Abstract
In this randomized, partially-blind study (clinicaltrials.gov; NCT00541970), the licensed formulation of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine (20 μg each of HPV-16/18 antigens) was found highly immunogenic up to 4 y after first vaccination, whether administered as a 2-dose (2D) schedule in girls 9–14 y or 3-dose (3D) schedule in women 15–25 y. This end-of-study analysis extends immunogenicity and safety data until Month (M) 60, and presents antibody persistence predictions estimated by piecewise and modified power law models. Healthy females (age stratified: 9–14, 15–19, 20–25 y) were randomized to receive 2D at M0,6 (N = 240 ) or 3D at M0,1,6 (N = 239). Here, results are reported for girls 9–14 y (2D) and women 15–25 y (3D). Seropositivity rates, geometric mean titers (by enzyme-linked immunosorbent assay) and geometric mean titer ratios (GMRs; 3D/2D; post-hoc exploratory analysis) were calculated. All subjects seronegative pre-vaccination in the according-to-protocol immunogenicity cohort were seropositive for anti-HPV-16 and −18 at M60. Antibody responses elicited by the 2D and 3D schedules were comparable at M60, with GMRs close to 1 (anti-HPV-16: 1.13 [95% confidence interval: 0.82–1.54]; anti-HPV-18: 1.06 [0.74–1.51]). Statistical modeling predicted that in 95% of subjects, antibodies induced by 2D and 3D schedules could persist above natural infection levels for ≥ 21 y post-vaccination. The vaccine had a clinically acceptable safety profile in both groups. In conclusion, a 2D M0,6 schedule of the HPV-16/18 AS04-adjuvanted vaccine was immunogenic for up to 5 y in 9–14 y-old girls. Statistical modeling predicted that 2D-induced antibodies could persist for longer than 20 y.
Keywords: administration schedule, female adolescents, human papillomavirus vaccine, immunogenicity, randomized controlled trial, safety, women
Abbreviations
- 2D
2-dose
- 3D
3-dose
- 95% CI
95% confidence interval
- AS04
Adjuvant System containing 50 µg 3-O-desacyl-4′-monophosphoryl lipid A (MPL) adsorbed on aluminum salt (500 µg Al3+)
- ATP-I
according-to-protocol cohort for immunogenicity
- CI
confidence interval
- CVT
Costa Rica HPV-16/18 Vaccine Trial
- ELISA
enzyme-linked immunosorbent assay
- EU
ELISA unit
- GMR
ratio of geometric mean antibody titers, GMT(s), geometric mean antibody titer(s)
- HPV
human papillomavirus
- M
month
- TVC
total vaccinated cohort
- MPL
3-O-desacyl-4′-monophosphoryl lipid A
- VLP(s)
virus-like particle(s)
Introduction
The human papillomavirus (HPV)-16/18 vaccine (Cervarix®, GSK group of companies) has been shown to be immunogenic, efficacious and to have a clinically acceptable safety profile in clinical studies.1-7 The licensed vaccine formulation contains 20 µg of HPV-16 L1 protein virus-like particles (VLPs) and 20 µg of HPV-18 L1 VLPs, formulated with the AS04 Adjuvant System of 3-O-desacyl-4′-monophosphoryl lipid A (MPL; 50 µg) adsorbed on aluminum hydroxide salt (500 µg Al(OH)3). The vaccine was first licensed as a 3-dose (3D) schedule to be given at months (M) 0,1 and 6. However, 3D regimens of HPV vaccines can be expensive to administer and challenging to complete, particularly in low income countries with limited access to healthcare services.8 Completion rates for the 3D schedule are also suboptimal in some higher income countries.9-12 Alternative vaccination schedules may improve coverage rates.
Evaluation of 2-dose (2D) schedules of HPV vaccines for preteen/adolescent girls was prompted by the observation that antibody titers to HPV vaccine types following administration of standard 3D schedules were approximately 2-fold higher in girls aged 9-15 y than in young women (15-25y),13,14 the age group in which vaccine efficacy has previously been demonstrated in clinical trials.2-4,15-18 We conducted a Phase I/II study to evaluate the immunogenicity and safety of 2D schedules of the HPV-16/18 AS04-adjuvanted vaccine in females aged 9-25 y, and showed that a 2D schedule of the licensed vaccine formulation given at M0,6 to girls aged 9-14 y was immunologically non-inferior one month after the last vaccine dose to the 3D schedule given to young women aged 15-25 y.19 Ratios of HPV-16 and −18 geometric mean antibody titers (GMTs) were also close to 1 at all time points up to 4 y after first vaccination.19,20 The current end-of-study analysis now extends the follow-up in this study to 5 y after first vaccination. Protocol-defined objectives for this 5-year follow-up were to describe the kinetics of observed HPV-16 and −18 antibody responses measured by enzyme-linked immunosorbent assay (ELISA) and to evaluate safety. Post-hoc exploratory objectives were to compare HPV-16 and −18 GMTs induced by the 2D M0,6 schedule in girls aged 9-14 y and the 3D schedule in women aged 15-25 y and to predict the duration of antibody persistence using statistical modeling.
Results
Study population
A total of 960 participants received at least one vaccine dose and are included in the total vaccinated cohort (TVC). The licensed vaccine formulation (20 µg each of HPV-16 and −18 L1 VLPs adjuvanted with AS04) was administered to 239 participants on a 3D M0,1,6 schedule and 240 participants on a 2D M0,6 schedule (Fig. 1). An alternative vaccine formulation (40 µg each of HPV-16 and −18 L1 VLPs adjuvanted with AS04) was administered to 241 participants on a 2D M0,6 schedule and 240 participants on a 2D M0,2 schedule. In the current Month 60 analysis, we report data for the licensed vaccine formulation only. Immunogenicity and safety data up to Month 24 for the alternative vaccine formulation have been reported previously and no added benefit over the standard formulation was observed.19
Figure 1.
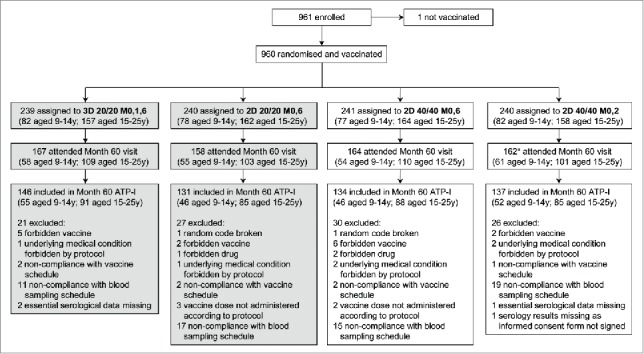
Flow of participants through the trial. 2D, 2-dose schedule; 3D, 3-dose schedule; 20/20, licensed HPV-16/18 AS04-adjuvanted vaccine formulation containing 20 μg each of HPV-16 and −18 L1 virus-like particles and adjuvanted with AS04; 40/40, alternative HPV-16/18 AS04-adjuvanted vaccine formulation containing 40 μg each of HPV-16 and −18 L1 virus-like particles and adjuvanted with AS04; ATP-I, according-to-protocol immunogenicity cohort; M, month; y, years. *Excluding one subject who attended the Month 60 visit but did not sign the informed consent form for this visit. This article focuses on subjects randomized to receive the HPV-16/18 AS04-adjuvanted licensed vaccine formulation (3D 20/20 M0,1,6 and 2D 20/20 M0,6 groups; shaded boxes). Disposition data are also shown for subjects randomized to receive the alternative HPV-16/18 AS04-adjuvanted vaccine formulation (2D 40/40 M0,6 and 2D 40/40 M0,2 groups) for completeness.
