Abstract
Chronic viral hepatitis B (CHB) is a major global health problem. A therapeutic vaccine for CHB comprised of yeast-derived recombinant HBsAg-anti-HBs immunogenic complexes (YIC) has been devloped by us. A series of clinical trials has shown its therapeutic efficacy in decreasing HBV viral load and converting serum HBeAg-positive to anti-HBe-positive status in a subpopulation of CHB patients. Herein, we present a study of the immuno-potentiating mechanisms of YIC revealed by live-cell imaging technology. We studied internalization and dissociation of YIC in cells in vitro, and antigen presentation and T cell stimulation in mice. We found that after YIC was internalized via the Fcγ receptors (FcγR) of antigen presenting cells, it was subsequently transferred through early and late endosomal into lysosomal compartments. The dissociation of YIC was mainly observed in the late endosome. Furthermore when YIC were injected into mice, the populations of IFN-γ- and TNF-α-producing CD8+ and CD4+ T cells were higher in the YIC group than in controls receiving antigen or antibody alone. These observations supplement the known mechanisms of YIC action as a therapeutic vaccine for CHB.
Keywords: cross-presentation, chronic hepatitis B, therapeutic vaccine, dendritic cells, HBsAg-anti-HBs complex
Introduction
Diseases caused by hepatitis B (HBV) have a worldwide distribution. Despite the success of HBV vaccines for prevention of infection, HBV-related diseases remain a serious worldwide problem. According to the World Health Organization (WHO), more than 2 billion people around the world are reported to have been infected with HBV. Approximately 360 million of these individuals are chronic carriers and at risk of HBV-associated diseases such as liver cirrhosis and hepatocellular carcinoma (HCC).1 Interferon and various antiviral drugs have shown efficacy in inhibiting replication of HBV. However, important unsolved problems remain: toxicity of the therapeutic agents; emergence of HBV mutants including drug-resistant viral strains; failures of viral clearance that necessitate long-term follow-up.
Compromised immune responses against HBV have been considered to be major contributors to virus persistence and the consequent pathogenesis of chronic liver disease. Key defects have been observed in cellular immunity, with dysfunction of dendritic cell (DC) and cytokine production.2,3 Multiple therapeutic approaches have been investigated to reverse the defects by boosting these host immune responses that are vital for the control of viral persistence.4,5
One strategy that we previously applied showed that YIC immunization could reverse the immunocompromisation to HBsAg that normally prevails in HBsAg-transgenic mice by evoking humoral and cellular immune responses to the antigen. Reduction of HBsAg level, induction of anti-HBsAg antibody in the serum and a cytolytic T cell response (CTL) with increased interferon-γ (IFN-γ) were detected in the YIC-immunized mice.6 A series of clinical trials based on this concept have been conducted, in which immunization with plasma-derived HBsAg complexed to HBIG (anti-HBs immunoglobulin) was shown to reduce the HBV load and to induce anti-HBeAg antibody in some patients.7-9 The clinical trials showed that YIC could promote the maturation of monocyte-derived DC (M-DCs) from patients by up-regulating the expression of HLA-II, B7 (CD80 & CD86) and CD40. In addition, YIC-stimulated DCs secreted higher levels of IL-12 and more efficiently activated T cells compared to HBsAg- or HBIG-stimulated DC from the same patient.7 A recent study reported that vaccination of mice with immune complex-loaded DCs significantly delayed tumor growth compared to vaccination with DCs loaded with the same amount of non-complexed antigen.10 Another study showed that immune complexes could activate DCs and enable them to efficiently prime antigen-specific CD8+ CTL responses in vivo.11
In this study, mechanisms involved in efficacy are revealed. We used confocal microscopy and a live-cell imaging system to investigate the processing and presentation of YIC in antigen presenting cells. An endocytic pathway of internalization and processing of HBIG-bound HBsAg that favours favors cross-presentation of antigen is suggested. This study provided additional support for our work using immune complexes as a therapeutic vaccine for chronic HBV infection.
Results
Facilitated entry of HBsAg complexed to antibody into DC2.4 cells is FcγR dependent
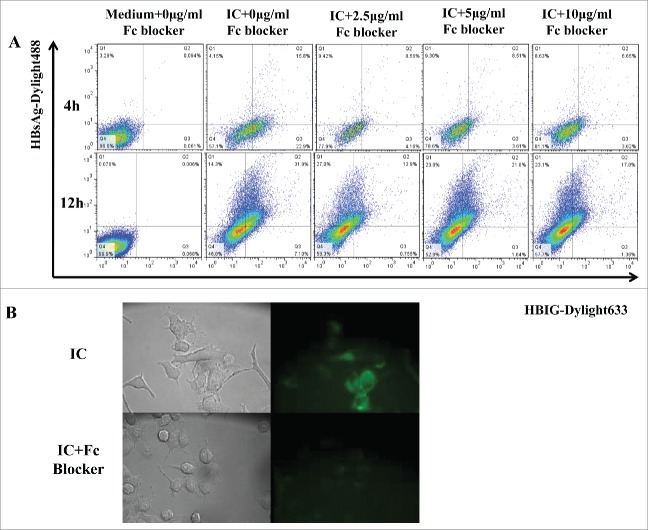
FcγRs comprise several types of receptors expressed on most cells of the hemopoietic lineages, including DCs, and play a pivotal role in linking the humoral and cellular arms of the immune response. To confirm that enhanced internalization of HBsAg when complexed to HBIG was FcγR dependent, Fc receptor blocker was used to inhibit entry into DC2.4. Based on immunofluorescence images, the blocking was observed to be most effective after 4 h and not effective after 12 h (Fig. 1A), some inhibition was also observed after 1 h (Fig. 1B). These results were consistent with previous studies.12
Figure 1.
