Abstract
Hepatitis A virus (HAV) has shifted from high to intermediate endemicity in Mexico, which may increase the risk of clinically significant HAV infections in older children, adolescents and adults. The objective of this study was to evaluate the cost-utility of single-dose or 2-dose universal infant HAV vaccination strategy in Mexico, compared with no vaccination. A previously published dynamic model estimated the expected number of HAV cases with each strategy, and a decision model was used to estimate the costs and quality-adjusted life-years (QALYs) expected with each strategy. The time horizon was 25 years (2012–2036) and the base case analysis was conducted from the perspective of the Mexican public health system. Costs and QALYs after the first year were discounted at 5% annually. Input data were taken from national databases and published sources where available. The single-dose HAV vaccination strategy had an incremental cost-utility ratio (ICUR) of Mexican peso (MXN) 2,270 per QALY gained, compared with no vaccination. The two-dose strategy had an ICUR of MXN 14,961/QALY compared with no vaccination, and an ICUR of MXN 78,280/QALY compared with the single-dose strategy. The estimated ICURs were below the threshold of 1 x Mexican gross domestic product per capita. When indirect costs were included (societal perspective), the single-dose HAV vaccination strategy would be expected to improve health outcomes and to be cost-saving. This analysis indicates that routine vaccination of toddlers against HAV would be cost-effective in Mexico using either a single-dose or a 2-dose vaccination strategy. GSK study identifier: HO-12-12877.
Keywords: cost-effectiveness, economic evaluation, hepatitis A, Mexico, vaccination
Introduction
Infection with hepatitis A virus (HAV) results in acute liver disease, which is usually self-limiting and resolves completely in >99% of cases.1 HAV is transmitted mainly by the faecal-oral route through contaminated food or water, or by direct contact with an infected person.1 The clinical presentation of HAV infection is strongly dependent on age. Young children typically have asymptomatic HAV infections, while in adolescents and adults, 70% of cases may be symptomatic.1,2 Fulminant hepatitis is rare but serious, occurring in <1% of HAV cases,2 and may result in death or the need for an urgent liver transplant.1,2
HAV endemicity can be categorised on the basis of seroprevalence data as high (≥90% have immunity by age 10 years), intermediate (≥50% have immunity by age 15 years), low (≥50% have immunity by age 30 years) or very low (<50% have immunity by age 30 years).3 Seroprevalence data from studies in Mexico4,5 and a systematic review conducted by the World Health Organization (WHO)3 indicate that Mexico has shifted from high to intermediate HAV endemicity. Intermediate endemicity increases the incidence of clinically significant HAV infections compared with a high endemicity pattern, as the proportion of older children, adolescents and adults who are susceptible to HAV infection is higher and these age groups are more likely to develop symptomatic disease.1 The economic burden of HAV disease is substantial, reflecting the high incidence of the disease. In 2009, the economic burden of HAV disease in Mexico was estimated at 75,972,381 Mexican pesos (MXN).6
Vaccines against HAV have been available since the early 1990s.3 WHO recommends that HAV vaccination should be integrated into national immunisation schedules for children aged ≥1 year, if indicated on the basis of acute HAV incidence, shift in endemicity from high to intermediate, and consideration of cost-effectiveness.1 National immunization programmes may consider inclusion of single-dose inactivated hepatitis A vaccines in immunization schedules. This option seems to be comparable in terms of effectiveness, and is less expensive and easier to implement than the classical 2-dose schedule. However, until further experience has been obtained with a single-dose schedule, in individuals at substantial risk of contracting hepatitis A, and in immunocompromised individuals, a 2-dose schedule is preferred.1
Universal HAV vaccination programmes have been introduced and have reduced the incidence of HAV in several countries, including the US,7 Israel.8,9 (2-dose programmes) and Argentina (single-dose program).10 The projections from a dynamic transmission model of HAV in Mexico indicated that universal HAV vaccination in infants could substantially reduce the HAV disease burden in Mexico.11
HAV vaccine in Mexico is part of the Basic National Medication Supply (Cuadro Básico y Catálogo de Medicamentos) from the Health Sector in Mexico.12 At present, HAV vaccination in Mexico has been implemented only in some programmes targeting children attending childcare units. The Mexican government is considering implementation of a program of universal infant HAV vaccination. Information on the potential economic impact of universal HAV vaccination will be needed to help healthcare decision-makers selecting the optimal HAV vaccination strategy in Mexico.
The objective of this study was to conduct an economic assessment of routine infant HAV vaccination in Mexico, estimating the cost-utility of HAV vaccination using a single dose or 2 doses compared with no vaccination.
Results
HAV cases and costs
Table 1 shows the estimated numbers of anicteric HAV infections, unreported icteric HAV infections and reported icteric HAV infections with various types of medical care projected with each of the 3 vaccination strategies. Compared with no vaccination, the single-dose HAV vaccination strategy would be expected to reduce the number of anicteric HAV infections by 67% and the number of icteric HAV infections (reported or unreported) by 36%. The two-dose HAV vaccination strategy would be expected to reduce the number of anicteric HAV infections by 72% and the number of icteric HAV infections (reported or unreported) by 55%. The projected reduction in total HAV infections was 57% with single-dose HAV vaccination and 67% with 2-dose HAV vaccination.
Table 1.
Projected HAV cases by presence of symptoms and resource use, and projected costs, for each vaccination strategy
| Projected HAV cases/costs | No vaccination | Single dose | Two doses |
|---|---|---|---|
| Total HAV infections | 46,841,960 | 20,188,993 | 15,494,178 |
| Anicteric infections | 31,664,073 | 10,456,707 | 8,723,659 |
| Asymptomatic | 15,832,057 | 5,228,353 | 4,361,830 |
| Symptomatic | 15,832,057 | 5,228,353 | 4,361,830 |
| Without medical resource use | 7,916,018 | 2,614,177 | 2,180,915 |
| With medical resource use | 7,916,018 | 2,614,177 | 2,180,915 |
| Icteric infections | 15,177,887 | 9,732,286 | 6,770,519 |
| Not reported | 14,154,963 | 9,076,373 | 6,314,215 |
| Without medical resource use | 758,894 | 486,614 | 338,526 |
| With medical resource use | 13,396,069 | 8,589,758 | 5,975,689 |
| Reported | 1,022,924 | 655,914 | 456,304 |
| Outpatient care only | 963,667 | 612,780 | 425,919 |
| Hospitalisation | 53,153 | 38,780 | 26,966 |
| Fulminant hepatitis | 6,103 | 4,354 | 3,419 |
| Alive after liver transplant | 275 | 201 | 158 |
| Alive without liver transplant | 2,119 | 1,506 | 1,181 |
| Death | 3,710 | 2,647 | 2,080 |
| Costs, base case (MXN) | |||
| Medical treatment costs | 12,556,191,424 | 7,855,880,009 | 6,275,142,125 |
| Vaccination costs | Not applicable | 5,098,632,701 | 9,432,470,554 |
| Total | 12,556,191,424 | 12,954,512,710 | 15,707,612,679 |
| Costs, societal perspective (MXN) | |||
| Medical treatment costs | 12,556,191,424 | 7,855,880,009 | 6,275,142,125 |
| Indirect costs | 4,178,903,736 | 2,185,832,885 | 1,817,758,851 |
| Vaccination costs | Not applicable | 5,098,632,701 | 9,432,470,554 |
| Total | 16,735,095,160 | 15,140,345,595 | 17,525,371,530 |
HAV, hepatitis A virus; MXN, Mexican peso. Data on costs are discounted.