For the licensed vaccine formulation, a total of 167 participants in the 3D group and 158 participants in the 2D group attended the Month 60 visit and of these, 146 (87%) and 131 (83%) participants, respectively, were included in the Month 60 according-to-protocol cohort for immunogenicity (ATP-I). Reasons for exclusion from the ATP-I are shown in Figure 1. Demographic characteristics and baseline serostatus for the 2D and 3D groups by age strata are shown in Table 1.
Table 1.
Summary of demographic characteristics and baseline serostatus by age stratum
| 3D M0,1,6 schedule |
2D M0,6 schedule |
|||
|---|---|---|---|---|
| Girls 9-14 years | Women 15-25 years | Girls 9-14 years | Women 15-25 years | |
| N = 58 | N = 109 | N = 55 | N = 103 | |
| Month 60 TVCAge (years) | ||||
| Mean (SD) | 12.4 (1.67) | 19.9 (3.12) | 12.5 (1.63) | 19.8 (3.21) |
| Median | 13.0 | 20.0 | 13.0 | 19.0 |
| Race, n (%) | ||||
| White - Caucasian / European Heritage | 58 (100) | 107 (98.2) | 52 (94.5) | 97 (94.2) |
| African Heritage / African American | 0 (0.0) | 1 (0.9) | 1 (1.8) | 1 (1.0) |
| American Indian or Alaskan Native | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (1.9) |
| Asian - East Asian Heritage | 0 (0.0) | 1 (0.9) | 1 (1.8) | 0 (0.0) |
| Asian - Japanese Heritage | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.0) |
| Other | 0 (0.0) | 0 (0.0) | 1 (1.8) | 2 (1.9) |
| Month 60 ATP-I | N = 55 | N = 91 | N = 46 | N = 85 |
| HPV-16 baseline serostatus, n (%) | ||||
| Seronegative | 48 (87.3) | 79 (86.8) | 45 (97.8) | 74 (87.1) |
| Seropositive* | 7 (12.7) | 12 (13.2) | 1 (2.2) | 11 (12.9) |
| HPV-18 baseline serostatus, n (%) | ||||
| Seronegative | 49 (89.1) | 76 (83.5) | 43 (93.5) | 73 (85.9) |
| Seropositive† | 6 (10.9) | 15 (16.5) | 3 (6.5) | 12 (14.1) |
2D, 2-dose schedule of the licensed HPV-16/18 AS04-adjuvanted vaccine formulation; 3D, 3-dose schedule of the licensed HPV-16/18 AS04-adjuvanted vaccine formulation; ATP-I, according-to-protocol immunogenicity cohort at Month 60; ELISA, enzyme-linked immunosorbent assay; N, number of subjects in the cohort; n(%); number (percentage) of subjects in the given category; SD, standard deviation; TVC, total vaccinated cohort at Month 60;
HPV-16 antibody titer equal to or above the ELISA cut-off of 8 ELISA unit(EU)/mL pre-vaccination.
HPV-18 antibody titer equal to or above the ELISA cut-off of 7 EU/mL pre-vaccination.
Observed persistence of antibody responses 5 y after first vaccination
In the Month 60 ATP-I, all participants who were seronegative prior to vaccination were seropositive for HPV-16 and −18 antibodies at Month 60 and the ratios of anti-HPV-16 and −18 GMTs for the 2D schedule administered to girls aged 9-14 y and the 3D schedule administered to women aged 15-25 y were close to 1 (Table 2). The kinetics of HPV-16 and −18 antibody responses for girls aged 9-14 y administered the 2D schedule followed a similar pattern to those observed for women aged 15-25 y administered the 3D schedule (Fig. 2). Antibody responses in both groups peaked one month after the last vaccine dose at Month 7 and gradually declined thereafter between Months 18 and 24 to reach a plateau that was sustained up to 5 y after administration of the first vaccine dose.
Table 2.
Observed HPV-16 and −18 antibody responses by ELISA at Month 60 for initially seronegative subjects in the ATP-I
| Antigen | Statistic | 3D M0,1,6 schedule Women 15-25 years | 2D M0,6 schedule Girls 9-14 years |
|---|---|---|---|
| HPV-16 | N | 79 | 45 |
| Seropositivity rate, n (%) | 79 (100) | 45 (100) | |
| GMT, EU/mL (95% CI) | 1454.5 (1187.2, 1782.1) | 1369.0 (1104.0, 1697.5) | |
| GMR* (3D/2D) (95% CI) | — | 1.06 (0.78, 1.45) | |
| HPV-18 | N | 76 | 43 |
| Seropositivity rate, n (%) | 76 (100) | 43 (100) | |
| GMT, EU/mL (95% CI) | 634.8 (497.9, 809.3) | 627.2 (476.1, 826.1) | |
| GMR* (3D/2D) (95% CI) | — | 1.01 (0.69, 1.48) |
Post-hoc exploratory analysis.
2D, 2-dose schedule of the licensed HPV-16/18 AS04-adjuvanted vaccine formulation; 3D, 3-dose schedule of the licensed HPV-16/18 AS04-adjuvanted vaccine formulation; 95% CI, exact 95% confidence interval; ATP-I, according-to-protocol immunogenicity cohort; ELISA, enzyme-linked immunosorbent assay; EU/mL, ELISA unit per milliliter; GMR, ratio of geometric mean antibody titers; GMT, geometric mean antibody titer; M, month; N, number of evaluable seronegative subjects in the Month 60 ATP-I; n (%), number (percentage) of seropositive subjects at Month 60.
Figure 2.
