Abstract
To evaluate the effect of a single course of high dose dexamethasone (HD-DXM) on CD28 and CTLA-4 expression in patients with newly-diagnosed primary immune thrombocytopenia (ITP). Twenty-8 ITP patients (18 females and 10 males, age range 18–65 years, median age 38.5 years) enrolled in this study and 26 healthy volunteers (19 women and 7 men, age range 16–66 years, median age 37 years) served as a control group. The patients were treated with HD-DXM (40 mg/day) for 4 consecutive days. CD28 and CTLA-4 expression was assessed by flow cytometry once-monthly for 6 months. Plasma levels of the cytokines IFN-γ and IL-10 were determined by enzyme-linked immunosorbent assay. One month after treatment, a platelet response was observed in 23 (82%) of the patients. The response rates over the next 5 months were 71%, 57%, 53%, 46%, and 39%, chronologically. We observed a significant decrease in CD28 expression after the first month (34.7 ± 4.8% vs. 44.5 ± 4.4% before treatment), after which the CD28 levels gradually increased. In contrast, CTLA-4 expression increased after the first month (3.2 ± 0.5% vs. 0.8 ± 0.4 before treatment), after which the CTLA-4 levels gradually decreased. Similar dynamic changes were seen in the levels of IFN-γ and IL-10. The dynamic changes of CD28 and CTLA-4 were consistent with those of IFN-γ and IL-10 and with the effectiveness of HD-DXM in the treatment of ITP. Our results suggest that a disturbed CD28/CTLA-4 balance may contribute to the immunopathogenesis of ITP.
Introduction
Primary immune thrombocytopenia (ITP) is an autoimmune disorder characterized by (1) a decreased platelet count due to the production of autoantibodies against the platelet proteins GPIIb/IIIa and/or GPIbα, (2) normal bone marrow, and (3) the absence of other causes of thrombocytopenia.1
The incidence of ITP is estimated to be 50–100 new cases per million persons annually, with children accounting for half of the cases. At least 70% of the cases involving children enter remission within 6 months, even without treatment. In addition, approximately one-third of the remaining chronic cases will gradually improve over time, and another one-third will display only mild thrombocytopenia (defined as a platelet count above 50,000).2 However, the remaining 510%– of the children with ITP will develop chronic severe refractory disease.3
Unlike children, adults with ITP usually have chronic disease with a probability of sustained remission of 20–40 percent.4 In the United States, the number of adults with chronic ITP is estimated to be 60,000, with women outnumbering men by approximately 2 to 1. Although the number of adults with ITP in China, or specifically in the XinJiang province, is not known, ITP has emerged as a major hemorrhagic disease in our hospital.
Corticosteroids are the conventional initial treatment for ITP.5-8 Corticosteroid regimens vary from 1 to 2 mg/kg of prednisone daily and exhibit a 70–85 percent initial response rate and a 30–40 percent sustained response rate. Unfortunately, serious side effects such as infections or metabolic alterations are common when long-term corticosteroid treatment is required to maintain the platelet count. Pulsed high-dose dexamethasone (HD-DXM), which has been used to treat multiple myeloma, sub-acute onset myositis, chronic inflammatory demyelinating polyradiculoneuropathy, rheumatoid arthritis, and recalcitrant necrobiotic xanthogranuloma, enables an early cessation of steroid treatment without a loss of response.9-13 Therefore, the latest international consensus report and practice guideline for ITP proposed HD-DXM therapy as one of the frontline options for ITP.14-15
The pathogenesis of ITP is complex, involving multiple imbalances in the immune system. These include a disturbed FcR balance, a reversal of IL-18/IL-18BP, and a dominance of Th1 cytokines. Several studies showed that each of these imbalances can be corrected by HD-DXM.16-17 Because HD-DXM induces a sustained reduction in immunoglobulin production and is tolerated well, HD-DXM should be a frontline treatment for ITP.
CD28 is a T cell costimulatory receptor that, when bound to its ligands CD80 and CD86 on antigen-presenting cells, activates the T cell during antigen presentation. CTLA-4 also binds to CD80 and CD86, but inactivates the T cell during antigen presentation. A balance of CD28 and CTLA-4 signaling is essential for the proper functioning of the immune system; an imbalance in CD28/CTLA-4 signaling is associated with many pathologies including allergy, cancer, spontaneous abortion, aplastic anemia, bronchial asthma, multiple sclerosis, and type 1 diabetes. We hypothesize that imbalanced CD28/CTLA-4 signaling also plays an important role in the immunopathogenesis of ITP and that HD-DXM elevates platelet counts by restoring the balance of CD28/CTLA-4 signaling.
Results
Clinical response to HD-DXM
Twenty-eight patients (18 females and 10 males, age range 18–65 years) were enrolled in this study. All of the patients finished the study and follow-up period. The initial ITP-related bleeding symptoms included petechiae, ecchymoses, epistaxis, gingival and genitourinary hemorrhage; specific characteristics are shown in Table 1. Positive responses to HD-DXM were observed in 23 (82%) of the patients one month after treatment. Over the following 5 months, the response rates were 71%, 57%, 53%, 46%, and 39%, chronologically (Fig. 1). The sustained response rate was defined as 39%, the response rate observed 6 months after treatment.
Table 1.