Table 1 also presents the projected costs for each strategy, from the perspective of the Mexican health authority (base case) and the societal perspective. Projected vaccination costs were higher for the 2-dose strategy than the single-dose strategy, as would be expected. Both vaccination strategies would be expected to reduce medical treatment costs and indirect costs, compared with no vaccination, and these reductions would be expected to be larger for the 2-dose strategy than for the single-dose strategy. These projected reductions in medical and indirect costs would partially offset the costs of vaccination.
Cost-utility analyses
Table 2 shows the results of the cost-utility analyses from the perspective of the Mexican health authority. Both vaccination strategies would be expected to have higher costs and higher health benefits (larger number of QALYs), compared with no vaccination. The single-dose HAV vaccination strategy had an incremental cost-utility ratio (ICUR) of MXN 2,270 per quality-adjusted life-year (QALY) gained, compared with no vaccination.
Table 2.
Cost-utility of HAV vaccination strategies from Mexican government perspective, base case
| Parameter | No vaccination | Single dose | Two doses |
|---|---|---|---|
| Cost (MXN) | 12,556,191,424 | 12,954,512,710 | 15,707,612,679 |
| QALYs lost | 374,689 | 199,210 | 164,040 |
| Cost-utility | Single dose versus no vaccination | Two doses vs. no vaccination | Two doses versus single dose |
| Incremental cost (MXN) | 398,321,286 | 3,151,421,255 | 2,753,099,969 |
| QALYs gained | 175,479 | 210,649 | 35,170 |
| ICUR (MXN/QALY gained) | 2,270 | 14,961 | 78,280 |
HAV, Hepatitis A virus; ICUR, incremental cost-utility ratio; MXN, Mexican peso; QALY, quality-adjusted life-year.
The two-dose HAV vaccination strategy had an ICUR of MXN 14,961 per QALY gained, compared with no vaccination. Compared with the single-dose strategy, the 2-dose vaccination strategy was more costly but more effective, with an ICUR of MXN 78,280 per QALY gained (Table 2).
Results from the societal perspective are shown in Table 3. When indirect costs were included, the single-dose HAV vaccination strategy was cost-saving and had higher health benefits (more QALYs gained) compared with no vaccination.
Table 3.
Cost-utility of HAV vaccination strategies from societal perspective
| Parameter | No vaccination | Single dose | Two doses |
|---|---|---|---|
| Cost (MXN) | 16,735,095,160 | 15,140,345,595 | 17,525,371,530 |
| QALYs lost | 374,689 | 199,210 | 164,040 |
| Cost-utility | Single dose vs. no vaccination | Two doses versus no vaccination | Two doses vs. single dose |
| Incremental cost (MXN) | −1,594,749,565 | 790,276,370 | 2,385,025,934 |
| QALYs gained | 175,479 | 210,649 | 35,170 |
| ICUR (MXN/QALY gained) | Dominant | 3,752 | 67,814 |
HAV, Hepatitis A virus; ICUR, incremental cost-utility ratio; MXN, Mexican peso; QALY, quality-adjusted life-year.
Sensitivity analysis
Table 4 summarizes the results of the one-way deterministic sensitivity analysis assessing the impact of transmission model parameters using a range of scenarios. The proportion of the mean rate of transmission caused by direct person-to-person transmission had a limited impact on the calculated ICUR, especially for the comparisons between either vaccination strategy or no vaccination. The single-dose strategy was dominant (cost-saving and higher benefits, i.e. more QALYs gained) when a different age-group split for the contact pattern in individuals aged less than 5 years was considered and when the annual waning rate of vaccine efficacy after one dose was assumed to be ≤1% from year 11 of the analysis onwards (Scenarios 0, 2 and 3), keeping the remaining parameters constant. The effect of the assumptions related to vaccine coverage for first and second doses was limited.
Table 4.
Results of the one-way deterministic sensitivity analysis on a range of scenarios for transmission model parameters
| ICUR (MXN/QALY gained) |
|||
|---|---|---|---|
| Parameter/Scenario | Single dose versus no vaccination | Two doses versus no vaccination | Two doses versus single dose |
| Base casea | 2,270 | 14,961 | 78,280 |
| Rate of transmission | |||
| 70% | 1,218 | 19,112 | 150,122 |
| 80% | 1,707 | 16,109 | 97,089 |
| 90% | 2,818 | 13,891 | 63,721 |
| 100% | 2,195 | 10,501 | 43,416 |
| First two age group split | |||
| 0–<3 and 3–<5 years | Dominant | 10,971 | 77,581 |
| Waning of vaccine protectionb | |||
| Scenario 0 | Dominant | 13,261 | 1,244,106 |
| Scenario 2 | Dominant | 14,615 | 142,740 |
| Scenario 3 | Dominant | 14,680 | 126,767 |
| Scenario 4 | 617 | 14,806 | 101,318 |
| Scenario 5 | 2,270 | 14,962 | 78,298 |
| Scenario 6 | 191 | 14,765 | 109,046 |
| First-dose coverage rate | |||
| 70% | 3,135 | 14,845 | 68,973 |
| 90% | 1,506 | 16,483 | 109,075 |
| Second-dose coverage ratec | |||
| 70% | 2,270 | 12,672 | 73,025 |
| 100% | 2,270 | 17,398 | 85,031 |
In the base case it was assumed that direct person-to-person transmission accounts for 85% of the mean rate of transmission. The first 2 age groups split for the contact pattern were 0–<1 and 1–<5 years. Annual waning rates of 1.62% for the first 10 years and 2.67% thereafter were considered for the single-dose HAV vaccination strategy. For the 2-dose HAV vaccination strategy the waning rate was 0.12% per year for the first 25 years.
See the description of each scenario in Table 6.
Of those receiving the first dose. HAV, hepatitis A virus; ICUR, incremental cost-utility ratio; MXN, Mexican peso; QALY, quality-adjusted life year.
Figure 1 shows tornado diagrams summarising the results of the univariate sensitivity analysis conducted for other parameters used in the decision model, for the single-dose HAV vaccination strategy compared with no vaccination (Fig. 1A), the 2-dose HAV vaccination strategy compared with no vaccination (Fig. 1B) and the 2-dose HAV vaccination strategy compared with the single-dose HAV vaccination strategy (Fig. 1C). The most influential variables included: the probability that an anicteric case is symptomatic; the disutility value for each non-fatal (either icteric or anicteric) symptomatic case; the annual discount rate for effectiveness; and the vaccine acquisition cost per dose.
Figure 1.
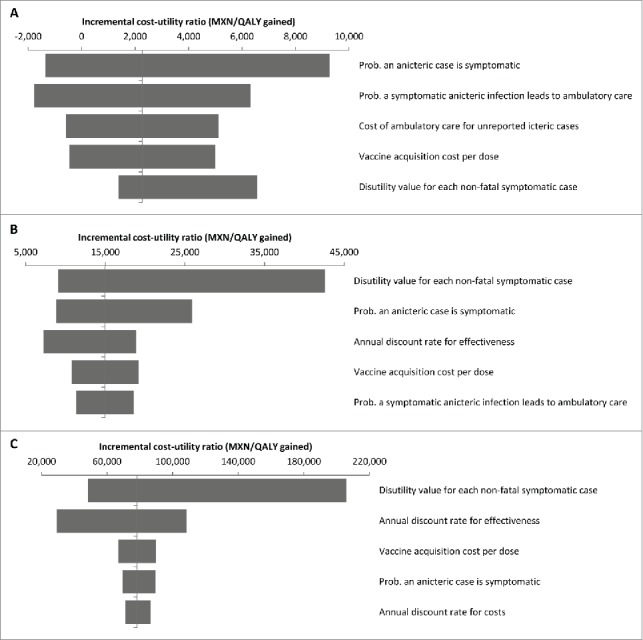
Tornado diagrams for the one-way deterministic sensitivity analysis for (A) single-dose HAV vaccination compared with no vaccination, (B) 2-dose HAV vaccination compared with no vaccination, and (C) 2-dose HAV vaccination compared with single-dose HAV vaccination. MXN, Mexican peso; Prob., probability; QALY, quality-adjusted life-year.
