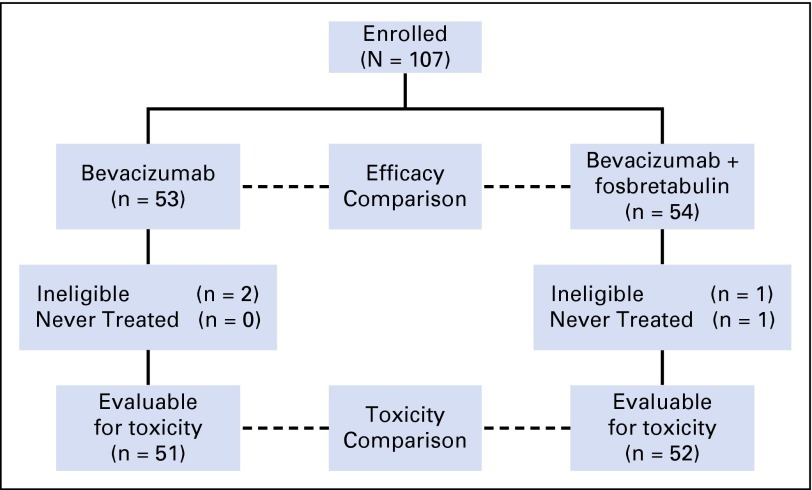
The study opened on March 21, 2011, and was closed to patient entry on April 22, 2013, after enrollment of 107 patients. The data cutoff for this analysis was March 3, 2014. All randomly assigned patients were included in the analysis of efficacy (intention to treat). On the combination arm, one patient never received protocol-directed therapy and another was ineligible. Two patients on the bevacziumab arm were ineligible. Thus, 103 eligible and treated patients were evaluable for toxicity (Fig 2). Patient demographic characteristics are outlined in Table 1. The arms were well balanced with regard to important prognostic factors.
Fig 2.
CONSORT diagram.
Table 1.
Demographic and Baseline Characteristics
| Characteristic | Bevacizumab | Bevacizumab + Fosbretabulin | Total | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age group, years | |||||
| 30-39 | 2 | 3.8 | 0 | 0.0 | 2 |
| 40-49 | 3 | 5.7 | 5 | 9.3 | 8 |
| 50-59 | 12 | 22.6 | 12 | 22.2 | 24 |
| 60-69 | 19 | 35.8 | 15 | 27.8 | 34 |
| 70-79 | 13 | 24.5 | 19 | 35.2 | 32 |
| ≥ 80 | 4 | 7.5 | 3 | 5.6 | 7 |
| Ethnicity | |||||
| Non-Hispanic | 46 | 86.8 | 49 | 90.7 | 95 |
| Hispanic/unknown/unspecified | 7 | 13.2 | 5 | 9.3 | 12 |
| Performance status | |||||
| 0 | 36 | 67.9 | 44 | 81.5 | 80 |
| 1 | 17 | 32.1 | 9 | 16.7 | 26 |
| 2 | 0 | 0.0 | 1 | 1.9 | 1 |
| Cell type | |||||
| Endometrioid | 3 | 5.7 | 2 | 3.8 | 5 |
| Serous | 45 | 84.9 | 46 | 85.2 | 91 |
| Clear cell | 1 | 1.9 | 3 | 5.6 | 4 |
| Mixed epithelial | 2 | 3.8 | 1 | 1.9 | 3 |
| Adenocarcinoma | 2 | 3.8 | 0 | 0.0 | 2 |