The enhanced entry of IC to DC2.4 is FcγR-dependent in Fc blocking assays. (A) DC2.4 cells were treated with different concentration of mouse BD Fc blocker for 15 min and washed with PBS 3 times. Then the cells were incubated with medium or IC (10 μg/ml) for 4 h or 12 h, washed with PBS and analyzed on a Calibur. Data were acquired by CellQuest and were analyzed with FlowJo software. (B) DC2.4 cells were cultured on glass-bottom 35-mm tissue culture dishes in complete medium. DC2.4 cells were treated or not with 5 μg/ml BD Fc blocker for 15 min, and cells were washed with PBS for 3 times. Then cells were incubated with IC for 1 h, followed by 3 washes with PBS. Images were acquired by Leica fluorescent microscopy. Data shown are representative from three 3 independent experiments.
IC co-localized with early endosome, late endosome, and lysosome but not with golgi body
Exogenous proteins that are internalized by a cell are degraded by lysosomal proteolysis. To examine the processing pathway of IC in DC2.4, flurorecence-labeled antigen (green) and IC (greygray) were incubated with cells for various durations. As depicted in Figure 2, IC was observed to co-localize with early endosomes, late endosomes and lysosomes (arrows indicate the co-localized signals), but not with the golgi bodies. The HBsAg was distributed with a somewhat similar pattern, but the lower intensity of fluorescence indicated that the amount internalized was much lower than for IC (Fig. 2). The IC were seen to have more intensive co-localization in lysosomal compartments than in other compartments, suggesting that this may be the main IC processing location.
Figure 2.
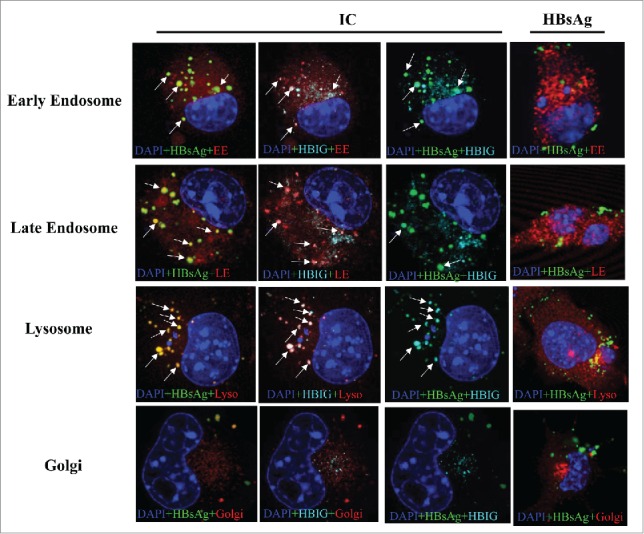
IC co-localization with cellular organelles and dissociations. DC2.4 cells were cultured on coverslips in 6-well dishes in complete medium. After 24 h, cells were labeled using CellLight reagents for early endosome (EE), late endosome (LE), lysosome (Lyso) and Golgi for 24 h. DC2.4 cells were further incubated with fluorescence conjugated IC (10 μg/ml) or fluorescence conjugated HBsAg (10 μg/ml) for 8 h before fixation. Nucleus was stained with DAPI (blue), HBsAg with green, HBIG with cyan, and organelles with red. Fluorescent images were acquired on an inverted Leica TCS SP5 confocal laser-scanning microscope with a 63× objective and analyzed by Leica Confocal Software. Multiple color images were acquired by scanning in sequential mode to avoid cross excitation. Images were processed and analyzed with Leica Confocal Software LCS and ImageJ. Arrows indicate the co-localized signals. ICs are co-localized with early endosome, late endosome, and lysosome but not with Golgi. Shown are representative images of at least three 3 independent experiments.
