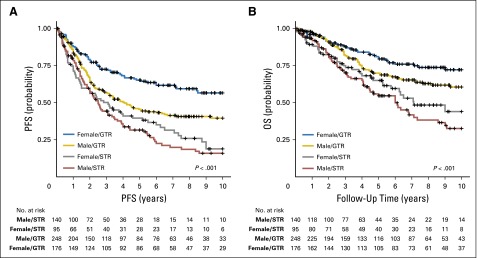
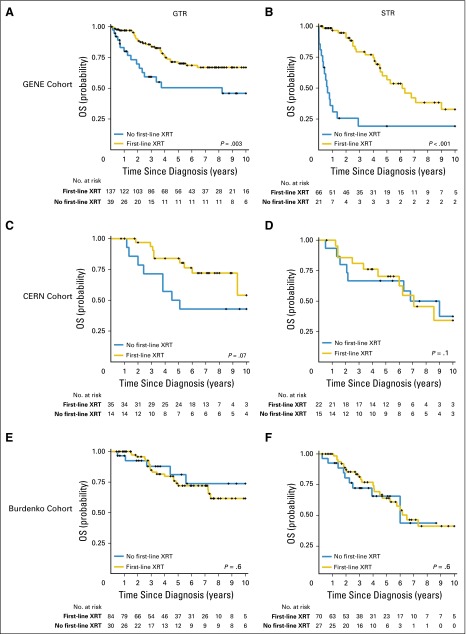
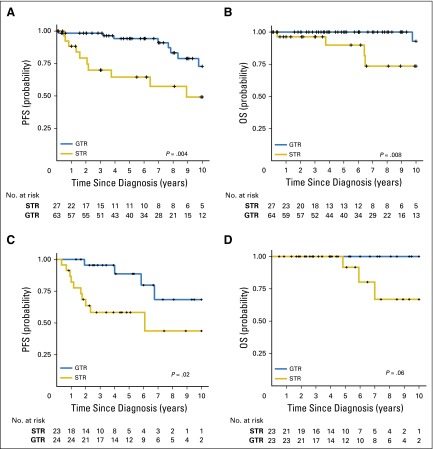
Vijay Ramaswamy
Vijay Ramaswamy
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Thomas Hielscher
Thomas Hielscher
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Stephen C Mack
Stephen C Mack
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Alvaro Lassaletta
Alvaro Lassaletta
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Tong Lin
Tong Lin
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Kristian W Pajtler
Kristian W Pajtler
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
David TW Jones
David TW Jones
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Betty Luu
Betty Luu
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Florence MG Cavalli
Florence MG Cavalli
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Kenneth Aldape
Kenneth Aldape
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Marc Remke
Marc Remke
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Martin Mynarek
Martin Mynarek
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Stefan Rutkowski
Stefan Rutkowski
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Sridharan Gururangan
Sridharan Gururangan
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Roger E McLendon
Roger E McLendon
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Eric S Lipp
Eric S Lipp
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Christopher Dunham
Christopher Dunham
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Juliette Hukin
Juliette Hukin
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
David D Eisenstat
David D Eisenstat
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Dorcas Fulton
Dorcas Fulton
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Frank KH van Landeghem
Frank KH van Landeghem
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Mariarita Santi
Mariarita Santi
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Marie-Lise C van Veelen
Marie-Lise C van Veelen
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Erwin G Van Meir
Erwin G Van Meir
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Satoru Osuka
Satoru Osuka
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Xing Fan
Xing Fan
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Karin M Muraszko
Karin M Muraszko
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Daniela PC Tirapelli
Daniela PC Tirapelli
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Sueli M Oba-Shinjo
Sueli M Oba-Shinjo
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Suely KN Marie
Suely KN Marie
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Carlos G Carlotti
Carlos G Carlotti
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Ji Yeoun Lee
Ji Yeoun Lee
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Amulya A Nageswara Rao
Amulya A Nageswara Rao
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Caterina Giannini
Caterina Giannini
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Claudia C Faria
Claudia C Faria
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Sofia Nunes
Sofia Nunes
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Jaume Mora
Jaume Mora
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Ronald L Hamilton
Ronald L Hamilton
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Peter Hauser
Peter Hauser
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Nada Jabado
Nada Jabado
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Kevin Petrecca
Kevin Petrecca
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Shin Jung
Shin Jung
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Luca Massimi
Luca Massimi
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Massimo Zollo
Massimo Zollo
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Giuseppe Cinalli
Giuseppe Cinalli
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
László Bognár
László Bognár
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Almos Klekner
Almos Klekner
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Tibor Hortobágyi
Tibor Hortobágyi
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Sarah Leary
Sarah Leary
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Ralph P Ermoian
Ralph P Ermoian
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
James M Olson
James M Olson
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Jeffrey R Leonard
Jeffrey R Leonard
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Corrine Gardner
Corrine Gardner
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Wieslawa A Grajkowska
Wieslawa A Grajkowska
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Lola B Chambless
Lola B Chambless
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Jason Cain
Jason Cain
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Charles G Eberhart
Charles G Eberhart
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Sama Ahsan
Sama Ahsan
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Maura Massimino
Maura Massimino
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Felice Giangaspero
Felice Giangaspero