| Mucinous | 0 | 0.0 | 2 | 3.7 | 2 |
| No. of prior regimens | |||||
| 1 | 30 | 56.6 | 22 | 40.7 | 52 |
| 2 | 14 | 26.4 | 22 | 40.7 | 36 |
| 3 | 9 | 17.0 | 10 | 18.5 | 19 |
| Prior radiation | |||||
| No | 53 | 100.0 | 53 | 98.1 | 106 |
| Yes | 0 | 0.0 | 1 | 1.9 | 1 |
| Prior immunotherapy | |||||
| No | 51 | 96.2 | 53 | 98.1 | 104 |
| Yes | 2 | 3.8 | 1 | 1.9 | 3 |
| Prior surgery | |||||
| No | 3 | 5.7 | 1 | 1.9 | 4 |
| Yes | 50 | 94.3 | 53 | 98.1 | 103 |
| Prior bevacizumab | |||||
| No | 48 | 90.6 | 49 | 90.7 | 97 |
| Yes | 5 | 9.4 | 5 | 9.3 | 10 |
| Measurable disease | |||||
| No | 14 | 26.4 | 12 | 22.2 | 26 |
| Yes | 39 | 73.6 | 42 | 77.8 | 81 |
| Platinum sensitivity, months | |||||
| Platinum resistant | 14 | 26.4 | 13 | 24.1 | 27 |
| Platinum sensitive, 6 to 12 months | 21 | 39.6 | 22 | 40.7 | 43 |
| Platinum sensitive > 12 months | 18 | 34.0 | 19 | 35.2 | 37 |
| Total | 53 | 100 | 54 | 100 | 107 |
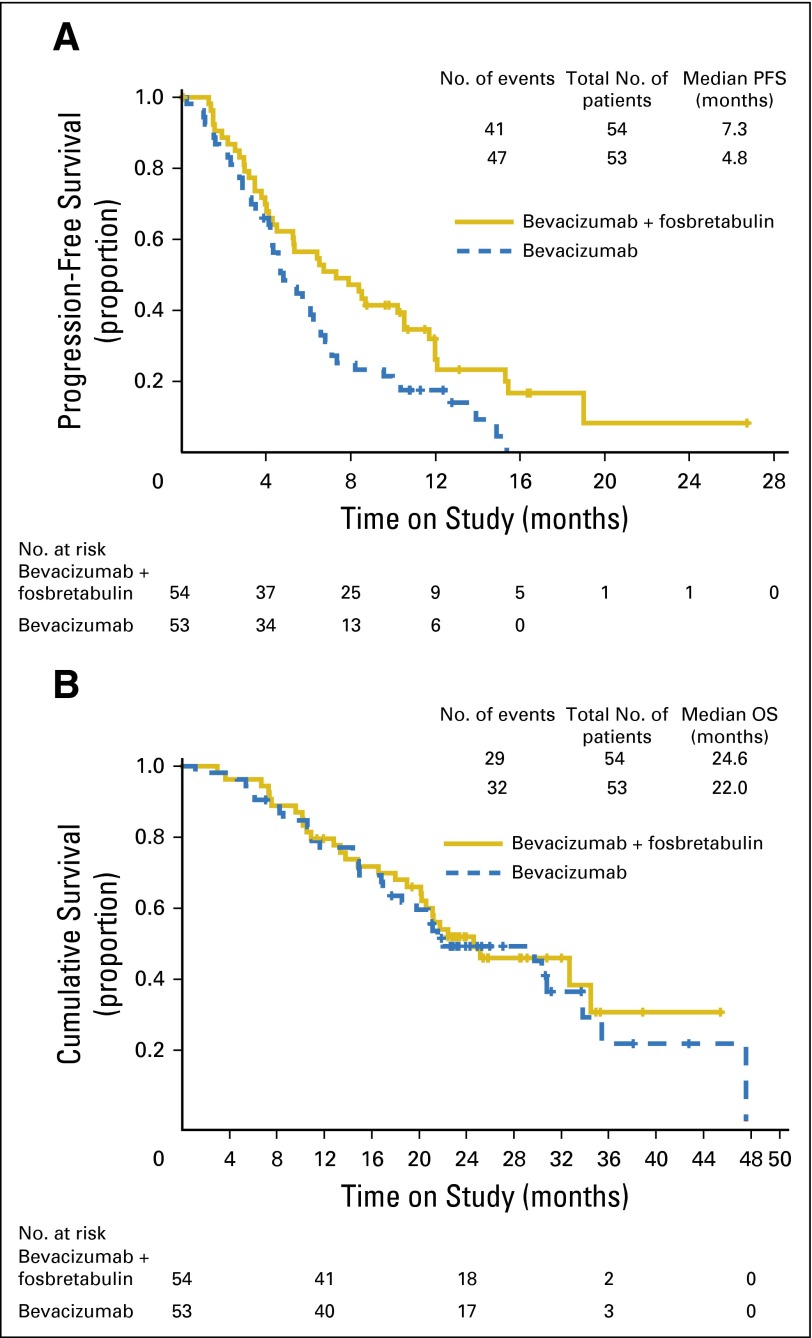
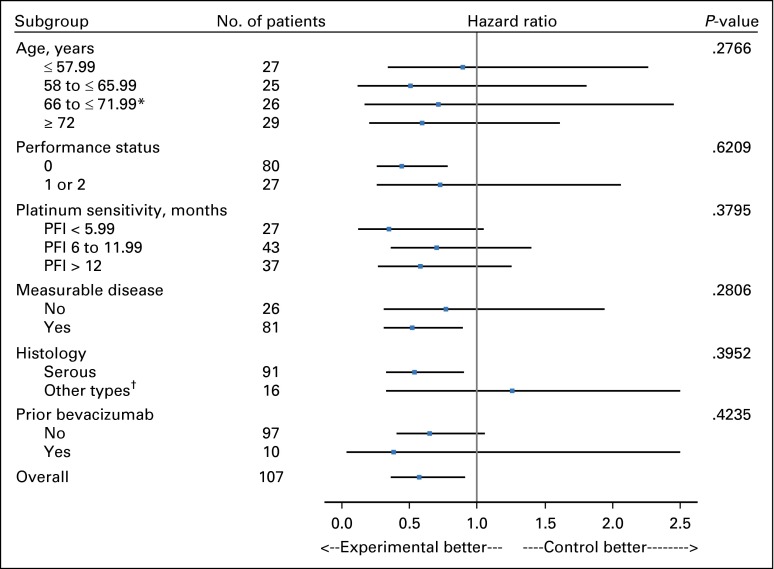
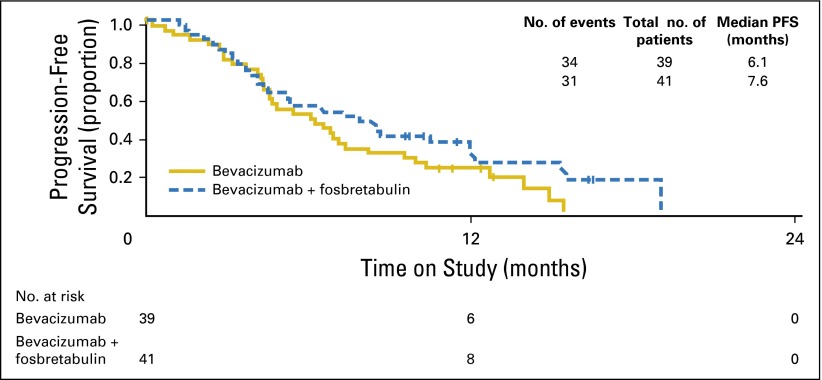
The final analysis was triggered after observing 88 PFS events. The standardized HR was Z2 = –1.66 to < –1.25. Adding fosbretabulin to bevacizumab seemed to prolong PFS compared with bevacizumab alone thereby meeting the primary objective of the study (median PFS, 4.8 months for bevacizumab and 7.3 months for bevacizumab plus fosbretabulin; HR, 0.69; two-sided 90% CI, 0.47 to 1.00; one-sided P = .05; Fig 3A). At the time of this data analysis, only 33 patients had died, making the evaluation of OS immature. On April 8, 2015, an