Clinical characteristics and responses of ITP patients
| Platelet counts(×109/L) |
||||
|---|---|---|---|---|
| Patient no. | Sex/Age | Bleeding symptoms | Before | After(one month) |
| 1 | F/59 | PT,GUH,GH | 6 | 141 |
| 2 | M/63 | PT,EC | 11 | 180 |
| 3 | F/46 | EC,GH | 10 | 21 |
| 4 | F/39 | EP | 8 | 124 |
| 5 | M/18 | EC,EP | 16 | 158 |
| 6 | F/21 | PT,EC,GH | 17 | 137 |
| 7 | F/37 | PT,GUH | 14 | 145 |
| 8 | M/23 | EC,EP | 14 | 54 |
| 9 | F/24 | PT | 7 | 139 |
| 10 | F/48 | EC,EP | 2 | 77 |
| 11 | M/37 | EC | 12 | 109 |
| 12 | F/49 | GH | 5 | 242 |
| 13 | M/29 | PT | 8 | 26 |
| 14 | F/45 | PT,EP | 11 | 190 |
| 15 | F/29 | PT,EP | 4 | 198 |
| 16 | M/56 | PT,GH | 8 | 186 |
| 17 | F/42 | EC,EP,GH | 15 | 231 |
| 18 | M/29 | PT,EC | 19 | 43 |
| 19 | F/44 | PT,GH | 7 | 104 |
| 20 | M/19 | PT | 17 | 74 |
| 21 | M/38 | EC,GH | 6 | 16 |
| 22 | F/21 | EC,GUH | 10 | 136 |
| 23 | F/39 | PT,EC | 15 | 28 |
| 24 | F/59 | EC,GUH,GH | 5 | 149 |
| 25 | F/23 | PT,EC,GH | 18 | 85 |
| 26 | F/47 | PT,GUH | 10 | 201 |
| 27 | M/31 | EC,EP | 2 | 132 |
| 28 | F/65 | PT | 4 | 174 |
| Median/mean | 10.04b | 125 |
p < 0.01 ITP before treatment vs. normal control.
p < 0.01 ITP one month after treatment vs. before treatment.
Abbreviations: PT = petechiae, EC = ecchymoses, EP = epistaxis, GUH = genitourinary hemorrhage,GH = gingival hemorrhage.
Figure 1.
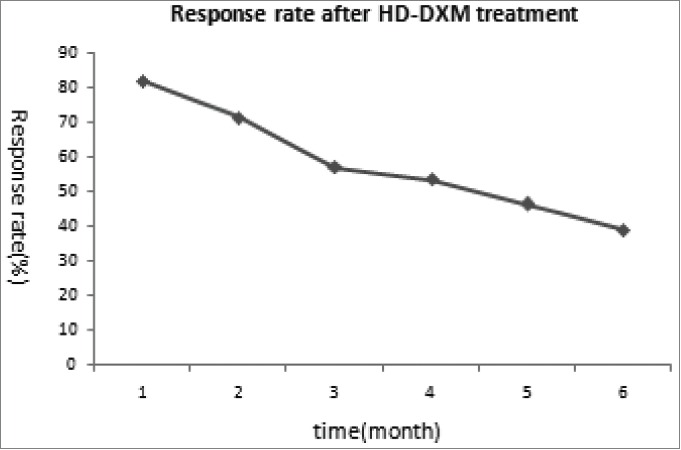
Clinical response of all the patients to HD-DXM.
Effect of HD-DXM treatment on CD28 and CTLA-4 expression
To determine the effect of HD-DXM treatment on CD28/CTLA-4 expression, we performed flow cytometry for these 2 receptors on circulating T cells from ITP patients before and every month after treatment. In addition, we performed the same analysis on T cells from healthy individuals. A significantly greater fraction of T cells expressed CD28, and a significantly lower fraction of T cells expressed CTLA-4, in the untreated ITP patients compared with the healthy controls (CD28: 44.5 ± 4.4% vs 32.0 ± 4.4%; CTLA-4: 0.8 ± 0.4% vs 3.3 ± 0.5%), consistent with the autoimmune cell activation occurring in ITP. HD-DXM treatment caused a significant decrease in the fraction of T cells expressing CD28 after one month (34.7 ± 4.8%), although this fraction increased gradually over the next 5 months (42.8+3.5% after the sixth month, Table 2). Conversely, HD-DXM treatment caused a significant increase in the fraction of T cells expressing CTLA-4 after one month (3.2 ± 0.5%), although this fraction decreased gradually over the next 5 months (1.2 ± 0.3% after the sixth month, Table 3). The flow cytometric data from a representative patient is shown in Figure 2. The changes in CD28 and CTLA-4 expression over time are shown in Figure 3.
Table 2.
Statistical data of CD28
| Time/month |
||||
|---|---|---|---|---|
| group | 0 | 1 | 6 | p2 |
| control | 32.0 ± 4.4 | 31.7 ± 4.6 | 32.3 ± 4.3 | 0.66 |
| HD-DXM | 44.5 ± 4.4 | 34.7 ± 4.8 | 42.8 ± 3.5 | – |
| P1 | 0.03 | 0.87 | 0.19 | |
P1: probability of no statistical difference between different groups at the same time.
P2: probability of no statistical difference at different times in the same group.
Table 3.
