Abstract
Conjugate vaccines play an important role in the prevention of infectious diseases such as those caused by the bacteria Haemophilus influenzae (Hi) type b (Hib), Neisseria meningitidis, and Streptococcus pneumoniae. Vaccines developed against these 3 pathogens utilize 3 main carrier proteins, non-toxic mutant of diphtheria toxin (CRM197), diphtheria toxoid (DT) and tetanus toxoid (TT). Current pediatric immunisation schedules include the administration of several vaccines simultaneously, therefore increasing the potential for immune interference (both positively and negatively) to the antigens administered. Knowledge of vaccine interactions is principally derived from clinical trials, these are reviewed here to explore immune interference which may result of from carrier-specific T-cell helper interactions, bystander interference and carrier induced epitopic suppression.
Keywords: conjugate, interactions, immunisation, infectious disease, pediatric, Vaccines
Introduction
The development of polysaccharide-protein conjugate vaccines has increased the ability to protect infants against multiple infectious diseases. Capsular polysaccharides alone elicit B-cell responses in the absence of T-cells (T-independent response) and result in a short-lived response. The conjugation of capsular polysaccharides to a protein carrier presents antigens to the immune system in a manner that results in differentiation of B cells with recruitment of T and B cells (T-dependent response), this has the advantage of being immunogenic in infancy and inducing immune memory. Conjugate vaccines have been developed for several bacterial pathogens such as Haemophilus influenzae type b (Hib), Neisseria meningitidis, and Streptococcus pneumoniae and involve the joining of a polysaccharide moiety to a protein molecule. In the development of vaccines against these bacteria only a few protein carriers have been utilised, such as tetanus toxoid (TT), diphtheria toxoid, non-toxic mutant of diphtheria toxoid (CRM197) and Haemophilus influenzae protein D. The administration of a conjugate vaccine results in antibody production against both the hapten and the protein carrier epitopes. The co-administration of multiple vaccines based on the same protein carrier (common antigenic epitopes) requires consideration due to potential interactions and consequent impact on immune responses to the vaccine antigens.
Successful childhood immunisation programmes, such as that in the UK (Table 1), involve simultaneous vaccine administration. This approach maximises the likelihood of children receiving age-appropriate vaccines in a timely manner, reduces the number of vaccination visits required and allows easier incorporation of new vaccines into the schedule. As the number of conjugate vaccines available increases, so does the dosage of carrier proteins and likelihood of immune interference with the immune response to conjugated and/or co-administered antigens. The interaction of co-administered vaccines can result in impairment or enhancement of the immune response to any of the vaccine antigens. The mechanisms of these interactions are poorly understood, difficult to predict and may be the result of carrier-specific T-helper cell interactions, T-cell bystander interferences or carrier induced epitopic suppression.1,2 Pediatric vaccine schedules are becoming more complex as new vaccines become available and vaccine trials are undertaken to address the potential for interference of antibody responses. It is mainly from these clinical trials that our knowledge of immune interactions has been derived. This article reviews some of the available data regarding the impact on the immune response with the co-administration of bacterial conjugate vaccines.
Table 1.
United Kingdom infant immunisation schedule as of 2015
| Age | Vaccines |
|---|---|
| 2 months | DTaP/IPV/Hib-TT + PCV13-CRM197 + Rotavirus |
| 3 months | DTaP/IPV/Hib-TT +MCC-CRM197/TT + Rotavirus |
| 4 months | DTaP/IPV/Hib-TT + PCV13-CRM197 |
| Between 12 and 13 months | Hib/MCC-TT + MMR+ PCV13-CRM197 |
D = Diphtheria; T = Tetanus; aP = acellular pertussis; IPV = Inactivated polio virus vaccine; Hib = Haemophilus influenzae type b; PCV13 = 13-valent pneumococcal conjugate vaccine; CRM197 = non-toxic mutant of diphtheria toxin.