Figure 2 displays the acceptability curves derived from the probabilistic sensitivity analysis. The strategy of no vaccination was the least costly in around 80% of simulations. The strategy of single-dose HAV vaccination was the preferred option if the willingness to pay for an additional QALY gained was between MXN 15,000 and MXN 70,000. For a willingness to pay of MXN 70,000 or more, the 2-dose HAV vaccination strategy became the preferred option. For a threshold value set at the gross domestic product (GDP) per capita in Mexico in 2012 (MXN 132,465),13 the probability that the 2-dose HAV vaccination scheme was the preferred option was 88%, whereas the corresponding value for the single-dose HAV vaccination strategy was only 12%.
Figure 2.
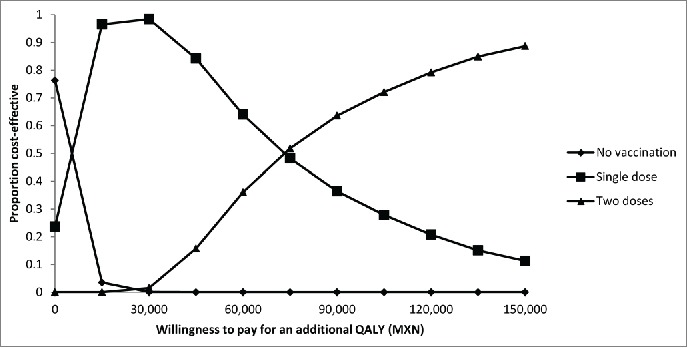
Cost-acceptability curves from the probabilistic sensitivity analysis. Costs expressed in Mexican pesos. MXN, Mexican peso; QALY, quality-adjusted life-year.
Discussion
In this analysis we have estimated the potential costs and health outcomes that would be expected for introduction of routine HAV vaccination in infants in Mexico using either a single-dose or a 2-dose vaccination strategy. To our knowledge, this is the first economic study assessing the potential impact of routine HAV vaccination in Mexican infants using projections of the number of icteric and anicteric HAV cases over time from a dynamic transmission model calibrated to epidemiological data in Mexico. HAV vaccination is not a part of Universal Mass Vaccination (UMV) in Mexico and hence there is no official registry for coverage. Therefore, in the absence of UMV in Mexico, we assumed no vaccination as baseline to calibrate the model to the epidemiological data. An earlier publication estimated the potential health benefits of routine infant HAV vaccination in Mexico using the same dynamic model.11 The present study builds on the earlier analysis by extending it to estimate the cost-utility of routine infant HAV vaccination.
The results presented here indicate that routine infant HAV vaccination would be expected to reduce the number of anicteric HAV cases by 67% for a single-dose vaccination strategy and 72% for a 2-dose vaccination strategy over 25 years, compared with no vaccination. The corresponding values for icteric HAV infections were 36% and 55%, respectively. The projected reduction in total HAV infections was 57% with single-dose HAV vaccination and 67% with 2-dose HAV vaccination. This is broadly consistent with the results of the earlier publication, as would be expected since the present analysis used the same model. The previous publication estimated a reduction in symptomatic HAV infections of 45% to 51% across the 4 different transmission scenarios considered with 70% coverage, and a reduction of 61% to 67% across the 4 different transmission scenarios considered with 90% coverage for a 2-dose strategy.11
The WHO recommends routine HAV vaccination for children aged ≥1 year, if indicated on the basis of acute HAV incidence, shift in endemicity from high to intermediate, and consideration of cost-effectiveness.1 Single-dose inactivated hepatitis A vaccines may be considered, as this schedule appears to have comparable effectiveness, lower costs and easier implementation, compared with the classical 2-dose schedule.1 The present results indicate that HAV vaccination with either a single-dose or a 2-dose strategy would increase both costs and health benefits (i.e., more QALYs gained) compared with no vaccination in Mexico. The estimated ICUR was MXN 2,270/QALY for the single-dose strategy and MXN 14,961/QALY for the 2-dose strategy, both well below the threshold of 1 x GDP per capita for Mexico (MXN 132,465 in 2012).13
Our results are consistent with previously published evaluations of HAV vaccination. In the US, the cost-utility of HAV vaccination using a 2-dose schedule has been estimated at US$9,100/QALY14 or US$40,000/QALY15 from the health system perspective. Both these estimates are much higher than our estimated ICUR of MXN 14,961/QALY for the 2-dose strategy (approximately US$3,850/QALY at an exchange rate of US$1=MXN 13.0112), reflecting the different socioeconomic and epidemiologic circumstances of Mexico and the US.
Studies in Latin America have also reported favorable cost-effectiveness and cost-utility for 2-dose HAV vaccination. In Chile, the cost-utility ratio of HAV vaccination from the health system perspective was estimated at US$281–503/QALY,16 well below per-capita Chilean GDP. An analysis from the societal perspective estimated the incremental cost-effectiveness ratio (ICER) at US$4,984/life-year gained in Chile,17 slightly higher than 1 x Chilean GDP per capita. Cost-benefit studies from the health system perspective have reported that 2-dose HAV vaccination is associated with a benefit-cost ratio of 2.26:1 in the Parana region of Brazil,18 indicating that HAV vaccination would be a cost-saving intervention.
Argentina has implemented a single-dose HAV vaccination schedule.10 Economic evaluation of this vaccination strategy indicated that the single-dose HAV vaccination strategy was dominant (lower costs and better health outcomes) from the societal perspective, compared with no vaccination.19 Addition of a second vaccine dose would be expected to increase both the costs and the health benefits (measured in QALYs), with an ICUR of US$551/QALY,19 and addition of a second dose was recommended by the study authors. These results are consistent with the pattern shown by our analysis, with the addition of a second vaccine dose expected to increase both costs and QALYs at an estimated ICUR of MXN 78,280 per QALY from the perspective of the Mexican health authority and MXN 67,814 per QALY from the societal perspective, below the threshold of 1 x Mexican GDP per capita. Adult inactivated hepatitis A (Havrix™) vaccination using a 2-dose strategy could offer long-term, possibly life-long, protection, with HAV antibody persistence reported for 15, 17 and 20 years after vaccination and model-based predictions estimating that seropositive anti-HAV levels would persist in ≥90% after 40 years.20-22 However, decisions on vaccine programmes must take into account other factors as well as the cost-effectiveness or cost-utility ratios of alternative interventions, such as restrictions on the available budget and implementability of the program.
