Abstract
Purpose
Survivors of childhood acute lymphoblastic leukemia (ALL) are at risk for low bone mineral density (BMD) and frail health, outcomes potentially modifiable by altering health behaviors and/or treating endocrine abnormalities. We evaluated associations between lifestyle and hormonal deficits with risk of low BMD and frailty among survivors of ALL.
Patients and Methods
Participants included 862 survivors of ALL (median age, 31.3 years [range, 18.4 to 59.7 years]) enrolled in the St Jude Lifetime Cohort study. Bone density was measured using quantitative computed tomography of L1 through L2 vertebrae; low BMD was defined as an age- and sex-standardized z score < −1. The presence of frailty or prefrailty was defined as having at least two of the following: low muscle mass, self-reported exhaustion, low energy expenditure, slow walking speed, and weakness. Hormonal deficiencies were determined according to medical history, medications, and laboratory findings (insulin-like growth factor 1, follicle-stimulating hormone, luteinizing hormone, and testosterone levels). Logistic regression was used to examine associations between lifestyle (smoking, alcohol consumption, and activity levels) and deficiencies in growth hormone (GHD) and/or sex steroids with low BMD and frailty.
Results
Thirty percent of survivors met criteria for low BMD, and 18.6% for frailty/prefrailty. After adjusting for body mass index, low BMD was associated with GHD (odds ratio [OR], 1.59; 95% CI, 1.02 to 2.13) and current smoking (OR, 1.71; 95% CI, 1.02 to 2.85) among men; and GHD (OR, 2.18; 95% CI, 1.26 to 3.78) and moderate alcohol consumption (OR, 2.09; 95% CI, 1.14 to 3.83) among women. After adjusting for current age, the odds of frailty/prefrailty were increased among men with GHD (OR, 2.97; 95% CI, 1.56 to 5.67) and those who smoked (OR, 3.26; 95% CI, 1.65 to 6.43); there were no significant associations among women.
Conclusion
The findings suggest that survivors of ALL should receive counseling regarding lifestyle and undergo screening for hormonal deficits to minimize the risk of low BMD and frailty.
INTRODUCTION
Contemporary treatments have improved the 5-year survival rates of pediatric acute lymphoblastic leukemia (ALL) to > 85%.1 However, survivors of ALL are at increased risk for phenotypes that suggest accelerated aging, such as deficits in bone mineral density (BMD)2-4 and frailty.5
Low BMD, defined as a BMD z score greater than one standard deviation (SD) below normative values, is of concern given the association with increased fracture risk, impaired mobility, and early mortality in the general population.6-8 Factors associated with low BMD among children treated for cancer include exposure to alkylating agents, methotrexate, and glucocorticoids9,10 and cranial or gonadal radiation.2,11 Radiation therapy likely impairs BMD by damaging the hypothalamic-pituitary axis, ovaries, or testes with resultant hormonal deficiencies.12 Lifestyle factors, such as smoking, alcohol consumption, and inactive lifestyle, are associated with low BMD in the general population.13-18 However, the contribution of these factors to BMD has not been extensively studied in survivors of ALL.
Among survivors of childhood cancer, frailty (an age-associated loss of fitness/function19) is a recently described phenomenon.5 Frailty is associated with chronic disease, falls and fractures, loss of independence and mortality in the elderly20,21 and in survivors of childhood cancer.5 Among elderly populations, frailty is influenced by lifestyle and genetics.19 However, among young adult survivors of ALL, reductions in physiologic reserve are more likely related to organ systems damage following treatment. Recent data indicate that untreated growth hormone deficiency (GHD) among survivors exposed to cranial radiation is associated with muscle weakness and low energy expenditure, suggesting that GHD may mediate the association between cranial radiation and frailty.22 The contribution of hormonal and lifestyle factors to the occurrence of frailty phenotype among survivors of ALL has not been well characterized.
In light of the high degree of morbidity and mortality associated with low BMD and frailty in the general population, the identification of factors that influence these health states among survivors is of particular importance, given that survivors are at risk for developing these outcomes decades earlier than expected.2-5 Unlike cancer therapies received in childhood, lifestyle choices are modifiable and hormonal deficiencies may be remediated by appropriate therapies. Therefore, the aims of this study were to: (1) evaluate associations between lifestyle and hormonal factors with risk of low BMD and presence of at least two components of the frailty phenotype among adult survivors of childhood ALL, and (2) determine if associations between hormonal status and low BMD or frailty mediated associations between cancer therapeutic exposures and these outcomes.