additional follow-up study was done after 61 deaths. After stratifying by prior bevacizumab, measurable disease, and PFI, the HR was 0.85 (two-sided 90% CI, 0.54 to 1.34) and the median OS was 22.0 months for the reference regimen and 24.6 months for the experimental regimen (Fig 3B). Because of an imbalance in the performance status, other analyses were conducted on PFS and OS, stratifying on performance status (0 v 1 or 2). The PFS HR was 0.66 (90% CI, 0.47 to 0.94), and the OS HR was 1.01 (90% CI, 0.65 to 1.57; Fig 4).
Fig 3.
(A) Progression-free survival (PFS) analysis by intention to treat. (B) Overall survival (OS) analysis by intention to treat.
Fig 4.
Progression-free survival for prespecified covariates. The upper bound has been truncated at a hazard ratio equal to 2.5. *The true upper bound for 66 to ≤72 is 2.69. †The true upper bound for Other types is 4.797 (with only 16 people in this group). PFI, platinum-free interval.
More patients with measurable disease who were treated with bevacizumab plus fosbretabulin responded to treatment (28.2% for bevacizumab [90% CI, 16.7% to 42.3%] v 35.7% for the combination [90% CI, 23.5% to 49.5%]; Table 2). This difference was not statistically significant. The asymptotic estimate of the relative probability of response (experimental to reference) was 1.27 (90% CI, 0.74 to 2.17; one-sided P = .24). The relative probability of responding by CA-125 level among evaluable patients on the combination arm compared to the reference arm was 1.41 (90% CI, 0.73 to 2.71; 33% on the combination arm v 24% on the reference arm).