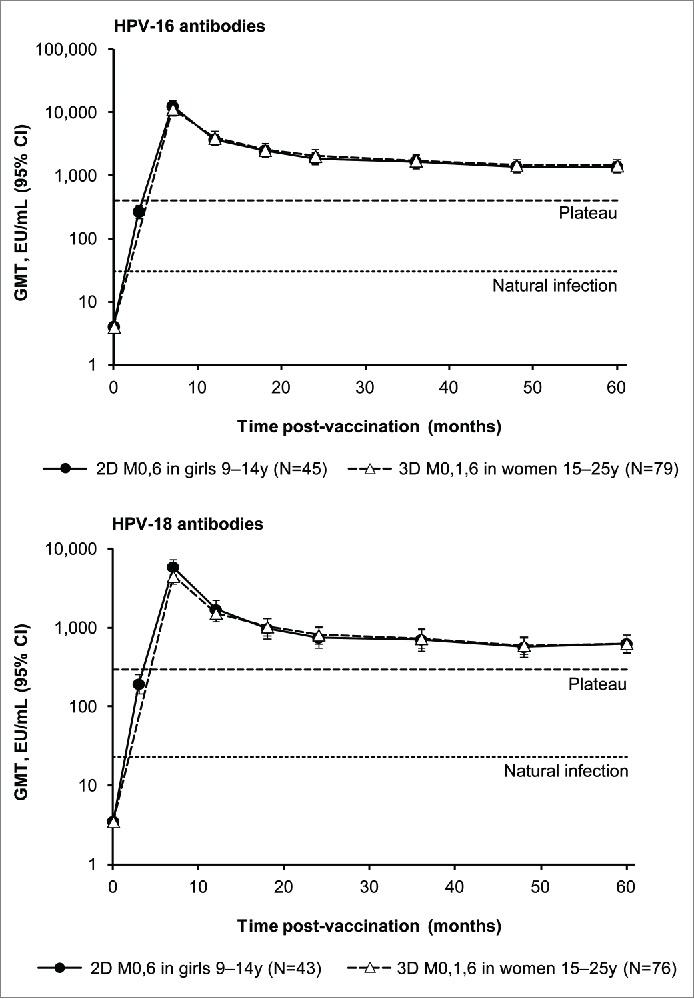
Observed HPV-16 and −18 geometric mean antibody titers (GMT) and corresponding 95% confidence intervals (CI) by enzyme-linked immunosorbent assay (ELISA) at each time point for subjects in the Month 60 according-to-protocol immunogenicity cohort (ATP-I) who were seronegative at baseline for the corresponding antigen. 2D, 2-dose schedule of the licensed HPV-16/18 AS04-adjuvanted vaccine formulation; 3D, 3-dose schedule of the licensed HPV-16/18 AS04-adjuvanted vaccine formulation. M, month; N, number of evaluable seronegative subjects in the Month 60 ATP-I; Plateau, GMTs at the plateau level (Month 45-50 time point) in women aged 15-25 y administered 3 doses of the HPV-16/18 AS04-adjuvanted vaccine at months 0, 1 and 6 in a previous trial (NCT00120848) were 397.8 and 297.3 ELISA unit (EU)/mL for HPV-16 and −18 antibodies, repectively.16 Natural infection, GMTs in women aged 15-25 y who had cleared a natural infection in a previous trial (NCT00122681) were 29.8 and 22.7 EU/mL for HPV-16 and −18 antibodies, repectively.1
HPV-16 and −18 GMTs are presented by pre-vaccination serostatus and by age stratum (9-25 y, 9-14 y or 15-25 y) in Supplemental Tables 1 and 2, respectively. Similar results were observed regardless of pre-vaccination serostatus for both HPV-16 and −18 antibodies. Within each age stratum, HPV-16 and HPV-18 GMTs appeared slightly higher after administration of the 3D schedule than after the 2D schedule.
For the 2D schedule administered to girls aged 9-14 years, vaccine induced anti-HPV-16 and −18 GMTs at Month 60 in the present study were, respectively, 45.9- and 27.6-fold higher than those induced by natural infection1 and 3.4- and 2.1-fold higher than the corresponding GMTs from the plateau phase (Months 45-50) of a reference study,16 in which efficacy of the HPV-16/18 AS04-adjuvanted vaccine was demonstrated against HPV-16 and −18 associated infections and histopathological lesions up to 6.4 y after first vaccination in women aged 15-25 y.21 For the 3D schedule administered to women aged 15-25 y, vaccine induced anti-HPV-16 and −18 GMTs at Month 60 in the present study were, respectively, 48.8- and 28.0-fold higher and 3.7- and 2.1-fold higher than these previously observed in natural infection and plateau benchmarks.
Predicted long-term persistence of antibody responses
Figure 3 depicts the predictions of long-term persistence of HPV-16 and −18 antibody responses for girls aged 9-14 y administered a 2D schedule or women aged 15-25 y administered a 3D schedule (modeled on the basis of 5 y of follow-up data from the current study). Data from 51 of 78 (65.4%) vaccinated girls aged 9-14 y in the 2D group and 95 of 157 (60.5%) of women aged 15-25 y in the 3D group are included. As a reference, this figure also depicts predicted GMTs for women aged 15-25 y administered 3 doses of the HPV-16/18 AS04-adjuvanted vaccine at M0,1,6, modeled on the basis of 6.4 y of follow-up data from a previous efficacy study,21 and GMTs measured in women who had cleared a natural infection in a previous study.1
Figure 3.
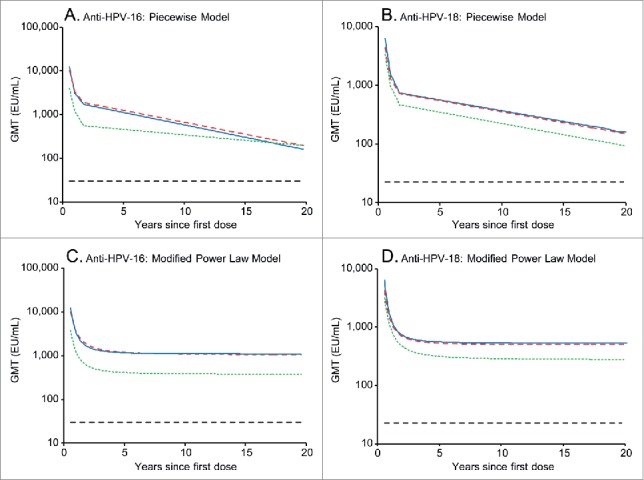
Predicted HPV-16 and −18 geometric antibody mean antibody titers (GMTs) over 20 y, as predicted by the piecewise model (panels A and B) and modified power law model (panels C and D). Blue solid line, predicted GMTs for girls aged 9-14 y administered 2 doses of the licensed HPV-16/18 AS04-adjuvanted vaccine at months 0 and 6, modeled on the basis of 5 y of follow-up data from the current study. Red dashed line, predicted GMTs for women aged 15-25 y administered 3 doses of the licensed HPV-16/18 AS04-adjuvanted vaccine at months 0, 1 and 6, modeled on the basis of 5 y of follow-up data from the current study. Green dotted line, predicted GMTs for women aged 15-25 y administered 3 doses of the licensed HPV-16/18 AS04-adjuvanted vaccine at months 0, 1 and 6, modeled on the basis of 6.4 y of follow-up data from a previous efficacy study (NCT00120848).21 Black dashed line, GMTs in women who had cleared a natural infection in a previous trial (NCT00122681) (29.8 and 22.7 ELISA unit (EU)/mL for anti-HPV-16 and −18, respectively).1 ELISA, enzyme-linked immunosorbent assay.