Statistical data of CTLA-4
| Time/month |
|||||
|---|---|---|---|---|---|
| group | 0 | 1 | 6 | p2 | |
| control | 3.3 ± 0.5 | 3.3 ± 0.4 | 3.3 ± 0.5 | 0.56 | |
| HD-DXM | 0.8 ± 0.4 | 3.2 ± 0.5 | 1.2 ± 0.3 | – | |
| P1 | <0.01 | 0.36 | 0.002 | ||
P1: probability of no statistical difference between different groups at the same time.
P2: probability of no statistical difference at different times in the same group.
Figure 2.
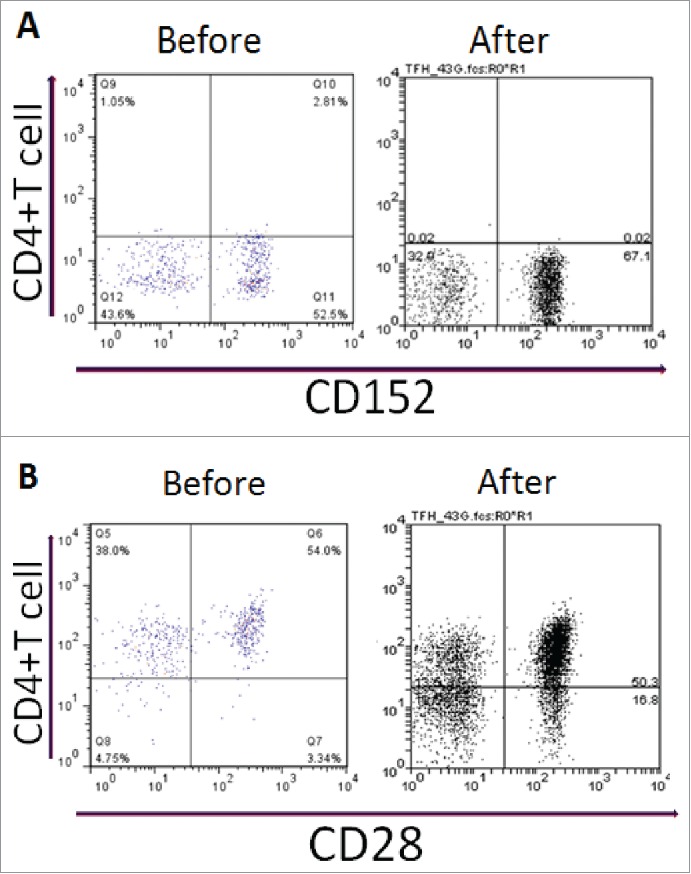
Expression of CD molecules on CD4+T cells from ITP patients before and after HD-DXM therapy. (A) representative scattergrams of surface expression of CD152 on CD4+T cells from an ITP patient before and after HD-DXM therapy, (B) representative scattergrams of surface expression of CD28 on CD4+T cells from an ITP patient before and after HD-DXM therapy.
Figure 3.

(A). Dynamic change Of CD28 on T-lymphocyte after HD-DXM treatment. (B). Dynamic change Of CTLA-4 on T-lymphocyte after HD-DXM treatment. (C). Plasma levels of IL-10 were elevated by HD-DXM. Panel D. Plasma levels of IFN-γwere reduced by HD-DXM.
Effect of HD-DXM treatment on plasma IL-10 and IFN-γlevels
To assess the effect of HD-DXM therapy on the T-helper1/T-helper2 (Th1/Th2) cell balance, we determined the levels of IL-10 (a Th2 cytokine) and IFN-γ (a Th1 cytokine) in plasma samples from the ITP patients and healthy controls. The untreated ITP patients contained a significantly lower level of plasma IL-10 and a significantly higher level of plasma IFN-γ compared with healthy controls (IL-10: 28.1 ± 8.2 vs 57.7 ± 10.1 pg/ml; IFN-γ: 32.6 ± 3.1 vs 10.9 ± 2.3 pg/ml), consistent with the dominance of Th1 cells over Th2 cells in ITP. The plasma levels of IL-10 increased significantly one month after HD-DXM treatment (56.7 ± 10.5 pg/ml) and decreased gradually thereafter (40.4 ± 7.9 pg/ml after the sixth month, Table 4). In contrast, the plasma levels of IFN-γ decreased significantly one month after HD-DXM treatment (10.7 ± 2.9 pg/ml) and increased gradually thereafter (25.2 ± 1.9 pg/ml after the sixth month, Table 5). The changes in plasma IL-10 and IFN-γ levels over time are shown in Figure 3.
Table 4.
Statistical data of IL-10 (pg/ml)
| Time/month |
||||
|---|---|---|---|---|
| group | 0 | 1 | 6 | p2 |
| Control | 57.7 ± 10.1 | 57.1 ± 11.0 | 58.2 ± 9.4 | 0.24 |
| HD-DXM | 28.1 ± 8.2 | 56.7 ± 10.5 | 40.4 ± 7.9 | – |
| P1 | 0.02 | 0.89 | 0.29 | |
P1: probability of no statistical difference between different groups at the same time.
P2: probability of no statistical difference at different times in the same group.
Table 5.