PATIENTS AND METHODS
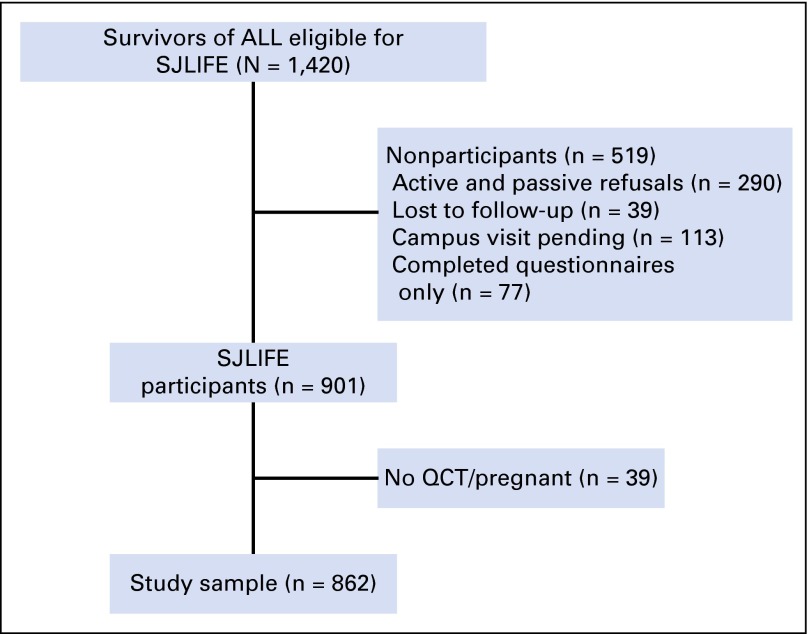
Survivors of childhood ALL participating in the St Jude Lifetime Cohort Study (SJLIFE) assessed before April 30, 2013, were eligible for these analyses. Eligibility criteria for SJLIFE include: prior therapy at St Jude Children’s Research Hospital (SJCRH) for a childhood malignancy, attained age of 18 years or older, and follow-up of 10 or more years from diagnosis.23 Although SJLIFE is a longitudinal study, all data used in these analyses were collected at participants’ baseline study visit. Of the 1,420 eligible survivors of ALL, 901 underwent clinical assessment at SJCRH, of whom 862 had BMD testing using quantitative computed tomography (QCT) and 846 had a functional assessment (Fig 1). All protocol documents were approved by the SJCRH Institutional Review Board and informed consent was obtained from all participants.
Fig 1.
Study diagram for participation in St Jude Lifetime Cohort Study (SJLIFE). ALL, acute lymphoblastic leukemia; QCT, quantitative computed tomography.
Volumetric BMD of L1 and L2 vertebral bodies was measured using a GE QCT Lightspeed 64 detector CT scanner (GE Healthcare, Milwaukee, WI) and analyzed with dose modulation techniques, and Mindways QCT calibration phantoms and software (Mindways Software, Inc., Austin, TX).24,25 Age- and sex-specific z scores were calculated using the manufacturer’s normative database.
Prefrailty was defined as having two and frailty as having at least three of the following: low muscle mass, self-reported exhaustion, low energy expenditure, slow walking speed, and weakness. Dual x-ray absorptiometry and height were used to determine relative lean muscle mass by dividing lean mass (excluding the head but including bone mineral content) by height in meters squared. Survivors with relative mass 1.5 SD below age-, sex-, and race-specific values from the National Health and Nutrition Examination Study (NHANES) were classified with low lean muscle mass.26 Exhaustion was measured using the vitality subscale of the Medical Outcomes Survey Short Form-36; those with scores 1.3 SD below the population mean were classified with exhaustion.27 Low energy expenditure was measured using the physical activity questionnaire from the National Health and Nutrition Examination Study. Men who expended < 383 kilocalories per week and women who expended < 270 kilocalories per week were classified with low activity.28 Slowness was assessed by asking participants to walk at their usual pace for 15 feet. Women shorter than 159 cm and men shorter than 173 cm were classified as slow if they took ≥ 7 seconds to complete the distance.28 Women ≥ 159 cm tall and men ≥ 173 cm tall were classified as slow if they took ≥ 6 seconds to complete the distance.28 Hand grip dynamometry (kilograms) in sitting with the forearm neutral and the elbow flexed 90 degrees was used to assess weakness. Muscle weakness was classified based on body mass index (BMI)-specific cut points.29
Information on treatment exposures was obtained from medical records. Hormonal deficiencies were identified during the SJLIFE clinical evaluation. For participants reporting medications for known endocrine deficiencies, or those that could interfere with laboratory measurements (eg, hormonal replacement therapies), classification of hormone deficiency was determined using both medical history and medication data.22 In individuals not receiving such medications or treatments, endocrine laboratory results were interpreted in relation to normative ranges and/or practice guidelines. A medical history of childhood or adult-onset GHD, ongoing treatment with GH, or plasma levels of insulin-like growth factor 1 (IGF-1) z score < −2 for age and sex at the time of the SJLIFE evaluation, were considered suggestive of GHD.30 Estradiol, luteinizing hormone, and follicle-stimulating hormone levels were measured in all women. Women with a prior diagnosis of premature ovarian insufficiency, or who experienced amenorrhea before the age of 40 years, were classified with premature ovarian insufficiency. Men were screened using morning plasma total testosterone, luteinizing hormone, and follicle-stimulating hormone levels. Men with total testosterone levels < 200 ng/dL were considered deficient.22
Data on lifestyle habits were collected using a structured questionnaire completed at the time of the SJLIFE evaluation. Alcohol intake was based on number of alcoholic drinks consumed during a typical day. Men who consumed between one and four drinks daily and women who consumed between one and three drinks daily were classified as moderate drinkers. Men and women who consumed more than five or four drinks daily, respectively, were considered risky drinkers. Smoking status was classified as current, past, or never. Survivors who met the Centers for Disease Control and Prevention guidelines for physical activity (150 minutes of moderate-intensity physical activity or 75 minutes of vigorous activity per week)31 were considered physically active.
