Abstract
Recent research suggests an involvement of pro-opiomelanocortin (POMC) gene products (e.g., beta-endorphin) in modulating cocaine-induced reward and addiction-like behaviors in rodents. In this study, we investigated whether chronic “binge” cocaine and its withdrawal altered POMC gene expression in the brain of rats. Male Fischer rats were treated with two different chronic (14-day) “binge” pattern cocaine administration regimens (3 injections at 1-h intervals, i.p.): steady-dose (45 mg/kg/day) and escalating-dose (90 mg/kg on the last day). Although there was no POMC mRNA alteration after chronic steady-dose cocaine, a significant decrease in POMC mRNA levels in the hypothalamus was found after chronic escalating-dose cocaine. In contrast, after acute (1-day) withdrawal from chronic “binge” escalating-dose regimen, but not steady-dose regimen, there were increased hypothalamic POMC mRNA levels that persisted into 14 days of protracted withdrawal. To study the role of the endogenous opioid systems in the cocaine withdrawal effects, we administered a single naloxone injection (1 mg/kg) that caused an elevated POMC mRNA levels observed 24 h later in cocaine naïve rats, but it did not lead to further increases in cocaine-withdrawn rats. Our results suggest that during withdrawal from chronic “binge” escalating-dose cocaine: (1) there was a persistent increase in hypothalamic POMC gene expression; and (2) hyposensitivity of the POMC gene expression to naloxone indicates altered opioidergic tone at or above the hypothalamic level.
Keywords: chronic “binge” cocaine, cocaine withdrawal, steady-dose, escalating-dose, POMC, hypothalamus
Introduction
Opioid receptor antagonists (especially the mu opioid receptor selective antagonists) have been found to reduce both the cocaine reinforcement using the self-administration model (Carroll et al., 1986; Ramsey and van Ree, 1991) and the rewarding action of cocaine using the conditioned place preference (CPP) model in rodents (Suzuki et al., 1992; Gerrits et al., 1995; Houdi et al., 1998). These early findings raise the possibility that cocaine may trigger the release of endogenous opioid peptides (e.g., beta-endorphin) and further suggest that these opioid neuropeptides play a functional role in the cocaine-induced behavior. Of interest, a recent study has found that the cocaine-induced CPP is reduced in beta-endorphin deficient mice, indicating a reduced rewarding effect of cocaine with less endogenous beta-endorphin (Marquez et al., 2008). Together, these studies suggest a modulatory role for the endogenous opioid peptide beta-endorphin in cocaine reward or reinforcement.
Pro-opiomelanocortin (POMC), a large peptide precursor, produces several biologically active neuropeptides, including beta-endorphin, adrenocorticotropic hormone (ACTH) and melanocortins. The presence of POMC neurons or cells was originally found to be mainly restricted to the rodent arcuate nucleus in the hypothalamus, nucleus of the solitary tract and pituitary (Mansour et al., 1995; Mercer et al., 2013). The opioid peptide beta-endorphin is distributed in the hypothalamus, and the dopaminergic mesocorticolimbic regions, probably from hypothalamic POMC neuronal projections, although it is still a topic of debate. Since activation of the mu opioid receptor by beta-endorphin is rewarding and modulates dopamine release in the nucleus accumbens (Spanagel et al., 1991), beta-endorphin may be involved in the motivational behavior and reinforcing effect of several drugs of abuse (Koob and Kreek, 2007; Roth-Deri et al., 2008). For instance, central administration of beta-endorphin via intra-cerebro-ventricular injection has been found to induce CPP in rats (Amalric et al., 1987). In line with above findings, we have recently found that POMC gene expression in the hypothalamus is increased by cocaine in the setting of drug-induced place conditioning (Zhou et al., 2012).
Since the early 1990’s, many laboratories (including our laboratory) have investigated the effect on opioid peptides and their receptors of drugs of abuse. Alterations of preproenkephalin, mu opioid receptor, preprodynorphin and kappa opioid receptor gene expression in mesolimbic brain areas of mice or rats after chronic cocaine exposure or across long-term withdrawal have been broadly studied (see reviews [Kreek et al., 2009; Le Merrer et al., 2009]). However, it is unclear if POMC gene expression in specific brain regions is altered by cocaine, particularly after chronic exposure and withdrawal.
To extend our research, we here report a set of experiments addressing the research question: are POMC mRNA levels in the hypothalamus or amygdala (where POMC expression is relatively abundant [Zhou et al., 2010]) altered after chronic cocaine administration or its withdrawal? As recent studies showing an involvement of different amygdalar neuropeptides (e.g., dynorphin, vasopressin and CRF) after drug withdrawal (e.g., Zhou and Kreek, 2014), the amygdala was included for the POMC mRNA measurement in the present study. On the basis of evidence implicating POMC-derived beta-endorphin in the rewarding property of cocaine, we predicted that POMC gene expression would be altered in animals after chronic exposure or during withdrawal.