IC dissociated first in the early endosome, followed by late endosome and lysosome
Live-cell imaging analysis was used to dynamically monitor internalization and the processing of IC in DC2.4 and representative videos are shown. Video sV4 was analyzed and animated using Imaris7.4.2 simulation software based on sV1 and sV2; videos sV3, sV5 and sV6 were analyzed and animated based on their corresponding original material. Green solid spheres represent HBsAg, pink solid spheres represent HBIG and red solid spheres represent other organelles. The black arrows indicate the co-localized signals which show as dual color spheres in enlarged images. For late endosomes, the traced purple line indicates the dynamic movement of the HBsAg that has dissociated from the IC and the green arrow indicates its final destination. These images showed that IC started to dissociate in the early endosome, followed by further dissociation in the late endosome and lysosome (Fig. 3). No IC was observed in the golgi bodies, which was consistent with the results from confocal microscopy.
Figure 3.
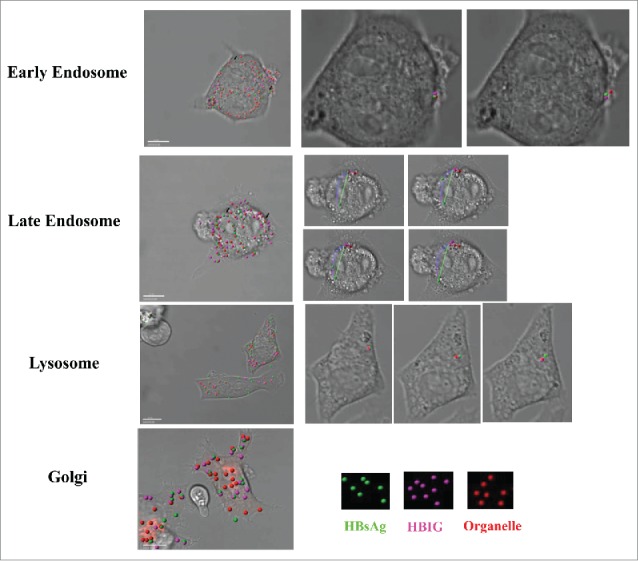
IC partially dissociated in the early endosome, late endosome and lysosome. For live imaging, DC2.4 cells were cultured on glass-bottom 35-mm tissue culture dishes in complete medium. After 24 h, cells were labeled using CellLight reagents for early endosome, late endosome, lysosome and golgi according to the manufacturer's instruction for 24 h. DC2.4 cells were incubated with IC (10μg/ml) for 1 h then washed with PBS 3 times. Cells were then maintained in HEPES solution in a humidified chamber at 37°C and 5% CO2 throughout the experiment. Cells were imaged using a Nikon A1R confocal laser-scanning microscope with a 60× objective. Images were captured at 5-min intervals for 1 h. Videos were processed and analyzed with Nikon NIS-Elements AR and Imaris7.4.2. Green solid spheres represent HBsAg, pink solid spheres represent HBIG and red solid spheres represent other organelles when the videos were analyzed and simulated with Imaris7.4.2. Representative co-localized signals were indicated with black arrows where the dual color sphere was further showed in enlarged images with colored arrows.. The traced purple line indicates dynamic movement of the dissociated HBsAg from the IC and the green arrow indicates its final destination at end of recording. Shown are representative images of at least three 3 independent experiments.
IC co-localized with MHC-I and MHC-II which were distributed on endosome, lysosome and ER
It was previously reported that IC bound antigen could be presented by MHC-II and cross-presented to MHC-I.11,12 We performed co-locations assays to test if HBsAg-HBIG IC could associate with both MHC-I and –II. As shown in Figure 4A, IC was co-localized strongly with MHC-II residing in endosomes, lysosomes and ER. As shown in Figure 4B, IC was also associated with MHC-I and co-localized in the endosomes and lysosomes, athough the MHC-I signal was much lower than that of MHC-II. This class I and II assocation of IC is consistent with previous studies demonstrating strong humoral and cell-mediated immune (CMI) responses, notably including CD8+ CTL responses.7
Figure 4.