A strength of the present study is that the number of icteric and anicteric HAV cases were based on the projections from a published dynamic transmission model, calibrated using Mexican data, which allows the analysis to account for the demographic and epidemiological population dynamics of HAV with and without vaccination.11 Another strength is the use of official data for study parameters where available. The base case coverage for the first HAV vaccine dose used in the model (80%) was taken from the reported coverage of the first dose of the measles, mumps and rubella (MMR) vaccine administered at the same age,23 and the vaccine acquisition cost and unit treatment costs were taken from official sources, as described in the Methods section. A further strength of the model is that its estimated frequency of reported symptomatic HAV infections for the period 1998–2013 is similar to that published by the Mexican authorities for the same period.24
Further, as per study of Rein et al.,15 our assumption that that 95% of the icteric cases (whether reported or not) received medical care seems reasonable since icteric cases show symptoms severe enough to seek medical treatment. This parameter was not tested in sensitivity analysis, but letting it change in typical range (90% to 100%) is expected to have a minimal effect in the ICUR. We have also assumed that that half of the anicteric cases are symptomatic and that half of them used medical resources. Since these parameters are subject to high uncertainty, we included both of them in the sensitivity analyses, allowing changing in a wide range (25% to 75%) (Fig. 1).
Our study has some limitations. Indirect costs such as lost productivity were not included in the base case analysis, and as shown by the additional analysis from the societal perspective this means that the base case under-estimated the potential benefits of HAV vaccination. We chose to include only direct medical costs in the base case because these are most relevant to public health authorities in Mexico. The expected ICUR for HAV vaccination was substantially more favorable if indirect costs were included in the analysis, and single-dose HAV vaccination became dominant over no vaccination (cost-saving and better health outcomes) when considered from the societal perspective. Outpatient care costs included only medical consultations, so other costs such as laboratory tests, imaging and medications were not included in the study. This would have under-estimated the total cost of medical treatment for HAV, and is another limitation of the study.
A further limitation is the lack of country-specific data for some parameters, such as the proportion of symptomatic HAV cases that would use some medical resource, the probability of fulminant hepatitis or the mortality rate from fulminant hepatitis. We took data for such parameters from published literature.15,25 Similarly, some cost data were taken from published literature in the absence of country-specific data, such as the cost of lifetime maintenance treatment after a liver transplant and the frequency of Family Medicine outpatient visits for symptomatic HAV cases. As the number of liver transplants performed in Mexico is very small, this parameter has little effect on the results. However, as most patients with symptomatic HAV receive only ambulatory care, the input data for this parameter may have an important effect on the results. Although the frequency of outpatient visits for anicteric symptomatic cases consuming health resources (one visit), icteric unreported cases (2 visits), and icteric reported cases (3 visits) were not gathered from local sources, these assumptions seem reasonable in the Mexican context and therefore were considered as transferable from the Rein et al. study.15 Estimates of the waning of the vaccine effect after 2 doses used in the dynamic model were informed by long-term follow-up data and a mathematical model.20,21 However, the rate of waning of vaccine efficacy after a single dose is not well known.11 Protective anti-HAV antibody levels can persist for almost 11 years after a single dose.26 Public health surveillance data from Argentina indicated that the introduction of single-dose HAV vaccination in 2005 resulted in a decrease of 88% in HAV incidence in 2006–2011, compared with 2000–2002, with no cases of fulminant hepatic failure or liver transplant due to HAV infection reported after March 2007.27 The waning scenario used in the base case corresponds to the most conservative scenario. The under-reporting factor was estimated at approximately 14 in Mexico,11 which is considerably higher than estimates for other countries such as Canada and the USA, where factors of 7.7 and 4.3, respectively, have been published.28,29 A higher under-reporting factor would be expected in Mexico, compared with the USA and Canada, as the USA and Canada have better surveillance systems than Mexico. Better data on these important issues of ambulatory care cost, vaccine waning and under-reporting would be valuable.
Another limitation is the lack of local data on health preferences. Studies reporting local preference values for health states are scarce in Latin America. To our knowledge, there are no published data regarding utility losses caused by non-fatal icteric or anicteric symptomatic HAV cases in Mexico. Applying a disutility value derived from a survey with Belgian population to those cases represents an important limitation, so the current estimates should be interpreted with some caution. However, mean utility losses may be not too different between Mexicans and Belgians because of the short duration of the symptoms. Other health outcomes (e.g., life years saved) markedly underestimate the real effect of vaccination given that only a small proportion of HAV infections are lethal. Background utilities for the healthy population were obtained from the study headed by Rein15 and correspond to data valid in the USA. This also adds a limitation, though this factor contributes less to the overall results as a consequence of the lower case-fatality rate of HAV.
In conclusion, the results of this analysis indicate that routine vaccination of toddlers against HAV would be cost-effective in Mexico using either a single-dose or a 2-dose vaccination strategy. The two-dose strategy was more costly but more effective than the single-dose strategy, with an estimated ICUR well below the threshold of 1 × Mexican GDP per capita. In the case of budgetary restrictions that preclude the adoption of a 2-dose strategy, the single-dose strategy could be a viable alternative. The economic analysis presented here should provide valuable information for public health authorities in Mexico making decisions with regard to a HAV vaccination program.
Methods
Design
The GSK study identifier for this analysis is HO-12-12877. This analysis compared the following 3 alternative interventions: no HAV vaccination in infants; routine HAV vaccination in infants with one dose at age 12 months; routine HAV vaccination in infants with 2 doses at age 12 months and 18 months, respectively.
The base case analysis was conducted from the perspective of the Mexican public health system (direct medical costs only) and considered the projected costs and outcomes in the total Mexican population of all ages. An additional analysis was conducted from the societal perspective, including indirect costs related to productivity losses due to absenteeism in adults or in parents with sick children. The time horizon of the study was 25 years, from 2012 to 2036, and both costs and benefits were discounted at an annual rate of 5% after the first year. All costs are expressed in 2012 MXN.
Model structure
A previously published dynamic model of HAV in Mexico, fully calibrated to epidemiological data in Mexico, was used to estimate epidemiological outcomes over time for each of the vaccination strategies considered.11 The dynamic model has been described elsewhere.11 Briefly, it is a deterministic, compartmental and age-stratified dynamic transmission model of HAV in Mexico that was developed and calibrated to country-specific demographic and epidemiological data. The original authors accounted for age-specific risk of HAV infection as a function of age-specific HAV prevalence and contact patterns between age groups, as well as the risk of HAV infection decreasing over recent decades due to improved hygiene and sanitation. The model allows projection of the impact of universal infant immunization with the 2-dose HAV vaccine at 12 and 18 months of age under different assumptions about duration of long-term vaccine protection and vaccination coverage after the first and second dose. The most plausible parameters and assumptions were selected for the base case analysis in the economic evaluation.
The effect of HAV vaccination in the dynamic model is an all-or-none effect on HAV infection in 97% of vaccinees after the first dose and 99% of vaccinees after the second dose.11,21,30-32 Vaccine protection was assumed to wane over time at a rate of 0.12% per year for the first 25 years and a rate of 0.62% per year thereafter in individuals who have received 2 doses, and at a rate of 1.62% per year for the first 10 years and 2.67% thereafter in individuals who have received one dose.11,19,20,21,33 For the base case, vaccine coverage was assumed to be 80% for the first dose,11 and in the 2-dose strategy it was assumed that 85% of those who received the first dose would also receive the second dose, as previously published.11
The economic analysis was conducted using a decision model, shown in Figure 3. The projected numbers of all HAV infections during the period 2012–2036 with each of the 3 alternative interventions considered (no vaccination, single-dose HAV vaccination or 2-dose HAV vaccination) were obtained from the dynamic model and entered into the decision model. HAV infections were grouped into icteric (symptoms with jaundice) and anicteric (symptoms without jaundice). Typical symptoms include dark urine, fatigue, itching, loss of appetite, low-grade fever, nausea and vomiting, and pale or clay-colored stools. The age-specific probability that a HAV infection becomes icteric is defined according to the equation published by Armstrong and Bell.29 It is important to make the distinction between icteric and anicteric infection because we assume that only icteric cases are reported, and thus the under-reporting factor applies only to those icteric infections.