Conjugate Vaccines Boost Antibody Levels to their Carrier Protein
Administration of a conjugate vaccine has the potential to boost the individual's immunity to the carrier protein; examples of this have been seen with CRM197 and TT conjugates. Boosting of diphtheria responses were observed in UK children, following immunisation with a CRM197 containing pneumococcal conjugate vaccine (PCV7, Prevenar®, Pfizer). Children were either vaccinated at 12 months of age with a meningococcal serogroup C (MCC) Hib conjugate (MCC/Hib) (conjugated to TT; Menitorix®, GSK) followed by vaccination one month later with PCV7 and MMR or all 3 vaccines concomitantly at 12 months of age.3 A significantly higher proportion (p < 0.001) of those children who received all 3 vaccines concomitantly achieved diphtheria toxoid antibody levels ≥0.1 or ≥1.0 IU/mL compared to those who received MCC/Hib alone, as shown in Figure 1. Despite significant differences in proportions of subjects achieving these levels, the anti-diphtheria toxoid geometric mean concentrations (GMCs) 4 weeks after PCV7 given with or without MCC/Hib were similar, confirming that there was no attenuation of the booster response to the CRM conjugate component by concomitant administration with another conjugate. MenAfriVac®, a meningococcal serogroup A conjugate vaccine utilizing TT as a carrier protein has been demonstrated to elicit robust TT antibody responses in a Phase I study4 and it is hoped that this conjugate vaccine will aid in the elimination of neonatal tetanus from sub-Saharan Africa.
Figure 1.
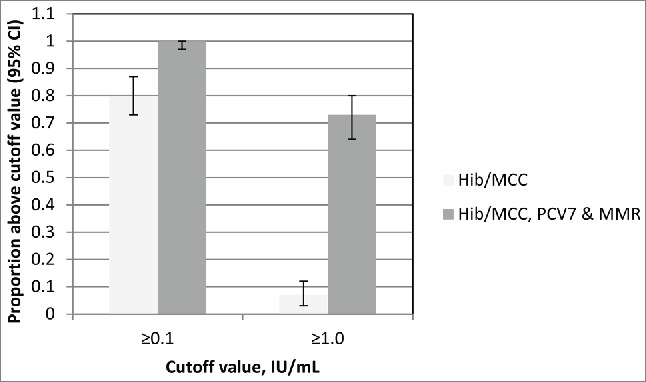
The proportions of subjects with Diphtheria toxoid antibody levels above 0.1 and 1.0 IU/mL at 1 month after vaccination according to whether MCC/Hib vaccine was given with or without PCV7 and MMR. Adapted from Miller et al. 2011.3
Carrier Specific Enhancement of T-Cell Help
Immune enhancement occurs when specific T-helper cells to one vaccine antigen increase the response to the same antigen in another vaccine. The enhancing effect from co-administration of conjugate vaccines is assumed to result from an increase of carrier driven T-helper frequency and T-cell mediated co-stimulatory signals.5 The most documented form of immune enhancement is in vaccines using TT as the conjugate protein co-administered with Hib-TT. The enhancing effect on Hib-TT from co-administration or combination with one or 2 additional polysaccharide-TT conjugates is assumed to result from an increase of carrier driven T-helper frequency and T-cell mediated co-stimulatory signals. Significant enhancement of anti-Hib IgG responses were observed in UK infants when DTaP5/IPV/Hib-TT (Pediacel®, Sanofi Pasteur) vaccine was co-administered with MCC-TT (NeisVac-C®, Baxter Bioscience), compared to the anti-Hib IgG responses in those who received MCC-CRM197 (either Meningitec® [now marketed by Neuron Biotech] or Menjugate® [now marketed by Novartis Vaccines]).6 Infants immunised with 3 doses of MCC and DTaP5/IPV/Hib-TT at 2, 3 and 4 months of age had blood samples taken 1 month following the final dose. In those who received MCC-TT, the Hib responses (as measured by anti-PRP IgG) was significantly higher than those who received one of the 2 available MCC-CRM vaccines, with the GMC of those receiving MCC-TT being 4.29 (95% CI 3.27–5.62) compared to 1.75 (95% CI 1.29–2.38) and 1.76 (95% CI 1.29–2.39) in those who received MCC-CRM vaccines Meningitec® and Menjugate®, respectively.
