Abstract
Dendritic structure is sensitive to changes in the environment during brain development. Accumulating evidence has demonstrated that early immune activation can significantly affect neuronal development. Our study concentrated on the morphological study of neural dendrites and spines in the hippocampal CA1 area using Diolistic labeling with Sholl analysis and fractal analysis. The results revealed that Bacille Calmette-Guérin (BCG) vaccination enhanced dendritic complexity, as reflected by the increased number of intersections, number of branch points and fractal dimension, and promoted neurite outgrowth. In addition, BCG increased the density and promoted the maturation of dendritic spines. The alterations in dendritic structure and spine morphology were observed at 2 and 4 w, but the differences were more apparent at 4 w than at 2 w. However, no significant difference was observed at 8 w. Furthermore, we observed that BCG increased the expression of hippocampal brain derived neurotrophic factor (BDNF) and insulin-like growth factor 1 (IGF-1). Hippocampal BDNF/IGF-1 was positively correlated with apical dendritic length, fractal dimension, and spine density. Taken together, we show in this study that neonatal BCG vaccination promotes dendritic development in developing hippocampal CA1 neurons, most likely by increasing the expression of BDNF and IGF-1 in the hippocampus.
Keywords: dendritic complexity, fractal dimension, neurotrophin, spine, sholl analysis
Abbreviations
- BCG
Bacillus Calmette-Guérin
- CNS
central nevers system
- BDNF
brain derived neurotrophic factor
- IGF-1
insulin-like growth factor 1
- AMPA
a-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor
Introduction
It is well-established that systemic immune activation plays a pivotal role in modulating the central nerves system (CNS). Early life immune activation can significantly affect neuronal development.1-3 The CNS has the capacity to transform sensory experience from the environment into changes in neuronal activity that, in turn, cause long-lasting alterations in dendritic morphology and synaptic connections.4,5 Previous research has shown that vaccination with complete Freund's adjuvant (CFA) alone can have a neuroprotective effect.6-9 The major immunogenic component of CFA is heat-killed Mycobacterium tuberculosis. Bacille Calmette-Guerin (BCG) is an attenuated live bovine tuberculosis bacillus that is closely related to M. tuberculosis. However, the neuroprotective role of the BCG vaccination was studied in adult mice in pathological status, but billions of infants have been vaccinated with BCG to protect against tuberculosis.10,11 Therefore, the correlation between neonatal vaccination and physiological neural development is worth exploring.
The acquisition of proper dendritic morphology is a crucial aspect of neuronal development that is required for the formation of a functional network. Dendritic arbors are complex neuronal structures that receive and process synaptic inputs. Arbor elaboration involves an early stage of dynamic dendrite elongation, branch addition, and retraction, followed by a later stage of slower branch dynamics.12,13 Therefore, the present study was designed to identify the dendritic development of developing hippocampal CA1 neurons in response to neonatal BCG vaccination.
We concentrated on studying the morphology of their dendritic length, dendritic complexity, the density and detailed shapes of their dendritic spines. To visualize dendritic structure, Diolistic labeling was used to analyze morphology, dendritic branching and dendritic spines in the hippocampus. The neuronal processes of growth and branching during neural circuit formation are regulated by multiple mechanisms. Manipulations that change the balance of the neurogenic niche have been reported to alter hippocampal structural development later in life.14 Therefore, we analyzed neurotrophins to identify the underlying mechanisms for the alterations of the dendritic morphology.
Results
Neonatal BCG vaccination promotes dendrite outgrowth in the hippocampus
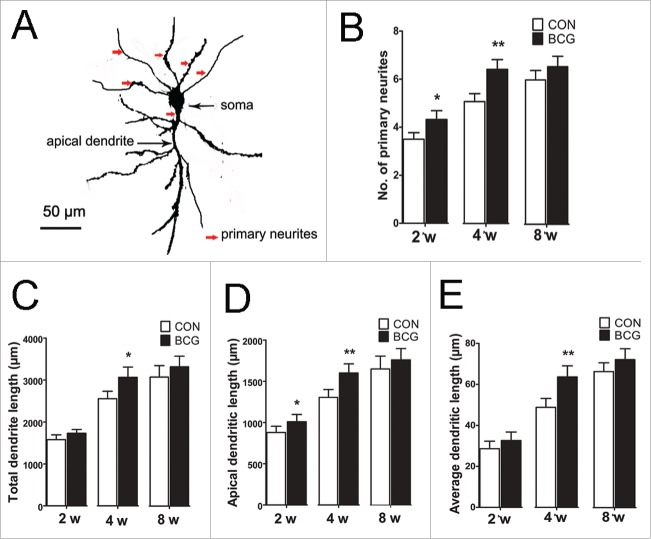
The proper dendritic structure of neurons is a crucial aspect of neuronal development that is required for the formation of a functional network.6 To observe the effects of neonatal BCG vaccination on the dendritic structure of hippocampal CA1 neurons, we assessed the dendritic length at 2, 4 and 8 w. Diolistic labeling was used to label neurons. Although both neurons and neuroglia were labeled with DiI, they could be clearly distinguished on the basis of their morphological features,15 moreover, the hippocaml areas could also be clearly distinguished. Our findings revealed that BCG increased the total dendritic length, the average dendritic length at 4 w (P < 0.05, Fig. 1C, E) and the apical dendritic length at 2 (P < 0.05) and 4 w (P < 0.01, Fig. 1D). In addition to the change in dendritic length, we found that the number of primary neurites in BCG rats was significantly higher at 2 (P < 0.05) and 4 w (P < 0.01) compared to the controls (Fig. 1B). Our results suggest that BCG application promotes neurite outgrowth by increasing both neurite elongation and the number of primary neurites in developing hippocampal CA1 neurons.
Figure 1.
Neonatal BCG vaccination regulates neurite outgrowth in CA1 area. (A) Representative image of a typical pyramidal cell used for analysis. The different compartments of the dendritic tree are indicated. (B) Histogram comparing the number of primary neurites of hippocampal pyramidal cells, which were increased by BCG at 2 and 4 w compared to controls. (C, D and E) BCG rats showed a significant increase in total dendritic length, apical dendritic length and average dendritic length at 4 w compared to controls. Additionally, BCG increased the apical dendritic length at 2 w. Data are presented as the mean ± SEM and analyzed by Student's t test. n = 9 neurons (3 neurons per pup, and 3 pups). *P < 0.05 and **P < 0.01 versus vehicle control.