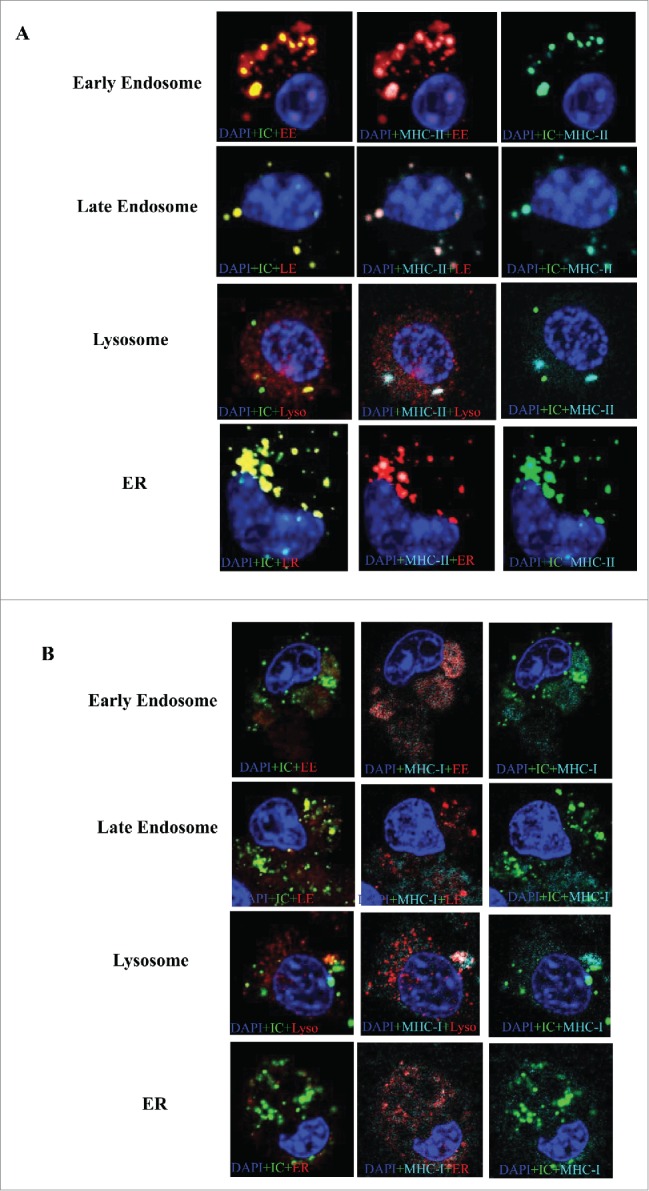
IC co-localized with MHC-I and MHC-II distributed among endosome, lysosome and ER. DC2.4 cells were cultured on coverslips in 6-well dishes (Falcon) in complete medium. After 24 h, cells were labeled using CellLight reagents for early endosome (EE), late endosome (LE), endoplasmic reticulum (ER) and golgi according the manufacturer's instruction. After 24 h, the cells were incubated with IC (Dylight conjugated HBsAg complexed to unlabeled HBIG) for 8 h. Then the cells were fixed with 4% formaldehyde for 15 min. Cells were then incubated in 1× PBS containing 1% fetal bovine serum and 0.1% TritonX-100 for permeabilization and blocking for 15 min at room temperature. Then the cells were stained with MHC-II (A) and MHC-I (B) conjugated to APC for 1 h in 1× PBS, followed by 2 washes. Nucleus was stained with DAPI(blue), IC with green, MHC-I/II with cyan and organelles with red. Fluorescent images were acquired on an inverted Leica TCS SP5 confocal laser-scanning microscope with a 63× objective, using Leica Confocal Software. Multiple color images were acquired by scanning in sequential mode to avoid cross excitation. Images were processed and analyzed with Leica Confocal Software LCS and ImageJ. Shown are representative images of at least three 3 independent experiments.
DC2.4 pulsed with IC had great potential to prime T cells
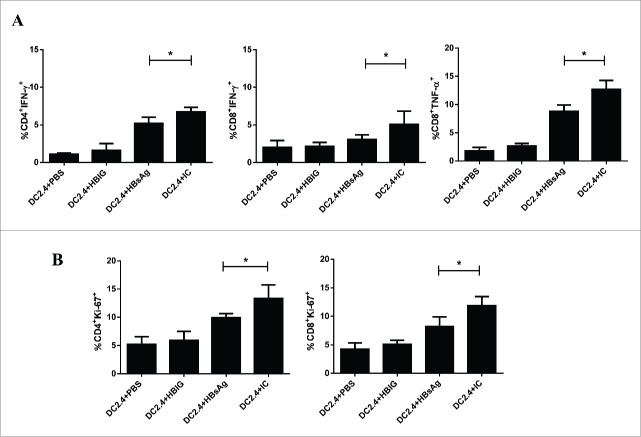
In order to analyze the biological functions of DC2.4 that had been stimulated with different forms of antigens, DC2.4 cells pulsed with PBS, HBIG, HBsAg or IC in vitro were adoptively transferred to syngeneic mice that had previously been immunized with HBsAg. Two days after the transfer, T cell proliferation and cytokine expression were analyzed. As shown in Figure 5A, we observed that higher levels of IFN-γ producing CD4+ and CD8+ T cells and of TNF-α producing CD8+ T cells could be isolated from recipients of IC-pulsed DC2.4 than from recipients of HBsAg pulsed DC2.4. However, the expression levels of IL-2, perforin and granzyme B were not significantly different between these two groups (data not shown). Nevertheless, both CD4+ and CD8+ T cells of mice that had received IC-pulsed DC2.4 showed higher levels of Ki67 expression compared to the group that had received HBsAg-pulsed DC2.4 (Fig. 5B), suggesting a more potent memory recall response.
Figure 5.
DC2.4 pulsed with IC had great potential to prime HBsAg specific T cells. DC2.4 cells were stimulated with PBS, HBIG, HBsAg and IC for 24 h, then the pulsed and unpulsed DC2.4 cells were transferred to mice immunized with HBsAg. Two days after adoptive transfer, cytokine expression (A) and T cell proliferation (B) of T cells in response to HBsAg stimulation were detected in cells from recipient mice using intracellular staining. Data shown are representative from three 3 independent experiments.