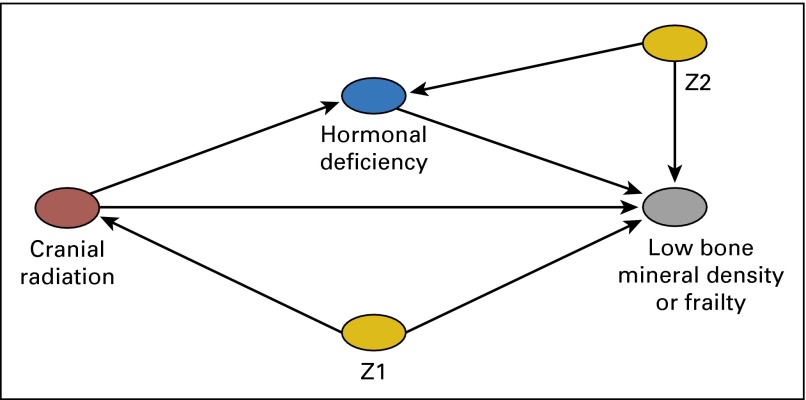
Demographic and treatment information were compared between participants and nonparticipants using χ2 statistics. In cross-sectional analysis, logistic regression was used to examine associations (odds ratios [OR] and 95% CIs) between lifestyle (smoking, alcohol consumption, and activity levels) and hormonal status (GHD, premature ovarian insufficiency, and testosterone deficiency) with low BMD or having at least two components of the frailty phenotype. Because the frequency of survivors with frailty was low, all analyses of the phenotype were limited to those with at least two components (ie, prefrail or frail). Multivariable models were stratified by sex and adjusted for attained age and BMI when appropriate. Because previous publications have suggested that associations between specific treatments and low BMD and frailty may be due to treatment-induced hormonal disturbances, we also assessed if the presence of hormonal deficiencies served to mediate associations between cranial radiation and the outcomes of interest using mediation analysis within a potential outcomes framework.32 Appendix Fig A1 illustrates a reduced-form structure of the hypothesized dependency assumptions for the relation between cranial radiation and low BMD or prefrailty/frailty. Specifically, we estimated the sex-specific controlled direct effect of cranial radiation on low BMD or frailty, where both the exposure-intermediate and exposure-outcome relations were modeled using logistic regression with adjustment for age at follow-up, smoking status, and alcohol use.33,34 The controlled direct effect represents the effect of cranial radiation on low BMD or frailty if hormonal deficiency were managed through intervention, and if the eliminated proportion represented the percentage of frailty cases that would be eliminated if GHD were resolved through intervention. Given limited sample size, we were unable to compute stable estimates for both mediation and moderation by hormonal deficiency; our analysis assumed no moderation by hormonal deficiency. Analyses were conducted with SAS software version 9.2 (SAS Institute, Inc., Cary, NC).
RESULTS
The median age at diagnosis among the 862 participants was 5.0 years (range, 0.2 to 19.5 years); the median age at follow-up was 31.3 years (range, 18.4 to 59.7 years). The median duration between diagnosis and follow-up was 25.1 years (range, 10.5 to 47.7 years). Of the 329 survivors with IGF-1 levels suggestive of GHD, only four were receiving replacement therapy. Approximately 11% of women had premature ovarian insufficiency, of whom 21.3% were taking hormone replacement therapy. Among men, 24.2% had low testosterone, of whom 34% were receiving treatment. When compared with nonparticipants, a higher proportion of participants were women (P < .01); of white, non-Hispanic descent (P < .01); and had received cranial or craniospinal irradiation (P < .05; Table 1).
Table 1.
Demographic and Treatment Characteristics of Study Participants
| Characteristic | No. of Participants (%) | No. of Nonparticipants (%) | P* |
|---|---|---|---|
| Total | 862 | 519 | |
| Sex | |||
| Female | 436 (51.0) | 222 (42.8) | |
| Male | 426 (49.0) | 297 (57.2) | .001 |
| Age at diagnosis, years | |||
| 0-4 | 422 (49.0) | ||
| 5-9 | 241 (28.0) | ||
| 10-14 | 140 (16.2) | ||
| 15-19 | 59 (6.8) | ||
| Age at follow-up, years | |||
| 18-29 | 373 (43.3) | 182 (35.1) | |
| 30-39 | 332 (38.5) | 200 (38.5) | |
| ≥ 40 | 157 (18.3) | 137 (26.4) | .001 |
| Race/ethnicity | |||
| White non-Hispanic | 762 (88.4) | 417 (80.4) | |
| Black non-Hispanic | 70 (8.1) | 64 (12.3) | |
| Other non-Hispanic | 7 (0.8) | 9 (1.7) | |
| Hispanic/Latino | 12 (1.4) | 8 (1.5) | |
| Other | 11 (1.3) | 21 (4.1) | .001 |
| Smoker | |||
| Past | 82 (9.5) | ||
| Current | 186 (21.6) | ||
| Never | 594 (68.9) | ||
| Body mass index | |||
| Underweight | 17 (2.0) | ||
| Normal | 240 (27.8) | ||
| Overweight | 238 (27.6) | ||
| Obese | 367 (42.6) | ||
| Growth hormone deficiency | |||
| Yes | 329 (38.2) | ||
| No | 463 (53.7) | ||
| Unknown | 70 (8.1) | ||
| Premature ovarian insufficiency | |||
| Yes | 47 (10.8) | ||
| No | 374 (85.8) | ||
| Unknown | 15 (3.4) | ||
| Testosterone deficiency | |||
| Yes | 103 (24.2) | ||
| No | 323 (75.8) | ||
| Radiotherapy | |||
| None | 337 (39.1) | 248 (47.8) | |
| CRT < 22 Gy | 194 (22.5) | 99 (19.1) | |
| CRT ≥ 22 Gy | 224 (26.0) | 108 (20.8) | |
| CRT + CS or TBI | 107 (12.4) | 64 (12.3) | .011 |
| High-dose methotrexate | |||
| Yes | 523 (60.7) | 317 (61.1) | |
| No | 339 (39.3) | 202 (38.9) | .88 |
| Methotrexate dose | 5,461 (12-51,367)† | ||
| Cyclophosphamide dose | 9,278 (300-10,889)† | ||
| Glucocorticoid dose‡ | 9,520 (82-27,360)† |
Abbreviation: CRT, cranial radiation; CS, craniospinal; TBI, total body radiation.