Neonatal BCG vaccination enhances hippocampal dendritic complexity
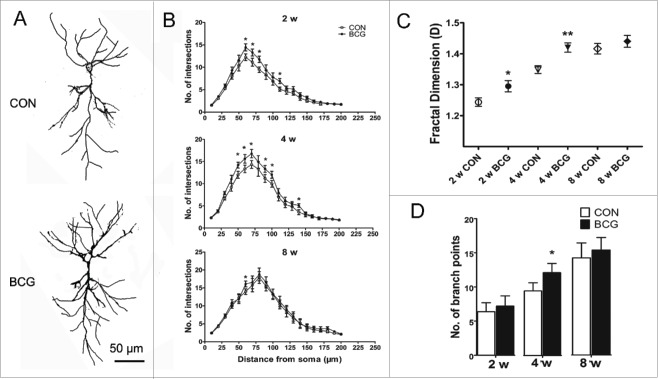
To investigate whether changes in dendritic complexity accompanied the changes in neurite outgrowth in hippocampal neurons, we used fractal and Sholl analysis, techniques that have been used to measure the complexity of pyramidal neurons.16,17 The pyramidal cell body, apical dendrites, basilar dendrites and even dendritic spines could be recognized by Diolistic labeling. Neonatal BCG vaccination resulted in a significant increase in dendritic complexity compared to the controls at 2, 4, and 8 w, using fractal dimension, the number of intersections and the number of branch points as indicators of morphological complexity (Fig. 2B-D). Moreover, we found an obvious difference in the number of intersections between the groups at the range of 50 to 100 μm distance from the soma, which is located in the stratum radiatum layer.
Figure 2.
Neonatal BCG vaccination enhances dendritic complexity in CA1 area. (A) Diolistic assay was used to visualize dendrites in pyramidal cells. Representative grayscale images of typical CON and BCG rat hippocampal neurons used for Sholl analysis and fractal analysis. (B) Graph plotting the dendritic complexity in relation to the distance to the cell body of hippocampal neurons in the DG under control conditions or BCG vaccination. The dendritic complexity of neurons expressing the indicated constructs was measured by Sholl analysis. Intersections at various radial distances (10–200 μm) from the center of the cell soma were significantly improved by BCG vaccination at 2 and 4 w. (C) Fractal dimension D of DG neurons in the different experimental groups. BCG increases the fractal dimension at 2 and 4 w. (D) The number of branch points were significantly increased in the BCG group at 4 w. Data are presented as the mean ± SEM and analyzed by Student's t test. n = 9 neurons (3 neurons per pup, and 3 pups). *P < 0.05 and **P < 0.01 vs. vehicle control.
Neonatal BCG vaccination changes hippocampal spine morphology
The plastic changes in spine morphology that reflect the dynamic state of its associated synapse are responsible, to some extent, for neuronal circuit remodeling.18 We have found that BCG vaccination enhances neurite outgrowth and dendritic complexity. Next, we asked whether the spine morphology was changed by BCG vaccination. As mentioned previously, Diolistic labeling was used to label spines of CA1 neurons. We showed that BCG vaccination increased spine area and spine head width at 2 (P < 0.05) and 4 w (P < 0.01); moreover, it also increased spine density at 4 w (P < 0.01, Fig. 3B, C, E). However, there was no significant change in spine length between the groups. Dendritic spines can have different morphology and be divided into either immature phenotypes with a small head width (filopodia, long thin) or mature phenotypes with a large head width (stubby, mushroom).19 Therefore, we assessed the density of the dendritic spines according to their specific morphology between the vaccinated and control groups. BCG caused a significant and selective increase in stubby and mushroom spines at 4 w (P < 0.05, Fig. 4C). There was no significant difference in the density of any subtype at 2 w and 8 w between the 2 groups. The data described above suggest that BCG vaccination promoted dendritic spine maturation in CA1 area.
Figure 3.
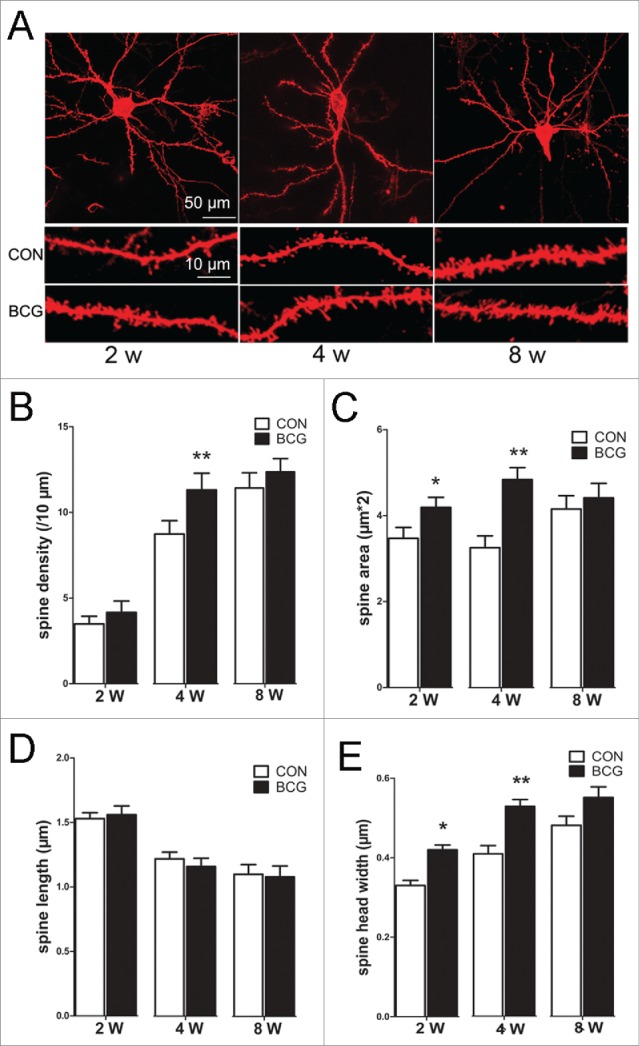
BCG improved the dendritic spine density, area and head width in hippocampal CA1 area. (A) Diolistic assay was used to visualize dendritic spines. Individual hippocampal neurons at 2, 4 and 8 w, respectively (up); representative sections of lateral dendrites in the CON and BCG groups at 2, 4 and 8 w, respectively. The magnified images were below. BCG vaccination increased the spine density at 4 w (B), and spine area (C) and spine head width (E) at 2 and 4 w compared with the controls. No profound alterations were observed in the spine length at the 3 time points (D). Data are presented as the mean ± SEM and analyzed by Student's t test. n = 9 neurons (3 dendritic segments per neuron, 3 neurons per pup, and 3 pups). *P < 0.05 versus vehicle control.
Figure 4.