Discussion
For around two 2 decades, we have been studying and developing YIC as a therapeutic vaccine for chronic hepatitis B patients. Our goal here has been to elucidate the mechanisms of the immune potentiating and therapeutic effects of HbsAg-HBIG immunogenic complexes.
In this study, we used confocal microscopy and live-cell imaging technology to actually look at the endocytic internalization pathway of HBsAb-bound HBsAg. Because we wanted to study the HBsAg-HBIG product being used in patients, we did not use a mouse anti-HBs antibody to substitute for human HBIG, and assume that the effects of IC resemble those which may occur in human. We not only confirmed that DC2.4 cells were robust in uptake of the HBsAg complexed to HBIG via the FcγR, but also we directly showed that the antibody complexed to HBsAg entered into the cellular endocytic pathway and may have protected the antigen from rapid degradation. A prolonged persistence of HBsAg may enhance the cross-presentation in APCs, a mechanism which is consistent with a recent study by others.13 Another recent study showed that prolonged antigen presentation by immune complex-binding dendric cells actually programs the proliferative capacity of memory CD8+ T cells.14 So the prolonged life of HBsAg in YIC may also enhance the function of specific memory CD8+ T cells.
Exogenous proteins are endocytosed and enter a vesicular pathway with successively more acidic and proteolytically active compartments consisting of early endosomes, late endosomes, and lysosomes. Exogenous proteins can also be internalized by phagocytosis with a similar pathway and degraded in phagolysosomes that are formed by the fusion of phagosomes and lysosomes.15 It is well known that peptides derived from exogenously acquired antigens can be presented on MHC-I of professional APCs. This process is known as a cross-presentation and CD8+ T cells can be primed by such antigens, a process known as cross-priming.16,17 We know from previous studies that IC can generate cross-priming and activate CD8+ T cells.11 Cross-priming serves to activate cytotoxic T lymphocytes for immune defense against viruses and tumors and plays an important role in effective vaccinations.18
From our dynamic analysis, although HBsAg and IC were processed with similar endocytic pathways, we showed that IC was progressively co-localized with early endosomes, late endosomes, and lysosomes after addition to cells (Figs. 2 and 3). Since the IC was internalized more efficiently, it may be that association with Fcγ receptors protected the HBsAg from rapid degradation, leading to more efficient antigen processing and presentation of HBsAg when compared with HBsAg alone. Although MHC-I expression was much lower than MHC-II (Fig. 4), IC was present together with both MHC-I and MHC-II molecules in the same cellular compartments. This would likely facilitate association of processed IC with pathways of cross-presentation. It was striking that IC seemed to also associate with MHC-I, even though it co-localized more with MHC-II. Possibly IC has a longer retention time in the endocytic pathway which could increase the chance of interaction with MHC-1 for the antigen cross-presentation. In addition, IC was observed to be co-localized with MHC-II in ER, which was inconsistent with traditional theory of antigen processing and presentation on MHC-II. However, recent study has given rise to an alternative hypothesis, that the ER can serve as the main intracellular membrane source to phagosomes, sustaining a process known as ER-mediated Phagocytosis. Antigen taken up by ER-mediated Phagocytosis can also enter the pathway for presentation on MHC-II.19,20 Evidently the IC internalization can involve mutiple complexed pathways.
Since adoptive transfer of IC-pulsed DC2.4 into mice was more effective than transfer of antigen-pulsed DC2.4, resulting in higher expression levels of IFN-γ and TNF-α by CD8+ T cells, enhanced presentation by DC may account for our previous observation that YIC was superior to antigen for induction of antigen-specific CTL in HBsAg transgenic mice.6 DC2.4 is a murine DC cell line derived from C57BL/6 and is well characterized and widely used for antigen presentation studies.21-24 It expresses MHC class I and class II molecules. It can present exogenous antigens on both MHC class I and class II molecules and efficiently mediate cross-presentation.21 This cell line is particularly useful for evaluating IC treatment effects in vitro and in vivo, considering the complex differentiation procedures needed for primary DCs and the limited numbers that are obtained. Antigen processing pathways are highly conserved between species and the effects we observed with the DC2.4 could also apply to mouse and human primary DCs. However, it would certainly be more convincing to demonstrate these effects with humans DCs, especially DCs from CHB patients. In addition, our data only showed the dynamic phenomena in a qualitative manner; it will be more informative in future to capture and analyze the whole dynamic internalization process quantitatively, comparing IC to HBsAg alone. These outstanding issues will be investigated in our future studies.
In conclusion, we have presented visual data on the processing of HBsAg-HBIG immunogenic therapeutic vaccine in dendritic cells. From these studies, we could see the intracellular pathways taken and the localization of IC in different compartments. The co-localization of IC with MHC-I molecules suggested there may be cross-presentation occuring in the processing and presentation of IC, which may need further studies. These studies have deepened our understanding of the mechanisms engaged by immunogenic complexes and allowed us to manipulate the immune response to break the immune tolerance that arises in HBV and other viral persistent infections.