χ2 test.
Median and range for cumulative drug dose (mg/m2).
Protocol prescribed doses were used to approximate cumulative dose exposure for glucocorticoids. The cumulative prednisone equivalent dose was calculated using the following equation: prednisone equivalent dose (mg/m2) = 1.0 (cumulative prednisone dose [mg/m2]) + 0.8 (cumulative methylprednisolone dose [mg/m2]) + 6.67 (cumulative dexamethasone dose [mg/m2]).
The mean BMD z score was −0.64 (± SD 1.08) for men, and −0.04 (± SD 1.18) for women. Among men, 36.6% had a BMD z score between −2.5 and −1, and 2.8% had z scores ≤ −2.5. Among women, 20.2% had BMD z scores between −2.5 and −1, and 0.7% had z scores ≤ −2.5. After adjusting for BMI, men with GHD (OR, 1.59; 95% CI, 1.02 to 2.49) or who were current smokers (OR, 1.71; 95% CI, 1.02 to 2.85) had increased odds of low BMD compared with those without GHD or who were nonsmokers (Table 2). Women with GHD (OR, 2.18; 95% CI, 1.26 to 3.78) and who consumed moderate levels of alcohol (OR, 2.09, 95% CI, 1.14 to 3.83) had increased odds of low BMD compared with women without GHD or who were never drinkers.
Table 2.
Multivariable Analyses of Lifestyle and Hormonal Factors Associated With Low BMD and Two or More Components of the Frailty Phenotype
| Variable | Low BMD, % | OR* | 95% CI | ≥ 2 Frailty Components, % | OR† | 95% CI |
|---|---|---|---|---|---|---|
| Men | ||||||
| Smoking | ||||||
| Never | 36.9 | 1.0 | 12.5 | 1.0 | ||
| Current | 48.8 | 1.71 | 1.02 to 2.85 | 26.8 | 3.26 | 1.65 to 6.43 |
| Past | 29.3 | 0.79 | 0.34 to 1.64 | 11.1 | 0.82 | 0.26 to 2.61 |
| Alcohol consumption | ||||||
| Never | 39.7 | 1.0 | 19.0 | 1.0 | ||
| Moderate | 41.3 | 0.98 | 0.60 to 1.60 | 16.4 | 0.83 | 0.42 to 1.66 |
| Risky | 33.3 | 0.65 | 0.37 to 1.13 | 8.6 | 0.39 | 0.15 to 1.01 |
| Growth hormone status | ||||||
| Normal | 35.5 | 1.0 | 9.8 | 1.0 | ||
| Insufficient/deficient | 43.5 | 1.59 | 1.02 to 2.13 | 25.2 | 2.97 | 1.56 to 5.67 |
| Testosterone status | ||||||
| Normal | 40.4 | 1.0 | 13.7 | 1.0 | ||
| Insufficient/deficient | 33.7 | 0.67 | 0.39 to 1.13 | 22.5 | 1.20 | 0.60 to 2.42 |
| Women | ||||||
| Smoking | ||||||
| Never | 19.6 | 1.0 | 27.3 | 1.0 | ||
| Current | 25.4 | 1.14 | 0.59 to 2.20 | 26.5 | 0.76 | 0.39 to 1.48 |
| Past | 14.7 | 0.66 | 0.23 to 1.89 | 39.4 | 1.56 | 0.70 to 3.46 |
| Alcohol consumption | ||||||
| Never | 15.4 | 1.0 | 30.3 | 1.0 | ||
| Moderate | 25.9 | 2.09 | 1.14 to 3.83 | 16.3 | 0.49 | 0.27 to 0.92 |
| Risky | 25.4 | 2.03 | 0.97 to 4.24 | 41.9 | 1.96 | 1.02 to 3.77 |
| Growth hormone status | ||||||
| Normal | 15.5 | 1.0 | 24.8 | 1.0 | ||
| Insufficient/deficient | 26.8 | 2.18 | 1.26 to 3.78 | 33.1 | 1.41 | 0.86 to 2.32 |
| Premature ovarian insufficiency | ||||||
| No | 19.1 | 1.0 | 27.2 | 1.0 | ||
| Yes | 28.9 | 1.61 | 0.76 to 3.39 | 36.6 | 1.12 | 0.53 to 2.42 |
Abbreviations: BMD, bone mineral density; OR, odds ratio.
Multivariable analyses adjusted for body mass index.
Multivariable analyses adjusted for age at follow-up.
Criteria for frailty (at least three components) and prefrailty (at least two components) were met by 3.6% and 18.6% of survivors, respectively. The most frequent frailty components observed among men were low energy expenditure (38.6%), self-reported exhaustion (16.6%), and low muscle mass (11.5%). Similarly, the most frequent frailty components among women were low energy expenditure (47.0%), self-reported exhaustion (30.7%), and low muscle mass (14.2%). After adjusting for current age, the odds of having at least two components of the frailty phenotype were increased among men with GHD (OR, 2.97; 95% CI, 1.56 to 5.66) and those who were current smokers (OR, 3.26; 95% CI, 1.65 to 6.43). Among women, the likelihood of demonstrating at least two frailty components was higher among risky drinkers (OR, 1.96; 95% CI, 1.02 to 3.77) and lower among moderate drinkers (OR, 0.49; 95% CI, 0.27 to 0.92) when compared with never drinkers. Survivors with low BMD did not have increased odds of having at least two components of the frailty phenotype compared with survivors with normal BMD (P > .05).