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Francesca R Buttarelli
Francesca R Buttarelli
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Roger J Packer
Roger J Packer
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Lyndsey Emery
Lyndsey Emery
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
William H Yong
William H Yong
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Horacio Soto
Horacio Soto
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Linda M Liau
Linda M Liau
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Richard Everson
Richard Everson
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Andrew Grossbach
Andrew Grossbach
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Tarek Shalaby
Tarek Shalaby
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Michael Grotzer
Michael Grotzer
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Matthias A Karajannis
Matthias A Karajannis
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
David Zagzag
David Zagzag
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Helen Wheeler
Helen Wheeler
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Katja von Hoff
Katja von Hoff
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Marta M Alonso
Marta M Alonso
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Teresa Tuñon
Teresa Tuñon
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Ulrich Schüller
Ulrich Schüller
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Karel Zitterbart
Karel Zitterbart
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Jaroslav Sterba
Jaroslav Sterba
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Jennifer A Chan
Jennifer A Chan
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Miguel Guzman
Miguel Guzman
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Samer K Elbabaa
Samer K Elbabaa
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Howard Colman
Howard Colman
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Girish Dhall
Girish Dhall
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Paul G Fisher
Paul G Fisher
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Maryam Fouladi
Maryam Fouladi
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Amar Gajjar
Amar Gajjar
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Stewart Goldman
Stewart Goldman
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Eugene Hwang
Eugene Hwang
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Marcel Kool
Marcel Kool
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Harshad Ladha
Harshad Ladha
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Elizabeth Vera-Bolanos
Elizabeth Vera-Bolanos
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Khalida Wani
Khalida Wani
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Frank Lieberman
Frank Lieberman
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Tom Mikkelsen
Tom Mikkelsen
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Antonio M Omuro
Antonio M Omuro
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Ian F Pollack
Ian F Pollack
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Michael Prados
Michael Prados
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
H Ian Robins
H Ian Robins
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Riccardo Soffietti
Riccardo Soffietti
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Jing Wu
Jing Wu
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Phillipe Metellus
Phillipe Metellus
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Uri Tabori
Uri Tabori
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Ute Bartels
Ute Bartels
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Eric Bouffet
Eric Bouffet
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Cynthia E Hawkins
Cynthia E Hawkins
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
James T Rutka
James T Rutka
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Peter Dirks
Peter Dirks
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Stefan M Pfister
Stefan M Pfister
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Thomas E Merchant
Thomas E Merchant
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Mark R Gilbert
Mark R Gilbert
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Terri S Armstrong
Terri S Armstrong
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Andrey Korshunov
Andrey Korshunov
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
David W Ellison
David W Ellison
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,
Michael D Taylor
Michael D Taylor