Figure 3.
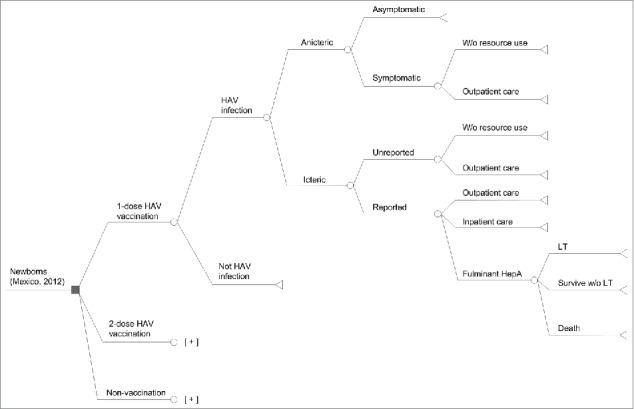
Decision model. HAV, hepatitis A virus; W/o: without; HepA: Hepatitis A; LT: Liver transplantation; [+] indicates that the structure for the single-dose schedule is repeated.
The model was developed in TreeAge Pro 2013 software
Icteric HAV infection may be reported to the health system or unreported. Unreported cases may require outpatient medical care, or no medical care. All reported cases were assumed to use medical resources. Reported cases may result in outpatient care, hospitalization or fulminant hepatitis. Fulminant hepatitis in turn may result in liver transplantation, survival without a liver transplant, or death.
Some anicteric cases may be symptomatic and would require outpatient care. Asymptomatic anicteric HAV infection was assumed to incur no medical costs and to have no impact on the patient's quality of life.
The base case assumed that 85% of the per-susceptible risk of HAV infection, e.g., of the rate of transmission resulted from direct person-to-person transmission (using the mean of the outcomes from the dynamic model with 80% and 90%11), and used the age stratification <1, 1–5, 6–11, 12–15, 16–19, 20–39 and 40+ years for contacts between individuals. The under-reporting factor for symptomatic cases was estimated from the dynamic model, with an estimated value of 14.8 (the mean of the estimates with 80% or 90% of the rate of transmission caused by direct person-to-person transmission).
The model parameters for transmission and the under-reporting factor of symptomatic icteric cases were estimated by simultaneously calibrating the model outcomes to epidemiological HAV data in Mexico (age-specific seroprevalence and incidence of reported symptomatic icteric HAV infections). Model calibration was performed by optimization, minimizing the weighted sum of squares of relative differences between model outcomes and observations.11
Input data: probabilities
The probability for a person infected with HAV to present with jaundice depends on the age. To estimate this probability, the formula proposed by Armstrong and Bell was used.29 The probability of jaundice due to an infection is as follows:
Probability of jaundice with HAV = 0.852 * (1-exp (−0.01244*age1.903))
exp = exponential; age = the age in years at half-year period (ie, 0.5, 1.5, 2.5 years, etc.).
For anicteric cases, it was assumed that 50% were symptomatic, and that 50% of symptomatic anicteric cases used medical resources (outpatient care by a general practitioner).15
The proportion of unreported icteric symptomatic cases that used medical resources was estimated by weighting the average use of resources between reported and unreported cases so that 95% of all icteric symptomatic cases used medical resources, as estimated in a previous publication.15 The probability that an icteric HAV infection is reported corresponds to the inverse value of the under-reporting factor.
The probability of hospitalization for an icteric symptomatic reported case was age-dependent, and was estimated from Mexican data on the number of hospitalizations due to HAV and the number of reported cases of HAV during the period 2004–2013.24,34 The probability of fulminant hepatitis was also age-dependent and was taken from a previous publication15 in the absence of data for Mexico. Remaining icteric symptomatic reported cases were assumed to require only ambulatory care.
The proportion of fulminant hepatitis cases receiving a liver transplant was estimated from data on the number of liver transplants performed in Mexico in 201335 and information on the age distribution and proportion of transplants attributable to HAV from Ellis et al.19 The probability of death from fulminant hepatitis in the population aged <60 years was 60%, based on a context of a shortage of liver transplantations,25 and 67% in people aged 60 years or more.15
Key probabilities are summarized in Table 5.
Table 5.
Key data input values
| Proportion of cases of HAV infection hospitalised in Mexico | ||
|---|---|---|
| Variable | Value | Source |
| Any age | 5.31% |
Calculated as number of HAV discharges reported34 divided by the frequency of HAV infections reported24 |
| <1 year | 12.16% | |
| 1–4 years | 2.91% | |
| 5–14 years | 4.04% | |
| 15–44 years | 10.87% | |
| 45–64 years | 12.79% | |
| ≥65 years | 17.66% | |
| Under-reporting factor for symptomatic cases | ||
| With 85% of the rate of transmission caused by direct person-to-person transmission | 14.83775a | 11 |
| Probability that a symptomatic and reported case results in fulminant hepatitis | ||
| 0–4 years | 0.38% | 15 |
| 5–14 years | 0.05% | |
| 15–39 years | 0.68% | |
| 40–59 years | 5.50% | |
| ≥60 years | 8.00% | |
| Waning vaccine efficacy for HAV vaccination, per year | ||
| 1st dose during first 10 years | 0.0162 | 11 |
| 1st dose after 10 years | 0.0267 | |
| 2nd dose during first 25 years | 0.0012 | |
| Direct costs (2012 values) | ||
| HAV infection hospitalization | MXN 43,509 | 38,39 |
| Fulminant hepatitis | MXN 76,872 | |
| Total direct medical cost per transplanted patientb | MXN 2,219,830 | |
| Mean cost for an anicteric symptomatic HAV case | MXN 482 | Equivalent to one Family Medicine outpatient visit. Applies only to those cases with resource use 15,38 |
| Mean cost for an icteric unreported HAV case | MXN 964 | Equivalent to 2 Family Medicine outpatient visits. Applies only to those cases with resource use 15,38 |
| Mean cost for an icteric reported HAV case | MXN 1,446 | Equivalent to 3 Family Medicine outpatient visits. Applies only to those cases with outpatient care 15,38 |
| Vaccine price (2012) | ||
| Price per dose for HAV vaccine | MXN 194.50 | 36 |
| Vaccine administration cost | ||
| Vaccine administration cost | MXN 13.17 | 37 US$1, converted to MXN using mean exchange rate during 2012.44 |
| Disutility due to HAV infection | ||
| Net disutility for each icteric or anicteric symptomatic HAV case | 0.019 | 42 |
| Utility value for each age group | ||
| 0–4 years | 0.94 | 15 |
| 5–17 years | 0.93 | |
| 18–34 years | 0.915 | |
| 35–44 years | 0.895 | |
| 45–64 years | 0.805 | |
| 65–74 years | 0.77 | |
| ≥75 years | 0.695 | |
Estimated from the dynamic model using the mean of the estimates with 80% and 90% of the rate of transmission caused by direct person-to-person transmission.
Total liver transplantation cost has 2 components, the cost of the liver transplantation procedure (MXN 1,082,844) plus the lifetime maintenance cost (MXN 1,136,986).19
HAV, hepatitis A virus; MXN, Mexican peso.