The piecewise model predicts that HPV-16 and −18 antibody titers will remain above those associated with natural infection in 95% of women for at least 21 y when the vaccine is administered as a 2D schedule to girls aged 9-14 y or a 3D schedule to women aged 15-25 y (Table 3). The modified power law predicts that antibody titers will always remain above those associated with natural infection in 95% of women (ie, life-long duration) for the 2D schedule administered to girls aged 9-14 y and the 3D schedule administered to women aged 15-25 y (Table 3).
Table 3.
Predicted HPV-16 and −18 antibody titers after 20 y and duration of persistence
| Predicted GMTs 20 y after first vaccine dose* |
Predicted duration of antibody persistence above natural infection levels† in 95% of women |
||||
|---|---|---|---|---|---|
| Antigen | Statistical model | 3D M0,1,6 15-25 years | 2D M0,6 9-14 years | 3D M0,1,6 15-25 years | 2D M0,6 9-14 years |
| HPV-16 | Piecewise | 189.7 EU/mL | 157.9 EU/mL | 22.0 years | 24.4 years |
| Modified power law | 1054.2 EU/mL | 1091.0 EU/mL | Always | Always | |
| HPV-18 | Piecewise | 149.1 EU/mL | 158.7 EU/mL | 21.5 years | 27.3 years |
| Modified power law | 497.4 EU/mL | 530.3 EU/mL | Always | Always | |
Post-hoc exploratory analysis.
2D, 2-dose schedule of the licensed HPV-16/18 AS04-adjuvanted vaccine formulation; 3D, 3-dose schedule of the licensed HPV-16/18 AS04-adjuvanted vaccine formulation; ELISA, enzyme-linked immunosorbent assay; EU/mL, ELISA unit per milliliter; GMT, geometric mean antibody titer; M, month.
For those subjects who receive the scheduled number of vaccine doses.
Natural infection, GMTs in women aged 15-25 y who had cleared a natural infection in a previous trial (NCT00122681) were 29.8 and 22.7 EU/mL for HPV-16 and −18 antibodies, repectively.1
Safety
All vaccine formulations and schedules evaluated in this study have been shown previously to have a clinically acceptable reactogenicity19 and safety profile up to Month 48.20 In this longer-term evaluation up to Month 60, the safety profile of the licensed formulation of the vaccine was comparable whether administered as a 2D or 3D schedule (Table 4).
Table 4.
Summary of safety and pregnancy outcomes in the total vaccinated cohort (TVC)
| 3D M0,1,6 schedule 9-25 years | 2D M0,6 schedule 9-25 years | |
|---|---|---|
| Safety Outcomes from Months 48 to 60* | ||
| Number of subjects in Month 60 TVC | 167 | 158 |
| Subjects with at least one event, n (%) [95% CI] | ||
| Serious adverse events | 2 (1.2) [0.1, 4.3] | 0 (0.0) [0.0, 2.3] |
| Medically significant conditions | 10 (6.0) [2.9, 10.7] | 9 (5.7) [2.6, 10.5] |
| New onset autoimmune diseases | 2 (1.2) [0.1, 4.3] | 0 (0.0) [0.0, 2.3] |
| Safety Outcomes from Months 0 to 60† | ||
| Number of subjects in TVC | 239 | 240 |
| Subjects with at least one event, n (%) [95% CI] | ||
| Serious adverse events | 15 (6.3) [3.6, 10.1] | 19 (7.9) [4.8, 12.1] |
| Medically significant conditions | 89 (37.2) [31.1, 43.7] | 92 (38.3) [32.2, 44.8] |
| New onset autoimmune diseases | 6 (2.5) [0.9, 5.4] | 5 (2.1) [0.7, 4.8] |
| Pregnancies from Months 0 to 60 | ||
| Number of subjects with pregnancies | 26 | 30 |
| Outcomes, n (%) | ||
| Ectopic pregnancy | 1 (3.8) | 0 (0.0) |
| Elective termination no apparent congenital anomaly | 6 (23.1) | 4 (13.3) |
| Elective termination congenital anomaly | 0 (0.0) | 1 (3.3) |
| Live infant no apparent congenital anomaly | 18 (69.2) | 22 (73.3) |
| Spontaneous abortion no apparent congenital anomaly | 1 (3.8) | 3 (10.0) |
2D, 2-dose schedule of the licensed HPV-16/18 AS04-adjuvanted vaccine formulation; 3D, 3-dose schedule of the licensed HPV-16/18 AS04-adjuvanted vaccine formulation; 95% CI, exact 95% confidence interval; n (%), number (percentage) of subjects with at least one event within the given category.
New events occurring since the previous reporting period for this trial.20
Events for the entire study period from Months 0 to 60.
Over the 5-year period from Months 0 to 60, 4 pregnancies which ended in spontaneous abortion were reported (1 [3.8%] in the 3D group and 3 [10.0%] in the 2D group) (Table 4). The women were aged 19-27 y at the time of the spontaneous abortion. All four of these pregnancies occurred at least 12 months after the last vaccine dose. The apparent difference in frequencies between groups is likely a chance finding due to the small number of events.
Discussion
In this study, we show that 2D of the HPV-16/18 AS04-adjuvanted vaccine administered at 0 and 6 months induced high HPV-16 and −18 antibody responses in girls aged 9-14 y, which are sustained up to 5 y after first vaccination. The kinetics of HPV-16 and −18 antibody responses in the current trial, regardless of whether the vaccine was administered on a 2D or 3D schedule, were similar to those observed in previous clinical trials with this vaccine, with GMTs peaking one month after administration of the last dose and then declining to reach a plateau at approximately 18-24 months after first vaccination.22-24 Compared with levels following natural infection, HPV-16 and −18 antibody levels in the vaccine groups were at least 25-fold higher. Antibody titers were also above those observed at the plateau phase in a previous trial in which 100% vaccine efficacy against CIN2+ was demonstrated up to 6.4 y after first vaccination in women aged 15-25 y who received 3D.21 The inclusion of the AS04 adjuvant system in the vaccine formulation may contribute to the high and sustained antibody titers induced by this vaccine.25
HPV-16 and −18 antibody titers elicited by the 2D schedule in girls aged 9-14 y were comparable to those elicited by the 3D schedule in young women aged 15-25 y, with GMT ratios being close to 1 at the 5-year time point. GMT ratios for these 2 groups of subjects have consistently been close to 1 at all previous evaluations, including one month after the last vaccine dose when non-inferiority was formally demonstrated.19,20 An appropriately powered Phase III trial of the HPV-16/18 vaccine (NCT01381575) with a larger sample size also demonstrated non-inferiority of HPV-16 and −18 antibody responses for a 2D M0,12 and 2D M0,6 schedule in girls aged 9-14 y compared with the 3D schedule in women aged 15-25 y one and 6 months after the last vaccine dose, respectively.26 Additionally, this larger study showed descriptively similar cross-reacting HPV-31 and −45 antibody titers and cell-mediated immune responses between 2D and 3D groups one month after the last vaccine dose.27 On the basis of data from the current study, and the larger Phase III study, a 2D schedule of the HPV-16/18 AS04-adjuvanted vaccine is now approved for girls aged 9–14 y in a number of countries, with flexibility around administration of the second vaccine dose from 5 to 13 months after first vaccination. A 2D schedule at 0 and 6 months of the quadrivalent HPV-6/11/16/18 vaccine is also approved for girls aged 9-13 y.