Statistical data of IFN-γ (pg/ml)
| time |
||||
|---|---|---|---|---|
| group | 0 | 1 | 6 | p2 |
| control | 10.9 ± 2.3 | 10.4 ± 2.2 | 11.4 ± 2.3 | 0.46 |
| HD-DXM | 32.6 ± 3.1 | 10.7 ± 2.9 | 25.2 ± 1.9 | – |
| P1 | <0.01 | 0.32 | 0.04 | |
P1: probability of no statistical difference between different groups at the same time.
P2: probability of no statistical difference at different times in the same group.
Correlation between CD28/CTLA-4 expression and platelet count
To investigate whether the CD28/CTLA-4 imbalance may contribute to the pathogenesis of ITP, we determined whether the HD-DXM treatment-mediated changes in CD28/CTLA-4 expression and platelet counts were correlated. We divided the fraction of T cells expressing CD28 by the fraction of T cells expressing CTLA-4, and plotted this ratio vs the platelet count. The two parameters were significantly and inversely correlated, with a correlation coefficient of −0.721 (Fig. 4). Hence, the HD-DXM treatment-mediated correction in the CD28:CTLA-4 imbalance correlated with the treatment-mediated increase in platelet counts.
Figure 4.

The scatter diagram of correlation between CD28/CTLA-4 and count of platelet. (correlation coefficient is −0.721, P < 0.05).
Discussion
The pathogenesis of ITP, an autoimmune disease characterized by decreased platelet counts and mucocutaneous hemorrhage, has not been fully elucidated. It is widely acknowledged that the low platelet counts are due primarily to autoantibodies against GPIIb/IIIa. The production of these autoantibodies by B cells depends on the state of the immune system, with T-helper cells playing an important role. In particular, Th1 cell dominance is frequently observed in ITP.18
The interaction of B7 (CD80 and CD86) with CD28 provides a costimulatory signal necessary for the activation of T cells. Semple and Freedman (1991) noted an elevated expression of CD80 on platelets from patients with ITP, suggesting that platelet-mediated costimulation of T cells could contribute to the immunopathogenesis of ITP.19 A number of studies have demonstrated that autoimmune diseases can be ameliorated by blocking the B7/CD28 interaction. CTLA-4 also binds to B7, but inhibits the T cell instead of costimulating it. T cell responses can be inhibited by administration of CTLA4-Ig, a fusion protein consisting of the extracellular domain of CTLA-4 and the Fc portion of human IgG1.20
We know that B7/CD28 signaling is required for the development of many diseases including allergy, cancer, spontaneous abortion, aplastic anemia, bronchial asthma, multiple sclerosis, and type 1 diabetes.21-26 Under normal circumstances, T cell activation requires both binding of the T cell receptor to the MHC-antigen complex and a secondary signal provided by costimulatory molecules. The primary costimulatory pathway for optimal T cell activation starts with the interaction between CD28 on the T cell and either of its 2 ligands B7–1 (CD80) and B7–2 (CD86) on antigen-presenting cells. However, CD80 and CD86 can also bind to CTLA-4, and this binding results in the inhibition of T cell activation. These results prompted us to study whether CD28/CTLA-4 signaling has an impact on the Th1/Th2 imbalance in ITP patients.
Although corticosteroids are widely considered to be the most appropriate frontline treatment for ITP, this choice is controversial because, in most cases, steroid tapering or withdrawal results in a decrease in platelet counts and the need for additional treatment. Recently, Cheng et al.27 reported that a single 4-day course of HD-DXM given as a frontline treatment for adult ITP patients induced an 85% initial response rate and a 42% sustained response rate. These rates are actually greater than the rates characteristic of corticosteroids (50–60% and 10–20%, respectively 28). Glucocorticoids including dexamethasone bind to and activate cytoplasmic receptors that subsequently translocate to the nucleus and regulate immune gene transcription in T cells.29, 30 Perhaps dexamethasone shifts the Th1/Th2 cytokine balance toward a Th2 phenotype and/or shifts the CD28/CTLA-4 balance toward CTLA-4.31 However, the reason why HD-DXM exhibits only a 40% sustained response rate is still unclear.
In this study, HD-DXM was given orally in a single 4-day course to ITP patients with active disorder and without any sort of prior treatment. HD-DXM displayed a favorable initial response rate, in agreement with previous reports.27,28,32,33 In this study, the expression of CD28 and CTLA-4 and the levels of Th1/Th2 cytokines (IFN-γ and IL-10) were investigated in ITP patients before and after HD-DXM treatment. HD-DXM down-regulated CD28 expression on T cells and decreased the plasma levels of the Th1 cytokine IFN-γ. Conversely, HD-DXM upregulated CTLA-4 expression on T cells and increased the plasma levels of the Th2 cytokine IL-10. Together, these results suggest that HD-DXM dampened T cell responses and shifted the Th1/Th2 balance toward Th2.
In accordance with previous reports, our data on plasma IFN-γ and IL-10 levels suggest that the Th1/Th2 balance in ITP is dominated by Th1 cells and that HD-DXM can restore the balance. During the final 5 months of the study, however, IL-10 levels decreased and IFN-γ levels increased. Similarly, CTLA-4 expression decreased and CD28 expression increased. The parallel changes of CD28/CTLA-4 expression and IL-10/IFN-γ levels partly confirm our hypothesis that CD28/CTLA-4 signaling plays an important role in the immunopathogenesis of ITP.