Table 3 summarizes the results of our mediation analysis. The proportion of cases of frailty that would be eliminated if GHD were resolved through intervention (ie, the magnitude of indirect effect through GHD) was 39% for men and 29% for women. For low BMD, the proportion eliminated was 20% for men and 47% for women.
Table 3.
Estimates of the Controlled Direct, Natural Indirect, and Total Effects of the Association Between Cranial Radiation and Frailty Mediated Through Growth Hormone Deficiency
| Marginal Total Effect | Controlled Direct Effect | ||||
|---|---|---|---|---|---|
| Outcome | OR* | 95% CI | OR* | 95% CI | Proportion Eliminated, %† |
| Frailty | |||||
| Men | 4.26 | 1.61 to 11.30 | 2.99 | 1.11 to 8.07 | 39.4 |
| Women | 1.66 | 0.94 to 2.94 | 1.51 | 0.83 to 2.76 | 28.6 |
| Low BMD | |||||
| Men | 2.49 | 1.33 to 4.66 | 2.20 | 1.16 to 4.16 | 20.0 |
| Women | 2.49 | 1.13 to 5.51 | 1.82 | 0.81 to 4.08 | 46.7 |
Abbreviations: BMD, bone mineral density; OR, odds ratio.
Odds ratios adjusted for age at follow-up, smoking status, and alcohol use.
Proportion of frailty cases that would be eliminated if growth hormone deficiency were resolved through intervention.34
DISCUSSION
To our knowledge, this study is among the first to investigate the contribution of lifestyle factors and hormonal deficiencies to frailty, and is among the largest to date to examine associations between modifiable risk factors and low BMD among long-term survivors of ALL. Our findings demonstrate an approximate prevalence of 30% of low BMD and 19% of at least two components of the frailty phenotype among survivors of ALL assessed at a median of 25 years from diagnosis. Male survivors who were current smokers or who had GHD had an increased odds of having low BMD or two or more components of the frailty phenotype. Among women, GHD was associated with an increased likelihood of low BMD, but not frailty. Consumption of alcohol at levels considered risky was also associated with an increased likelihood of having at least two components of the frailty phenotype among women.
Our results indicate an association between GHD and low BMD among both male and female survivors of ALL. Growth hormone is known to be an important contributor to bone growth and development in childhood and adolescence.35 We also observed that the odds of having at least two components of the frailty phenotype were more than three-fold higher among men with a history of GHD compared with those without. However, we did not observe an association between GHD and frailty among women. In addition, the results of our mediation analysis demonstrated that GHD was only a modest mediator of the association between cranial radiation and low BMD or frailty. These data suggest that remediation of GHD would eliminate 39% and 29% of cases of prefrailty/frailty among men and women, respectively, and 20% and 47% of cases of low BMD among men and women, respectively. Thus, cranial radiation appears to have either a strong direct effect on these outcomes, or the effect is mediated through other pathways. Nevertheless, these data suggest that untreated GHD contributes to deficits in bone density and physiologic reserve among survivors of ALL. Whereas GH replacement therapy in adult-onset deficiency improves body composition and BMD,36,37 it is unknown whether such benefits would be realized among survivors of ALL with a high frequency of additional comorbidities and uncertain safety profile in this population.38 Future research evaluating the impact of GH replacement on BMD, muscle strength and mass, and fatigue among survivors is required.
We found that men who smoked had an increased odds of having both low BMD and at least two components of the frailty phenotype, a finding that is consistent with a Childhood Cancer Survivor Study investigation reporting increased odds of fracture among men who smoked.39 Moreover, our findings are consistent with studies of men without cancer demonstrating associations between smoking and reduced BMD,40,41 sarcopenia,42,43 and fatigue.44 We also observed a two-fold increased likelihood of low BMD among women who consumed moderate levels of alcohol. In contrast, the likelihood of demonstrating at least two components of the frailty phenotype was lower among female moderate drinkers when compared with nondrinkers. Increasing alcohol intake is associated with reduced bone density in older women17 and excessive alcohol intake is a well-known cause of secondary osteoporosis.15 However, the relationship between alcohol and frailty is less clear. Whereas in some studies alcohol consumption has been associated with an increased risk of frailty, a study of more than 20,000 women reported that moderate alcohol consumption was associated with decreased risk of frailty.45 Although it is unlikely that alcohol in and of itself is beneficial for muscle,46 moderate alcohol consumption may be associated with additional factors or behaviors that promote maintenance of physiologic reserve. Hence, our finding of a protective association between moderate alcohol consumption and frailty among women suggests that there are unidentified factors influencing the frailty risk among survivors that warrant further investigation. Finally, it is unclear why sex-specific differences were observed in the associations between lifestyle factors and our phenotypes of interest. It is well recognized in the field of survivorship that sex-specific differences in susceptibility to acute47 and late effects48 following cancer therapy exist, and that these differences are often speculated to be the result of hormonal variations. However, inherent differences in developmental and aging patterns of both bone and muscle between the sexes may have contributed to the sex-specific associations observed in this study.