We applied 2 statistical models, using the 5-year data observed in the current study (which included approximately 60% of vaccinated subjects for the relevant age strata in each vaccine group), to predict how long vaccine-induced antibodies are likely to persist. For both models, predictions of persistence were similar for girls aged 9-14 y administered the 2D schedule and women aged 15-25 y administered the 3D schedule, which was expected given the similarity of observed antibody responses over 5 y for these 2 groups. The effect of age upon the magnitude of initial HPV-16 and −18 antibody responses is well documented,13,28 but there is no evidence of an interaction between age and time that might indicate different antibody decay rates between age groups.29 Using the modified power law, which assumes a progressive decay of antibody and antibody-producing B-cells while assuming that the proportion of memory B-cells remains stable,30 lifelong persistence of vaccine-induced antibody titers above those associated with natural infection is predicted for 95% of the population. The piecewise model assumes a linear decay of serum antibody levels from Month 21 onwards and provides a more conservative estimation of long-term antibody responses.29 Using the piecewise model, it is predicted that vaccine-induced antibody titers will be sustained above those associated with natural infection for at least 21 y for 95% of the population.
This study was not designed to assess efficacy, but using the principle of immunobridging we infer that protection against HPV infection and cervical disease in adolescent girls given a 2D schedule will be similar to that previously observed in women aged 15-25 y given a 3D schedule. The large Phase III efficacy trial (PATRICIA) conducted with a 3D schedule of the HPV-16/18 AS04-adjuvanted vaccine in women aged 15-25 y showed very high vaccine efficacy against grade 3 or greater cervical intraepithelial neoplasia in HPV-naive women, irrespective of HPV type in the lesion.3 Proof-of principle for the efficacy of fewer than 3 vaccine doses comes from a post-hoc analysis of the Costa Rica HPV-16/18 Vaccine Trial (CVT), conducted in women aged 18-25 y.31 While this comparison was not randomized, similar vaccine efficacy was observed against 12-month persistent HPV-16 and −18-associated infection in women who received only 2 or even one dose of the 3 scheduled vaccine doses compared with those women who received all 3 doses. Immunological evaluation from the CVT showed that both anti-HPV-16 and −18 GMTs (by ELISA) among women who received 2 vaccine doses separated by 6 months were non-inferior to those in women who received the complete 3D schedule 4 y after first vaccination and that anti-HPV-16 and −18 GMTs in women who received 2 vaccine doses were at least 24- and 14-fold higher than those observed in natural infection.32
We previously showed that both the 2D and 3D schedules had a clinically acceptable reactogenicity profile in this study,19 and no safety concerns were raised during this 5-year follow-up. Safety findings were generally in accordance with a pooled analysis of data from completed or ongoing clinical studies of the HPV-16/18 AS04-adjuvanted vaccine, which show that it has an acceptable benefit-risk profile in adolescent girls and adult women.7
A strength of our study is that this is the longest period of follow-up for a 2D schedule of an HPV vaccine, providing confidence in the persistence of responses with a reduced dose schedule. A limitation is that the study was designed and powered to evaluate non-inferiority of antibody responses at Month 7 only. Results from exploratory comparisons of GMT ratios at subsequent time points should be interpreted with caution as there was no adjustment for multiplicity and the clinical relevance of any difference was not accounted for in the planning of the exploratory analysis. Although the statistical modeling predicts that administration of a 2D schedule of the HPV-16/18 AS04-adjuvanted vaccine to preteen/adolescent girls will provide long-term persistence of HPV-16 and −18 antibodies, which may protect them from the consequences of HPV-16 and −18 infection for most of their sexually active lives, these conclusions can only be considered as informative until long-term observational data are available. The study was conducted in healthy females of predominantly Caucasian ethnicity, and it is not known if the predictions regarding long-term antibody kinetics can be extrapolated to other populations.
In conclusion, the durable immune response elicited by a 2D schedule of the HPV-16/18 vaccine in preteen/adolescent girls is predicted to provide long-lasting protection against HPV infection and subsequent development of high-grade cervical lesions and cancer. A 2D schedule is likely to offer logistical and economic advantages over a 3D schedule, which in turn may facilitate greater vaccination coverage rates for adolescent girls, with the potential to substantially reduce the global burden of cervical cancer.
Methods and Participants
Study design, participants and ethics
The design of this Phase I/II, partially-blind, controlled, randomized, parallel group trial has been reported previously.19,20 The study was conducted at 21 centers in Canada and Germany. It was initiated in October 2007 and data for the Month 60 analysis were collected up to March 2013. Briefly, healthy girls and young women aged 9-25 y at the time of first vaccination in the study were stratified by age (9-14, 15-19, 20-25 y) and randomized (1:1:1:1) to receive 3 doses of the licensed HPV-16/18 AS04-adjuvanted vaccine formulation (containing 20 µg each of HPV-16 and −18 L1 VLPs adjuvanted with AS04) at 0, 1 and 6 months, 2 doses of the licensed vaccine formulation at 0 and 6 months, 2 doses of an alternative vaccine formulation (containing 40 µg each of HPV-16 and −18 L1 VLPs adjuvanted with AS04) at 0 and 6 months, or 2 doses of the alternative vaccine formulation at 0 and 2 months.
The trial was approved by the appropriate Independent Ethics Committee for each center and was conducted according to the Declaration of Helsinki and Good Clinical Practice. The trial is registered with www.clinicaltrials.gov (registration number NCT00541970). A summary of the protocol is available at www.gsk-clinicalstudyregister.com (GSK study ID 110659). All participants provided written informed consent, or informed assent with written consent from a parent or legal representative (if below the legal age of consent).
Study data up to 4 y after first vaccination (including primary and secondary endpoints) have been published previously.19,20 Here, we extend immunogenicity and safety data through 5 y after first vaccination and present statistical modeling predictions of antibody persistence. We focus on results for girls aged 9–14 y who received the 2D schedule of the licensed vaccine formulation and women aged 15–25 y who received the 3D schedule of the licensed vaccine formulation.
Vaccines, randomization and masking
Each 0.5 mL dose of the licensed vaccine formulation (Cervarix®, GSK group of companies) contained 20 μg of HPV-16 and 20 μg of HPV-18 L1 VLPs adjuvanted with AS04 and each 0.5 mL vaccine dose of the alternative vaccine formulation contained 40 μg of HPV-16 and 40 μg of HPV-18 L1 VLPs adjuvanted with AS04. AS04 is a GSK proprietary Adjuvant System containing MPL (50 µg) adsorbed on aluminum hydroxide salt (500 µg Al(OH)3). Vaccine doses were administered by intramuscular injection in the deltoid region of the non-dominant arm.
The randomization schedule was generated by GSK Vaccines using validated software. The study was observer-blind within the 2D schedule groups, with blinding maintained to Month 7, as reported previously.19 The study was open within the 3D group.