Input data: resource use and cost
The price of HAV vaccine was obtained from the Mexican Institute for Social Security (IMSS) for 2012,36 and the cost of administration was assumed to be the same as for pneumococcal vaccination, since both are given by intramuscular injection, at US$ 1 per dose37 (MXN 13.17, average exchange rate in 2012).
It was assumed that 5% of icteric symptomatic HAV cases would incur no medical cost, with 2 medical consultations in ambulatory care (Family Medicine) required for icteric symptomatic unreported cases and 3 for icteric symptomatic reported cases.15 Anicteric symptomatic cases were assumed to require one consultation in Family Medicine.15 The unit cost for a consultation at the Family Medicine Service was obtained from the IMSS official list of unit costs by level of care for 2012.38 Hospitalization costs for the 3 diagnosis-related groups (DRG) codes associated with HAV infection were obtained from IMSS,39 updated to 2012 values.38 The cost of a liver transplant (DRG 006) was obtained from IMSS,39 and the cost of lifetime maintenance treatment was 1.05 times the cost of the transplant.19 Key cost data are summarized in Table 5.
Input data: work absenteeism
An additional analysis was conducted from the societal perspective, including indirect costs associated with work absenteeism. It was assumed that HAV cases could result in work absenteeism in people aged 14 years or more who were economically active, and in parents who have to take time away from work to care for a child aged <14 years with HAV infection. Therefore, the indirect costs were calculated as the product of 3 factors: (i) the labor participation rate, (ii) the duration of work loss attributable to stages of HAV infection, and (iii) the average daily income.
For children aged 0 to 13 years, we applied a labor participation rate of 36.5%, which corresponds to the probability of a child having either both parents, or the unique parent in a monoparental home, working.40,41 The labor participation rates of subjects aged more than 14 years were obtained from the same sources and are as follows: 28%, 61.7%, 71.7%, 72.1%, 63.8%, and 33.6% for age-groups 14–19 years, 20–29 years, 30–39 years, 40–49 years, 50–59 years, and 60 years and older, respectively.
All anicteric symptomatic patients as well as those unreported icteric cases were assumed to incur 3 days of work absenteeism. For icteric patients, cases requiring outpatient care in children aged 0–12 years incurred 3.7 days of work absenteeism, and cases requiring outpatient care in people aged 13 years or more incurred 10 days of work absenteeism. Hospitalized cases without fulminant hepatitis, and cases of fatal or non-fatal fulminant hepatitis without liver transplantation, incurred 33.2 days of work absenteeism. Liver transplantation incurred 153.2 days of work absenteeism.15
The income lost due to a day of work absenteeism was MXN 190.36, calculated from the average income for the employed population in Mexico and average hours worked per day in 2012.41
Health outcomes
The model estimated the frequency of icteric and anicteric HAV cases, differentiated by report status (reported or unreported), symptom status (symptomatic or asymptomatic), medical resources used (ambulatory care, hospitalization with or without fulminant hepatitis), and fulminant hepatitis cases differentiated by outcome (death, liver transplant, survival without liver transplant).
In the absence of country-specific data regarding health-state preferences for HAV infection and its potential complications in Mexico, the number of QALYs lost was estimated using a disutility value of 0.019 for each non-fatal icteric or anicteric symptomatic HAV case,42 and applying a factor related to age for the life-years lost due to premature death caused by fulminant hepatitis.15 Asymptomatic anicteric cases were assumed to incur no QALY losses.
Cost-utility analysis
The ICUR was calculated as the incremental cost per incremental QALY gained for one intervention compared with another.
The threshold value for the ICUR was taken as 1 × GDP per capita in Mexico, as recommended by the Mexican General Health Council.43 According to data from the International Monetary Fund (IMF), this was MXN 132,465 in 2012.13
Sensitivity analysis
The robustness of the model results were tested using deterministic and probabilistic sensitivity analyses. First, a one-way deterministic sensitivity analysis assessed the impact of transmission model parameters using a range of scenarios. Specifically, 4 scenarios related to the percentage of the rate of transmission caused by direct person-to-person transmission (70%, 80%, 90%, and 100%), one alternative scenario for the age group split in children aged less than 5 years (0 to <3 years and 3–5 years), 6 scenarios based on varying profiles of waning vaccine protection (Table 6), 2 alternative scenarios for the first-dose vaccine coverage (70% and 90%), and 2 alternative scenarios for the percentage of subjects receiving the first dose who also received the second dose (70% and 100%).
Table 6.
Scenarios for loss of vaccine effect over time (waning) used in sensitivity analysis
| Scenario | Annual Waning Rate of HAV Vaccine Protection |
||
|---|---|---|---|
| After 1 single dose administered at 12 months of age |
After 2 doses administered at 12 and 18 months of age | ||
| First 10 years | After 10 years | First 25 years | |
| Scenario 0* | 0 | 0 | 0 |
| Scenario 1 (Base case) | 0.0162 | 0.0267 | 0.0012 |
| Scenario 2 | 0.0162 | 0.0062 | 0.0012 |
| Scenario 3 | 0.0162 | 0.0100 | 0.0012 |
| Scenario 4 | 0.0162 | 0.0175 | 0.0012 |
| Scenario 5 | 0.0162 | 0.0267 | 0.0012 |
| Scenario 6 | 0.0162 | 0.0150 | 0.0012 |
No waning.
HAV, hepatitis A virus.
A series of tornado diagrams summarized the univariate sensitivity analysis conducted for the rest of the parameters used in the decision model. In this case, the vaccine price was varied by ±10% from the base-case value, meanwhile other costs were assumed to vary by ± 20%. Disutility was varied from 0.006 to 0.032, and the annual discount rates from 3% to 7% for costs and 0% to 7% for QALYs. The probabilities were varied by ± 25% (in relative terms). The exceptions were the probability that an anicteric case is symptomatic and the probability that a symptomatic anicteric infection leads to (ambulatory) care, with both of them varied in a wider range (0.25 to 0.75).
A further analysis was also conducted from the societal perspective.
The probabilistic sensitivity analysis performed 1,000 second-order Monte Carlo simulations to produce cost-utility acceptability curves. The vaccine acquisition and administration costs and disutility were varied using a uniform distribution. Medical costs were modeled using an approximate gamma distribution assuming a standard deviation of 10%. For discount rates, a triangular distribution was used. Probabilities were varied using an approximate β distribution, assuming a standard deviation of 12.5% or 25%. The annual frequency of liver transplantations attributable to HAV was modeled using a Poisson distribution.
Disclosure of Potential Conflicts of Interest
JAG and LRM are employees of the GSK group of companies and own restricted shares in the GSK group of companies. FCR (or his institution) received funding from GSK México, S.A. de C.V. to conduct the study. He and his institution receive funding to develop pharmacoeconomic models and adapt cost-effectiveness models for vaccines for GSK México S.A. de C.V., Novartis Farmacéutica S.A. de C.V., Merck Sharp & Dohme Comercializadora S. de R.L. de C.V and PFIZER, S.A. de C.V. PA was an employee of the GSK group of companies at the time of the study.
Acknowledgments
The authors would like to thank Thierry Van Effelterre for critical review, Desmond Curran and Rodrigo DeAntonio for critical review of the study report and the manuscript, and María Yolanda Cervantes for critical review. Medical writing services were provided by Carole Nadin on behalf of GSK Vaccines, and publication co-ordination was provided by Ingrid Leal (GSK Vaccines), Vinicius Costa (GSK Vaccines) and Uta Gomes (on behalf of GSK Vaccines).