We did not find an association between either premature ovarian insufficiency or testosterone deficiency with either low BMD or the presence of at least two components of the frailty phenotype among adult survivors of ALL. This was unexpected because declining levels of sex steroids are associated with osteoporosis,49 sarcopenia, frailty, and functional decline50 in older populations. The lack of association between sex hormone deficiency and BMD and frailty in our cohort may be because, unlike GHD, sex hormone deficiencies are more likely to be screened for and treated if identified. One-third of men classified as testosterone deficient in this study were receiving hormone replacement at their SJLIFE evaluation, whereas just over 20% of women with premature ovarian insufficiency were on replacement therapy at follow-up. Furthermore, it is likely that some survivors not taking a hormone replacement at the time of assessment may have done so previously. Although we considered reanalyzing our data excluding those patients receiving hormone replacements at their evaluation, the high frequency of women taking oral contraceptives limited detailed investigation. Nevertheless, results were not found to differ appreciably for men once those survivors receiving androgen replacement were removed from analyses.
A strength of the current study was the use of the SJLIFE cohort, a large, well-characterized population of cancer survivors with data on BMD, strength, mobility, body composition, hormonal status, and lifestyle.23 However, interpretation of our findings is limited by the cross-sectional design of this analysis. In particular, mediation analyses assume a temporal order among the variables. Although BMD and frailty were measured many years after cancer treatment, lifestyle behaviors and hormonal status were measured at SJLIFE assessment, and therefore, the status of the modifiable factors before the occurrence of each outcome is unknown. Accordingly, caution is warranted when interpreting findings, and further prospective longitudinal studies are required to confirm the direction of associations. This study was also limited by our reliance on plasma IGF-1 levels for identifying GHD in place of dynamic endocrine testing and our assumption that all preexisting hormonal deficits were valid and persistent at the SJLIFE assessment, which may have resulted in misclassification of exposure for some participants.
In summary, survivors of ALL are at risk for phenotypes that suggest accelerated aging. We found that GHD negatively impacted BMD and increased the likelihood of prefrailty/frailty among men. Accordingly, future studies among survivors of ALL that examine the use of GH replacement therapy for improving bone density and physiologic reserve are warranted. Furthermore, we found that smoking was associated with increased odds of having both low BMD and at least two components of the frailty phenotype among men. These findings highlight the importance of survivor health education programs that discourage smoking initiation and support cessation. Overall, survivors of ALL should receive counseling regarding lifestyle habits and undergo screening for hormonal deficits to minimize the risk of low BMD and frailty.
Appendix
Fig A1.
Reduced-form directed acyclic graph illustrating dependency assumptions for the relations between cranial radiation and low bone mineral density or frailty and hypothesized mediation by hormonal deficiency. Note: The direct effect of cranial radiation on low bone mineral density or frailty was estimated after adjusting for hormonal deficiency (the intermediate). Z1 and Z2 represent covariate vectors on biasing pathways for the exposure-outcome relation and the intermediate-outcome relation, respectively.
Footnotes
Listen to the podcast by Dr Klepin at www.jco.org/podcasts
Supported by St Jude Children’s Research Hospital Cancer Center Support Grant No. 5P30CA021765-33, the St Jude Lifetime Cohort Study Grant No. U01 CA195547, and American Lebanese Syrian Associated Charities.
Presented at the 47th Congress of the International Society of Paediatric Oncology, Cape Town, South Africa, October 8-11, 2015.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Carmen L. Wilson, Wassim Chemaitilly, Sue C. Kaste, Deo Kumar Srivastava, Yutaka Yasui, Kirsten K. Ness
Provision of study materials or patients: Ching-Hon Pui, Leslie L. Robison, Melissa M. Hudson
Collection and assembly of data: Carmen L. Wilson, Kendra E. Jones, Leslie L. Robison, Melissa M. Hudson, Kirsten K. Ness
Data analysis and interpretation: Carmen L. Wilson, Wassim Chemaitilly, Kendra E. Jones, Deo Kumar Srivastava, Rohit P. Ojha, Ching-Hon Pui, Kirsten K. Ness
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Modifiable Factors Associated With Aging Phenotypes Among Adult Survivors of Childhood Acute Lymphoblastic Leukemia
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Carmen L. Wilson
No relationship to disclose
Wassim Chemaitilly
Honoraria: Novo Nordisk
Consulting or Advisory Role: Novo Nordisk
Kendra E. Jones
Employment: Roche
Sue C. Kaste
No relationship to disclose
Deo Kumar Srivastava
No relationship to disclose
Rohit P. Ojha
No relationship to disclose
Yutaka Yasui
No relationship to disclose
Ching-Hon Pui
No relationship to disclose
Leslie L. Robison
No relationship to disclose
Melissa M. Hudson
No relationship to disclose
Kirsten K. Ness
No relationship to disclose
REFERENCES