Immunological evaluation
Blood samples for serologic evaluation were drawn prior to first vaccination (Month 0), at Month 3 (2D groups only), and at Months 7, 12, 18, 24, 36, 48 and 60. Antibodies to HPV-16 and −18 were measured by ELISA, as described previously.33 Seropositivity was defined as an antibody titer greater than the assay cut-off. For time points from Month 0 through Month 48, the assay cut-off was 8 ELISA unit (EU)/mL for HPV-16 and 7 EU/mL for HPV-18. The assay used to measure HPV-16 and −18 antibody concentrations at the designated laboratory was recently improved to increase precision, consequently for the Month 60 time point the assay cut-off changed from 8 EU/mL to 19 EU/mL for HPV-16 and from 7 EU/mL to 18 EU/mL for HPV-18.
Safety evaluation
Serious adverse events, adverse events leading to withdrawal, other medically significant conditions (ie, adverse events prompting emergency room or physician visits that were not related to common diseases), new onset chronic diseases including new onset autoimmune diseases and pregnancies occurring through Month 60 were documented. Pregnancies were followed until delivery. As described previously,34 all adverse events reported during the trial were compared with a pre-defined list of potential chronic diseases derived from the Medical Dictionary for Regulatory Activities. Determination of whether a chronic disease was of new onset was based on blinded review of the reported symptoms and the subject's pre-vaccination medical history by a physician from GSK. A separate list, restricted to potential autoimmune events which excluded allergy-related events or isolated signs and symptoms and events not considered to be autoimmune in origin, was used to identify new onset autoimmune diseases among events identified as new onset chronic diseases.
Statistical methods
The sample size justification for this study has been reported previously.19 The TVC included all vaccinated subjects and the Month 60 TVC included all vaccinated subjects with data at Month 60. The Month 60 ATP-I included all evaluable subjects (ie, those meeting all eligibility criteria, complying with the procedures defined in the protocol, with no elimination criteria during the trial) for whom data concerning immunogenicity endpoints were available. This included subjects who returned for blood sampling at Month 60 and for whom assay results were available for antibodies against at least one study vaccine antigen component after vaccination. Analyses were performed using SAS 9.2 and PROC StatXact 8.1.
Seroconversion and seropositivity rates with exact 95% confidence interval (CI) and GMTs (with 95% CI) for HPV-16 and −18 antibodies were calculated by pre-vaccination serostatus. GMTs were computed by taking the anti-log of the mean of the log titer transformations; antibody titers below the cut-off of the assay were given an arbitrary value of half the cut-off in this calculation. HPV-16 and −18 antibody GMTs in the present study were compared descriptively with those observed in women aged 15–25 y in a previous study who had cleared a natural infection (29.8 and 22.7 EU/mL, respectively),1 as well as with those measured at the plateau phase at Months 45–50 in another study (397.8 and 297.3 EU/mL, respectively),16 in which vaccine efficacy was demonstrated in women aged 15–25 y.21 The proportion of participants with at least one report of a serious adverse event, medically significant condition, new onset chronic disease, and new onset autoimmune disease were calculated with exact 95% CI.
In a post-hoc exploratory analysis, anti-HPV-16 and −18 titers at Month 60 were compared between 2D and 3D schedules for initially seronegative subjects in the Month 60 ATP-I by calculating the ratio of GMTs with exact 95% CI (3D schedule in women aged 15-25 y divided by the 2D schedule in girls aged 9-14 y).
In post-hoc exploratory analyses of the persistence of vaccine-induced antibodies, 2 different mixed effects models (the modified power law and the piecewise models) were fitted to the individual HPV-16 and −18 antibody titers measured at each time point up to Month 60 in participants in the total vaccinated cohort who had received the scheduled number of doses of the HPV-16/18 AS04-adjuvanted vaccine and for whom results were available at all the post-vaccination time points, as previously described.29,30 The piecewise model fitted the data on 3 non-overlapping time intervals, corresponding to the observed decay of humoral antibodies. Each piece of the model used a linear function, and 3 break points (Months 7, 12 and 21) were selected on the basis of Akaike's Information Criterion.35 The modified power law model includes B-cell dynamics to estimate antibody decay over time after vaccination, in which 2 populations of B-cells (activated and memory B-cells) are involved, accounting for the long-term persistence of a memory B-cell subpopulation and a long-term antibody plateau.30 The piecewise and power law models were fitted using, respectively, a MIXED and a NLMIXED SAS procedure.
Disclosure of Potential Conflicts of Interest
B.R. received grants, travel support and speaker's fees through her institution and her Professional Corporation from the GSK group of companies outside of the submitted work. T.S. has received honoraria from the GSK group of companies during the conduct of the study and personal fees from the GSK group of companies outside of the submitted work. L.F. has received travel payments from the GSK group of companies during the conduct of the study. Outside of the submitted work she has received consultancy fees, lecture payments, fees for developing educational presentations and travel payments from the GSK group of companies, Merck, Sanofi, Pfizer and Novartis. She is a member of Colchester Research Group which received payment from the GSK group of companies, Merck, Sanofi, Pfizer and Novartis. K.P. received fees from the GSK group of companies to conduct the study. K.S. received payments from the GSK group of companies during the conduct of the study and outside of the submitted work. The institution of P.H. received grants/grants pending from the GSK group of companies during the conduct of the study and outside of the submitted work. P.H. also received payment for expert testimony from the GSK group of companies outside of the submitted work. P.S., F.T. and F.S. are employees of the GSK group of companies. F.T. and F.S. also own restricted shares/stock options in the GSK group of companies. M.D. and U.B. have nothing to disclose.
Acknowledgments
The authors thank the study participants and their families, as well as the study investigators and their staff members who are not named as authors but who substantially contributed to the HPV-048 study at months 60. The authors would like to thank the global and regional clinical teams of GSK Vaccines for their overall contribution to the design and conduct of the study, the Research and Development team for the laboratory analyses, the data management and statistical teams for their contribution to the data collection and analysis and the elaboration of the statistical report, the scientific writers for their assistance to the writing of the clinical protocol and the clinical study report. They also acknowledge Julie Taylor (Peak Biomedical, UK, on behalf of GSK Vaccines) for writing assistance and Bruno Baudoux (Business & Decisions Life Sciences, Belgium, on behalf of GSK Vaccines) for editorial assistance and manuscript coordination.
Authors' Contributions
T.S. was one of the coordinating investigators and together with B.R., L.F., K.P., M.D., U.B., K.S. and P.H. participated in the recruitment and/or follow-up of subjects. T.S. designed the study in collaboration with GSK Vaccines. At GlaxoSmithKline (India), P.S. contributed toward data analyses and interpretation, and prepared the statistical analysis report. F.S. and F.T. supervised the conduct of the study at GSK Vaccines (Belgium), and together with T.S. critically reviewed the study report. All authors reviewed and commented upon the drafts of the manuscript and gave final approval to submit for publication. All authors had full access to the data. The authors received no financial support or other form of compensation for the development of the manuscript.