Another example of enhanced Hib responses was seen in a clinical trial utilizing a 10-valent pneumococcal conjugate vaccine (PCV10, Synflorix®, GSK) developed utilizing non-typeable Hib protein D as the carrier protein for 8 of the 10 pneumococcal polysaccharides, with serotypes 18C and 19F conjugated to TT and DT, respectively. Protein D was chosen, in part, to avoid carrier induced epitopic suppression and bystander interference, both of which are known to occur with CRM and TT conjugates. The anti-Hib IgG GMC and percentage of subjects with anti-Hib concentrations ≥1.0 µg/mL were significantly higher in PCV10 recipients compared with PCV7 recipients when co-administered with DTaP3-HBV-IPV-/Hib-TT (Infanrix hexaTM, GSK). The GMC of those vaccinated with PCV10 was 2.132 µg/mL (95% CI 1.925–2.362) compared to 1.176 µg/mL (95% CI 0.973–1.423) in those vaccinated with PCV7, suggesting an enhancement of the anti-Hib response by the TT carrier used for serotype 18C in PCV10.7
Carrier-Induced Epitopic Suppression
The presence of pre-existing immunity to a carrier protein has the potential to suppress the subsequent immune response to an antigen conjugated to the same carrier; this has been termed carrier induced epitopic suppression (CIES). The reduced responses to conjugated antigens can result from one or more of several mechanisms, the presence of pre-existing antibodies to the carrier protein preventing access of antigen-specific B cells to their epitopes by steric hindrance, or promotion of anti-carrier B-cell responses over anti-polysaccharide B-cell responses. The presence of a carrier protein may result in competition for resources; for example, the presence of dominant carrier protein-specific B cells can deprive antigen specific B cells of T cell help resulting in reduced antigen induced antibodies. Finally, the carrier generated regulatory T cells may interfere with anti-antigen responses.
In clinical trials, CIES related to TT conjugates has been reported. Burrage and colleagues8 investigated the potential interaction between MCC vaccines which contained either TT or CRM197 as a protein carrier and diphtheria and tetanus vaccines given as booster doses at school entry (DT) or school leaving (Td). Children received MCC-TT a month before, after, or at same time as their DT or Td booster vaccine. Immune responses were indicative of protection to MenC in all 3 groups, however an adverse effect on the immunogenicity of MCC-TT was observed prior to and to a lesser extent with concomitant administration of DT or Td vaccine (Fig. 2).8
Figure 2.
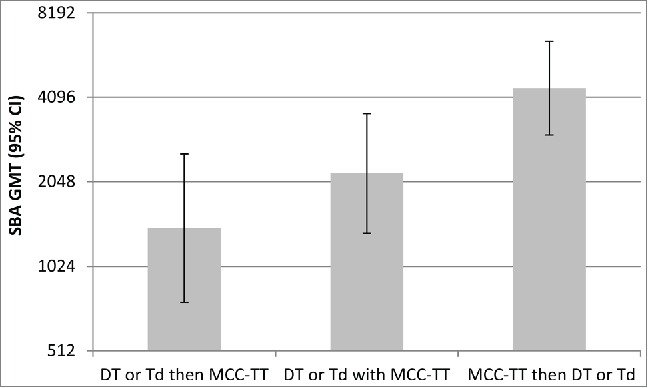
Serogroup C serum bactericidal antibody titres (95% CI) in children whom had received MCC-TT a month before, after or at the same time as their DT or Td booster vaccineAdapted from Burrage et al. 2002.8 DT = pre-school booster of diphtheria and tetanus; Td = school leavers dose of Tetanus and diphtheria reflecting lower dose of diphtheria.
Dagan et al.2 compared the co-administration of one of 2 pneumococcal polysaccharide conjugate vaccines (containing serotypes 6B, 14, 19F and 23F) conjugated to either TT or DT with 2 other vaccines, diphtheria-tetanus-pertussis and Hib-TT in infants.
The anti-Hib antibody response was significantly impaired when Hib-TT was administered simultaneously with pneumococcal vaccine conjugated to TT. Dosage of the TT carrier protein was shown to influence Hib responses. Comparison of the Hib responses of 4 vaccination groups, each receiving a different pneumococcal TT conjugate containing varying amounts of pneumococcal polysaccharide and hence carrier protein showed the reduction in anti-Hib antibodies to be dose-dependent. Anti-Hib antibody concentrations decreased with increasing TT content (Fig. 3) with significant differences in the anti-Hib responses of the 4 groups (p = 0.0121).2 The same observation was not made in those who received a diphtheria toxoid conjugate where the reduced response observed was TT carrier specific. The reduced responses observed by Dagan et al. (1998) was suggested to be due to TT overload which interferes with the anti-Hib response mediated by the clonal dominance of B cells specific for carrier epitopes.2 Optimal doses of TT will rapidly stimulate the activation and proliferation of carrier-specific B cells clones that efficiently take up the conjugate and present it to T cells, there are less polysaccharide-specific B cells clones so they will not reach the same frequency as carrier specific B cell clones. The decrease in polysaccharide response will therefore be proportional to the intensity of the immune response to the carrier.