Materials and Methods
Cells and cell cultures
Murine dendritic cell line, DC2.4 was cultured at 37°C in 5% CO2 in complete medium consisting of DMEM, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% fetal bovine serum (FCS).
Antibodies and reagents
Plasma-derived HBsAg was purchased from GuiKang Biochem Co., Ltd. (Shanghai, China). Human high-titer anti-HBs immunoglobulin (HBIG) was purchased from Bayer (NY, USA). HBsAg was labeled with Dylight 488 (green) and HBIG was labeled with Dylight 633 (cyan) according to the manufacturer's instruction (Thermo, Rockford, USA). Purified rat anti-mouse CD16/CD32 (Mouse BD Fc Block™) was purchased from BD Biosciences. Anti-LAMP1 antibody (Cy3 ®) (ab67283) was purchased from Abcam (Cambridge, USA). CellLight™ Early Endosomes-RFP (C10587), CellLight® Late Endosomes-RFP (C10589), CellLight™ ER-RFP (C10591), CellLight™ Golgi-RFP (C10593), CellLight™ Lysosomes-RFP (C10597) were purchased from Invitrogen Molecular (Carlsbad, USA). The fluorescent conjugated HBsAg and HBIG were characterized by Nano-Drop detection of the OD280nm value and OD493nm (for Dylight488) or OD627nm (for Dylight633). Dye:protein (F/P) molar ratios was calculated according to the manufacture's instruction. F/P ratio at 4–7 was optimal for the conjugation. The conjugated HBsAg and HBIG were mixed at an appropriate ratio (described in US patent 6,221,664 B1 and European patent 913157) and incubated at 37°C for 2 h, then kept at 4°C for 24 h. After centrifugation at 12,000 rpm for 30 min, the supernatant was removed and the deposit was resuspended.
Flow cytometric analysis
DC2.4 cells were grown to subconfluency on 24-well dishes in complete medium. After incubation with IC (10 μg/ml) for different durations, cells were washed with PBS and analyzed on a Calibur (BD Biosciences). For Fc blocking assay, DC2.4 cells were treated with mouse BD Fc blockers for 15 min before being washed with PBS 3 times. After, cells were further incubated with IC (10 μg/ml) for different times before being washed with PBS and analyzed on a Calibur (BD Biosciences). Data were acquired by CellQuest (BD Biosciences) and analyzed with FlowJo software (Tree Star Inc.).
Immunofluorescence microscopy
DC2.4 cells were cultured on coverslips in 6-well dishes (Falcon, Rockford, USA) in complete medium. After 24 h, cells were labeled using CellLight reagents (Molecular Probe, Invitrogen) according the manufacturer's instructions for 24 h. Then DC2.4 cells were incubated with IC (10 μg/ml) for different times and then fixed with 4% formaldehyde for 15 min. For fixed-image analysis, cells were further incubated in 1× PBS containing 1% fetal bovine serum and 0.1% TritonX-100 for permeabilization and blocking followed by staining with conjugate antibodies for 30 min in 1× PBS and 2 washes. Nuclei were stained with DAPI (Sigma-Aldrich, St Louis, MO, USA) for 5 min, followed by 5 washes with PBS. Fluorescence images were acquired on an inverted Leica TCS SP5 confocal laser-scanning microscope with a 63× objective. Multiple color images were acquired by scanning in sequential mode to avoid cross excitation. Images were processed and analyzed with Leica Confocal Software LCS and ImageJ.
Live cell imaging analysis
For live imaging, DC2.4 cells were cultured on glass-bottom 35-mm tissue culture dishes (NEST Biotechnology Co.Ltd, Rahway, NJ, USA) in complete medium. After 24 h, cells were labeled using CellLight reagents (Molecular Probe, Invitrogen) according to the manufacturer's instruction for 24 h. Then DC2.4 cells were incubated with IC (10 μg/ml) for 1 h and washed with PBS 3 times then kept in HEPES solution in a humidified chamber at 37°C and 5% CO2 throughout the experiment. Cells were recorded using a Nikon A1R confocal laser-scanning microscope with a 60× objective. Images were captured at 5-min intervals for 1 h. Images were processed and analyzed with Nikon NIS-Elements AR and Imaris7.4.2 (Bitplane AG, Zurich).