Experimental procedures
Experiment 1. Withdrawal from chronic (14-day) escalating-dose “binge” pattern cocaine administration and interactions with naloxone in rats
1.1. Animals
Male Fischer 344 rats (190–220 g; Charles River Labs, Kingston, NY) were housed individually in a stress-minimized facility with free access to food and water. In order to minimize stress, noise and animal handling unrelated to the experimental protocol were kept to a bare minimum. Prior to the beginning of the experiment, animals were adapted to a standard 12-h light/dark cycle (lights on from 9:00 h to 21:00 h) for 7 days. The protocol was approved by the Rockefeller University Animal Care and Use Committee.
Before cocaine or saline administration, all rats were handled and received three daily intraperitoneal (i.p.) injections of saline (3 ×1 ml/kg/day) at 9:30, 10:30 and 11:30 h for 7 days in order to minimize injection-induced stress when the experiment began on day 8 (a method validated in earlier studies, see [e.g., Zhou et al., 2004]). Fischer rats were selected because this inbred strain self-administers cocaine at a high level after cocaine self-administration behavior is established, with high anxiety phenotype (Kosten and Ambrosio, 2002).
1.2. Procedure of escalating-dose “binge” pattern cocaine administration and withdrawal with naloxone pretreatment
The “binge” pattern regimen of drug administration (cocaine or saline) consisted of i.p. injections three times daily with two 1-hour intervals, beginning 30 min after the light cycle (9:30, 10:30, and 11:30) in the home cage (Branch et al., 1992). This dosing schedule was selected to mimic the pattern often observed in human cocaine abusers with relation to the circadian rhythm of rest and activity during the day, and with respect to repeated administrations over several hours. For animals treated with cocaine, the drug doses were increased after every three days, with the same volume of saline (3 ml/kg/day). The volume of injection was kept constant through the entire experiment and only the concentration of cocaine was escalated. Therefore, the cocaine-treated rats received initial cocaine dosing at 45 (3 × 15) mg/kg/day on days 1–3, 60 (3 × 20) mg/kg/day on days 4–6, 75 (3 × 25) mg/kg/day on days 7–9, and then 90 (3 × 30) mg/kg/day on days 10–14. As previously reported, this dosing schedule models the cocaine dose range self-administered by rats given long access (6 to 10 hours) to the drug (Koob and Kreek, 2007). For animals treated with saline, the volume was 3 ml/kg/day through the all experimental days.
The experiment paradigm contained three phases: chronic 14-day escalating-dose “binge” pattern cocaine exposure, 1-day acute withdrawal and 14-day chronic withdrawal (Fig. 1A).
Figure 1.
Timelines for cocaine administration regimens with antagonist applications.
In experiment 1.1 chronic (14 days) escalating-dose “binge” cocaine, rats received saline (n = 8) or escalating-dose “binge” cocaine (from 45 up to 90 mg/kg/day) (n = 8) injections for 14 days and were then sacrificed at 12:00 h on day 14, 30 min after the last saline or “binge” cocaine injection. During exposure to 90 mg/kg/day dose of cocaine, two animals died with seizures, resulting in an n = 6 for the cocaine-treated group.
In experiment 1.2 acute (1 day) cocaine withdrawal with naloxone, rats received saline or “binge” cocaine (from 45 up to 90 mg/kg/day) injections for 14 days and were then sacrificed at 17:00 h on day 15, 1 day after the last saline or “binge” cocaine injection. On day 14 (1 day before sacrifice for sampling), an i.p injection of saline or naloxone (1 mg/kg) was administered 30 min after the last saline or “binge” cocaine injection. In this experiment, rats were assigned to one of four treatment groups: (1) Acute (1-day) cocaine withdrawal: cocaine injections for 14 days with one saline administration 30 min after the last “binge” cocaine, followed by 1-day withdrawal, n = 7 (During exposure to 90 mg/kg/day dose of cocaine, one rat died with seizures resulting in an n = 6); (2) Saline control: saline injections for 14 days with one saline administration 30 min after the last “binge” saline, followed by 1-day withdrawal, n = 6; (3) Naloxone+Acute cocaine withdrawal: cocaine injections for 14 days with one naloxone administration (1 mg/kg) 30 min after the last “binge” cocaine, followed by 1-day withdrawal, n = 7; and (4) Naloxone: saline injections for 14 days with one naloxone administration (1 mg/kg) 30 min after the last “binge” saline, followed by 1-day withdrawal, n = 6. The naloxone dose chosen was based on our pilot study, in which a single 1 mg/kg dose was observed to moderately increase POMC in cocaine naïve rats after 24 hours of naloxone injection.
In experiment 1.3 chronic (14 days) cocaine withdrawal, rats received saline or “binge” cocaine (from 45 up to 90 mg/kg/day) injections for 14 days and were then sacrificed at 17:00 h on day 28, 14 days after the last “binge” cocaine or saline injection. In this experiment, rats were assigned to one of two treatment groups: (1) Chronic (14-day) cocaine withdrawal: cocaine injections for 14 days followed by 14-day withdrawal, n = 7 (during exposure to 90 mg/kg/day dose of cocaine, one died with seizures, resulting in an n = 6); and (2) Saline control: saline administrations for 14 days followed by 14-day withdrawal, n = 6.