Figure 3.
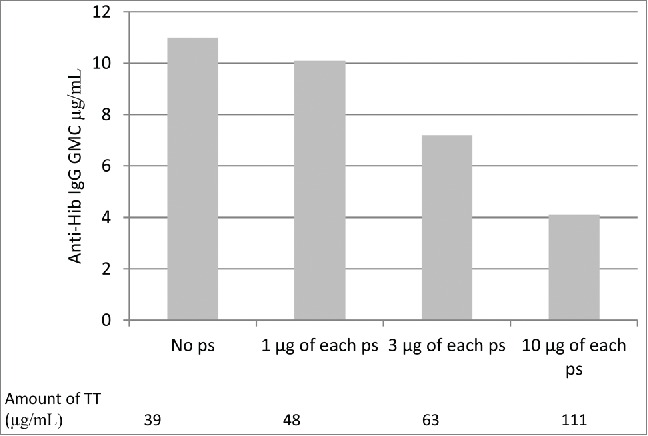
Geometric mean concentrations of anti-Hib IgG when co-administered with increasing amounts of pneumococcal conjugate vaccine utilising TT as a carrier protein. Adapted from Dagan et al. 1998.2
Bystander Interference
The co-administration and/or combinations of vaccines containing a given conjugate protein can induce interference that extends to unrelated antigens that are part of the combinations in use.5 This has been termed bystander interference and may influence responses to non-conjugated antigens administered simultaneously, or even sequentially.9 The mechanism suggested for this interference is competition for limited resources within the lymph nodes. Vaccines differ in their vulnerability to bystander interference, and Hib responses appear to be particularly vulnerable to these effects. Conjugate vaccines utilizing CRM197 when co-administered with DTaP/Hib vaccines consistently demonstrate reduced anti-Hib IgG responses compared to schedules where CRM197 is not co-administered. The addition of further CRM197 conjugate vaccines may reduce the Hib responses further.
Comparison of the anti-Hib response in UK infants simultaneously vaccinated at 2, 3, and 4 months of age with DTaP5/IPV/Hib-TT (Pediacel®, Sanofi Pasteur) and MCC (either MCC-TT, NeisVacC® or MCC-CRM, Menjugate®)10 were compared to those who received DTaP5/IPV/Hib-TT (Pediacel®, Sanofi Pasteur), MCC-CRM (Meningitec® [now marketed by Neuron Biotech]) and PCV7 (Prevenar7®, Pfizer).11 Hib responses were shown to be reduced in those who received Pediacel along with MCC-CRM compared to those who received MCC-TT and further reduced when MCC-CRM and PCV7 were co-administered (Fig. 4).
Figure 4.
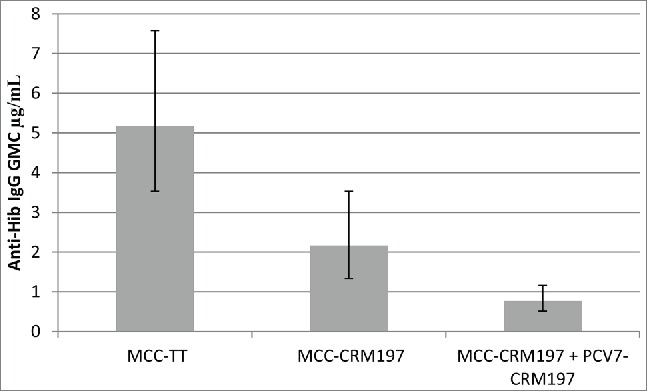
Hib IgG responses in those who received Pediacel together with MCC-TT or MCC-CRM or MCC-CRM and PCV7. Adapted from Kitchin et al. 2007,10 Moss et al. 2010.11
Vaccines containing the same antigens can also have different effects on responses to the same antigens, e.g. Hib IgG responses. Grimprel et al.12 compared Hib IgG responses of those who received DTaP5-IPV-Hib (Pediacel®, Sanofi Pasteur) or DTaP3-IPV-Hib (Infanrix-IPV® + Hib, GSK) at the same time as PCV7 (Prevenar®, Pfizer). Following three doses, the Hib IgG GMC in those who received DTaP5-IPV-Hib was significantly higher than those who received DTaP3-IPV-Hib, with GMCs of 1.38 (95%CI 1.14–1.66) and 0.59 (95% CI 0.49–0.71), respectively. Although both vaccines contain the same amount of Hib antigen, the results described here show that co-administration of DTaP5-IPV-Hib with MCC-TT enhances the Hib response whereas the co-administration of 2 CRM conjugates, such as MCC-CRM197 and PCV-CRM197, results in bystander interference which spreads to combined antigens such as Hib.