ITP is an autoimmune disorder whose pathophysiology may involve multiple possible mechanisms: (1) CD40-CD40L interactions, which contribute to the failure of immune tolerance, have been shown to play an important role in the pathogenesis of ITP. (2) Rituximab, the B cell-depleting antibody, has been shown to increase T-regulatory cell numbers and thus dampen T cell responses. These include Th1 cells reactive against GPIIb/IIIa, and therefore Rituximab can be therapeutically beneficial for ITP. (3) Decreased indoleamine 2,3-dioxygenase expression in dendritic cells, which can be corrected by CTLA-4-Ig treatment, may play a critical role in ITP. (4) CD28, CD80 and CD86 costimulatory molecules, which are over-expressed in ITP patients, may play an important role in ITP pathogenesis.18 (5) A disturbed FcγR balance might play a role in the pathogenesis of ITP, and HD-DXM therapy may shift the monocyte FcγR balance toward inhibitory FcγRIIb in patients with ITP.32 Although the pathogenesis of ITP can have multiple contributors, HD-DXM appears to address only those that involve CD28/CTLA-4 signaling. This may explain why HD-DXM alone exhibited a sustained response rate of 40%, whereas the combination of HD-DXM and Rituximab exhibited a sustained response rate 63%. 34
In this study we not only determined the expression of CD28, CTLA-4, IFN-γ and IL-10 in ITP patients, but also found a potential relationship between CD28/CTLA-4 signaling and Th1/Th2 balance. The fact that these 2 parameters changed hand-in-hand during the initial response to HD-DXM, as well as during the 5-month reversion back to their original states, provides new ideas in the pathogenesis and treatment of ITP.
This study has limitations. The ITP patients varied widely in age, severity of symptoms, and basic immunization status; these variations may affect the results and are worthy of further exploration. Moreover, the underlying mechanism of impaired IL-10 secretion in ITP is currently unknown.
In recent years, the B7/CD28 family has expanded. PD-1 and other newly-described costimulatory molecules have been shown to be involved in autoimmune diseases. Therefore, it is important to elaborate whether PD-1/PD-L1 signaling is involved in the immune regulation of CD28, CTLA-4, IFN-γ and IL-10 in ITP patients.
In summary, an increased CD28 expression and a decreased CTLA-4 expression in untreated ITP patients suggest that a disturbed CD28/CTLA-4 balance contributes to the pathogenesis of ITP. The disturbed CD28/CTLA-4 balance may then contribute to the disturbed Th1/Th2 balance and ultimately to the development of ITP. HD-DXM treatment may reduce inflammation in ITP by restoring the balance of CD28/CTLA-4, reducing the extent of autoimmune cell activation. The role of CD28 and CTLA-4 in ITP remains to be elaborated, but these 2 proteins are likely involved in the efficacy of HD-DXM treatment.
Materials & Methods
Research Subjects
Twenty-eight patients (18 females and 10 males, age range: 18–65 y, median age: 38.5 years) that were enrolled in this study were studied by the Department of Hematology, the first Affiliated Hospital of XinJiang Medical University, Urumqi, China, from January 2011 to May 2012. All the subjects were diagnosed according to the international criteria35 and their curative effect was also evaluated. None of the individuals had previously been treated with immunosuppressants or platelet transfusions. Cases were excluded if they were complicated by hypertension, diabetes, active infection, pregnancy, cardiovascular diseases or connective tissue diseases (i.e., systemic lupus erythematosus). More detailed information, regarding the ITP patients and their clinical characteristics and responses are presented in Table 1.
All patients were treated using HD-DXM 40 mg/d for 4 consecutive days. Initial response was evaluated at the end of the first month after commencement of the treatment, and every month thereafter for another 5 successive months. The response was evaluated on the basis of the under-mentioned criteria: response, defined as an increase in the platelet count of at least 30,000/mm3 and a platelet count of more than 50,000/mm3 by month 1 after the commencement of treatment; sustained response, defined as a platelet count of more than 50,000 per mm3 6 months after the initial treatment. Blood samples were collected before and every month after HD-DXM for 6 successive months. 26 healthy volunteers (19 women and 7 men, age range: 16–66 years, median age: 37 years) served as a control group, in which the differences in the age and gender between these 2 groups were not statistically significant. The study was approved by the Medical Ethical Committee of the first Affiliated Hospital of XinJiang Medical University, and informed consent was obtained from all subjects.
Reagents and instruments
APC-mouse-anti-human IgG1, APC-mouse-anti-human CD3 monoclonal antibody, FITC-mouse-anti-human IgG1, FITC-mouse-anti-human CD4 monoclonal antibody, PE-mouse-anti-human IgG1, PE-mouse-anti-human CD28 monoclonal antibody, hemolytic agent, fixation and permeabilization, were all purchased from Becton, Dickinson and Company. Flow cytometry was bought from Becton, Dickinson and Company. Coulter Ih750 Fully Automatic Haemocytometer Analyzer was purchased from Beckman Coulter. Inc. and finally IFN-γ and IL-10 kit were purchased from eBioscience, San Diego, CA, USA.