Trademark Statement
Cervarix is a registered trade mark of the GSK group of companies.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
Funding
GlaxoSmithKline Biologicals SA funded this study (clinicaltrials.gov registration number NCT00541970) and was involved in all stages of study conduct, including analysis of the data. GlaxoSmithKline Biologicals SA also took in charge all costs associated with the development and publication of this manuscript.
References
- 1.Paavonen J, Jenkins D, Bosch FX, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter DL, Kitchener HC, Castellsagué X, et al.. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet 2007; 369: 2161-70; PMID:17602732; http://dx.doi.org/ 10.1016/S0140-6736(07)60946-5 [DOI] [PubMed] [Google Scholar]
- 2.Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, Kitchener H, Castellsagué X, Teixeira JC, Skinner SR, et al.. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 2009; 374: 301-14; PMID:19586656; http://dx.doi.org/ 10.1016/S0140-6736(09)61248-4 [DOI] [PubMed] [Google Scholar]
- 3.Lehtinen M, Paavonen J, Wheeler CM, Jaisamrarn U, Garland SM, Castellsagué X, Skinner SR, Apter D, Naud P, Salmeron J, et al.. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 2012; 13: 89-99; PMID:22075171; http://dx.doi.org/ 10.1016/S1470-2045(11)70286-8 [DOI] [PubMed] [Google Scholar]
- 4.Wheeler CM, Castellsagué X, Garland SM, Szarewski A, Paavonen J, Naud P, Salmeron J, Chow SN, Apter D, Kitchener H, et al.. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 2012; 13: 100-10; PMID:22075170; http://dx.doi.org/ 10.1016/S1470-2045(11)70287-X [DOI] [PubMed] [Google Scholar]
- 5.Verstraeten T, Descamps D, David MP, Zahaf T, Hardt K, Izurieta P, Dubin G, Breuer T. Analysis of adverse events of potential autoimmune aetiology in a large integrated safety database of AS04 adjuvanted vaccines. Vaccine 2008; 26: 6630-8; PMID:18845199; http://dx.doi.org/ 10.1016/j.vaccine.2008.09.049 [DOI] [PubMed] [Google Scholar]
- 6.Descamps D, Hardt K, Spiessens B, Izurieta P, Verstraeten T, Breuer T, Dubin G. Safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine for cervical cancer prevention: a pooled analysis of 11 clinical trials. Hum Vaccin 2009; 5: 332-40; PMID:19221517; http://dx.doi.org/ 10.4161/hv.5.5.7211 [DOI] [PubMed] [Google Scholar]
- 7.Angelo MG, David MP, Zima J, Baril L, Dubin G, Arellano F, Struyf F. Pooled analysis of large and long-term safety data from the human papillomavirus-16/18-AS04-adjuvanted vaccine clinical trial programme. Pharmacoepidemiol Drug Saf 2014; 23: 466-79; PMID:24644063; http://dx.doi.org/ 10.1002/pds.3554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldie SJ, Gaffikin L, Goldhaber-Fiebert JD, Gordillo-Tobar A, Levin C, Mahe C, Wright TC. Cost-effectiveness of cervical-cancer screening in five developing countries. N Engl J Med 2005; 353: 2158-68; PMID:16291985; http://dx.doi.org/ 10.1056/NEJMsa044278 [DOI] [PubMed] [Google Scholar]
- 9.Deleré Y, Böhmer MM, Walter D, Wichmann O. HPV vaccination coverage among women aged 18-20 years in Germany three years after recommendation of HPV vaccination for adolescent girls: results from a cross-sectional survey. Hum Vaccin Immunother 2013; 9: 1706-11; http://dx.doi.org/ 10.4161/hv.24904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stokley S, Jeyarajah J, Yankey D, Cano M, Gee J, Roark J, Curtis RC, Markowitz L. Human papillomavirus vaccination coverage among adolescents, 2007-2013, and post-licensure vaccine safety monitoring, 2006-2014-United States. MMWR Morb Mortal Wkly Rep 2014; 63: 620-4; PMID:25055185 [PMC free article] [PubMed] [Google Scholar]
- 11.Public Health England Annual HPV vaccine coverage in England: 2012 to 2013. Published by Public Health England on 10 December 2013 Available at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/266190/HPV_AnnualDataTable2012_13_SHA_acc2.pdf. Accessed 11December2014 [Google Scholar]
- 12.Colucci R, Hryniuk W, Savage C. HPV vaccination programs in Canada. Are we hitting the mark? Cancer Advocacy Coalition of Canada. Report Card on Cancer in Canada 2008; 11: 7-10 [Google Scholar]
- 13.Pedersen C, Petaja T, Strauss G, Rumke HC, Poder A, Richardus JH, Spiessens B, Descamps D, Hardt K, Lehtinen M, et al.. Immunization of early adolescent females with human papillomavirus type 16 and 18 L1 virus-like particle vaccine containing AS04 adjuvant. J Adolesc Health 2007; 40: 564-71; PMID:17531764; http://dx.doi.org/ 10.1016/j.jadohealth.2007.02.015 [DOI] [PubMed] [Google Scholar]
- 14.Block SL, Nolan T, Sattler C, Barr E, Giacoletti KE, Marchant CD, Castellsague X, Rusche SA, Lukac S, Bryan JT, et al.. Comparison of the immunogenicity and reactogenicity of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in male and female adolescents and young adult women. Pediatrics 2006; 118: 2135-45; PMID:17079588; http://dx.doi.org/ 10.1542/peds.2006-0461 [DOI] [PubMed] [Google Scholar]
- 15.Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, Zahaf T, Innis B, Naud P, De Carvalho NS, et al.. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet 2004; 364: 1757-65; PMID:15541448; http://dx.doi.org/ 10.1016/S0140-6736(04)17398-4 [DOI] [PubMed] [Google Scholar]
- 16.Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli-Martins CM, Jenkins D, Schuind A, Costa Clemens SA, Dubin G. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 2006; 367: 1247-55; PMID:16631880; http://dx.doi.org/ 10.1016/S0140-6736(06)68439-0 [DOI] [PubMed] [Google Scholar]
- 17.FUTURE II Study Group . Prophylactic efficacy of a quadrivalent human papillomavirus (HPV) vaccine in women with virological evidence of HPV infection. J Infect Dis 2007; 196: 1438-46; PMID:18008221; http://dx.doi.org/ 10.1086/522864 [DOI] [PubMed] [Google Scholar]
- 18.Ault KA, FUTURE II Study Group . Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet 2007; 369: 1861-8; PMID:17544766; http://dx.doi.org/ 10.1016/S0140-6736(07)60852-6 [DOI] [PubMed] [Google Scholar]