More than one dose of some conjugate vaccines are required, for example in the UK infant immunisation schedule from 2006 to 2013 infants received 2 doses of one of the 3 different manufacturers' MCC vaccines; 2 conjugated to CRM and one to TT. Analysis of MenC serum bacterial antibody (SBA) responses in UK infants showed that MCC vaccines with different carrier proteins are not interchangeable.13 Those infants vaccinated with MCC-CRM followed by MCC-TT had significantly reduced MenC and Hib responses compared to those vaccinated with 2 doses of MCC-CRM or MCC-TT, or MCC-TT followed by MCC-CRM (Fig. 5).13 While none of the previously reported phenomena explain the reason for these reduced responses, immune interaction between the carrier proteins is the most likely explanation.
Figure 5.
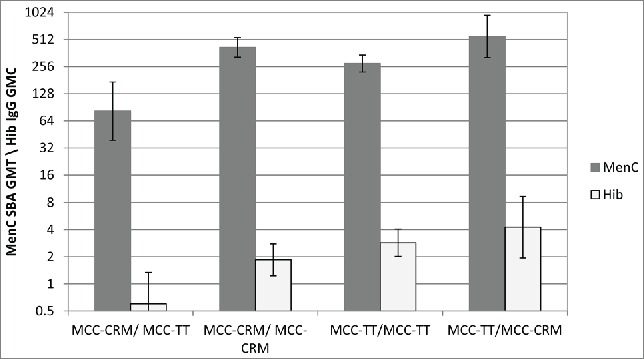
Meningococcal serogroup C SBA GMTs and Hib IgG GMCs given with different combinations of MCC vaccine. Adapted from Ladhani et al. 2015.13
Conclusion
Immune responses following co-administration of conjugate vaccines needs to be evaluated prior to incorporation into national immunisation campaigns. Specific clinical trials are required to evaluate the interference and enhancement effects and to increase our understanding of these phenomena. The trials discussed above provide evidence on the interactions of co-administered vaccines, some of which demonstrate negative impacts of simultaneous administration. The main concern from the observed vaccine interactions is the clinical relevance and impact on individual protection. In those trials in which a negative impact from simultaneous vaccine administration, the levels achieved by the majority of the study cohorts were above the protective thresholds for the antigen in question and therefore the observed immune interferences are not expected to have an effect on population effectiveness of the vaccine being studied.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Dagan R, Poolman JT, Zepp F. Combination vaccines containing DTPa-Hib: impact of IPV and coadministration of CRM197 conjugates. Expert Rev Vaccines 2008; 7:97-115; PMID:18251697; http://dx.doi.org/ 10.1586/14760584.7.1.97 [DOI] [PubMed] [Google Scholar]
- 2.Dagan R, Eskola J, Leclerc C, Leroy O. Reduced response to multiple vaccines sharing common protein epitopes that are administered simultaneously to infants. Infect Immun 1998; 66:2093-8; PMID:9573094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller E, Andrews N, Waight P, Findlow H, Ashton L, England A, Stanford E, Matheson M, Southern J, Sheasby E, et al.. Safety and immunogenicity of coadministering a combined meningococcal serogroup C and Haemophilus influenzae type b conjugate vaccine with 7-valent pneumococcal conjugate vaccine and measles, mumps, and rubella vaccine at 12 months of age. Clin Vaccine Immunol 2011; 18(3):367-72; PMID:21191076; http://dx.doi.org/ 10.1128/CVI.00516-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kshirsagar N, Mur N, Thatte U, Gogtay N, Viviani S, Preziosi M-P, Elie C, Findlow H, Carlone G, Borrow R, et al.. Safety and immunogenicity of a new meningococcal group A conjugate vaccine in healthy Indian adults. Vaccine 2007; 25S:A101-7; http://dx.doi.org/ 10.1016/j.vaccine.2007.04.050 [DOI] [PubMed] [Google Scholar]