Table 2.
Response to Therapy and Patient Outcomes
| Characteristic | Bevacizumab | Bevacizumab + Fosbretabulin | Total | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Response | |||||
| Partial response | 11 | 20.8 | 15 | 27.8 | 26 |
| Stable disease | 24 | 45.3 | 20 | 37.0 | 44 |
| Progressive disease | 4 | 7.5 | 4 | 7.4 | 8 |
| Indeterminate | 0 | 0.0 | 3 | 5.6 | 3 |
| Nonmeasurable | 14 | 26.4 | 12 | 22.2 | 26 |
| Alive/cause of death | |||||
| Alive without progression | 6 | 11.3 | 13 | 24.1 | 19 |
| Alive with progression | 32 | 60.4 | 23 | 42.6 | 55 |
| Dead from disease | 14 | 26.4 | 16 | 29.6 | 30 |
| Dead, neither drug-related nor disease-related | 0 | 0.0 | 1 | 1.9 | 1 |
| Dead, undetermined cause | 1 | 1.9 | 1 | 1.9 | 2 |
| Total | 53 | 100 | 54 | 100 | 107 |
NOTE. The relative probability of responding on the bevacizumab + fosbretabulin arm compared with the bevacizumab only arm (among patients with measurable disease) was 1.27 (90% CI, 0.74 to 2.17; one-sided P = .24). The odds ratio for responding on the bevacizumab + fosbretabulin arm compared with the bevacizumab only arm was 1.41 (90% CI, 0.58 to 3.47; one-sided P = .31). Measurable disease overall response rate for bevacizumab was 28.2% (90% CI, 16.7% to 42.3%) among 39 patients; overall response rate for bevacizumab + fosbretabulin was 35.7% (90% CI, 23.5% to 49.5%) among 42 patients.
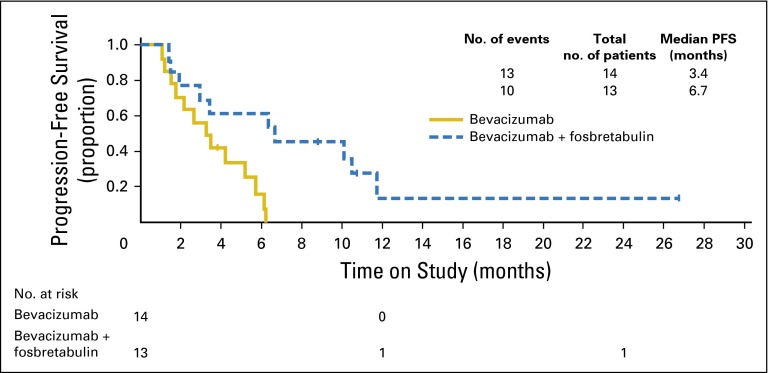
In a post hoc hypothesis-generating exploratory analysis, the median PFS for those patients having a PFI ≤ 182 days (platinum-resistant recurrent ovarian cancer; n = 27) in the bevacizumab arm was 3.4 months compared with 6.7 months in the group treated with a fosbretabulin combination (HR, 0.57; 90% CI, not reliable; Appendix Fig A1 [online only]). There also seemed to be an improvement in PFS in the platinum-sensitive (PFI > 182 days; n = 80) cohort (HR, 0.67; 90% CI, 0.43 to 1.03; Appendix Fig A2 [online only]).
Treatment emergent AEs are listed in Table 3. AEs for hypertension (grade > 3) seemed to be more common among those treated with bevacizumab plus fosbretabulin (35% v 20%; relative risk, 1.77; 95% CI, 0.90 to 3.45). There was one grade 3 thromboembolic event in the combination arm, one intestinal perforation was observed in the bevacizumab arm, and there were no treatment-related deaths.
Table 3.