Authors' Contributions
FCR provided substantial scientific input, method selection and development, development of the economic modeling, data mining and literature review, model input, assessment of robustness of results and substantial scientific input to the study report. JAG provided substantial scientific input, data mining and literature review, populating the model and determination of model settings, acquisition, model input, substantial scientific input to the study report, data verification and accuracy and supervision of the study/ research group. PA provided substantial scientific input, method selection and development, populating the model and determination of model settings, model input, statistical support for analysis and reporting of data, assessment of robustness of results, critical review of the study report, acquisition of funding, data verification and accuracy, and administrative support. LRM provided substantial scientific input, development of the questionnaire survey, data acquisition and critical review of the study report. All authors provided intellectual input into the manuscript and approved the final version. All authors read and approved the final manuscript.
Trademark Statement
Havrix™ is a trademark of the GSK group of companies
Funding
The study was funded by one or more of the companies within the GSK group of companies. The sponsor was involved in all stages of the study, conduct and analysis. GlaxoSmithKline Biologicals SA also paid all costs associated with the development and the publication of the present manuscript.
References
- 1.WHO position paper on hepatitis A vaccines - June 2012. Wkly Epidemiol Rec 2012; 87:261-76; PMID:22905367 [PubMed] [Google Scholar]
- 2.Brundage SC, Fitzpatrick AN. Hepatitis A. Am Fam Physician 2006; 73:2162-8; PMID:16848078 [PubMed] [Google Scholar]
- 3.Jacobsen KH, Wiersma ST. Hepatitis A virus seroprevalence by age and world region, 1990 and 2005. Vaccine 2010; 28:6653-7; PMID:20723630; http://dx.doi.org/ 10.1016/j.vaccine.2010.08.037 [DOI] [PubMed] [Google Scholar]
- 4.Tapia-Conyer R, Santos JI, Cavalcanti AM, Urdaneta E, Rivera L, Manterola A, Potin M, Ruttiman R, Tanaka KJ. Hepatitis A in Latin America: a changing epidemiologic pattern. Am J Trop Med Hyg 1999; 61:825-9; PMID:10586919 [DOI] [PubMed] [Google Scholar]
- 5.Valdespino JL, Ruiz-Gómez J, Olaiz-Fernández G, Arias-Toledo E, Conde-González CJ, Palma O, Sepúlveda J. Seroepidemiology of hepatitis A in Mexico. A detector of social inequity and monitor of immunization policies. Salud Publica Mex 2007; 49:S377-85; http://dx.doi.org/ 10.1590/S0036-36342007000900009 [DOI] [Google Scholar]
- 6.Rivas-Oropeza I, Valencia-Mendoza A, Sánchez-González G. Carga financiera por hepatitis A en México. Value Health 2009; 12:A516; http://dx.doi.org/ 10.1016/S1098-3015(10)75446-2 [DOI] [Google Scholar]
- 7.Daniels D, Grytdal S, Wasley A. Surveillance for acute viral hepatitis - United States, 2007. MMWR Surveill Summ 2009; 58:1-27; PMID:19478727 [PubMed] [Google Scholar]
- 8.Dagan R, Leventhal A, Anis E, Slater P, Ashur Y, Shouval D. Incidence of hepatitis A in Israel following universal immunization of toddlers. JAMA 2005; 294:202-10; PMID:16014594; http://dx.doi.org/ 10.1001/jama.294.2.202 [DOI] [PubMed] [Google Scholar]
- 9.Chodick G, Heymann AD, Ashkenazi S, Kokia E, Shalev V. Long-term trends in hepatitis A incidence following the inclusion of Hepatitis A vaccine in the routine nationwide immunization program. J Viral Hepat 2008; 15 Suppl 2:62-5; PMID:18837837; http://dx.doi.org/ 10.1111/j.1365-2893.2008.01032.x [DOI] [PubMed] [Google Scholar]
- 10.Vacchino MN. Incidence of hepatitis A in Argentina after vaccination. J Viral Hepat 2008; 15(Suppl 2):47-50; PMID:18837834; http://dx.doi.org/ 10.1111/j.1365-2893.2008.01029.x [DOI] [PubMed] [Google Scholar]
- 11.Van Effelterre T, De Antonio-Suarez R, Cassidy A, Romano-Mazzotti L, Marano C. Model-based projections of the population-level impact of hepatitis A vaccination in Mexico. Hum Vaccin Immunother 2012; 8:1099-108; PMID:22854667; http://dx.doi.org/ 10.4161/hv.20549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Consejo de Salubridad General Cuadro Básico y Catálogo de Medicamentos del Sector Salud en México, Edición 2013. 2013. [cited 2014October17]. Available from: http://www.csg.gob.mx/contenidos/CB2013/medicamentos/med2013 [Google Scholar]
- 13.International Monetary Fund World Economic Outlook Database, October 2013. 2013. Available from: http://www.imf.org/external/pubs/ft/weo/2013/02/weodata/weorept.aspx?sy=2011&ey=2013&scsm=1&ssd=1&sort=country&ds=.&br=1&c=273&s=NGDPPC&grp=0&a=&pr.x=40&pr.y=15 [Google Scholar]
- 14.Jacobs RJ, Greenberg DP, Koff RS, Saab S, Meyerhoff AS. Regional variation in the cost effectiveness of childhood hepatitis A immunization. Pediatr Infect Dis J 2003; 22:904-14; PMID:14551492; http://dx.doi.org/ 10.1097/01.inf.0000091295.53969.6a [DOI] [PubMed] [Google Scholar]
- 15.Rein DB, Hicks KA, Wirth KE, Billah K, Finelli L, Fiore AE, Hoerger TJ, Bell BP, Armstrong GL. Cost-effectiveness of routine childhood vaccination for hepatitis A in the United States. Pediatrics 2007; 119:e12-e21; PMID:17200237; http://dx.doi.org/ 10.1542/peds.2006-1573 [DOI] [PubMed] [Google Scholar]
- 16.Valenzuela MT, Jacobs RJ, Arteaga O, Navarrete MS, Meyerhoff AS, Innis BL. Cost-effectiveness of universal childhood hepatitis A vaccination in Chile. Vaccine 2005; 23:4110-9; PMID:15964479; http://dx.doi.org/ 10.1016/j.vaccine.2005.03.021 [DOI] [PubMed] [Google Scholar]
- 17.Quezada A, Baron-Papillon F, Coudeville L, Maggi L. Universal vaccination of children against hepatitis A in Chile: a cost-effectiveness study. Rev Panam Salud Publica 2008; 23:303-12; PMID:18510790; http://dx.doi.org/ 10.1590/S1020-49892008000500002 [DOI] [PubMed] [Google Scholar]
- 18.Zahdi MR, Maluf I Jr, Maluf EM. Hepatitis A: the costs and benefits of the disease prevention by vaccine, Parana, Brazil. Braz J Infect Dis 2009; 13:257-61; PMID:20231986; http://dx.doi.org/ 10.1590/S1413-86702009000400003 [DOI] [PubMed] [Google Scholar]