- 5.Dagan R, Poolman J, Siegrist CA. Glycoconjugate vaccines and immune interference: A review. Vaccine 2010; 28(34):5513-23; PMID:20600514; http://dx.doi.org/ 10.1016/j.vaccine.2010.06.026 [DOI] [PubMed] [Google Scholar]
- 6.Southern J, Borrow R, Andrews N, Morris R, Waight P, Hudson M, Balmer P, Findlow H, Findlow J, Miller E. Immunogenicity of a reduced schedule of meningococcal group C conjugate vaccine given concomitantly with the Prevenar and Pediacel vaccines in healthy infants in the United Kingdom. Clin Vaccine Immunol 2009; 16(2):194-9; PMID:19091990; http://dx.doi.org/ 10.1128/CVI.00420-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knuf M, Szenborn L, Moro M, Petit C, Bermal N, Bernard L, Dieussaert I, Schuerman L. Immunogenicity of routinely used childhood vaccines when coadministered with the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV). Pediatr Infect Dis J 2009; 28:S97-S108; PMID:19325452; http://dx.doi.org/ 10.1097/INF.0b013e318199f61b [DOI] [PubMed] [Google Scholar]
- 8.Burrage M, Robinson A, Borrow R, Andrews N, Southern J, Findlow J, Martin S, Thornton C, Goldblatt D, Corbel M, et al.. Effect of vaccination with carrier protein on response to meningococcal C conjugate vaccines and value of different immunoassays as predictors of protection. Infect Immun. 2002; 70:4946-54; PMID:12183540; http://dx.doi.org/ 10.1128/IAI.70.9.4946-4954.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Insel RA. Potential alterations in immunogenicity by combining or simultaneously administering vaccine components. Ann N Y Acad Sci 1995; 754:35-47; PMID:7625671; http://dx.doi.org/ 10.1111/j.1749-6632.1995.tb44436.x [DOI] [PubMed] [Google Scholar]
- 10.Kitchin NR, Southern J, Morris R, Hemme F, Thomas S, Watson MW, Cartwright K, Miller E. Evaluation of a diphtheria-tetanus-acellular pertussis-inactivated poliovirus-Haemophilus influenzae type b vaccine given concurrently with meningococcal group C conjugate vaccine at 2, 3 and 4 months of age. Arch Dis Child. 2007; 92:11-6; PMID:16670121; http://dx.doi.org/ 10.1136/adc.2005.076109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moss SJ, Fenton AC, Toomey J, Grainger A, Borrow R, Balmer P, Smith J, Gennery AR. Immunogenicity of a heptavalent conjugate pneumococcal vaccine administered concurrently with a combination diphtheria, tetanus, five-component acellular pertussis, inactivated polio, and Haemophilus influenzae type B vaccine and a meningococcal group C conjugate vaccine at 2, 3, and 4 months of age. Clin Vaccine Immunol 2010; 17:311-6; PMID:20042517; http://dx.doi.org/ 10.1128/CVI.00315-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grimprel E, Wysocki J, Boisnard F, Thomas S, Mwawasi G, Reynolds D. Immunogenicity and safety of fully liquid DTaP5-IPV-Hib compared with DTaP3-IPV/Hib when both coadministered with a heptavalent pneumococcal conjugate vaccine (PCV7) at 2, 3, 4, and 12 to 18 months of age: a phase III, single-blind, randomised, controlled, multicentre study. Vaccine 2011; 29:7370-8; PMID:21807056; http://dx.doi.org/ 10.1016/j.vaccine.2011.07.078 [DOI] [PubMed] [Google Scholar]
- 13.Ladhani SN, Andrews NJ, Waight P, Hallis B, Matheson M, England A, Findlow H, Bai X, Borrow R, Burbidge P, et al.. Interchangeability of meningococcal group C conjugate vaccines with different carrier proteins in the United Kingdom infant immunisation schedule. Vaccine 2015; 33:648-55; PMID:25510388; http://dx.doi.org/ 10.1016/j.vaccine.2014.12.018 [DOI] [PubMed] [Google Scholar]