Treatment Emergent Adverse Events
| Site | No. of Events for Bevacizumab-Only Arm | No. of Events for Bevacizumab + Fosbretabulin–Arm | ||||||
|---|---|---|---|---|---|---|---|---|
| Grade 2 | Grade 3 | Grade 4 | Grade 5 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | |
| Blood/lymphatics | 7 | 0 | 0 | 0 | 3 | 1 | 0 | 0 |
| GI | 10 | 6 | 0 | 0 | 12 | 10 | 0 | 1* |
| General/administration site | 13 | 2 | 0 | 0 | 17 | 3 | 0 | 0 |
| Infections/infestations | 19 | 3 | 0 | 0 | 18 | 3 | 0 | 0 |
| Investigation site | 11 | 4 | 0 | 0 | 6 | 5 | 0 | 0 |
| Metabolism/nutrition | 11 | 7 | 1 | 0 | 7 | 11 | 0 | 0 |
| Musculoskeletal/connective tissue | 14 | 2 | 0 | 0 | 6 | 2 | 0 | 0 |
| Nervous system | 6 | 1 | 0 | 0 | 7 | 3 | 0 | 0 |
| Renal/urinary | 5 | 0 | 0 | 0 | 4 | 2 | 0 | 0 |
| Respiratory/thoracic/mediastinal | 10 | 2 | 0 | 0 | 5 | 1 | 0 | 0 |
| Vascular disorders | 13 | 10 | 0 | 0 | 13 | 18† | 1 | 0 |
Grade 5 GI was gastric obstruction, considered not related to treatment.
Includes 17 for hypertension and one for a thromboembolic event.
DISCUSSION
Bevacizumab is the first targeted agent approved to treat ovarian cancer worldwide.15 In the United States, its approval is based on the AURELIA trial (AURELIA: A Study of Avastin [Bevacizumab] Added to Chemotherapy in Patients With Platinum-resistant Ovarian Cancer), in which adding bevacizumab to one of three single chemotherapeutic agents improved median PFS by 3.3 months (range, 3.4 to 6.7 months; HR, 0.48; 95% CI, 0.38 to 0.60). Because all patients received cytotoxic chemotherapy in addition to bevacizumab, toxicity was substantial. In an attempt to improve the therapeutic ratio, noncytotoxic combinations are emerging. In almost every instance, one partner of these targeted therapy doublets is an antiangiogenesis agent, frequently one that targets VEGF.16
This randomized phase II trial comparing bevacizumab plus fosbretabulin to bevacizumab alone provides further evidence regarding the safety and efficacy of nonchemotherapy-containing antivascular combinations. Inducing tumor vascular collapse with a VDA and concurrently preventing vessel regrowth with an established anti-VEGF compound reduced the risk of tumor progression by an estimated 31.5% among women with recurrent ovarian cancer. In addition, the odds ratio for responding to bevacizumab plus fosbretabulin compared with bevacizumab alone was 1.41 (90% CI, 0.58 to 3.47). A post hoc follow-up analysis of more mature OS data indicated an HR of 0.85 (unadjusted 90% CI, 0.54 to 1.34). This degree of activity is potentially important, especially when balanced against the frequency and severity of the attributable AEs. With more attention to the management of treatment-related hypertension, the rate of hypertension will likely decrease. Many lessons were learned in this trial about managing BP with this combination. Importantly, future trials should be even more vigilant in monitoring and treating BP, although serious hypertensive events were not seen in this study.
The advantages of this trial included its multicenter design and lean sample size. Its drawbacks included the lack of a placebo and blinded independent review of radiologic measurements of PFS and overall response rate as well as translational end points. Going forward, it will be important to compare nonchemotherapy targeted agents such as bevacizumab plus fosbretabulin to potentially more toxic and less efficacious chemotherapy options. However, the median PFS seen in this trial, which did not include chemotherapy, was much shorter than that in the historical literature, especially in the platinum-sensitive cohort. Thus, a major opportunity would be to add fosbretabulin to chemotherapy, as in the AURELIA regimen, which would create a chemotherapy plus bevacizumab plus fosbretabulin combination. Finally, administration of fosbretabulin once per week might increase its efficacy, given its short biologic effect, and should be studied.
Acknowledgment
We thank Daniele A. Sumner for her assistance in editing the manuscript, Sandra Dascomb for data management, and Kim Blaser, a member of the NRG Oncology/Gynecologic Oncology Group Foundation Publication Committee. OXiGENE provided study drug and limited financial support for the study. We also thank the following gynecologic oncology institutions that participated in this study: Duke University Medical Center, University of Mississippi Medical Center, University of California at Los Angeles Health System, University of Iowa Hospitals and Clinics, Indiana University Hospital, University of California Irvine Medical Center, Cleveland Clinic Foundation, Washington University School of Medicine, Memorial Sloan Kettering Cancer Center, University of Massachusetts Memorial Health Care, Fox Chase Cancer Center, Women's Cancer Center of Nevada, University of Oklahoma Health Sciences Center, University of Chicago Medical Center, Yale University, Women & Infants Hospital, The Hospital of Central Connecticut, Georgia Center for Oncology Research and Education, St. Joseph's Hospital and Medical Center, and Community Clinical Oncology Program.