Body weight decreased after 14 days of chronic escalating-dose “binge” cocaine (t = 306, d.f. = 10, p < 0.0001), and rats withdrawn for 1 day from chronic cocaine continued to lose body weight with naloxone treatment (t = 480, d.f. = 10, p < 0.0001) or without naloxone treatment (t = 294, d.f. = 10, p < 0.0001). The 14-day withdrawal groups were still significantly lower than saline controls (Table 1) (t = 37, d.f. = 10, p < 0.0001).
Table 1.
Effects of chronic (14-day) escalating-dose (45 up to 90 mg/kg/day) “binge” cocaine, acute (1-day) withdrawal (WD) and chronic (14-day) WD with opioid receptor antagonist naloxone (NXN, 1 mg/kg) on body weight (g) and plasma corticosterone (ng/ml) levels.
| 14-day-dose | Escalating Cocaine | 1-day | WD | 1-day + | WDNaloxone | 14-day | WD | |
|---|---|---|---|---|---|---|---|---|
| Saline | Cocaine | Saline | Cocaine | Saline | Cocaine | Saline | Cocaine | |
| Body weight | 244±2 | 177±3** | 249±2 | 173±3** | 265±3 | 170±4** | 276±3 | 239±5* |
| Corticosterone | 15±5 | 159±52** | 129±37 | 102±41 | 127±26 | 100±38 | 122±32 | 98±32 |
p<0.05,
p<0.01 vs. Saline control. n=6–7.
All rats were rapidly decapitated after brief exposure to CO2 (within 15 sec), and the hypothalamus and amygdala were collected for subsequent mRNA analyses.
1.3. Preparation of RNA extracts
In each experiment, rats were sacrificed by decapitation after a brief exposure to CO2. Each animal brain was removed from the skull and placed in a chilled rat brain matrix (ASI Instruments, Houston, TX). A coronal slice containing the hypothalamus or amygdala was removed from the matrix and placed on an ice-cold petri dish. Dissection was carried out using forceps and razor blades under a dissecting microscope. Two coronal slices were made: the first was between Bregma −1.80 mm to −2.80 mm for the hypothalamus and amygdala; and the second between Bregma −2.80 mm to −3.80 mm for the amygdala, according to The Rat Brain in Stereotaxic Coordinates (Paxinos and Watson, Academic Press, New York, 1986). The two regions were immediately homogenized in guanidinium thiocyanate buffer and then extracted with acidic phenol and chloroform. After the final 70% ethanol precipitation step, each extract was resuspended in DEPC-treated H2O and stored at −80 °C.
1.4. POMC mRNA measurements using solution hybridization ribonuclease (RNase) protection-trichloroacetic acid (TCA) precipitation assay
The protocol for the solution hybridization RNase protection-TCA precipitation assay has been described in detail in earlier reports (e.g., Zhou et al., 2004). A 538-bp fragment from the rat POMC cDNA was cloned in both the sense and antisense orientations into the polylinker region of either pSP64 or pSP65 plasmids (Promega, Madison, WI). 33P-labeled cRNA antisense probes and unlabeled cRNA sense standards were synthesized using SP6 transcription system. The plasmid pS/E (a pSP65 derivative) was used to synthesize riboprobe for the 18S rRNA to determine total RNA. A single full-length transcript from each plasmid had been confirmed by a denaturing agarose gel (1.0 M formaldehyde).
RNA extracts were dried in 1.5 ml Eppendorf tubes and resuspended in 30 μl of 2 × TESS (10 mM N-Tris[hydroxy-methyl]methyl-2-aminoethane sulfonic acid, pH 7.4; 10 mM ethylenediaminetetraacetic acid [EDTA]; 0.3 M NaCl; 0.5% sodium dodecyl sulfate [SDS]) containing 33P-labeled POMC cRNA probe with 150 K to 300 K cpm. After covered with mineral oil, the samples hybridized overnight for 10–12 hours at 75 °C. During RNase treatment, 250 μl of a buffer (0.3 M NaCl; 5 mM EDTA; 10 mM Tris-HCl [pH 7.5]) containing 40 μg/ml RNase A (Worthington Biochemical, Freehold, NJ) and 2 μg/ml RNase T1 (Calbiochem, San Diego, CA) was added and each sample was incubated at 30 °C for 1 hour. TCA precipitation was effected by the addition of 1 ml of a solution containing 5% TCA and 0.75% sodium pyrophosphate. Precipitates were finally collected onto a filter in sets of 24 using a cell harvester (Brandel, Gaithersburg, MD) and were then measured in a scintillation counter with liquid scintillant (Beckman, Palo Alto, CA).