Quantitation and determination
Specimen collection
Six ml of fasting peripheral venous blood were collected from all the ITP patients and control patients. Two ml of the 6 ml were anticoagulated by EDTA and was used to detect CD4+ and CD28+. The collection of non-hemolytic serum samples were assembled from another 2 ml centrifugal blood (2500 r/min) and cryo-preserved at −80°C. This 2 ml sample of blood was used to detect cytokines. The remaining 2 ml of blood sample was used to count the amount of platelets.
Quantitation of CD28/CTLA-4 on the surface of CD4 (+) T Lymphocytes
APC-CD3 20 ul, FITC-CD4 20 ul and PE-CD28 were added to test tubes while and in the isotype control tubes isotype but irrelevant monoclonal antibodies (APC-mouse-anti-human IgG1, FITC-mouse-anti-human IgG1,PE-mouse-anti-human IgG1) were added. The contents in the test tubes and the isotype controls were thoroughly mixed, and incubated for 30 mins at 4°C in the absence of light. After incubation, a hemolytic agent was added, and then incubated again for a further 10 min at 25°C. The formed precipitates were isolated by gradient centrifugation (1000 r/min, 10 mins) and washed with 10 ml Phosphate Buffered Saline (PBS), then centrifuged (1000 r/min, 10 min) again to remove the supernatant. This step was repeated 2 times and subsequently thoroughly mixed with 300 ul PBS and 200 ul 4% paraformaldehyde. After the temperature was fixed at 4°C in a darkroom the determination of CD28 using flow cytometry ((FACS Vantage, equipped with Coherent argon ion laser and Cell Quest 3.1) was performed. APC-CD3 and SSC functions as the abscissa and ordinate of a scatter plot, respectively, and CD4 cell population was obtained. After adjusting the fluorescence compensation and resetting the coordinate lines using negative control, we then acquired 20 000–50 000 cells CTLA-4 was quantified in the same manner.
Cytokines determination
IFN-γ and IL-10 in the supernatant were determined by ELISA in the guidance of the manufacturer's instructions. A standard of known concentration and a sample of unknown concentration were added to 96-well plates for determination. The analytes were incubated in the presence of the antibody biotin. After incubation, avidin-HRP was added and the resulting sample was washed. Then the unbound enzyme conjugates were removed after another incubation and washing step. Finally A and B substrates were added for the purpose of combination with conjugates, and the colored reaction was stopped with stop solution. The depth of the color is proportional to the concentration of cytokines in the analyte. Absorbance was measured at 450 nm and the concentration of cytokines is proportional to the value of A450. According to this, a standard curve was drawn and the concentration of cytokines were calculated.
Statistical analysis
Statistical Product and Service Solutions (SPSS17.0) were used to analyze the data. The results were expressed as mean ± standard deviation. The differences between group means (log-transformed data) were determined by using ANOVA firstly, then the differences between any 2 groups were determine by using SNK test. P < 0.05 was considered to be statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Project Number
The Natural Science Foundation of Xinjiang Uygur Autonomous Region [project number: 2014211C043] and The National Natural Science Foundation [project number: 81360086].
References
- 1.George JN, el-Harake MA, Raskob GE. Chronic idiopathic thrombocytopenic purpura. New Eng J Med 1994; 331(18); PMID:7935660; http://dx.doi.org/ 10.1056/nejm199411033311807 [DOI] [PubMed] [Google Scholar]
- 2.Watts RG. Idiopathic thrombocytopenic purpura: a 10-year natural history study at the childrens hospital of alabama. Clin Pediat 2004; 43(8):691-702; PMID:15494875; http://dx.doi.org/ 10.1177/000992280404300802 [DOI] [PubMed] [Google Scholar]
- 3.Journeycake JM. Childhood immune thrombocytopenia: role of rituximab, recombinant thrombopoietin, and other new therapeutics. Hematology Am Soc Hematol Educ Program. 2012; 2012:444-9; PMID:23233617; http://dx.doi.org/ 10.1182/asheducation-2012.1.444 [DOI] [PubMed] [Google Scholar]
- 4.Stevens W, Koene H, Zwaginga JJ, Vreugdenhil G. Chronic idiopathic thrombocytopenic purpura: present strategy, guidelines and new insights. Netherlands J Med 2006; 64(10):356-63. [PubMed] [Google Scholar]