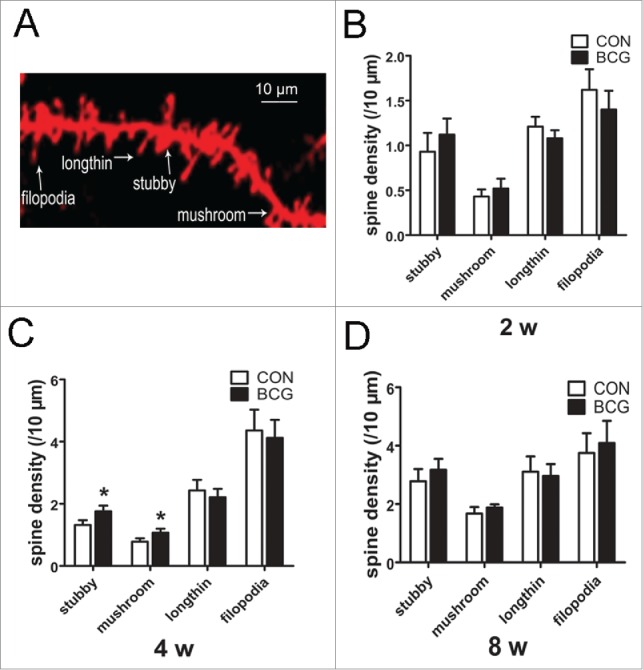
BCG promoted dendritic spine maturation. (A) Representative photo micrograph depicts the different morphological subtypes of dendritic spines in relation to the dendritic shaft. (B, C and D) Graph showing the density of spines within each category. BCG increased the density of stubby and mushroom spines at 4 w (C). There was no significant difference in the density of any subtype at 2 w and 8 w between the 2 groups (B and D). Data are presented as the mean ± SEM and analyzed by Student's t test. n = 9 neurons (3 dendritic segments per neuron, 3 neurons per pup, and 3 pups). For all graphs, *P < 0.05 vs. vehicle control.
Neonatal BCG vaccination increases the expression of BDNF and IGF-1 in the hippocampus
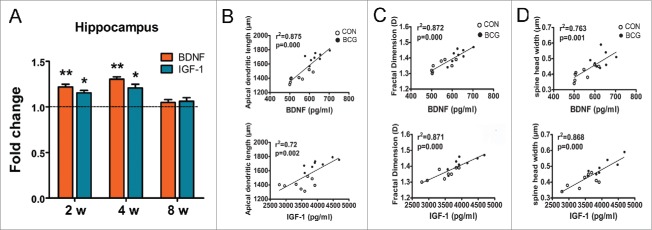
The neuroprotective role of BCG has been extensively studied in an adult neurodegenerative disease mouse model, which has shown that BCG increased the levels of circulating neurosupportive cytokines/chemokines that may have a supportive effect on neurons.8,9 However, there are many differences in the BCG administered in our study compared with previous research. Neurotrophins are essential regulators for multiple aspects of neuronal development and function.20 According to these reports, it is unclear whether the neurotrophin expression was regulated by BCG vaccination. Our data revealed that BCG vaccination increased the levels of BDNF (P < 0.01) and IGF-1 (P < 0.05, Fig. 5A) at 2 and 4 w. As shown in Figure 5B, the association between apical dendritic length/fractal dimension/spine density and BDNF/IGF-1 were positively correlated. The specific data were shown in Table 1. Interestingly, the results showed that the correlation coefficient was higher at 4 w than the other 2 time points.
Figure 5.
BCG increased the levels of BDNF and IGF-1 in the hippocampus, and there was a positive correlation between apical dendritic length/fractal dimension/spine density and the hippocampal BDNF/IGF-1. The levels of BDNF and IGF-1 in the hippocampus were normalized analyzed at 2, 4 and 8 w. BCG increased the levels of BDNF and IGF-1 in the hippocampus at 2 and 4 w (A). Data are presented as the mean ± SEM normalized to control and analyzed by Student's t test. n = 6 for each group. (B) Pearson correlation analysis was assessed using the apical dendritic length/fractal dimension/spine density and the hippocampal BDNF/IGF-1 at 4 w.
Table 1.
Correlation analysis between the apical dendritic length/fractal dimension/spine density and BDNF/IGF-1
| Apical dendritic length |
Fractal dimension (D) |
Spine density |
||||
|---|---|---|---|---|---|---|
| R2 | P | R2 | P | R2 | P | |
| 2 w | 0.769/0.686 | 0.000/0.003 | 0.626/0.724 | 0.010/0.002 | 0.627/0.641 | 0.009/0.007 |
| 4 w | 0.875/0.720 | 0.000/0.002 | 0.872/0.871 | 0.000/0.000 | 0.849/0.72 | 0.000/0.002 |
| 8 w | 0.375/0.490 | 0.152/0.054 | 0.610/0.540 | 0.012/0.031 | 0.572/0.503 | 0.021/0.047 |
Discussion
In this study, we examine the effects of neonatal BCG vaccination on the dendritic development of hippocampal CA1 neurons in early postnatal rats. Our results showed a marked increase of dendritic complexity, as reflected by the increased number of intersections, number of branch points and fractal dimension. Moreover, there was a significant increase in the number of primary neurites and dendritic length in BCG rats. In addition to the change in dendritic morphology, we found that BCG enhanced the number of spines and promoted their maturation. Furthermore, we found that BCG increased the levels of hippocampal BDNF and IGF-1. Hippocampal BDNF/IGF-1 was positively correlated with apical dendritic length/fractal dimension/spine density.
Proper dendrite growth and branching are crucial for nervous system function. Accumulating evidence has indicated that dendritic growth is remarkably dynamic and responsive to environmental signals.21,22 Dendrites grow through a multistage process of extension and branching. Dendritic arbors develop in a highly choreographed manner. Apical dendrites of pyramidal neurons form from the leading process of migrating neurons while basal dendrites sprout from the cell body in a characteristic order. For most neuronal types, dendritic growth is slow at first but then dramatically increases with a transient overproduction of dendrites to achieve the mature dendritic arborisation.22,23 Changes in the dendritic characteristics examined (number of intersections, number of branch points and fractal dimension) were collectively grouped as dendritic complexity in this study. Our data showed that BCG enhanced the dendritic complexity, which served to increase the available surface area for synaptic contact and the capacity for neuronal connectivity.24 Additionally, BCG increased the dendritic length and number of primary neurites; greater dendritic length and a greater number of primary neurites increase the number of possible synaptic contacts and the information that may be received by a dendrite.25 Alterations in dendritic characteristics indicated that BCG promoted the process of dendritic development. As a result, it would increase the responses to incoming synaptic information.
The formation, elimination and remodeling of dendritic spines represent a continuous process that shapes the organization of synaptic networks during development. Spines are first formed in early postnatal life, shaped by experience, and maintained into adulthood.26 In addition to the change of dendritic growth, we found alterations of spine morphology in BCG rats. Specifically, BCG resulted in an increase of stubby spines and mushroom spines with large spine head width, suggesting an increase and an enhancement of synaptic connections under these conditions. Indeed, spine head width is correlated with the size of the postsynaptic density and the number of a-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptors.27 It has been shown that, upon maturation, the proportion of mushroom spines with a large spine head width increases, while the prorportion of long thin spines with a small spine head width decreases.28 Our results are supported by these findings. In general, the vast majority of studies examining neuronal structure have addressed the growth-promoting effects of neonatal BCG vaccination on dendritic development.