- 19.Ellis A, Ruttimann RW, Jacobs RJ, Meyerhoff AS, Innis BL. Cost-effectiveness of childhood hepatitis A vaccination in Argentina: a second dose is warranted. Rev Panam Salud Publica 2007; 21:345-56; PMID:17761046; http://dx.doi.org/ 10.1590/S1020-49892007000500002 [DOI] [PubMed] [Google Scholar]
- 20.Van Herck K, Jacquet JM, Van Damme P. Antibody persistence and immune memory in healthy adults following vaccination with a two-dose inactivated hepatitis A vaccine: long-term follow-up at 15 years. J Med Virol 2011; 83:1885-91; PMID:21915861; http://dx.doi.org/ 10.1002/jmv.22200 [DOI] [PubMed] [Google Scholar]
- 21.Van Herck K. Crasta PD, Messier M, Hardt K, Van Damme P. Seventeen-year antibody persistence in adults primed with two doses of an inactivated hepatitis A vaccine. Hum Vaccin Immunother 2012; 8:323-7; PMID:22327499; http://dx.doi.org/ 10.4161/hv.18617 [DOI] [PubMed] [Google Scholar]
- 22.Van Damme P, Theeten H, Van Der Meeren O, Crasta P, Van Herck K. Does hepatitis A vaccine confer life-long protection? 5th Northern European Conference on Travel Medicine, Bergen, 5–8 June 2014 [Google Scholar]
- 23.SINAVE/DGE/SALUD Perfil epidemiológico de la Infancia en México 2010. Junio; 2011. [Google Scholar]
- 24.Sistema Único de Información para la Vigilancia Epidemiológica (SUIVE)/Dirección General de Epidemiología/Secretaría de Salud Anuarios de morbilidad, 1998-2013. 2014. [cited 2014October25]. Available from: http://www.dgepi.salud.gob.mx/anuario/html/anuarios.html [Google Scholar]
- 25.Ciocca M. Clinical course and consequences of hepatitis A infection. Vaccine 2000; 18(Suppl 1):S71-4; PMID:10683554; http://dx.doi.org/ 10.1016/S0264-410X(99)00470-3 [DOI] [PubMed] [Google Scholar]
- 26.Ott JJ, Wiersma ST. Single-dose administration of inactivated hepatitis A vaccination in the context of hepatitis A vaccine recommendations. Int J Infect Dis 2013; 17:e939-44; PMID:23791857; http://dx.doi.org/ 10.1016/j.ijid.2013.04.012 [DOI] [PubMed] [Google Scholar]
- 27.Vizzotti C, Gonzalez J, Gentile A, Rearte A, Ramonet M, Canero-Velasco MC, Perez Carrega ME, Uruena A, Diosque M. Impact of the single-dose immunization strategy against hepatitis A in Argentina. Pediatr Infect Dis J 2014; 33:84-8; PMID:24352191; http://dx.doi.org/ 10.1097/INF.0000000000000042 [DOI] [PubMed] [Google Scholar]
- 28.Pham B, Chen MH, Tricco AM, Anonychuk A, Krahn M, Bauch CT. Use of a catalytic model to estimate hepatitis A incidence in a low-endemicity country: implications for modeling immunization policies. Med Decis Making 2012; 32:167-75; PMID:21393559; http://dx.doi.org/ 10.1177/0272989X11398489 [DOI] [PubMed] [Google Scholar]
- 29.Armstrong GL, Bell BP. Hepatitis A virus infections in the United States: model-based estimates and implications for childhood immunization. Pediatrics 2002; 109:839-45; PMID:11986444; http://dx.doi.org/ 10.1542/peds.109.5.839 [DOI] [PubMed] [Google Scholar]
- 30.Abarca K, Ibanez I, Perret C, Vial P, Zinsou JA. Immunogenicity, safety, and interchangeability of two inactivated hepatitis A vaccines in Chilean children. Int J Infect Dis 2008; 12:270-7; PMID:17988917; http://dx.doi.org/ 10.1016/j.ijid.2007.08.006 [DOI] [PubMed] [Google Scholar]
- 31.Noskova AV, Gorbunov MA, Pavlova LI, El'shina GA, Sharipova IS, Karpovich LG, Kalashnikova TI, Bektimirov TA, Rikhmer VV, El'nikov VS. Comparative study of the reactivity, safety and immunogenicity of “Havrix” inactivated hepatitis A vaccine. Vopr Virusol 2000; 45:42-4; PMID:11200647 [PubMed] [Google Scholar]
- 32.Poovorawan Y, Kosuwon P, Sutra S, Theamboonlers A, Vimolket T, Safary A. Comparison of the reactogenicity and immunogenicity of two different dose levels of hepatitis A vaccine in healthy children and adolescents. Asian Pac J Allergy Immunol 1998; 16:111-7; PMID:9876949 [PubMed] [Google Scholar]
- 33.Summary of Product Characteristics Havrix Junior Monodose vaccine. 2011. August 22 [cited 2011November3]. Available from: http://www.medicines.org.uk/emc/medicine/2040/SPC/ [Google Scholar]
- 34.Dirección General de Información en Salud (DGIS ). Base de datos de egresos hospitalarios por morbilidad en Instituciones Públicas, 2004-2013. 2014. [cited 2014October25]. Available from: http://www.sinais.salud.gob.mx [Google Scholar]
- 35.Centro Nacional de Trasplantes Estado actual de donación y trasplantes en México. Reporte anual 2013; 2013 [Google Scholar]
- 36.Portal de compras del Instituto Mexicano del Seguro Social (IMSS ). 2013. [cited 2013December20]. Available from: http://compras.imss.gob.mx/ [Google Scholar]
- 37.Muciño-Ortega E, Mould-Quevedo JF, Farkouh R, Strutton D. Economic evaluation of an infant immunization program in Mexico, based on 13-valent pneumococcal conjugated vaccines. Value Health 2011; 14:S65-S70; PMID:21839902; http://dx.doi.org/ 10.1016/j.jval.2011.05.025 [DOI] [PubMed] [Google Scholar]
- 38.Instituto Mexicano del Seguro Social Costos unitarios por nivel de atención médica para el año 2012. Diario Oficial de la Federación, Miércoles 9 de mayo de 2012; 2012 [Google Scholar]
- 39.Instituto Mexicano del Seguro Social Grupos Relacionados con el Diagnóstico 2013. [cited 2013December20]. Available from: http://www.imss.gob.mx/profesionales/Documents/GRD_IMSS.pdf [Google Scholar]
- 40.Instituto Nacional de Estadística y Geografía Mujeres y Hombres en México 2011. Instituto Nacional de las Mujeres. México: INEGI; 2012; xxxii, 218 p.:il [Google Scholar]
- 41.Instituto Nacional de Estadística y Geografía Encuesta Nacional de Ocupación y Empleo. Consulta interactiva de datos para los cuatro trimestres del año 2012. 2012. Available from: http://www.inegi.org.mx/sistemas/olap/proyectos/bd/consulta.asp?p=27608&c=27221&s=est&cl=4# [Google Scholar]
- 42.Luyten J, Marais C, Hens N, De Schrijver K, Beutels P. Imputing QALYs from single time point health state descriptions on the EQ-5D and the SF-6D: a comparison of methods for hepatitis a patients. Value Health 2011; 14:282-90; PMID:21296602; http://dx.doi.org/ 10.1016/j.jval.2010.10.004 [DOI] [PubMed] [Google Scholar]
- 43.Consejo de Salubridad General Guía de Evaluación de Insumos para la Salud. Edición 2011. Mexico: 2011 [Google Scholar]
- 44.Banco de México. Tipos de Cambio Peso/Dólar durante el Año 2012. 2014. [cited 2014December20]. Available from: http://www.banxico.org.mx/portal-mercado-cambiario/index.html [Google Scholar]