GLOSSARY TERMS
- angiogenesis:
the process involved in the generation of new blood vessels. Although this is a normal process that naturally occurs and is controlled by so-called on and off switches, blocking tumor angiogenesis (antiangiogenesis) disrupts the blood supply to tumors, thereby preventing tumor growth.
Appendix
Fig A1.
Progression-free survival (PFS) among platinum-resistant patients by treatment. The hazard ratio was 0.57 (log-rank P = .01) for comparison of the experimental level to the reference level by treatment stratified by measurable disease status (yes/no) and prior bevacizumab use (yes/no), using a Cox proportional hazards model. The CI is questionable, which may be the result of the small number of patients within some strata and is therefore not available.
Fig A2.
Progression-free survival (PFS) among platinum-sensitive patients with a PFI of more than 6 months. The hazard ratio was 0.67 (90% CI, 0.43 to 1.03; log-rank P = .14) for comparison of the experimental level to the reference level by treatment stratified by measurable disease status (yes/no), prior bevacizumab use (yes/no), and platinum sensitivity (> 12 months/ ≤ 12 months) using a Cox proportional hazards model. PFI, platinum-free interval.
Table A1.
CA-125 Response by Treatment Among All Patients
| CA-125 Response | Bevacizumab | Bevacizumab + Fosbretabulin | Total | ||
|---|---|---|---|---|---|
| Frequency | % | Frequency | % | ||
| Full response | 1 | 1.89 | 1 | 1.85 | 2 |
| Partial response | 8 | 15.09 | 8 | 14.81 | 16 |
| Stable disease | 19 | 35.85 | 11 | 20.37 | 30 |
| Progressive disease | 2 | 3.77 | 0 | 0.00 | 2 |
| Indeterminate* | 8 | 15.09 | 7 | 12.96 | 15 |
| Not evaluable† | 15 | 28.30 | 27 | 50.00 | 42 |
| Total | 53 | 54 | 107 | ||
Abbreviation: CA-125, cancer antigen 125.
Patients who were indeterminate were evaluable but did not submit enough samples to determine their response.
Patients who were not evaluable had CA-125 values less than 2× upper limit of normal (ULN) or did not submit their baseline sample within 2 weeks of beginning therapy.
Table A2.
CA-125 Response by Treatment Among CA-125 Evaluable Patients
| CA-125 Response | Bevacizumab | Bevacizumab + Fosbretabulin | Total | ||
|---|---|---|---|---|---|
| Frequency | % | Frequency | % | ||
| Full response | 1 | 2.63 | 1 | 3.70 | 2 |
| Partial response | 8 | 21.05 | 8 | 29.63 | 16 |
| Stable disease | 19 | 50.00 | 11 | 40.74 | 30 |
| Progressive disease | 2 | 5.26 | 0 | 0.00 | 2 |
| Indeterminate | 8 | 21.05 | 7 | 25.93 | 15 |
| Total | 38 | 27 | 65 | ||
NOTE. The relative probability of responding by cancer antigen 125 (CA-125) among evaluable patients on the combination arm compared with the reference arm is 1.41 (90% CI, 0.73 to 2.71). Proportion responding was 23.7% (90% CI, 13.0% to 37.7%) on the bevacizumab arm and 33.3% (90% CI, 18.6% to 50.9%) on the combination arm.
Abbreviation: CA-125, cancer antigen 125.
Table A3.