Adoptive transfer of DC2.4 to mice immunized with HBsAg
Female 6–8 weeks old C57BL/6 wild type mice were purchased from the Shanghai SLAC Laboratory Animal Co. Ltd. (Shanghai, China). All mice were kept under specific pathogen-free conditions at Fudan University and handled according to the animal welfare guidelines for experimental animals. In total 24 mice were immunized with HBsAg (2 μg/animal) for three 3 times with 2-week intervals. DC2.4 cells were pulsed either with PBS, HBIG, HBsAg (10 μg/ml) or IC for 24 h, then arrested with mitomycin C for 30 min, followed by extensive washing in PBS. The pulsed and unpulsed DC2.4 (2 × 106 cells/animal) were injected to HBsAg immunized mice (6 mice/group) via a tail vein. Single-cell splenocyte suspensions were prepared from recipient mice on day 2 after the adoptive transfer according to following protocol. In brief, the spleen was taken out and homogenized by the black rubber end of a 2-ml syringe. The homogenized cell suspension was centrifuged at 300 x g for 5 min. The supernatant was removed and the red blood cells were lysed by lysing reagent. Then the cells were washed with PBS and resuspended in RPMI1640 supplemented with 10% FCS. The splenocytes were stimulated with HBsAg (10 μg/ml) in vitro for 24 h or 48 h in the presence of BD GolgiPlug (BD Biosciences, USA). The stimulated cells were fixed with 4% paraformaldehyde at 4°C for 15 min, permeabilized with 0.1% saponin (Sigma-Aldrich) and immunostained with CD4-FITC, CD8a-APC, IFN-r-PE, Ki-67-PE, TNF-αalpha-PE-Cy7, IL-2-PerCP-Cy5.5, Perforin-PE, GranzymeB-Alex647. Data were acquired using BD LSR Fortessa and FACSDiva software (BD Biosciences) and analyzed using FlowJo (TreeStar, San Carlos, CA, USA). Fluorescent-conjugated anti-mouse monoclonal antibodies were purchased from eBioscience (CA, USA) and BioLegend (CA, USA). Results are presented as means with standard error of the mean (SEM). Student's t-test analysis was used for data analysis. Analyses were performed with GraphPad Prism 5 software (GraphPad, La Jolla, CA). A value of p ≤ 0.05 was considered statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgements
We wish to thank Dr. Douglas Lowrie for his English editing and proof readings.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
Funding
This work was partly supported by the National Science and Technology Projects of Major Infectious Diseases of China (2008ZX10002–003, 2012ZX10002002004 and 2012ZX10002002).
References
- 1.Weekly epidemiological record World health organization genebra. Suica, CH; 2009. [Google Scholar]
- 2.Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol 2005; 5:215-29; PMID:15738952; http://dx.doi.org/ 10.1038/nri1573 [DOI] [PubMed] [Google Scholar]
- 3.Chisari FV, Ferrari C. Hepatitis B Virus Immunopathogenesis. Ann Rev Immunol 1995; 13:29-60; PMID:7612225; http://dx.doi.org/ 10.1146/annurev.iy.13.040195.000333 [DOI] [PubMed] [Google Scholar]
- 4.Mancini-Bourgine M, Fontaine H, Bréchot C, Pol S, Michel M-L. Immunogenicity of a hepatitis B DNA vaccine administered to chronic HBV carriers. Vaccine 2006; 24:4482-9; PMID:16310901; http://dx.doi.org/ 10.1016/j.vaccine.2005.08.013 [DOI] [PubMed] [Google Scholar]
- 5.Buchmann P, Dembek C, Kuklick L, Jäger C, Tedjokusumo R, von Freyend MJ, Drebber U, Janowicz Z, Melber K, Protzer U. A novel therapeutic hepatitis B vaccine induces cellular and humoral immune responses and breaks tolerance in hepatitis B virus (HBV) transgenic mice. Vaccine 2013; 31:1197-203; PMID:23306359; http://dx.doi.org/ 10.1016/j.vaccine.2012.12.074 [DOI] [PubMed] [Google Scholar]
- 6.Zheng B, Ng M, He L, Yao X, Chan K, Yuen K, Wen Y. Therapeutic efficacy of hepatitis B surface antigen-antibodies-recombinant DNA composite in HBsAg transgenic mice. Vaccine 2001; 19:4219-27; PMID:11457548 [DOI] [PubMed] [Google Scholar]
- 7.Yao X, Zheng B, Zhou J, Xu D-Z, Zhao K, Sun S-H, Yuan Z-H, Wen Y-M. Therapeutic effect of hepatitis B surface antigen-antibody complex is associated with cytolytic and non-cytolytic immune responses in hepatitis B patients. Vaccine 2007; 25:1771-9; PMID:17224217; http://dx.doi.org/ 10.1016/S0264-410X(01)00158-X [DOI] [PubMed] [Google Scholar]