Interestingly, we found that the significant difference in the number of intersections was predominantly found at distances between 50 to 100 μm from the soma. The direction of the apical dendritic growth was toward the stratum molecular layer. The position of the intersections ranging between 50 to 100 μm was located in the stratum radiatum, which implies that the major influence of neonatal BCG vaccination on the dendritic structure was in the stratum radiatum.
It should be noted that the alterations in the dendritic structure and spine morphology were at 2 and 4 w; however, there was no obvious difference in these data points at 8 w. These results strongly suggest that BCG vaccination promotes the dendritic development of a pool of transient dendrites, which was in accord with the transient increase of the BDNF and IGF-1 in the hippocampus. In addition to the transient increase of neurotrophins, for those vaccinated with BCG in infancy, there is little reason to expect that the vaccination would be a persistent influence on neuronal development later in life because the BCG-induced immune responses, as assessed by skin sensitivity to tuberculin, wane within a few months or, at most, a few years in humans.29
The development of proper dendritic morphology depends on the interplay between genetic programming and extracellular signals.22,30,31 It is now well established that neurotrophins are important mediators of neuronal development, where they modulate dendritic growth and remodeling, membrane receptor trafficking, neurotransmitter release and synapse formation and function.20,32-34 In our study, neutrophins had a physiological role in the dendritic architecture in the hippocampus. This observation is in line with previous work showing that BDNF regulates dendritic architecture during the early development of hippocampal neurons.34 IGF-1 is most abundant in growing projection neurons that become the largest neurons equipped with the most extensive dendritic trees in the brain.35 Moreover, IGF-1 is also highly expressed in early postnatal development when dendritic process growth is most exuberant.36 The changes in spine morphology observed here were also in accord with the role of BDNF and IGF-1 in regulating the structure of dendritic spines in hippocampal neurons.37,38 However, many different extracellular molecules have been associated with neurite extension or restriction.12,39,40 BCG-induced T-cell responses, and activated T cells can secrete many neurotrophins, such as nerve growth factor (NGF), neurotrophins-3 (NT-3), and NT-4/5, in addition to BDNF and IGF-1.41-44 These neurotrophins may also participate in neuronal development.
Thus, the most sophisticated question for further study is to demonstrate why neonatal BCG vaccination resulted in the increased expression of BDNF and IGF-1 in the hippocampus. It has been reported that early life events can significantly affect the development of neural processes via their specific impact on cytokine and neurotrophin expression.45,46 Previous research has shown that neurotrophins, as a bridge between the immune system and neuroplasticity, results from the integration of multiple mechanisms.47 BCG vaccination has long been known to be a potent inducer of IFN-γ-secreting Th1 responses and Tregs.15,48,49 The increase in the levels of circulating immune factors (e.g., cytokines, chemokines or other factors), many of which can enter the CNS. These immune factors may promote microglia activation and proliferation and thus increasing the expression of neurotrophins. The interplay of cytokines and neurotrophins is complex. Neural traffic factors can be secreted by several types of immune cells, including T cells, microglia, macrophages and mast cells,50,51 particularly after exposing these cells to various cytokines, including TNF-a, IL-1β and IL-6.52,53 Once secreted, these neurotrophins can serve as mediators of the beneficial effects of immunity on neural development. Taken together, BCG induced an increase in circulating neurosupportive cytokines/neurotrophins that may have a supportive effect on neurons. However, how this or other pro-inflammatory cytokines affect neurotrophins remains to be elucidated.
Several studies have reported the neuroprotective role of BCG in neurodegenerative disease.8,9,54,55 In clinical trials, there was a relatively higher dosage of BCG, which reached 106 CFU or more, administered to adult mice with pathological changes in the CNS. However, our research was performed at physiological status and imitated BCG levels for the human infants' vaccine. BCG was administered in a single dose of 105 CFU at P0 in our study. It has been reported that BCG vaccination limited the loss of striatal dopamine (DA) and dopamine transporter (DAT) and prevented an increase in activated microglia in the substantia nigra in the model of neurodegenerative disease.8,9 Our data showed that BCG increased the dendritic complexity and promoted neurite growth and the maturation of spines. These differences may result from differences in the ratio of living/dead BCG in the inocula, inducing different type of T cells, different levels of Tregs,56 quantitative and qualitative differences in the induced cytokines and neurotrophins, differences in the kinetics of their biodistribution and replication, or other factors. In spite of these differences, the positive effect on CNS of BCG vaccination were both demonstrated in pathological and physiological statuses.
Taken together, the results of the present study demonstrate for the first time that neonatal BCG vaccination promoted neuronal dendritic development in early postnatal rats. The observed changes of dendritic arborization and of dendritic spine morphology, together with a positive correlation between apical dendritic length/fractal dimension/spine density and BDNF/IGF-1, may reflect enhanced neuronal networks. Moreover, this enhancement of BCG on dendritic development may be especially relevant to mechanisms of increased BDNF and IGF-1 induced by BCG vaccination.
Material and Methods
Animals
Newborn litters of Sprague Dawley (SD) rats were purchased from the Sun Yat-Sen University Laboratory Animal Center (Guangzhou, China) and were housed in a specific pathogen-free facility. All procedures were approved by the Sun Yat-Sen University Institute Research Ethics Committee.
Newborn pups were randomly assigned to experimental groups and matched control groups. Rats administered with BCG were named the BCG group. The control groups received injections of sterile phosphate-buffered saline (PBS) using the same protocol as the BCG group.
Vaccination
The immune procedure of BCG imitated that of the human infants' vaccine project.57 BCG was administered in a single dose at P0. Freeze-dried living BCG (D2-PB302 strain, Biological Institute of Shanghai, Shanghai, China) was suspended in sterile PBS. Newborn pups were injected intradermally on the back with 50 μl/rat of BCG suspension containing 105 colony forming units (CFU), or 50 μl sterile PBS according to a previously described procedure.58
Tissue preparation
Slices were prepared using a previously described method.59 Briefly, anesthetized SD rats were transcardially perfused with 25 mM PBS (pH = 7.2) for 3 minutes at 25 ml/min followed by 1.5% paraformaldehyde in 25 mM PBS for 20 minutes. The brains were removed from the skull and post-fixed for 1 hour in 1.5% paraformaldehyde in PBS. Coronal brain slices (200 μm free floating) were cut on a vibratome (Leica VT 1000S, Bannockburn, IL). Slices were rinsed and stored in 0.1 M phosphate buffer (PB) for DiI delivery.