Joint Frequency Distribution of Response by RECIST Against Response by CA-125
| RECIST Response | CA-125 Response | ||||||
|---|---|---|---|---|---|---|---|
| Full Response | Partial Response | Stable Disease | Progressive Disease | Indeterminate | Not Evaluable | Total | |
| Complete response | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| — | — | — | — | — | — | ||
| 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| Partial response | 2 | 6 | 7 | 0 | 1 | 10 | 26 |
| 7.69 | 23.08 | 26.92 | 0.00 | 3.85 | 38.46 | ||
| 100.00 | 37.50 | 23.33 | 0.00 | 6.67 | 23.81 | ||
| Stable disease | 0 | 6 | 19 | 1 | 3 | 15 | 44 |
| 0.00 | 13.64 | 43.18 | 2.27 | 6.82 | 34.09 | ||
| 0.00 | 37.50 | 63.33 | 50.00 | 20.00 | 35.71 | ||
| Increasing disease | 0 | 0 | 0 | 0 | 5 | 3 | 8 |
| 0.00 | 0.00 | 0.00 | 0.00 | 62.50 | 37.50 | ||
| 0.00 | 0.00 | 0.00 | 0.00 | 33.33 | 7.14 | ||
| Indeterminate | 0 | 0 | 0 | 0 | 1 | 2 | 3 |
| 0.00 | 0.00 | 0.00 | 0.00 | 33.33 | 66.67 | ||
| 0.00 | 0.00 | 0.00 | 0.00 | 6.67 | 4.76 | ||
| Nonmeasurable | 0 | 4 | 4 | 1 | 5 | 12 | 26 |
| 0.00 | 15.38 | 15.38 | 3.85 | 19.23 | 46.15 | ||
| 0.00 | 25.00 | 13.33 | 50.00 | 33.33 | 28.57 | ||
| Total | 2 | 16 | 30 | 2 | 15 | 42 | 107 |
NOTE. Percentages are conditional on row or column. Bold numbers indicate cases along the diagonal that are concordant.
Abbreviation: CA-125, cancer antigen 125.
Footnotes
Presented at the 15th Biennial Meeting of the International Gynecologic Cancer Society, Melbourne, Australia, November 8-11, 2014.
Supported by Grants No. CA27469 from the National Cancer Institute to Gynecologic Oncology Group [GOG] Administrative Office, No. CA37517 to the GOG Statistical Office, No. 1U10CA180822 to NRG Oncology, and No. U10CA180868 to NRG Operations.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
The authors are solely responsible for the content and preparation.
Clinical Trials Information: NCT01305213.
AUTHOR CONTRIBUTIONS
Conception and design: Bradley J. Monk, Michael W. Sill, Jai Balkissoon, Carol Aghajanian
Administrative support: Bradley J. Monk
Provision of study materials or patients: Bradley J. Monk, Joan L. Walker, Gregory Sutton, Lainie P. Martin, Jeanne M. Schilder
Collection and assembly of data: Michael W. Sill, Joan L. Walker, Christopher J. Darus, Gregory Sutton, Lainie P. Martin
Data analysis and interpretation: Michael W. Sill, Krishnansu S. Tewari, Lainie P. Martin, Jeanne M. Schilder, Robert L. Coleman, Carol Aghajanian
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Randomized Phase II Evaluation of Bevacizumab Versus Bevacizumab Plus Fosbretabulin in Recurrent Ovarian, Tubal, or Peritoneal Carcinoma: An NRG Oncology/Gynecologic Oncology Group Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Bradley J. Monk
Consulting or Advisory Role: GlaxoSmithKline, Merck, TESARO, Genentech, AstraZeneca, Gradalis, Verastem, Cerulean Pharma, Amgen, Vermillion, Immunogen, Bayer, NuCana BioMed, INSYS Therapeutics, Clovis Oncology, OXiGENE, Pfizer
Speakers’ Bureau: Genentech, AstraZeneca, Janssen, Myriad Genetics
Research Funding: Novartis (Inst), Amgen (Inst), Genentech (Inst), Eli Lilly (Inst), Janssen (Inst), Array BioPharma (Inst), TESARO (Inst), Morphotek (Inst)
Michael W. Sill
No relationship to disclose
Joan L. Walker
Research Funding: Genentech (Inst)
Christopher J. Darus
Research Funding: Amgen (Inst), AstraZeneca (Inst), Gradalis (Inst), Gynecologic Oncology Group partners (Inst), PharmaMar (Inst), AbbVie (Inst)
Travel, Accommodations, Expenses: Merck
Gregory Sutton
No relationship to disclose
Krishnansu S. Tewari
Honoraria: Genentech
Consulting or Advisory Role: Genentech
Lainie P. Martin
Consulting or Advisory Role: Immunogen
Research Funding: AbbVie (Inst), Clovis Oncology (Inst), Merck (Inst), Millennium Pharmaceuticals (Inst), Regeneron Pharmaceuticals (Inst), Sanofi (Inst), TetraLogic Pharmaceuticals (Inst), Teva (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: Immunogen
Jeanne M. Schilder
Employment: Eli Lilly
Stock or Other Ownership: Eli Lilly
Travel, Accommodations, Expenses: Eli Lilly
Robert L. Coleman