The procedure to measure POMC mRNA levels involved a comparison of values obtained from experimental samples (brain extracts) to those obtained for a set of POMC calibration standards. The calibration standards had known amounts of an in vitro POMC sense transcript whose concentration was determined by optical absorbance at 260 nm. The set of POMC calibration standards included those with no added sense transcript (0) and those that contained between 1.25 and 80 pg of the POMC sense transcript. A new POMC standard curve was generated each time experimental samples were analyzed and all extracts of a particular tissue were assayed for the POMC mRNA levels as a group in a single assay. Total cellular RNA concentrations were measured by hybridization of diluted extracts to a 33P-labeled probe complementary to 18S rRNA at 75 °C. The 18S calibration standards for this curve contained 10 μg of E. coli tRNA plus either 0.0, or from 2.5 to 40 ng of total RNA from rat brains whose concentration was determined by optical absorbance at 260 nm.
1.5. Radioimmunoassays
At the time of decapitation, truck blood from each rat was collected in EDTA-containing tubes, placed on ice, and spun in a centrifuge at 4 °C. Plasma was separated and stored at −40 °C for corticosterone measurements by radioimmunoassay. Corticosterone levels were assayed using a rat corticosterone 125I kit from MP Biomedicals (Costa Mesa, CA). All values were determined in duplicate in a single assay.
Experiment 2. Withdrawal from chronic (14-day) steady-dose “binge” pattern cocaine administration in rats
2.1. Animals
A new cohort of rats is identical to those used in Experiment 1.
2.2. Procedure for steady-dose “binge” pattern cocaine administration and withdrawal
The “binge” pattern of drug administration (cocaine or saline), injection route, injection volume and time points were identical to those in Experiment 1. For animals treated with cocaine, the dose was 3 × 15 mg/kg every day, and there was no animal loss with this dose. The experiment paradigm is shown in Fig. 1B.
In separate experiments, animals were subjected to acute (1 day) or chronic (14 days) steady-dose “binge” cocaine (3× 15 mg/kg, n = 6), while control animals received equivalent treatment with saline (n = 6). Animals were sacrificed at 12:00 h either 30 min (Experiment 2.1 after acute cocaine and Experiment 2.2 after chronic cocaine), or at 17:00 h 1 day (Experiment 2.3 after chronic cocaine) or 14 days (Experiment 2.4 after chronic cocaine) following their last cocaine injection.
Body weight decreased after 14 days of chronic steady-dose binge cocaine (t = 102, d.f. = 10, p < 0.0001), and rats withdrawn for 1 day from chronic cocaine continued to lose body weight significantly (t = 204, d.f. = 10, p < 0.0001). The 14-day withdrawal groups were no longer different from saline controls (Table 2).
Table 2.
Effects of chronic (14-day) steady-dose (45 mg/kg/day) “binge” cocaine, acute (1-day) withdrawal (WD) and chronic (14-day) WD on body weight (g) and plasma corticosterone (ng/ml) levels.
| 14-day | Steady-dose Cocaine | 1-day | WD | 14-day | WD | |
|---|---|---|---|---|---|---|
| Saline | Cocaine | Saline | Cocaine | Saline | Cocaine | |
| Body weight | 233±2 | 187±4** | 236±2 | 183±3** | 255±3 | 239±8 |
| Corticosterone | 12±4 | 117±37* | 119±38 | 112±40 | 120±29 | 110±28 |
p<0.05 or
p<0.01 vs. Saline control. n=6–7.
The hypothalamus and amygdala were collected for subsequent mRNA analyses, as well as plasma for corticosterone analyses, as described in the above Experiment 1 section.
Data analysis
In Experiment 1.2, group differences in the POMC mRNA levels were analyzed using two-way ANOVA for acute or chronic withdrawal (cocaine, saline) and for antagonist (naloxone, saline) followed by Newman-Keuls post-hoc tests. In other experiments, differences between two groups were analyzed using a two-tailed student’s t-test for each measure. The accepted level of significance for all tests was p < 0.05. All statistical analyses were performed using Statistica (version 5.5, StatSoft Inc, Tulsa, OK).
Results
Experiment 1. Effects of 14-day escalating-dose “binge” cocaine with naloxone, and of its 1 or 14 -day withdrawal
1A. POMC mRNA levels in the hypothalamus or amygdala
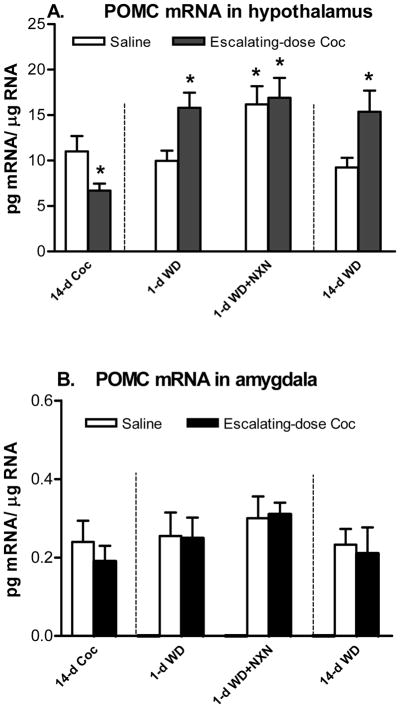
In the hypothalamus, there was a significant decrease in POMC mRNA levels after 14 days of escalating-dose cocaine administration (t = 4.81, d.f. = 12, p < 0.05) (Fig. 2A). For 1-day withdrawal, two-way ANOVA showed a significant effect of naloxone (F (1,20) = 6.30, p < 0.05) on the POMC mRNA levels. Newman-Keuls post hoc tests showed a significant difference between saline and 1-day withdrawal groups (p < 0.05), and between saline and naloxone + 1-day withdrawal groups (p < 0.05) (Fig. 2A). For 14-day withdrawal, a Student’s t-test was carried out on the POMC mRNA levels and showed a significant difference between saline and 14-day withdrawal groups (t = 6.49, d.f. = 10, p < 0.05) (Fig. 2A).