- 1.Pui CH, Mullighan CG, Evans WE, et al. Pediatric acute lymphoblastic leukemia: Where are we going and how do we get there? Blood. 2012;120:1165–1174. doi: 10.1182/blood-2012-05-378943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nysom K, Holm K, Michaelsen KF, et al. Bone mass after treatment for acute lymphoblastic leukemia in childhood. J Clin Oncol. 1998;16:3752–3760. doi: 10.1200/JCO.1998.16.12.3752. [DOI] [PubMed] [Google Scholar]
- 3.Brennan B, Shalet SM. Reduced bone mineral density at completion of chemotherapy for a malignancy. Arch Dis Child. 1999;81:372. doi: 10.1136/adc.81.4.372a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tillmann V, Darlington AS, Eiser C, et al. Male sex and low physical activity are associated with reduced spine bone mineral density in survivors of childhood acute lymphoblastic leukemia. J Bone Miner Res. 2002;17:1073–1080. doi: 10.1359/jbmr.2002.17.6.1073. [DOI] [PubMed] [Google Scholar]
- 5.Ness KK, Krull KR, Jones KE, et al. Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: A report from the St Jude Lifetime cohort study. J Clin Oncol. 2013;31:4496–4503. doi: 10.1200/JCO.2013.52.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312:1254–1259. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller PD, Siris ES, Barrett-Connor E, et al. Prediction of fracture risk in postmenopausal white women with peripheral bone densitometry: Evidence from the National Osteoporosis Risk Assessment. J Bone Miner Res. 2002;17:2222–2230. doi: 10.1359/jbmr.2002.17.12.2222. [DOI] [PubMed] [Google Scholar]
- 8.Vega E, Ghiringhelli G, Mautalen C, et al. Bone mineral density and bone size in men with primary osteoporosis and vertebral fractures. Calcif Tissue Int. 1998;62:465–469. doi: 10.1007/s002239900462. [DOI] [PubMed] [Google Scholar]
- 9.Mandel K, Atkinson S, Barr RD, et al. Skeletal morbidity in childhood acute lymphoblastic leukemia. J Clin Oncol. 2004;22:1215–1221. doi: 10.1200/JCO.2004.04.199. [DOI] [PubMed] [Google Scholar]
- 10.Gnudi S, Butturini L, Ripamonti C, et al. The effects of methotrexate (MTX) on bone. A densitometric study conducted on 59 patients with MTX administered at different doses. Ital J Orthop Traumatol. 1988;14:227–231. [PubMed] [Google Scholar]
- 11.Hesseling PB, Hough SF, Nel ED, et al. Bone mineral density in long-term survivors of childhood cancer. Int J Cancer Suppl. 1998;11:44–47. [PubMed] [Google Scholar]
- 12.Gurney JG, Kaste SC, Liu W, et al. Bone mineral density among long-term survivors of childhood acute lymphoblastic leukemia: Results from the St. Jude Lifetime Cohort Study. Pediatr Blood Cancer. 2014;61:1270–1276. doi: 10.1002/pbc.25010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chastin SF, Mandrichenko O, Helbostadt JL, et al. Associations between objectively-measured sedentary behaviour and physical activity with bone mineral density in adults and older adults, the NHANES study. Bone. 2014;64:254–262. doi: 10.1016/j.bone.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Bonaiuti D, Shea B, Iovine R, et al. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. 2002;(3):CD000333. doi: 10.1002/14651858.CD000333. [DOI] [PubMed] [Google Scholar]
- 15.Kanis JA, Johansson H, Johnell O, et al. Alcohol intake as a risk factor for fracture. Osteoporos Int. 2005;16:737–742. doi: 10.1007/s00198-004-1734-y. [DOI] [PubMed] [Google Scholar]
- 16.De Laet C, Kanis JA, Odén A, et al. Body mass index as a predictor of fracture risk: A meta-analysis. Osteoporos Int. 2005;16:1330–1338. doi: 10.1007/s00198-005-1863-y. [DOI] [PubMed] [Google Scholar]
- 17.Hannan MT, Felson DT, Dawson-Hughes B, et al. Risk factors for longitudinal bone loss in elderly men and women: The Framingham Osteoporosis Study. J Bone Miner Res. 2000;15:710–720. doi: 10.1359/jbmr.2000.15.4.710. [DOI] [PubMed] [Google Scholar]
- 18.Kanis JA, Johnell O, Oden A, et al. Smoking and fracture risk: A meta-analysis. Osteoporos Int. 2005;16:155–162. doi: 10.1007/s00198-004-1640-3. [DOI] [PubMed] [Google Scholar]
- 19.Xue QL. The frailty syndrome: Definition and natural history. Clin Geriatr Med. 2011;27:1–15. doi: 10.1016/j.cger.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hope AA, Gong MN, Guerra C, et al. Frailty before critical illness and mortality for elderly Medicare beneficiaries. J Am Geriatr Soc. 2015;63:1121–1128. doi: 10.1111/jgs.13436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang SF, Lin PL. Frail phenotype and mortality prediction: A systematic review and meta-analysis of prospective cohort studies. Int J Nurs Stud. 2015;52:1362–1374. doi: 10.1016/j.ijnurstu.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Chemaitilly W, Li Z, Huang S, et al. Anterior hypopituitarism in adult survivors of childhood cancers treated with cranial radiotherapy: A report from the St Jude Lifetime Cohort study. J Clin Oncol. 2015;33:492–500. doi: 10.1200/JCO.2014.56.7933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hudson MM, Ness KK, Nolan VG, et al. Prospective medical assessment of adults surviving childhood cancer: Study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort study. Pediatr Blood Cancer. 2011;56:825–836. doi: 10.1002/pbc.22875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cann CE. Quantitative CT for determination of bone mineral density: A review. Radiology. 1988;166:509–522. doi: 10.1148/radiology.166.2.3275985. [DOI] [PubMed] [Google Scholar]