- 5.Sakamoto K, Nakasone H, Tsurumi S, Sasaki K, Mitani K, Kida M, Hangaishi A, Usuki K, Kobayashi A, Sato K, et al. Prednisone versus high-dose dexamethasone for untreated primary immune thrombocytopenia. A retrospective study of the Japan Hematology & Oncology Clinical Study Group. J Thromb Thrombolysis 2014; 37(3):279-86; PMID:23686644 [DOI] [PubMed] [Google Scholar]
- 6.Nakazaki K, Hosoi M, Hangaishi A, Ichikawa M, Nannya Y, Kurokawa M. Comparison between pulsed high-dose dexamethasone and daily corticosteroid therapy for adult primary immune thrombocytopenia: A retrospective study. Intern Med 2012; 51: 859-863; http://dx.doi.org/ 10.2169/internalmedicine.51.7005 [DOI] [PubMed] [Google Scholar]
- 7.Mazzucconi MG, Fazi P, Bernasconi S, De Rossi G, Leone G, Gugliotta L, Vianelli N, Avvisati G, Rodeghiero F, Amendola A, et al.. Therapy with high-dose dexamethasone (HD-DXM) in previously untreated patients affected by idiopathic thrombocytopenic purpura: a GIMEMA experience. Blood 2007; 109(4):1401-7; PMID:17077333 [DOI] [PubMed] [Google Scholar]
- 8.Cheng Y, Wong RS, Soo YO, Chui CH, Lau FY, Chan NP, Wong WS, Cheng G. Initial Treatment of Immune Thrombocytopenic Purpura with High-Dose Dexamethasone. N Engl J Med 2003; 349:831-6; PMID:12944568 [DOI] [PubMed] [Google Scholar]
- 9.Alexanian R, Dimopoulos MA, Delasalle K, Barlogie B. Primary dexamethasone treatment of multiple myeloma. Blood. 1992; 80:887-890. [PubMed] [Google Scholar]
- 10.van de Vlekkert J, Hoogendijk JE, de Haan RJ, Algra A, van der Tweel I, van der Pol WL, Uijtendaal EV, de Visser M, Dexa Myositis Trial . Oral dexamethasone pulse therapy versus daily prednisolone in sub-acute onset myositis, a randomised clinical trial. Neuromuscul Disord 2010; 20(6):382-9; PMID: 20423755; http://dx.doi.org/ 10.1016/j.nmd.2010.03.011 Epub 2010 Apr 25 [DOI] [PubMed] [Google Scholar]
- 11.van Schaik IN, Eftimov F, van Doorn PA, Brusse E, van den Berg LH, van der Pol WL, Faber CG, van Oostrom JC, Vogels OJ, Hadden RD, et al.. Pulsed high-dose dexamethasone versus standard prednisolone treatment for chronic inflammatory demyelinating polyradiculoneuropathy (PREDICT study): a double-blind, randomised, controlled trial. Lancet Neurol 2010; 9(3):245-53; PMID:20133204; http://dx.doi.org/ 10.1016/S1474-4422(10)70021-1 Epub 2010 Feb 2. [DOI] [PubMed] [Google Scholar]
- 12.Kroot EJ, Huisman AM, Van Zeben J, Wouters JM, Van Paassen HC. Oral pulsed dexamethasone therapy in early rheumatoid arthritis: a pilot study. Ann NY Acad Sci 2006; 1069:300-6. [DOI] [PubMed] [Google Scholar]
- 13.Chave TA, Chowdhury MM, Holt PJ. Recalcitrant necrobiotic xanthogranuloma responding to pulsed high-dose oral dexamethasone plus maintenance therapy with oral prednisolone. Br J Dermatol 2001; 144(1):158-61. [DOI] [PubMed] [Google Scholar]
- 14.Provan D, Stasi R, Newland AC, Blanchette VS, Bolton-Maggs P, Bussel JB, Chong BH, Cines DB, Gernsheimer TB, Godeau B, et al.. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood 115:168-186, 2010. [DOI] [PubMed] [Google Scholar]
- 15.Neunert C, Lim W, Crowther M, Cohen A, Solberg L Jr, Crowther MA. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood 117:4190-4207, 2011. [DOI] [PubMed] [Google Scholar]
- 16.Liu XG, Ma SH, Sun JZ, Ren J, Shi Y, Sun L, Dong XY, Qin P, Guo CS, Hou M, et al.. High-dose dexamethasone shifts the balance of stimulatory and inhibitory Fcreceptors on monocytes in patients with primary immune thrombocytopenia BLOOD 2011; 117:6; PMID:21131591 [DOI] [PubMed] [Google Scholar]
- 17.Shan NN, Zhu XJ, Wang Q, Wang CY, Qin P, Peng J, Hou M. HouHigh-dose dexamethasone regulates interleukin-18 and interleukin-18 binding protein in idiopathic thrombocytopenic purpura haematologica. Haematologica 2009; 94(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou B, Zhao H, Yang RC, Han ZC. Multi-dysfunctional pathophysiology in ITP. Crit Rev Oncol Hematol 2005; 54(2):107-16; http://dx.doi.org/ 10.1016/j.critrevonc.2004.12.004 [DOI] [PubMed] [Google Scholar]
- 19.Semple JW, Freedman J. Increased antiplatelet T helper lymphocyte reactivity in patients with autoimmune thrombocytopenia. Blood 1991; 78(10); PMID:1840468 [PubMed] [Google Scholar]
- 20.Peng J, Liu C, Liu D, Ren C, Li W, Wang Z, Xing N, Xu C, Chen X, Ji C, et al.. Effects of B7-blocking agent and/or CsA on induction of platelet-specific T-cell anergy in chronic autoimmune thrombocytopenic purpura. Blood 2003; 101(7):2721-6; PMID:12446456; http://dx.doi.org/ 10.1182/blood-2002-06-1666 [DOI] [PubMed] [Google Scholar]