- 19.Romanowski B, Schwarz TF, Ferguson LM, Peters K, Dionne M, Schulze K, Ramjattan B, Hillemanns P, Catteau G, Dobbelaere K, et al.. Immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose schedule compared to the licensed 3-dose schedule: Results from a randomized study. Hum Vaccin 2011; 7: 1374-86; PMID:22048171; http://dx.doi.org/ 10.4161/hv.7.12.18322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romanowski B, Schwarz TF, Ferguson LM, Ferguson M, Peters K, Dionne M, Schulze K, Ramjattan B, Hillemanns P, Behre U, et al.. Immune response to the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose or 3-dose schedule up to 4 years after vaccination: Results from a randomized study. Hum Vaccin Immunother 2014; 10: 1155-65; PMID:24576907; http://dx.doi.org/ 10.4161/hv.28022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The GlaxoSmithKline Vaccine HPV-007 Study Group . Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet 2009; 374: 1975-85; PMID:19962185; http://dx.doi.org/ 10.1016/S0140-6736(09)61567-1 [DOI] [PubMed] [Google Scholar]
- 22.Petaja T, Pedersen C, Poder A, Strauss G, Catteau G, Thomas F, Lehtinen M, Descamps D. Long-term persistence of systemic and mucosal immune response to HPV-16/18 AS04-adjuvanted vaccine in preteen/adolescent girls and young women. Int J Cancer 2011; 129: 2147-57; PMID:21190190; http://dx.doi.org/ 10.1002/ijc.25887 [DOI] [PubMed] [Google Scholar]
- 23.Schwarz TF, Huang LM, Medina DM, Valencia A, Lin TY, Behre U, Catteau G, Thomas F, Descamps D. Four-year follow-up of the immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine when administered to adolescent girls aged 10-14 years. J Adolesc Health 2012; 50: 187-94; PMID:22265115; http://dx.doi.org/ 10.1016/j.jadohealth.2011.11.004 [DOI] [PubMed] [Google Scholar]
- 24.Naud PS, Roteli-Martins CM, De Carvalho NS, Teixeira JC, de Borba PC, Sanchez N, Zahaf T, Catteau G, Geeraerts B, Descamps D. Sustained efficacy, immunogenicity, and safety of the HPV-16/18 AS04-adjuvanted vaccine : final analysis of a long-term follow-up study up to 9.4 years post-vaccination. Hum Vaccin Immunother 2014; 10: 2147-62; PMID:25424918; http://dx.doi.org/ 10.4161/hv.29532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giannini SL, Hanon E, Moris P, Van Mechelen M, Morel S, Dessy F, Fourneau MA, Colau B, Suzich J, Losonksy G, et al.. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine 2006; 24: 5937-49; PMID:16828940; http://dx.doi.org/ 10.1016/j.vaccine.2006.06.005 [DOI] [PubMed] [Google Scholar]
- 26.Puthanakit T, Huang L, Tang R, Schwarz T, Esposito S, Frenette L, McNeil S, Rheault P, Horn M, Klar M, et al.. Non-inferiority of HPV-16/18 AS04-adjuvanted vaccine administered as 2-dose schedules in girls (9-14 years) versus 3 doses in women (15-25 years): a randomised trial. Abstract presented at ESPID 2014 >May 6-10, 2014 Dublin, Ireland Available at: http://espid.meetingxpert.net/espid_945/poster_95279/program.aspx. Accessed 11December2014 [Google Scholar]
- 27.Puthanakit T, Schwarz T, Esposito S, Frenette L, McNeil S, Rheault P, Horn M, Poncelet S, Suryakiran P, Hezareh M, et al.. Immune responses to a 2-dose schedule of the HPV-16/18 AS04-adjuvanted vaccine in girls (9-14) versus 3 doses in women (15-25): a randomised trial. Abstract presented at EUROGIN 2013 November 3-6, 2013 Florence, Italy Available at: http://www.eurogin.com/2013/images/pdf/EUROGIN-2013-Abstracts-Part-2.pdf. Accessed 11December2014 [Google Scholar]
- 28.Schwarz TF, Spaczynski M, Schneider A, Wysocki J, Galaj A, Perona P, Poncelet S, Zahaf T, Hardt K, Descamps D, et al.. Immunogenicity and tolerability of an HPV-16/18 AS04-adjuvanted prophylactic cervical cancer vaccine in women aged 15-55 years. Vaccine 2009; 27: 581-7; PMID:19022320; http://dx.doi.org/ 10.1016/j.vaccine.2008.10.088 [DOI] [PubMed] [Google Scholar]
- 29.David MP, Van Herck K, Hardt K, Tibaldi F, Dubin G, Descamps D, Van Damme P. Long-term persistence of anti-HPV-16 and −18 antibodies induced by vaccination with the AS04-adjuvanted cervical cancer vaccine: modeling of sustained antibody responses. Gynecol Oncol 2009; 115: S1-S6; PMID:19217149; http://dx.doi.org/ 10.1016/j.ygyno.2009.01.011 [DOI] [PubMed] [Google Scholar]
- 30.Fraser C, Tomassini JE, Xi L, Golm G, Watson M, Giuliano AR, Barr E, Ault KA. Modeling the long-term antibody response of a human papillomavirus (HPV) virus-like particle (VLP) type 16 prophylactic vaccine. Vaccine 2007; 25: 4324-33; PMID:17445955; http://dx.doi.org/ 10.1016/j.vaccine.2007.02.069 [DOI] [PubMed] [Google Scholar]
- 31.Kreimer AR, Rodriguez AC, Hildesheim A, Herrero R, Porras C, Schiffman M, Gonzalez P, Solomon D, Jimenez S, Schiller JT, et al.. Proof-of-Principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. J Natl Cancer Inst 2011; 103: 1444-51; PMID:21908768; http://dx.doi.org/ 10.1093/jnci/djr319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Safaeian M, Porras C, Pan Y, Kreimer A, Schiller JT, Gonzalez P, Lowy DR, Wacholder S, Schiffman M, Rodriguez AC, et al.. Durable antibody responses following one dose of the bivalent human papillomavirus L1 virus-like particle vaccine in the Costa Rica Vaccine Trial. Cancer Prev Res (Phila) 2013; 6: 1242-50; PMID:24189371; http://dx.doi.org/ 10.1158/1940-6207.CAPR-13-0203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dessy FJ, Giannini SL, Bougelet CA, Kemp TJ, David MP, Poncelet SM, Pinto LA, Wettendorff MA. Correlation between direct ELISA, single epitope-based inhibition ELISA and pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine. Hum Vaccin 2008; 4: 425-34; PMID:18948732; http://dx.doi.org/ 10.4161/hv.4.6.6912 [DOI] [PubMed] [Google Scholar]
- 34.Einstein MH, Baron M, Levin MJ, Chatterjee A, Edwards RP, Zepp F, Carletti I, Dessy FJ, Trofa AF, Schuind A, et al.. Comparison of the immunogenicity and safety of CervarixTM and Gardasil® human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18-45 years. Hum Vaccin 2009; 5: 705-19; PMID:19684472; http://dx.doi.org/ 10.4161/hv.5.10.9518 [DOI] [PubMed] [Google Scholar]
- 35.Pan W. Akaike's information criterion in generalized estimating equations. Biometrics 2001; 57: 120-5; PMID:11252586; http://dx.doi.org/ 10.1111/j.0006-341X.2001.00120.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