Honoraria: National Comprehensive Cancer Network
Consulting or Advisory Role: Clovis Oncology, Genentech, Esperance Pharmaceuticals, National Comprehensive Cancer Network, Department of Defense Congressionally Directed Medical Research Programs
Research Funding: AstraZeneca/MedImmune, Esperance Pharmaceuticals, OncoMed Pharmaceuticals, Array BioPharma, Clovis Oncology, Amgen, Johnson & Johnson, Merrimack Pharmaceuticals
Travel, Accommodations, Expenses: Millennium Pharmaceuticals, Merck, Amgen, AstraZeneca/MedImmune, Array BioPharma, Merrimack Pharmaceuticals, Gradalis
Jai Balkissoon
Employment: Pharmaceutical Product Development International
Carol Aghajanian
Consulting or Advisory Role: AstraZeneca
Travel, Accommodations, Expenses: AstraZeneca, AbbVie
REFERENCES
- 1.Eskander RN, Tewari KS. Incorporation of anti-angiogenesis therapy in the management of advanced ovarian carcinoma–mechanistics, review of phase III randomized clinical trials, and regulatory implications. Gynecol Oncol. 2014;132:496–505. doi: 10.1016/j.ygyno.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 2.Burger RA, Sill MW, Monk BJ, et al. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: A Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:5165–5171. doi: 10.1200/JCO.2007.11.5345. [DOI] [PubMed] [Google Scholar]
- 3.Mita MM, Sargsyan L, Mita AC, et al. Vascular-disrupting agents in oncology. Expert Opin Investig Drugs. 2013;22:317–328. doi: 10.1517/13543784.2013.759557. [DOI] [PubMed] [Google Scholar]
- 4.Kim S, Peshkin L, Mitchison TJ. Vascular disrupting agent drug classes differ in effects on the cytoskeleton. PLoS One. 2012;7:e40177. doi: 10.1371/journal.pone.0040177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Siemann DW, Bibby MC, Dark GG, et al: Differentiation and definition of vascular-targeted therapies. Clin Cancer Res 11:416-420, 2005. [PubMed] [Google Scholar]
- 6.Wu XY, Ma W, Gurung K, et al. Mechanisms of tumor resistance to small-molecule vascular disrupting agents: Treatment and rationale of combination therapy. J Formos Med Assoc. 2013;112:115–124. doi: 10.1016/j.jfma.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 7.Liu L, Su X, Mason RP. Dynamic contrast enhanced fluorescent molecular imaging of vascular disruption induced by combretastatin-A4P in tumor xenografts. J Biomed Nanotechnol. 2014;10:1545–1551. doi: 10.1166/jbn.2014.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nathan P, Zweifel M, Padhani AR, et al. Phase I trial of combretastatin A4 phosphate (CA4P) in combination with bevacizumab in patients with advanced cancer. Clin Cancer Res. 2012;18:3428–3439. doi: 10.1158/1078-0432.CCR-11-3376. [DOI] [PubMed] [Google Scholar]
- 9.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 10. U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute: Common Terminology Criteria for Adverse Events (CTCAE). Version 4.0, published May 28, 2009 (v4.03 published June 14, 2010)
- 11.Rustin GJ, Vergote I, Eisenhauer E, et al. Definitions for response and progression in ovarian cancer clinical trials incorporating RECIST 1.1 and CA 125 agreed by the Gynecological Cancer Intergroup (GCIG) Int J Gynecol Cancer. 2011;21:419–423. doi: 10.1097/IGC.0b013e3182070f17. [DOI] [PubMed] [Google Scholar]
- 12. Schoenfeld DA. Sample-size formula for the proportional-hazards regression model. Biometrics 39:499–503, 1983. [PubMed]
- 13.Wieand S, Schroeder G, O’Fallon JR. Stopping when the experimental regimen does not appear to help. Stat Med. 1994;13:1453–1458. doi: 10.1002/sim.4780131321. [DOI] [PubMed] [Google Scholar]
- 14. Jennison C, Turnbull BW: Group Sequential Methods With Applications to Clinical Trials. Boca Raton, FL, Chapman & Hall/CRC, 2000, pp 49. [Google Scholar]
- 15.Pujade-Lauraine E, Hilpert F, Weber B, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32:1302–1308. doi: 10.1200/JCO.2013.51.4489. [DOI] [PubMed] [Google Scholar]
- 16.Liu JF, Barry WT, Birrer M, et al. Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: A randomised phase 2 study. Lancet Oncol. 2014;15:1207–1214. doi: 10.1016/S1470-2045(14)70391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]