- 8.Xu D-Z, Zhao K, Guo L-M, Li L-J, Xie Q, Ren H, Zhang J-M, Xu M, Wang H-F, Huang W-X, et al.. A randomized controlled phase IIb trial of antigen-antibody immunogenic complex therapeutic vaccine in chronic hepatitis B patients. PloS one 2008; 3:e2565; PMID:18596958; http://dx.doi.org/ 10.1371/annotation/8b913538-74f4-4560-b700-0936a8e35847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wen Y, Wu X, Hu D, Zhang Q, Guo S. Hepatitis B vaccine and anti-HBs complex as approach for vaccine therapy. Lancet 1995; 345:1575-6; PMID:7791465; http://dx.doi.org/ 10.1016/S0140-6736(95)91126-X [DOI] [PubMed] [Google Scholar]
- 10.Schuurhuis D, van Montfoort N, Ioan-Facsinay A, Jiawan R, Camps M, Nouta J, Melief C, Verbeek J, Ossendorp F. Immune complex-loaded dendritic cells are superior to soluble immune complexes as antitumor vaccine. J Immunol 2006; 176:4573-80; PMID:16585547; http://dx.doi.org/ 10.4049/jimmunol.176.8.4573 [DOI] [PubMed] [Google Scholar]
- 11.Schuurhuis D, Ioan-Facsinay A, Nagelkerken B, van Schip J, Sedlik C, Melief C, Verbeek J, Ossendorp F. Antigen-antibody immune complexes empower dendritic cells to efficiently prime specific CD8+ CTL responses in vivo. J Immunol 2002; 168:2240-6; PMID:11859111; http://dx.doi.org/ 10.4049/jimmunol.168.5.2240 [DOI] [PubMed] [Google Scholar]
- 12.Regnault A, Lankar D, Lacabanne V, Rodriguez A, Théry C, Rescigno M, Saito T, Verbeek S, Bonnerot C, Ricciardi-Castagnoli P, et al.. Fcgamma receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J Exp Med 1999; 189:371-80; PMID:9892619; http://dx.doi.org/ 10.1084/jem.189.2.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Montfoort N, Camps M, Khan S, Filippov D, Weterings J, Griffith J, Geuze H, van Hall T, Verbeek J, Melief C, et al.. Antigen storage compartments in mature dendritic cells facilitate prolonged cytotoxic T lymphocyte cross-priming capacity. Proc Natl Acad Sci U S A 2009; 106:6730-5; PMID:19346487; http://dx.doi.org/ 10.1073/pnas.0900969106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.León B, Ballesteros-Tato A, Randall TD, Lund FE. Prolonged antigen presentation by immune complex–binding dendritic cells programs the proliferative capacity of memory CD8 T cells. J Exp Med 2014; 211:1637-55; PMID:25002751; http://dx.doi.org/ 10.1084/jem.20131692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huotari J, Helenius A. Endosome maturation. EMBO J 2011; 30:3481-500; PMID:21878991; http://dx.doi.org/ 10.1038/emboj.2011.286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bevan M. Cross-priming. Nat Immunol 2006; 7:363-5; PMID:16550200; http://dx.doi.org/ 10.1038/ni0406-363 [DOI] [PubMed] [Google Scholar]
- 17.Wagner C, Grotzke J, Cresswell P. Intracellular events regulating cross-presentation. Front Immunol 2012; 3:138; PMID:22675326; http://dx.doi.org/ 10.3389/fimmu.2012.00138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurts C. Molecular and cell-biological mechanisms of antigen cross-presentation. Front Immunol 2013; 4:51; PMID:23450983; http://dx.doi.org/ 10.3389/fimmu.2013.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desjardins M. ER-mediated phagocytosis: a new membrane for new functions. Nat Rev Immunol 2003; 3:280-91; PMID:12669019; http://dx.doi.org/ 10.1038/nri1053 [DOI] [PubMed] [Google Scholar]
- 20.Watts C. Phagocytosis: How the Phagosome Became the Phag-ER-some. Curr Biol 2002; 12:R666-R8; PMID:12361588; http://dx.doi.org/ 10.1016/S0960-9822(02)01163-6 [DOI] [PubMed] [Google Scholar]
- 21.Shen Z, Reznikoff G, Dranoff G, Rock KL. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. The J Immunol 1997; 158:2723-30; PMID:9058806 [PubMed] [Google Scholar]
- 22.Zou L, Zhou J, Zhang J, Li J, Liu N, Chai L, Li N, Liu T, Li L, Xie Z, et al.. The GTPase Rab3b/3c-positive recycling vesicles are involved in cross-presentation in dendritic cells. Proc Nat Acad Sci U S A 2009; 106:15801-6; PMID:19717423; http://dx.doi.org/ 10.1073/pnas.0905684106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imai J, Hasegawa H, Maruya M, Koyasu S, Yahara I. Exogenous antigens are processed through the endoplasmic reticulum-associated degradation (ERAD) in cross-presentation by dendritic cells. Int Immunol 2005; 17:45-53; PMID:15546887; http://dx.doi.org/ 10.1093/intimm/dxh184 [DOI] [PubMed] [Google Scholar]
- 24.Shakushiro K, Yamasaki Y, Nishikawa M, Takakura Y. Efficient scavenger receptor-mediated uptake and cross-presentation of negatively charged soluble antigens by dendritic cells. Immunology 2004; 112:211-8; PMID:15147564; http://dx.doi.org/ 10.1111/j.1365-2567.2004.01871.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.