Figure 2.
Effects of chronic (14-day) escalating-dose (45 up to 90 mg/kg/day) “binge” cocaine (Coc), acute (1-day) withdrawal (WD) and chronic (14-day) WD with opioid receptor antagonist naloxone (NXN, 1 mg/kg) on POMC mRNA levels in the hypothalamus (A) and amygdala (B). * p<0.05 vs. Saline control. n=6–7.
In the amygdala, there was no significant effect on POMC mRNA levels after chronic cocaine, its 1-day withdrawal with naloxone or 14-day withdrawal (Fig. 2B).
1B. Plasma corticosterone levels
There was a significant increase after 14 days of escalating-dose regimen (t = 10.2, d.f. = 9, p < 0.01) (Table 1). For 1- or 14-day withdrawal, there was no significant effect after 1-day withdrawal with naloxone or 14-day withdrawal.
Experiment 2. Effects of 14-day steady-dose “binge” cocaine and of its 1 or 14 -day withdrawal
2A. POMC mRNA levels in the hypothalamus or amygdala
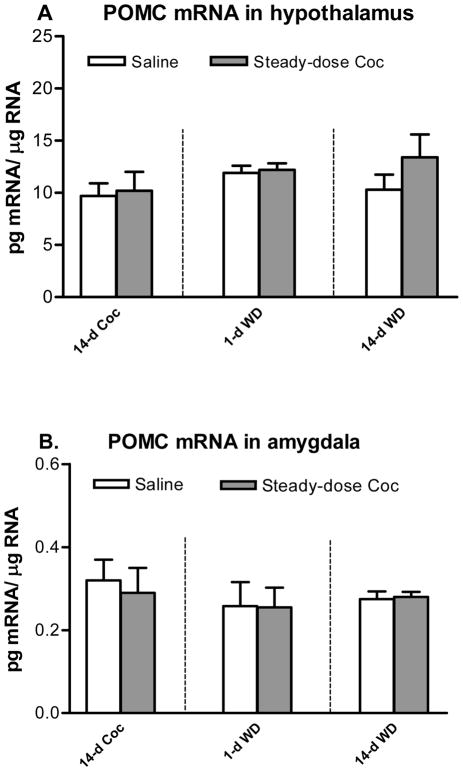
After 14 days of chronic steady-dose “binge” cocaine (Fig. 3A), no difference from the controls was found on POMC mRNA levels in the hypothalamus. After 1- or 14-day withdrawal from chronic steady-dose cocaine, there was no significant change in POMC mRNA levels in the hypothalamus.
Figure 3.
Effects of chronic (14-day) steady-dose (45 mg/kg/day) “binge” cocaine (Coc), acute (1-day) withdrawal (WD) and chronic (14-day) WD on POMC mRNA levels in the hypothalamus (A) and amygdala (B). n=6–7.
As shown in Fig. 3B, no difference from the controls was found on POMC mRNA levels in the amygdala after either chronic steady-dose cocaine or its 1- or 14-day withdrawal.
2B. Plasma corticosterone levels
There was a significant increase after 14 days of steady-dose regimen (t= 6.8, d.f. = 9, p < 0.05) (Table 2). For 1- or 14-day withdrawal, there was no significant effect on plasma corticosterone levels.
Discussion
The present study was designed to examine the gene expression of an important hypothalamic opioid peptide gene, POMC, after chronic cocaine and withdrawal. In fact, we identified that chronic exposure and withdrawal from different doses of cocaine corresponded to significant differences in hypothalamic POMC mRNA levels. Chronic escalating-dose cocaine decreased the POMC mRNA levels in the hypothalamus (but not the amygdala). It seems dose-dependent, as the steady-dose cocaine regimen with a relative low dose did not alter the POMC mRNA levels at the same time point. It seems time-dependent, as acute “binge” cocaine at 45 mg/kg dose did not alter hypothalamic POMC mRNA levels after 1 day exposure (Zhou et al., 2004). After acute (1-day) withdrawal, there was a significant increase in POMC mRNA levels in the hypothalamus of the escalating-dose cocaine-treated rats, which was also not seen in the steady-dose cocaine-treated animals. This increase seems long lasting: POMC mRNA levels were still found to be higher after 14 days of protracted withdrawal.
This effect on the hypothalamic POMC seemed to be region-specific, as no effect on the POMC mRNA levels was found in the amygdala (where POMC expression is relatively abundant [Zhou et al., 2010]). The inclusion of the amygdalar POMC was based on recent studies showing gene expression alterations of other neuropeptides in this region (including CRF, vasopressin, dynorphin after drug withdrawal [Zhou and Kreek, 2014].