- 21.Zhou W-H, Dong L, Du M-R, Zhu X-Y, Li D-J. Cyclosporin A improves murine pregnancy outcome in abortion-prone matings: involvement of CD80/86 and CD28/CTLA-4. Reproduction 2008; 135(3):385-95; http://dx.doi.org/ 10.1530/rep-07-0063 [DOI] [PubMed] [Google Scholar]
- 22.van Wijk F, Nierkens S, de Jong W, Wehrens EJM, Boon L, van Kooten P, Knippels LM, Pieters R. The CD28/CTLA-4-B7 signaling pathway is involved in both allergic sensitization and tolerance induction to orally administered peanut proteins. J Immunol 2007; 178(11):6894-900; PMID: 17513738 [DOI] [PubMed] [Google Scholar]
- 23.He G-S, Zhou L, Wu D-P, Sun A-N, Miao M, Wang X-L, Chang WR, Zhu ZL, Jin ZM, Qiu HY, et al.. Role of CD28/CTLA-4 co-stimulators in immune pathophysiology of aplastic anemia. Zhonghua xue ye xue za zhi 2007; 28(9):590-3; PMID:18246813 [PubMed] [Google Scholar]
- 24.Chen YQ, Shi HZ. CD28/CTLA-4-CD80/CD86 and ICOS-B7RP-1 costimulatory pathway in bronchial asthma. Allergy. 2006; 61(1):15-26; http://dx.doi.org/ 10.1111/j.1398-9995.2006.01008.x [DOI] [PubMed] [Google Scholar]
- 25.Chitnis T, Khoury SJ. Role of costimulatory pathways in the pathogenesis of multiple sclerosis and experimental autoimmune encephalomyelitis. J Allergy Clin Immunol 2003; 112(5):837-50; PMID:14610467 [DOI] [PubMed] [Google Scholar]
- 26.Pawlowski P, Urban M, Stasiak-Barmuta A. Surface expression of costimulatory molecules CD28/CTLA-4 on peripheral blood T lymphocytes in the course of type 1 diabetes mellitus in children and adolescents. Endokrynologia, diabetologia i choroby przemiany materii wieku rozwojowego 2004; 10(2):81-5; PMID:15504310 [PubMed] [Google Scholar]
- 27.Cheng Y, Wong RSM, Soo YOY, Chui CH, Lau FY, Chan NP, Wong WS, Cheng G. Initial treatment of immune thrombocytopenic purpura with high-dose dexamethasone. N Engl J Med 2003; 349(9):831-6; PMID:12944568; http://dx.doi.org/ 10.1056/NEJMoa030254 [DOI] [PubMed] [Google Scholar]
- 28.Mazzucconi MG, Fazi P, Bernasconi S, De Rossi G, Leone G, Gugliotta L, Vianelli N, Avvisati G, Rodeghiero F, Amendola A, et al.. Therapy with high-dose dexamethasone (HD-DXM) in previously untreated patients affected by idiopathic thrombocytopenic purpura: a GIMEMA experience. Blood. 2007; 109(4):1401-7; http://dx.doi.org/ 10.1182/blood-2005-12-015222 [DOI] [PubMed] [Google Scholar]
- 29.Adcock IM, Shirasaki H, Gelder CM, Peters MJ, Brown CR, Barnes PJ. The effects of glucocorticoids on phorbol ester and cytokine stimulated transcription factor activation in human lung. Life sciences. 1994; 55(14):1147-53; PMID:8090056; http://dx.doi.org/ 10.1016/0024-3205(94)00243-6 [DOI] [PubMed] [Google Scholar]
- 30.Cines DB, McMillan R. Management of adult idiopathic thrombocytopenic purpura. Annual Review of Medicine. Annu Rev Med 56 2005. p. 425-42. [DOI] [PubMed] [Google Scholar]
- 31.Agarwal SK, Marshall GD Jr.. Role of CD28/B7 costimulation in the dexamethasone-induced suppression of IFN-gamma. J Interf Cytok Res 2000; 20(11); PMID:11096449; http://dx.doi.org/ 10.1089/10799900050198363 [DOI] [PubMed] [Google Scholar]
- 32.Liu X-g, Ma S-h, Sun J-z, Ren J, Shi Y, Sun L, Dong XY, Qin P, Guo CS, Hou M, et al.. High-dose dexamethasone shifts the balance of stimulatory and inhibitory Fc gamma receptors on monocytes in patients with primary immune thrombocytopenia. Blood. 2011; 117(6):2061-9; http://dx.doi.org/ 10.1182/blood-2010-07-295477 [DOI] [PubMed] [Google Scholar]
- 33.Shan N-n, Zhu X-j, Wang Q, Wang C-y, Qin P, Peng J, Hou M. High-dose dexamethasone regulates interleukin-18 and interleukin-18 binding protein in idiopathic thrombocytopenic purpura. Haematologica-the Hematol J 2009; 94(11):1603-7; http://dx.doi.org/ 10.3324/haematol.2009.007708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zaja F, Baccarani M, Mazza P, Bocchia M, Gugliotta L, Zaccaria A, Vianelli N, Defina M, Tieghi A, Amadori S, et al.. Dexamethasone plus rituximab yields higher sustained response rates than dexamethasone monotherapy in adults with primary immune thrombocytopenia. Blood 2010; 115(14):2755-62; http://dx.doi.org/ 10.1182/blood-2009-07-229815 [DOI] [PubMed] [Google Scholar]
- 35.Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, Bussel JB, Cines DB, Chong BH, Cooper N, et al.. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood 2009; 113(11):2386-93; PMID:19005182; http://dx.doi.org/ 10.1182/blood-2008-07-162503 [DOI] [PubMed] [Google Scholar]