Diolistic labeling
Dendrite and spine morphology of hippocampal neurons were assessed by quantifying them in neurons labeled with the fluorescent dye (1-1′-Dioctadecyl-3,3,3′,3′- tetramethylindocarbocyanine perchlorate) Dil (CM-DiI, Sigma-Aldrich, USA) in 2, 4 and 8 w old pups.60 The gene gun bullets were prepared according to a previously described method.59 Briefly, 8 mg of gold particles (1.0μm in diameter, Sigma-Aldrich, USA) were mixed with 2 mg of DiI (CM-DiI, Sigma-Aldrich, USA) and dissolved in 300 μl methylene chloride. After drying, the coated particles were collected in 2 ml water, vortexed on a sonicator for 5 minutes, and immediately transferred to gene gun tubing of 1 mm diameter (BioRad, USA). The tube was allowed to settle for 1 hour before slowly withdrawing the remaining liquid. Then, the tubing was dried under a constant nitrogen flow for 30 minutes. The tube was cut into small sections (2 cm in length) and stored in a desiccated container at 4°C for up to 1 month. For particle delivery, slices were transferred to a Petri dish and most of the PB was removed. The DiI-coated particles were delivered using the Helios Gene Gun system (BioRad, USA) at a pressure of 150–180 psi helium gas pressure. It is useful to insert a membrane filter with a 3 μm pore size (Millipore, Billerica, MA) between the gene gun and the brain sections to prevent clusters of large particles from landing on the tissue, which causes non-specific labeling and prevents single-cell resolution. After delivery, the slices were incubated overnight in 0.1 M PB at 4°C to allow the dye to diffuse along the neuronal processes. Last, the sections were rinsed 3 times with PB before being mounted on slides and cover slipped with 65% glycerin in 0.1 M PB.
Photography and confocal imaging
Image acquisition and analysis were performed systematically by an observer who was blind with respect to the treatment. On the same day, the brain sections were imaged on a confocal microscope (LSM 710, Carl Zeiss, Germany) with 20X and 63X objectives. Images of the complete dendritic profiles of DiI impregnated cells were captured using a 20X lens with appropriate optical zoom to fill the field of view with the cell. The z-stack depth was established and the dendritic profile was scanned at 1.0 μm increments along the z-axis. The images were used to analyze dendritic complexity and length using fractal and Sholl analysis. The neurites and soma could be clearly displayed using Diolistic labeling. The areas in the hippocampus could be easily distinguished depending on the certain arrangement of soma and the direction of the apical dendrite toward with 10X objectives. Although both neurons and neuroglia were labeled with DiI, they could be clearly distinguished on the basis of their morphological features. Pyramidal cells were identified from their large and triangular cell bodies and their lengthy axonal projections; apical dendrites, several lateral and basilar dendrites could also be recognized. In the CA1 area, the apical dendrite of pyramidal cell extends toward the interior (stratum moleculare) and the basilar dendrites extend toward the exterior (stratum oriens). At least 3 neurons of the hippocampal CA1 region per rat were randomly selected by the same investigator when they met the following criteria:1) neuron strictly located in CA1, triangular soma shape and apical dendritic extension toward the pial surface, and numerous dendritic spines, 2) neuron located within the middle of the thickness of the section, 3) neuron distinct from other neurons and 4) soma and branches were not obscured by vessels or adjacent neurons. Neurons were reconstructed by one investigator blinded to group identity. Spine morphology was measured separately for portions of 3 to 5 selected secondary dendrites per neuron and imaged using a 63× objective by acquiring 3-dimensional stacks that were z-sectioned at 0.5 μm (1024 × 1024 pixels, x-y scaling = 0.0952 μm/pixel).
Sholl analysis
The dendritic morphological analysis was performed using Image J (National Institute of Health;http://rsb.info.nih.gov/ij/). A 3D reconstruction of the entire dendritic processes of each neuron was made from the z-series stacks of confocal images to analyze dendritic tree development in the hippocampus. The 2D projection images were then traced using the NIH Image J program with the Neuron J plugin. Sholl analysis was performed using the NIH Image J Sholl analysis plugin as described previously.61 Briefly, Sholl analysis was performed on maximum intensity projections of each image by generating a series of 11 concentric circles of increasing radii (10 μm intervals) centered at the cell body, using the Image J Concentric Circles plugin. The number of dendrite intersections at each circle for each image was counted,16 and the number of dendrite crossings at each circle for each condition was calculated by averaging the number of crossings from every image.
Fractal analysis
Images of the DiI labeled hippocampal neurons were analyzed by fractal analysis, as it has been shown to be a robust technique for quantifying the complexity of the neuronal dendritic tree.62 The fractal dimension of each image was determined by the box-counting method, based on previous investigations17,62 showing this technique is the most of the used to analyze the fractality of neuronal dendritic arborisation patterns. For this analysis, neurons were projected at the same scale, converted to binary files and skeletonised so that all processes throughout the neuron profile were one pixel in diameter. Low intensity pixels representing background signal were manually removed. The present study deals only with the quantitative analysis of dendritic arborisation patterns, it was necessary to remove the cell bodies and axons from the digitized images of the neurons. After their removal, the images were then analyzed using the NIH Image J program with the fractal box counter tool. The calculated fractal dimension (D) had a range of 1 ≤ D ≤ 2 using the fractal box counter. Generally, the higher the fractal dimension, the more complex the dendritic pattern.
Spine analysis
Spine morphology was measured separately for portions of 3 to 5 selected secondary dendrites per neuron and imaged using a 63× objective by acquiring 3-dimensional stacks that were z-sectioned at 0.5 μm (1024 × 1024 pixels, x-y scaling = 0.0952 μm/pixel). At least 3 neurons per rat were collected. All segments were imaged from the secondary branches in the apical dendritic tree and of a similar distance from the cell body across genotypes. Dendritic spine morphology was analyzed using the Imaris software package (Version 7.0, Bitplane Inc., St Paul, MN). Spine density was determined by manually identifying spines. Spine area, length and spine head width were measured automatically in the 3D reconstructive stacks, which can classify spines according to their spine head width.
Enzyme-linked immunosorbent assay (ELISA)
The levels of BDNF and IGF-1 in the hippocampus were measured at 2, 4, and 8 w using ELISA kits (Cusabio Life Science Co., Ltd.) according to an optimized manufacturer's protocol. Samples were homogenized on ice in buffer (pH 7.6) containing (in mM): 50.0 Tris–HCl, 150 NaCl, 5.0 CaCl2, 0.02% NaN3, and 1% Triton X-100. The samples were then centrifuged at 17,000 x gravity for 30 minutes at 4°C. The total hippocampal homogenate concentration was adjusted to 4.5 mg/ml using an Enhanced BCA Protein Assay Kit (Beyotime) according to a previous report.63 The prepared plates were analyzed by the microplate reader (BIO-TEk ELx800, USA) at 450 nm.