Since many of cocaine’s effects are mediated by transiently increased synaptic dopamine levels due to inhibition of dopamine re-uptake at nerve terminals, dopamine receptors may play a role in modulating the POMC mRNA changes. Dopamine has been reported to reduce POMC mRNA levels in primary cell cultures of rat hypothalamus in a dose dependent manner (L’Hereault and Barden, 1991). In contrast, an increase in POMC mRNA levels has been reported in the rat treated with non-selective dopamine receptor antagonists for 14 days (e.g., Autelitano et al., 1987). With selective dopamine receptor antagonists, the POMC mRNA levels in the hypothalamus were found to increase after dopamine D2 (but not D1) receptor antagonist treatment (Zhou et al., 2004). An early microdialysis study from our laboratory has shown that following withdrawal from chronic “binge” pattern cocaine administration, the baseline of dopamine levels in extracellular fluid is significantly lower than that before cocaine exposure in both the nucleus accumbens and caudate putamen of rats (Maisonneuve et al., 1995). In the present study, there were increases in hypothalamic POMC mRNA levels during chronic withdrawal from chronic escalating-dose cocaine. On the basis of the above literature, we predicted that the POMC mRNA increase across 14-day cocaine withdrawal may be in part due to relative dopamine deficiency during cocaine withdrawal. However, there are other mechanisms potentially involved. For example, studies have reported that POMC mRNA and POMC peptides in the hypothalamus are under stimulatory modulation by serotonin (Locatelli et al., 1983; L’Hereault and Barden, 1991a). Both the mu and kappa opioid receptors are found in the arcuate nucleus of the hypothalamus (Mansour et al., 1995), and the opioid agonists or antagonists have inhibitory or stimulatory effects, respectively, on POMC neurons in the hypothalamus both in vivo and in vitro (Nikolarakis et al., 1987; L’Hereault and Barden, 1991b; Markowitz et al., 1992; Wardlaw et al., 1996). Although the mechanisms are complex and not fully understood, our findings indicate that POMC-producing neurons may adapt to chronic cocaine withdrawal, as the increased POMC gene expression persisted into 2 weeks of protracted withdrawal, with a long-lasting feature.
After a single injection of an opioid receptor antagonist naloxone (1 mg/kg), there was an increase in rat hypothalamic POMC mRNA levels after 1 day. This increase was not seen after 2 days of naloxone treatment (data not shown). Our results clearly show that the blockade of opioid receptors transiently increased POMC gene expression in the hypothalamus, indicating an inhibition of POMC activity by endogenous opioids. Of particular interest, POMC gene expression that increased by cocaine withdrawal was insensitive to opioid receptor blockade, since we observed no further effect of naloxone on POMC mRNA levels during cocaine withdrawal.
The alterations in POMC mRNA levels reported here could be the result of enhanced POMC promoter activity, leading to an increased POMC gene transcriptional rate. This increase in POMC gene expression could further lead to an increase in beta-endorphin and other POMC-derived peptides’ biosynthesis and/or release from the arcuate nucleus (e.g., melanocortins). It is possible that the large quantities of this psychostimulant led to a dysregulation of stress pathways that enhanced hypothalamic stress responsive gene expressions, including POMC. Thereby, the enhanced POMC expression, with resultant increases in beta-endorphin biosynthesis and/or release, could contribute to the persistent increases in the cocaine seeking and/or taking behaviors observed during protracted withdrawal. In accordance with this notion, pretreatment with beta-endorphin and opioid receptor antagonists reinstated and attenuated cocaine seeking during cocaine withdrawal, respectively (e.g., Burattini et al., 2008; Simmons and Self, 2009; Sticht et al., 2010).
Early studies showed that melanocortin MC4 receptor blockade in the nucleus accumbens reduced cocaine CPP and self-administration in rats (e.g., Hsu et al., 2005), suggesting that endogenous melanocortins stimulated cocaine rewarding via the MC4 activation, which is consistent with a recent report on alcohol (Shelkar et al., 2014). The role of endogenous melanocortins in regulation of cocaine withdrawal, however, is still not clear. The melanocortins are well-known hypothalamic feeding peptides modulating hedonic feeding behaviors and body weight (e.g., Friedman, 1997; Cowley et al., 2001). There was a persistent body weight loss during 14-day withdrawal from chronic escalating-dose cocaine exposure, suggesting that enhanced melanocortin activity resultant from enhanced POMC expression, could contribute to the long-lasting decrease in body weight. Beta-endorphin and melanocortin (both derived from POMC) could be interactively involved in cocaine behaviors, and could play different roles in different phases of cocaine seeking.
Caution should be used when interpreting the results of increased POMC mRNA expression, as the change in mRNA levels may not predict a higher POMC biosynthesis rate with higher peptide levels. With this limitation, the study of the POMC gene expression data in different brain regions at different time points of protracted cocaine withdrawal could provide useful information about a potential involvement of hypothalamic POMC gene products (beta-endorphin, melanocortin or ACTH) in cocaine withdrawal and/or relapse.