1Vijay Ramaswamy, Stephen C. Mack, Alvaro Lassaletta, Betty Luu, Florence M.G. Cavalli, Uri Tabori, Ute Bartels, Eric Bouffet, Cynthia E. Hawkins, James T. Rutka, Peter Dirks, and Michael D. Taylor, The Hospital for Sick Children; Vijay Ramaswamy, Kenneth Aldape, James T. Rutka, and Michael D. Taylor, University of Toronto; Kenneth Aldape, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario; Christopher Dunham and Juliette Hukin, British Columbia Children’s Hospital; Juliette Hukin, University of British Columbia, Vancouver, British Columbia; David D. Eisenstat, Dorcas Fulton, and Frank K.H. van Landeghem, University of Alberta, Edmonton; Jennifer A. Chan, University of Calgary, Calgary, Alberta; Nada Jabado and Kevin Petrecca, McGill University, Montreal, Quebec, Canada; Thomas Hielscher, Kristian W. Pajtler, David T.W. Jones, Marcel Kool, Stefan M. Pfister, and Andrey Korshunov, German Cancer Research Center; Stefan M. Pfister, University Hospital Heidelberg, Heidelberg; Marc Remke, University Hospital Düsseldorf, Düsseldorf; Martin Mynarek, Stefan Rutkowski, and Katja von Hoff, University Medical Center Hamburg-Eppendorf, Hamburg; Ulrich Schüller, Ludwig-Maximilians-Universität, Munich, Germany; Stephen C. Mack, Cleveland Clinic Foundation, Cleveland; Maryam Fouladi, University of Cincinnati, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tong Lin, Amar Gajjar, Thomas E. Merchant, and David W. Ellison, St Jude Children’s Research Hospital, Memphis; Lola B. Chambless, Vanderbilt Medical Center, Nashville, TN; Sridharan Gururangan, Roger E. McLendon, and Eric S. Lipp, Duke University, Durham; Jing Wu, University of North Carolina at Chapel Hill, Chapel Hill, NC; Mariarita Santi, Children’s Hospital of Philadelphia; Lyndsey Emery, Hospital of the University of Pennsylvania, Philadelphia; Ronald L. Hamilton and Ian F. Pollack, University of Pittsburgh School of Medicine; Frank Lieberman, University of Pittsburgh Medical Center, Pittsburgh, PA; Erwin G. Van Meir and Satoru Osuka, Emory University, Atlanta, GA; Xing Fan and Karin M. Muraszko, University of Michigan Medical School, Ann Arbor; Tom Mikkelsen, Henry Ford Health System, Detroit, MI; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Seattle Children’s Hospital; Sarah Leary, Ralph P. Ermoian, and James M. Olson, Fred Hutchinson Cancer Research Center; Sarah Leary, Ralph P. Ermoian, and James M. Olson, University of Washington, Seattle, WA; Jeffrey R. Leonard and Corrine Gardner, Washington University School of Medicine and St Louis Children’s Hospital; Miguel Guzman and Samer K. Elbabaa, St Louis University School of Medicine, St Louis, MO; Charles G. Eberhart and Sama Ahsan, John Hopkins University School of Medicine, Baltimore; Mark R. Gilbert, National Cancer Institute, Bethesda, MD; Roger J. Packer and Eugene Hwang, Children’s National Medical Center, Washington, DC; William H. Yong, Horacio Soto, Linda M. Liau, and Richard Everson, David Geffen School of Medicine at University of California, Los Angeles; Girish Dhall, Children's Hospital Los Angeles, Los Angeles; Paul G. Fisher, Stanford University Medical Center, Stanford; Michael Prados, University of California San Francisco, San Francisco, CA; Andrew Grossbach, University of Iowa Hospitals and Clinics, Iowa City, IA; Matthias A. Karajannis and David Zagzag, New York University Langone Medical Center; Antonio M. Omuro, Memorial Sloan-Kettering Cancer Center, New York, NY; Howard Colman, Huntsman Cancer Institute, University of Utah Health System, Salt Lake City, UT; Amulya A. Nageswara Rao and Caterina Giannini, Mayo Clinic, Rochester, MN; Stewart Goldman, Lurie Children’s Hospital, Chicago, IL; Harshad Ladha, Elizabeth Vera-Bolanos, Khalida Wani, and Mark R. Gilbert, The University of Texas MD Anderson Cancer Center; Terri S. Armstrong, University of Texas Health Science Center, Houston, TX; H. Ian Robins, University of Wisconsin, Madison, WI; Marie-Lise C. van Veelen, Erasmus University Medical Center, Rotterdam, the Netherlands; Daniela P.C. Tirapelli, Sueli M. Oba-Shinjo, Suely K.N. Marie, and Carlos G. Carlotti, University of Sao Paulo, São Paulo, Brazil; Ji Yeoun Lee, Seoul National University College of Medicine, Seoul; Shin Jung, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hospital and Medical School, Hwasun-gun, Chonnam, South Korea; Claudia C. Faria, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria; Sofia Nunes, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Jaume Mora, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona; Marta M. Alonso, University Hospital of Navarra, Pamplona; Teresa Tuñon, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Peter Hauser, Semmelweis University, Budapest; László Bognár, Almos Klekner, and Tibor Hortobágyi, University of Debrecen, Debrecen, Hungary; Luca Massimi, Catholic University Medical School; Francesca R. Buttarelli, Sapienza University of Rome, Rome; Massimo Zollo and Giuseppe Cinalli, University of Naples; Giuseppe Cinalli, Ospedale Santobono-Pausilipon, Naples; Maura Massimino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan; Felice Giangaspero, Sapienza University of Rome–Policlinico Umberto I and IRCCS Neuromed, Pozzilli; Riccardo Soffietti, University of Turin, Turin, Italy; Wieslawa A. Grajkowska, The Children’s Memorial Health Institute, Warsaw, Poland; Jason Cain, The Hudson Institute of Medical Research, Clayton, Victoria; Helen Wheeler, Kolling Institute of Medical Research, The University of Sydney, Sydney, New South Wales, Australia; Tarek Shalaby and Michael Grotzer, University Children’s Hospital of Zurich, Zurich, Switzerland; Karel Zitterbart and Jaroslav Sterba, Masaryk University, Brno, Czech Republic; and Phillipe Metellus, Centre Hospitalier Clairval, Marseille, France.
1,✉