Statistical Analysis
Data are presented as the mean ± SEM. Differences between groups were evaluated using Student's t test using SPSS 13.0 (SPSS for Windows). The analysis of the correlation between the apical dendritic length/fractal dimension and BDNF/IGF-1 was performed using the Pearson correlation analysis.Statistical significance was set at P < 0.05, and analyses were performed in Graph Pad Prism 5.0 (GraphPad Software).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
Authors thank Prof. Huaiyu Gu and Prof. Juntao Zou for their technical assistance. Thanks also to technician Qunfang Yuan for technical guidance and the Elsevier Language Editing Services for the English edited.
Funding
This work was supported by National Natural Science Foundation of China (No. 31371130), and Science and Technology Planning Project of Guangdong Province, China (No. 2009B080701089) and Medical Scientific Research Foundation of Guangdong Province, China.
References
- 1.Bitanihirwe BK, Peleg-Raibstein D, Mouttet F, Feldon J, Meyer U. Late prenatal immune activation in mice leads to behavioral and neurochemical abnormalities relevant to the negative symptoms of schizophrenia. Neuropsychopharmacology 2010; 35:2462-78; PMID:20736993; http://dx.doi.org/ 10.1038/npp.2010.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ito HT, Smith SE, Hsiao E, Patterson PH. Maternal immune activation alters nonspatial information processing in the hippocampus of the adult offspring. Brain Behav Immun 2010; 24:930-41; PMID:20227486; http://dx.doi.org/ 10.1016/j.bbi.2010.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen YH, Kuo TT, Chu MT, Ma HI, Chiang YH, Huang EY. Postnatal systemic inflammation exacerbates impairment of hippocampal synaptic plasticity in an animal seizure model. Neuroimmunomodulation 2013; 20:223-32; PMID:23736043; http://dx.doi.org/ 10.1159/000348440 [DOI] [PubMed] [Google Scholar]
- 4.Cheadle L, Biederer T. Activity-dependent regulation of dendritic complexity by semaphorin 3A through Farp1. J Neurosci 2014; 34:7999-8009; PMID:24899721; http://dx.doi.org/ 10.1523/JNEUROSCI.3950-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci 2005; 6:877-88; PMID:16261181; http://dx.doi.org/ 10.1038/nrn1787 [DOI] [PubMed] [Google Scholar]
- 6.Benner EJ, Mosley RL, Destache CJ, Lewis TB, Jackson-Lewis V, Gorantla S, Nemachek C, Green SR, Przedborski S, Gendelman HE. Therapeutic immunization protects dopaminergic neurons in a mouse model of Parkinson's disease. Proc Natl Acad Sci U S A 2004; 101:9435-40; PMID:15197276; http://dx.doi.org/ 10.1073/pnas.0400569101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones TB, Ankeny DP, Guan Z, McGaughy V, Fisher LC, Basso DM, Popovich PG. Passive or active immunization with myelin basic protein impairs neurological function and exacerbates neuropathology after spinal cord injury in rats. J Neurosci 2004; 24:3752-61; PMID:15084655; http://dx.doi.org/ 10.1523/JNEUROSCI.0406-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yong J, Lacan G, Dang H, Hsieh T, Middleton B, Wasserfall C, Tian J, Melega WP, Kaufman DL. BCG vaccine-induced neuroprotection in a mouse model of Parkinson's disease. PLoS One 2011; 6:0016610; PMID:21304945; http://dx.doi.org/ 10.1371/journal.pone.0016610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lacan G, Dang H, Middleton B, Horwitz MA, Tian J, Melega WP, Kaufman DL. Bacillus Calmette-Guerin vaccine-mediated neuroprotection is associated with regulatory T-cell induction in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease. J Neurosci Res 2013; 91:1292-302; PMID:23907992; http://dx.doi.org/ 10.1002/jnr.23253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barreto ML, Pereira SM, Ferreira AA. BCG vaccine: efficacy and indications for vaccination and revaccination. J Pediatr 2006; 82:S45-54; PMID:16826312; http://dx.doi.org/ 10.2223/JPED.1499 [DOI] [PubMed] [Google Scholar]
- 11.Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, Mosteller F. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. Jama 1994; 271:698-702; PMID:8309034; http://dx.doi.org/ 10.1001/jama.1994.03510330076038 [DOI] [PubMed] [Google Scholar]
- 12.Fields RD, Nelson PG. Activity-dependent development of the vertebrate nervous system. Int Rev Neurobiol 1992; 34:133-214; PMID:1587715 [DOI] [PubMed] [Google Scholar]
- 13.Rasakham K, Schmidt HD, Kay K, Huizenga MN, Calcagno N, Pierce RC, Spires-Jones TL, Sadri-Vakili G. Synapse density and dendritic complexity are reduced in the prefrontal cortex following seven days of forced abstinence from cocaine self-administration. PLoS One 2014; 9:e102524; PMID:25072653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J, Harte-Hargrove LC, Siao CJ, Marinic T, Clarke R, Ma Q, Jing D, Lafrancois JJ, Bath KG, Mark W, et al.. proBDNF negatively regulates neuronal remodeling, synaptic transmission, and synaptic plasticity in hippocampus. Cell Rep 2014; 7:796-806; PMID:24746813; http://dx.doi.org/ 10.1016/j.celrep.2014.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui ZJ, Zhao KB, Zhao HJ, Yu DM, Niu YL, Zhang JS, Deng JB. Prenatal alcohol exposure induces long-term changes in dendritic spines and synapses in the mouse visual cortex. Alcohol Alcohol 2010; 45:312-9; PMID:20543181; http://dx.doi.org/ 10.1093/alcalc/agq036 [DOI] [PubMed] [Google Scholar]
- 16.Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat 1953; 87:387-406; PMID:13117757 [PMC free article] [PubMed] [Google Scholar]
- 17.Milosevic NT, Ristanovic D. Fractality of dendritic arborization of spinal cord neurons. Neurosci Lett 2006; 396:172-6; PMID:16364544; http://dx.doi.org/ 10.1016/j.neulet.2005.11.031 [DOI] [PubMed] [Google Scholar]
- 18.Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci 2007; 30:79-97; PMID:17280523; http://dx.doi.org/ 10.1146/annurev.neuro.30.051606.094222 [DOI] [PubMed] [Google Scholar]