In summary, our results showed that chronic escalating-dose cocaine and its withdrawal can dynamically modulate POMC gene expression in the hypothalamus of rats: after chronic cocaine exposure at high doses, there was a decreased POMC gene expression; whereas the rats were under cocaine withdrawal condition, the POMC gene expression was enhanced by acute withdrawal and persisted into protracted withdrawal. With a long-lasting neuroadaptation during cocaine withdrawal, a relative excess in opioid activity (as reflected by increased POMC mRNA levels) may lead to enhanced opioid activity at basal levels and/or in response to drugs or environments. Therefore, our data further suggest the persistent increase of hypothalamic POMC gene expression may be involved in cocaine taking and/or seeking behaviors during withdrawal.
Acknowledgments
This work was supported by Dr. Miriam and Sheldon G. Adelson Medical Research Foundation (MJK).
Footnotes
Disclosure/Conflict of Interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Amalric M, Cline EJ, Martinez JL, Bloom FE, Koob GF. Rewarding properties of beta-endorphin as measured by conditioned place preference. Psychopharmacology. 1987;91:14–19. doi: 10.1007/BF00690919. [DOI] [PubMed] [Google Scholar]
- Autelitano DJ, Clements JA, Nikolaidis I, Canny BJ, Funder JW. Concomitant dopaminergic and glucocorticoid control of pituitary proopiomelanocortin messenger ribonucleic acid and beta-endorphin levels. Endocrinology. 1987;121:1689–1696. doi: 10.1210/endo-121-5-1689. [DOI] [PubMed] [Google Scholar]
- Branch AD, Unterwald EM, Lee SE, Kreek MJ. Quantitation of preproenkephalin mRNA levels in brain regions from male Fischer rats following chronic cocaine treatment using a recently developed solution hybridization assay. Brain Res Mol Brain Res. 1992;14:231–238. doi: 10.1016/0169-328x(92)90178-e. [DOI] [PubMed] [Google Scholar]
- Burattini C, Burbassi S, Aicardi G, Cervo L. Effects of naltrexone on cocaine- and sucrose-seeking behaviour in response to associated stimuli in rats. Int J Neuropsycho-pharmacol. 2008;11:103–109. doi: 10.1017/S1461145707007705. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lac ST, Walker MJ, Kragh R, Newman T. Effects of naltrexone on intravenous cocaine self-administration in rats during food satiation and deprivation. J Pharmacol Exp Ther. 1986;238:1–7. [PubMed] [Google Scholar]
- Friedman JM. The alphabet of weight control. Nature. 1997;385:119–120. doi: 10.1038/385119a0. [DOI] [PubMed] [Google Scholar]
- Gerrits MA, Patkina N, Zvartau EE, van Ree JM. Opioid blockade attenuates acquisition and expression of cocaine-induced place preference conditioning in rats. Psychopharmacology. 1995;119:92–98. doi: 10.1007/BF02246059. [DOI] [PubMed] [Google Scholar]
- Houdi AA, Bardo MT, Van Loon GR. Opioid mediation of cocaine-induced hyperactivity and reinforcement. Brain Res. 1989;497:195–198. doi: 10.1016/0006-8993(89)90989-x. [DOI] [PubMed] [Google Scholar]
- Hsu R, Taylor JR, Newton SS, Alvaro JD, Haile C, Han G, Hruby VJ, Nestler EJ, Duman RS. Blockade of melanocortin transmission inhibits cocaine reward. Eur J Neurosci. 2005;21:2233–2242. doi: 10.1111/j.1460-9568.2005.04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Ambrosio E. HPA axis function and drug addictive behaviors: insights from studies with Lewis and Fischer 344 inbred rats. Psychoneuroendocrinology. 2002;27:35–69. doi: 10.1016/s0306-4530(01)00035-x. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Zhou Y, Butelman ER, Levran O. Opiate and cocaine addiction: from bench to clinic and back to the bench. Cur Opin Pharmacol. 2009;9:74–80. doi: 10.1016/j.coph.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Merrer L, Becker JAJ, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiol Rev. 2009;89:1379–1412. doi: 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L’Hereault S, Barden N. Monoaminergic regulation of proopiomelanocortin messenger RNA concentrations in primary cell cultures of rat hypothalamus. Mol Brain Res. 1991a;9:327–332. doi: 10.1016/0169-328x(91)90080-h. [DOI] [PubMed] [Google Scholar]
- L’Hereault S, Barden N. Regulation of proopiomelanocortin messenger RNA concentrations by opioid peptides in primary cell cultures of rat hypothalamus. Mol Brain Res. 1991b;10:115–121. doi: 10.1016/0169-328x(91)90101-3. [DOI] [PubMed] [Google Scholar]
- Locatelli V, Petraglia F, Penalva A, Panarai AE. Effect of dopaminergic drugs on hypothalamic and pituitary immunoreactive beta-endorphin concentrations in the rat. Life Sci. 1983;33:1711–1717. doi: 10.1016/0024-3205(83)90728-2. [DOI] [PubMed] [Google Scholar]