- 25.Cann CE, Genant HK. Precise measurement of vertebral mineral content using computed tomography. J Comput Assist Tomogr. 1980;4:493–500. doi: 10.1097/00004728-198008000-00018. [DOI] [PubMed] [Google Scholar]
- 26.Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-Ray absorptiometry body composition reference values from NHANES. PLoS One. 2009;4:e7038. doi: 10.1371/journal.pone.0007038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 28.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M157. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 29.Mathiowetz V, Kashman N, Volland G, et al. Grip and pinch strength: Normative data for adults. Arch Phys Med Rehabil. 1985;66:69–74. [PubMed] [Google Scholar]
- 30.Molitch ME, Clemmons DR, Malozowski S, et al. Evaluation and treatment of adult growth hormone deficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1587–1609. doi: 10.1210/jc.2011-0179. [DOI] [PubMed] [Google Scholar]
- 31.Physical Activity Guidelines Advisory Committee . Physical Activity Guidelines Advisory Committee Report. Washington, DC: U.S., Department of Health and Human Services; 2008. [DOI] [PubMed] [Google Scholar]
- 32.Pearl J. The causal mediation formula—A guide to the assessment of pathways and mechanisms. Prev Sci. 2012;13:426–436. doi: 10.1007/s11121-011-0270-1. [DOI] [PubMed] [Google Scholar]
- 33.Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: Theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18:137–150. doi: 10.1037/a0031034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.VanderWeele TJ. Policy-relevant proportions for direct effects. Epidemiology. 2013;24:175–176. doi: 10.1097/EDE.0b013e3182781410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saggese G, Baroncelli GI, Bertelloni S, et al. Effects of long-term treatment with growth hormone on bone and mineral metabolism in children with growth hormone deficiency. J Pediatr. 1993;122:37–45. doi: 10.1016/s0022-3476(05)83484-5. [DOI] [PubMed] [Google Scholar]
- 36.Elbornsson M, Götherström G, Bosæus I, et al. Fifteen years of GH replacement improves body composition and cardiovascular risk factors. Eur J Endocrinol. 2013;168:745–753. doi: 10.1530/EJE-12-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elbornsson M, Götherström G, Bosæus I, et al. Fifteen years of GH replacement increases bone mineral density in hypopituitary patients with adult-onset GH deficiency. Eur J Endocrinol. 2012;166:787–795. doi: 10.1530/EJE-11-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chemaitilly W, Robison LL. Safety of growth hormone treatment in patients previously treated for cancer. Endocrinol Metab Clin North Am. 2012;41:785–792. doi: 10.1016/j.ecl.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Wilson CL, Dilley K, Ness KK, et al. Fractures among long-term survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer. 2012;118:5920–5928. doi: 10.1002/cncr.27626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ortego-Centeno N, Muñoz-Torres M, Jódar E, et al. Effect of tobacco consumption on bone mineral density in healthy young males. Calcif Tissue Int. 1997;60:496–500. doi: 10.1007/s002239900270. [DOI] [PubMed] [Google Scholar]
- 41.Winther A, Dennison E, Ahmed LA, et al. The Tromsø Study: Fit Futures: A study of Norwegian adolescents’ lifestyle and bone health. Arch Osteoporos. 2014;9:185. doi: 10.1007/s11657-014-0185-0. [DOI] [PubMed] [Google Scholar]
- 42.Szulc P, Duboeuf F, Marchand F, et al. Hormonal and lifestyle determinants of appendicular skeletal muscle mass in men: The MINOS study. Am J Clin Nutr. 2004;80:496–503. doi: 10.1093/ajcn/80.2.496. [DOI] [PubMed] [Google Scholar]
- 43.Castillo EM, Goodman-Gruen D, Kritz-Silverstein D, et al. Sarcopenia in elderly men and women: The Rancho Bernardo study. Am J Prev Med. 2003;25:226–231. doi: 10.1016/s0749-3797(03)00197-1. [DOI] [PubMed] [Google Scholar]
- 44.Corwin EJ, Klein LC, Rickelman K. Predictors of fatigue in healthy young adults: Moderating effects of cigarette smoking and gender. Biol Res Nurs. 2002;3:222–233. doi: 10.1177/109980040200300407. [DOI] [PubMed] [Google Scholar]
- 45.Woods NF, LaCroix AZ, Gray SL, et al. Frailty: Emergence and consequences in women aged 65 and older in the Women’s Health Initiative Observational Study. J Am Geriatr Soc. 2005;53:1321–1330. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]
- 46.Reilly ME, Mantle D, Richardson PJ, et al. Studies on the time-course of ethanol’s acute effects on skeletal muscle protein synthesis: Comparison with acute changes in proteolytic activity. Alcohol Clin Exp Res. 1997;21:792–798. [PubMed] [Google Scholar]
- 47.Meeske KA, Ji L, Freyer DR, et al. Comparative toxicity by sex among children treated for acute lymphoblastic leukemia: A report from the Children’s Oncology Group. Pediatr Blood Cancer. 2015;62:2140–2149. doi: 10.1002/pbc.25628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Armstrong GT, Sklar CA, Hudson MM, et al. Long-term health status among survivors of childhood cancer: Does sex matter? J Clin Oncol. 2007;25:4477–4489. doi: 10.1200/JCO.2007.11.2003. [DOI] [PubMed] [Google Scholar]
- 49.Iannuzzi-Sucich M, Prestwood KM, Kenny AM. Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J Gerontol A Biol Sci Med Sci. 2002;57:M772–M777. doi: 10.1093/gerona/57.12.m772. [DOI] [PubMed] [Google Scholar]
- 50.Cappola AR, Bandeen-Roche K, Wand GS, et al. Association of IGF-I levels with muscle strength and mobility in older women. J Clin Endocrinol Metab. 2001;86:4139–4146. doi: 10.1210/jcem.86.9.7868. [DOI] [PubMed] [Google Scholar]