- 19.Nimchinsky EA, Sabatini BL, Svoboda K. Structure and function of dendritic spines. Annu Rev Physiol 2002; 64:313-53; PMID:11826272; http://dx.doi.org/ 10.1146/annurev.physiol.64.081501.160008 [DOI] [PubMed] [Google Scholar]
- 20.Spedding M, Gressens P. Neurotrophins and cytokines in neuronal plasticity. Novartis Found Symp 2008; 289:222-33; PMID:18497106 [DOI] [PubMed] [Google Scholar]
- 21.McAllister AK. Cellular and molecular mechanisms of dendrite growth. Cereb Cortex 2000; 10:963-73; PMID:11007547; http://dx.doi.org/ 10.1093/cercor/10.10.963 [DOI] [PubMed] [Google Scholar]
- 22.Jan YN, Jan LY. Branching out: mechanisms of dendritic arborization. Nat Rev Neurosci 2010; 11:316-28; PMID:20404840; http://dx.doi.org/ 10.1038/nrn2836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koester SE, O'Leary DD. Functional classes of cortical projection neurons develop dendritic distinctions by class-specific sculpting of an early common pattern. J Neurosci 1992; 12:1382-93; PMID:1556599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghiretti AE, Moore AR, Brenner RG, Chen LF, West AE, Lau NC, Van Hooser SD, Paradis S. Rem2 is an activity-dependent negative regulator of dendritic complexity in vivo. J Neurosci 2014; 34:392-407; PMID:24403140; http://dx.doi.org/ 10.1523/JNEUROSCI.1328-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jan YN, Jan LY. Dendrites. Genes Dev 2001; 15:2627-41; PMID:11641269; http://dx.doi.org/ 10.1101/gad.916501 [DOI] [PubMed] [Google Scholar]
- 26.Yoshihara Y, De Roo M, Muller D. Dendritic spine formation and stabilization. Curr Opin Neurobiol 2009; 19:146-53; PMID:19523814; http://dx.doi.org/ 10.1016/j.conb.2009.05.013 [DOI] [PubMed] [Google Scholar]
- 27.Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci 2009; 10:647-58; PMID:19693029; http://dx.doi.org/ 10.1038/nrn2699 [DOI] [PubMed] [Google Scholar]
- 28.De Simoni A, Griesinger CB, Edwards FA. Development of rat CA1 neurones in acute versus organotypic slices: role of experience in synaptic morphology and activity. J Physiol 2003; 550:135-47; PMID:12879864; http://dx.doi.org/ 10.1113/jphysiol.2003.039099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menzies D. What does tuberculin reactivity after bacille Calmette-Guerin vaccination tell us? Clin Infect Dis 2000; 31:S71-4; PMID:11010826; http://dx.doi.org/ 10.1086/314075 [DOI] [PubMed] [Google Scholar]
- 30.Parrish JZ, Emoto K, Kim MD, Jan YN. Mechanisms that regulate establishment, maintenance, and remodeling of dendritic fields. Annu Rev Neurosci 2007; 30:399-423; PMID:17378766; http://dx.doi.org/ 10.1146/annurev.neuro.29.051605.112907 [DOI] [PubMed] [Google Scholar]
- 31.Urbanska M, Blazejczyk M, Jaworski J. Molecular basis of dendritic arborization. Acta Neurobiol Exp 2008; 68:264-88; PMID:18511961 [DOI] [PubMed] [Google Scholar]
- 32.Kelamangalath L, Smith GM. Neurotrophin treatment to promote regeneration after traumatic CNS injury. Front Biol 2013; 8:486-95; PMID:25419214; http://dx.doi.org/ 10.1007/s11515-013-1269-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takahashi T, Shimizu K, Shimazaki K, Toda H, Nibuya M. Environmental enrichment enhances autophagy signaling in the rat hippocampus. Brain Res 2014; 10:113-23; PMID:25451096; http://dx.doi.org/ 10.1016/j.brainres.2014.10.026 [DOI] [PubMed] [Google Scholar]
- 34.McAllister AK, Katz LC, Lo DC. Neurotrophin regulation of cortical dendritic growth requires activity. Neuron 1996; 17:1057-64; PMID:8982155; http://dx.doi.org/ 10.1016/S0896-6273(00)80239-1 [DOI] [PubMed] [Google Scholar]
- 35.Bondy CA. Transient IGF-I gene expression during the maturation of functionally related central projection neurons. J Neurosci 1991; 11:3442-55; PMID:1658250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bartlett WP, Li XS, Williams M, Benkovic S. Localization of insulin-like growth factor-1 mRNA in murine central nervous system during postnatal development. Dev Biol 1991; 147:239-50; PMID:1879610; http://dx.doi.org/ 10.1016/S0012-1606(05)80021-1 [DOI] [PubMed] [Google Scholar]
- 37.Shimada A, Mason CA, Morrison ME. TrkB signaling modulates spine density and morphology independent of dendrite structure in cultured neonatal Purkinje cells. J Neurosci 1998; 18:8559-70; PMID:9786964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanaka J, Horiike Y, Matsuzaki M, Miyazaki T, Ellis-Davies GC, Kasai H. Protein synthesis and neurotrophin-dependent structural plasticity of single dendritic spines. Science 2008; 319:1683-87; PMID:18309046; http://dx.doi.org/ 10.1126/science.1152864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Powell EM, Meiners S, DiProspero NA, Geller HM. Mechanisms of astrocyte-directed neurite guidance. Cell Tissue Res 1997; 290:385-93; PMID:9321702; http://dx.doi.org/ 10.1007/s004410050945 [DOI] [PubMed] [Google Scholar]
- 40.Powell EM, Fawcett JW, Geller HM. Proteoglycans provide neurite guidance at an astrocyte boundary. Mol Cell Neurosci 1997; 10:27-42; PMID:9361286; http://dx.doi.org/ 10.1006/mcne.1997.0629 [DOI] [PubMed] [Google Scholar]
- 41.Barouch R, Appel E, Kazimirsky G, Braun A, Renz H, Brodie C. Differential regulation of neurotrophin expression by mitogens and neurotransmitters in mouse lymphocytes. J Neuroimmunol 2000; 103:112-21; PMID:10696906; http://dx.doi.org/ 10.1016/S0165-5728(99)00233-7 [DOI] [PubMed] [Google Scholar]
- 42.Hammarberg H, Lidman O, Lundberg C, Eltayeb SY, Gielen AW, Muhallab S, Svenningsson A, Lindå H, van Der Meide PH, Cullheim S, et al.. Neuroprotection by encephalomyelitis: rescue of mechanically injured neurons and neurotrophin production by CNS-infiltrating T and natural killer cells. J Neurosci 2000; 20:5283-91; PMID:10884312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moalem G, Gdalyahu A, Shani Y, Otten U, Lazarovici P, Cohen IR, Schwartz M. Production of neurotrophins by activated T cells: implications for neuroprotective autoimmunity. J Autoimmun 2000; 15:331-45; PMID:11040074; http://dx.doi.org/ 10.1006/jaut.2000.0441 [DOI] [PubMed] [Google Scholar]