- Maisonneuve IM, Ho A, Kreek MJ. Chronic administration of cocaine “binge” alters basal extracellular levels in male rats: an in vivo microdialysis study. J Pharmacol Exp Ther. 1995;272:652–657. [PubMed] [Google Scholar]
- Mansour A, Fox CA, Akil H, Watson SJ. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci. 1995;18:22–29. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- Markowitz CE, Berkowitz KM, Jaff SB, Wardlaw SL. Effect of opioid receptor antagonism on proopiomelanocortin peptide levels and gene expression in the hypothalamus. Mol Cell Neurosci. 1992;3:184–190. doi: 10.1016/1044-7431(92)90037-3. [DOI] [PubMed] [Google Scholar]
- Marquez P, Baliram R, Dabaja I, Gajawada N, Lutfy K. The role of beta-endorphin in acute motor stimulatory and rewarding actions of cocaine in mice. Psychopharmacology. 2008;197:443–48. doi: 10.1007/s00213-007-1053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer AJ, Hentges ST, Meshul CK, Low MJ. Unraveling the central proopio-melanocortin neural circuits. Front Neurosci. 2013;7:19–25. doi: 10.3389/fnins.2013.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolarakis KE, Almeida OFX, Herz A. Feedback inhibition of opioid peptide release in the hypothalamus of the rat. Neuroscience. 1987;23:143–148. doi: 10.1016/0306-4522(87)90278-8. [DOI] [PubMed] [Google Scholar]
- Picetti R, Ho A, Butelman ER, Kreek MJ. Dose preference and dose escalation in extended-access cocaine self-administration in Fischer and Lewis rats. Psychopharmacology (Berl) 2010;211:313–323. doi: 10.1007/s00213-010-1899-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey NF, van Ree JM. Intracerebroventricular naltrexone treatment attenuates acquisition of intravenous cocaine self-administration in rats. Pharmacol Biochem Behav. 1991;40:807–810. doi: 10.1016/0091-3057(91)90090-o. [DOI] [PubMed] [Google Scholar]
- Roth-Deri I, Green-Sadan T, Yadid G. Beta-endorphin and drug-induced reward and reinforcement. Prog Neurobiol. 2008;86:1–21. doi: 10.1016/j.pneurobio.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Shelkar GP, Kale AD, Singh U, Singru PS, Subhedar NK, Kokare DM. Alpha-melanocyte stimulating hormone modulates ethanol self-administration in posterior ventral tegmental area through melanocortin-4 receptors. Addict Biol. 2014 doi: 10.1111/adb.12126. in press. [DOI] [PubMed] [Google Scholar]
- Simmons D, Self DW. Role of mu- and delta-opioid receptors in the nucleus accumbens in cocaine-seeking behavior. Neuropsychopharmacology. 2009;34:1946–1957. doi: 10.1038/npp.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Bals-Kubik R, Shippenberg TS. Beta-endorphin-induced locomotor stimulation and reinforcement are associated with an increase in dopamine release in the nucleus accumbens. Psychopharmacology. 1991;104:51–56. doi: 10.1007/BF02244553. [DOI] [PubMed] [Google Scholar]
- Sticht M, Mitsubata J, Tucci M, Leri F. Reacquisition of heroin and cocaine place preference involves a memory consolidation process sensitive to systemic and intra-ventral tegmental area naloxone. Neurobiol Learn Mem. 2010;93:248–260. doi: 10.1016/j.nlm.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Shiozaki Y, Masukawa Y, Nagase H. Role of mu and kappa-opioid receptors in cocaine-induced conditioned place preference. Jpn J Pharmacol. 1992;58:435–442. doi: 10.1254/jjp.58.435. [DOI] [PubMed] [Google Scholar]
- Wardlaw SL, Kim J, Sobieszczyk S. Effect of morphine on proopiomelanocortin gene expression and peptide levels in the hypothalamus. Mol Brain Res. 1996;41:140–147. doi: 10.1016/0169-328x(96)00084-8. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Spangler R, Yuferov V, Schlussman SD, Ho A, Kreek MJ. Effects of selective D1- or D2-like dopamine receptor antagonists with acute “binge” pattern cocaine on corticotropin-releasing hormone and proopiomelanocortin mRNA levels in the hypothalamus. Mol Brain Res. 2004;130:61–67. doi: 10.1016/j.molbrainres.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Proudnikov D, Yuferov V, Kreek MJ. Drug-induced and genetic alterations in stress-responsive systems: Implications for specific addictive diseases. Brain Res. 2010;1314:235–252. doi: 10.1016/j.brainres.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Kruyer A, Ho A, Kreek MJ. Cocaine place conditioning increases pro-opiomelanocortin gene expression in rat hypothalamus. Neurosci Lett. 2010;530:59–63. doi: 10.1016/j.neulet.2012.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Kreek MJ. Alcohol: a stimulant activating brain stress responsive systems with persistent neuroadaptation. Neuropharmacology. 2014;87:51–58. doi: 10.1016/j.neuropharm.2014.05.044. [DOI] [PubMed] [Google Scholar]