- 44.Kerschensteiner M, Gallmeier E, Behrens L, Leal VV, Misgeld T, Klinkert WE, Kolbeck R, Hoppe E, Oropeza-Wekerle RL, Bartke I, et al.. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J Exp Med 1999; 189:865-70; PMID:10049950; http://dx.doi.org/ 10.1084/jem.189.5.865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goshen I, Kreisel T, Ounallah-Saad H, Renbaum P, Zalzstein Y, Ben-Hur T, Levy-Lahad E, Yirmiya R. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology 2007; 32:1106-15; PMID:17976923; http://dx.doi.org/ 10.1016/j.psyneuen.2007.09.004 [DOI] [PubMed] [Google Scholar]
- 46.Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun 2011; 25:181-213; PMID:20970492; http://dx.doi.org/ 10.1016/j.bbi.2010.10.015 [DOI] [PubMed] [Google Scholar]
- 47.Calabrese F, Rossetti AC, Racagni G, Gass P, Riva MA, Molteni R. Brain-derived neurotrophic factor: a bridge between inflammation and neuroplasticity. Front Cell Neurosci 2014; 8:430; PMID:25565964; http://dx.doi.org/ 10.3389/fncel.2014.00430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fedatto PF, Sergio CA, Paula MO, Gembre AF, Franco LH, Wowk PF, Ramos SG, Horn C, Marchal G, Turato WM, et al.. Protection conferred by heterologous vaccination against tuberculosis is dependent on the ratio of CD4(+) /CD4(+) Foxp3(+) cells. Immunology 2012; 137:239-48; PMID:22891805; http://dx.doi.org/ 10.1111/imm.12006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Q, Shen HH. Neonatal bacillus Calmette-Guerin vaccination inhibits de novo allergic inflammatory response in mice via alteration of CD4+CD25+ T-regulatory cells. Acta Pharmacol Sin 2009; 30:125-33; PMID:19060917; http://dx.doi.org/ 10.1038/aps.2008.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elkabes S, DiCicco-Bloom EM, Black IB. Brain microglia/macrophages express neurotrophins that selectively regulate microglial proliferation and function. J Neurosci 1996; 16:2508-21; PMID:8786427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakajima K, Honda S, Tohyama Y, Imai Y, Kohsaka S, Kurihara T. Neurotrophin secretion from cultured microglia. J Neurosci Res 2001; 65:322-31; PMID:11494368; http://dx.doi.org/ 10.1002/jnr.1157 [DOI] [PubMed] [Google Scholar]
- 52.Gadient RA, Cron KC, Otten U. Interleukin-1 beta and tumor necrosis factor-alpha synergistically stimulate nerve growth factor (NGF) release from cultured rat astrocytes. Neurosci Lett 1990; 117:335-40; PMID:2094822; http://dx.doi.org/ 10.1016/0304-3940(90)90687-5 [DOI] [PubMed] [Google Scholar]
- 53.Schulte-Herbruggen O, Nassenstein C, Lommatzsch M, Quarcoo D, Renz H, Braun A. Tumor necrosis factor-alpha and interleukin-6 regulate secretion of brain-derived neurotrophic factor in human monocytes. J Neuroimmunol 2005; 160:204-9; PMID:15710474; http://dx.doi.org/ 10.1016/j.jneuroim.2004.10.026 [DOI] [PubMed] [Google Scholar]
- 54.Sewell DL, Reinke EK, Co DO, Hogan LH, Fritz RB, Sandor M, Fabry Z. Infection with Mycobacterium bovis BCG diverts traffic of myelin oligodendroglial glycoprotein autoantigen-specific T cells away from the central nervous system and ameliorates experimental autoimmune encephalomyelitis. Clin Diagn Lab Immunol 2003; 10:564-72; PMID:12853387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ben-Nun A, Mendel I, Sappler G, Kerlero de Rosbo N. A 12-kDa protein of Mycobacterium tuberculosis protects mice against experimental autoimmune encephalomyelitis. Protection in the absence of shared T cell epitopes with encephalitogenic proteins. J Immunol 1995; 154:2939-48; PMID:7876560 [PubMed] [Google Scholar]
- 56.Burl S, Adetifa UJ, Cox M, Touray E, Ota MO, Marchant A, Whittle H, McShane H, Rowland-Jones SL, Flanagan KL. Delaying bacillus Calmette-Guerin vaccination from birth to 4 1/2 months of age reduces postvaccination Th1 and IL-17 responses but leads to comparable mycobacterial responses at 9 months of age. J Immunol 2010; 185:2620-8; PMID:20644160; http://dx.doi.org/ 10.4049/jimmunol.1000552 [DOI] [PubMed] [Google Scholar]
- 57.Marchant A, Goetghebuer T, Ota MO, Wolfe I, Ceesay SJ, De Groote D, Corrah T, Bennett S, Wheeler J, Huygen K, et al.. Newborns develop a Th1-type immune response to Mycobacterium bovis bacillus Calmette-Guerin vaccination. J Immunol 1999; 163:2249-55; PMID:10438968 [PubMed] [Google Scholar]
- 58.Kiros TG, Power CA, Wei G, Bretscher PA. Immunization of newborn and adult mice with low numbers of BCG leads to Th1 responses, Th1 imprints and enhanced protection upon BCG challenge. Immunotherapy 2010; 2:25-35; PMID:20635889; http://dx.doi.org/ 10.2217/imt.09.80 [DOI] [PubMed] [Google Scholar]
- 59.Staffend NA, Meisel RL. DiOlistic labeling in fixed brain slices: phenotype, morphology, and dendritic spines. Curr Protoc Neurosci 2011; 2; PMID:21462159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Erion JR, Wosiski-Kuhn M, Dey A, Hao S, Davis CL, Pollock NK, Stranahan AM. Obesity elicits interleukin 1-mediated deficits in hippocampal synaptic plasticity. J Neurosci 2014; 34:2618-31; PMID:24523551; http://dx.doi.org/ 10.1523/JNEUROSCI.4200-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duan X, Chang JH, Ge S, Faulkner RL, Kim JY, Kitabatake Y, Liu XB, Yang CH, Jordan JD, Ma DK, et al.. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell 2007; 130:1146-58; PMID:17825401; http://dx.doi.org/ 10.1016/j.cell.2007.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fernandez E, Jelinek HF. Use of fractal theory in neuroscience: methods, advantages, and potential problems. Methods 2001; 24:309-21; PMID:11465996; http://dx.doi.org/ 10.1006/meth.2001.1201 [DOI] [PubMed] [Google Scholar]
- 63.Selenica ML, Alvarez JA, Nash KR, Lee DC, Cao C, Lin X, Reid P, Mouton PR, Morgan D, Gordon MN. Diverse activation of microglia by chemokine (C-C motif) ligand 2 overexpression in brain. J Neuroinflammation 2013; 10:1742-2094; PMID:23866683; http://dx.doi.org/ 10.1186/1742-2094-10-86 [DOI] [PMC free article] [PubMed] [Google Scholar]