ABSTRACT
The mitochondrion is an important power generator in most eukaryotic cells. To preserve its function, many essential nuclear-encoded factors play specific roles in mitochondrial RNA metabolic processes, including RNA editing. RNA editing consists of post-transcriptional deamination, which alters specific nucleotides in transcripts to mediate gene expression. In plant cells, many pentatricopeptide repeat proteins (PPRs) participate in diverse organellar RNA metabolic processes, but only PLS-type PPRs are involved in RNA editing. Here, we report a P-type PPR protein from Arabidopsis thaliana, P-type PPR-Modulating Editing (PPME), which has a distinct role in mitochondrial nad1 RNA editing via RNA binding activity. In the homozygous ppme mutant, cytosine (C)-to-uracil (U) conversions at both the nad1-898 and 937 sites were abolished, disrupting Arg300-to-Trp300 and Pro313-to-Ser313 amino acid changes in the mitochondrial NAD1 protein. NAD1 is a critical component of mitochondrial respiration complex I; its activity is severely reduced in the homozygous ppme mutant, resulting in significantly altered growth and development. Both abolished RNA editing and defective complex I activity were completely rescued by CaMV 35S promoter- and PPME native promoter-driven PPME genomic fragments tagged with GFP in a homozygous ppme background. Our experimental results demonstrate a distinct role of a P-type PPR protein, PPME, in RNA editing in plant organelles.
KEYWORDS: Arabidopsis, complex I activity, editing, mitochondria, nad1, PPR
Introduction
The mitochondrion, originating from endosymbiosis of α-proteobacteria in ancestral host cells, is the energy factory for most eukaryotic cells. It plays vital roles in diverse cellular processes via oxidative respiration and various metabolic pathways within most eukaryotic cells.1,2 The mitochondrion has its own genome, which encodes a small number of proteins essential for mitochondrial gene expression and functional respiration; however, most mitochondrial genes involved in gene expression regulation in this organelle were transferred to the nuclear genome during evolution.3 Therefore, translocation of these nuclear factors back into the mitochondrion is critical for mitochondrial biosynthesis and the modulation of mitochondrial RNA metabolism.4
RNA metabolism in mitochondria of plants is complex and unique compared to that of organisms in other kingdoms, particularly at the post-transcriptional level, and it involves extensive RNA editing, 5′ and 3′ trimming, intron splicing, degradation, and translation.5-8 Recent studies of several nuclear-encoded protein families, including the mitochondrial transcription termination factors (mTERFs) 9,10 and pentatricopeptide repeat (PPR) families, have revealed their unique roles in Arabidopsis mitochondrial RNA metabolism.
PPR proteins in eukaryotic organisms belong to one of the largest protein families, and they are essential for transcription and RNA metabolism in both the nucleus and organelles.11 Notably, land plants contain large PPR families compared with other species; for example, the Arabidopsis genome contains over 400 PPR-encoding genes.12 Similarly to tetratricopeptide repeat (TPR) proteins, PPR proteins contain various numbers of tandem, degenerate, 35-amino-acid helical repeat motifs (PPR motifs) and other C-terminal motifs.13 PPRs are classified into P and PLS subgroups based on the architecture of these motifs. P-subgroup proteins exclusively contain 35-amino-acid tandem repeats (P motif); alternatively, PLS-subgroup proteins contain P, S (short, 31 amino acids), and L (long, 35-36 amino acids) motifs, as well as additional conserved C-terminal motifs, such as E and E+ and DYW.12 These additional motifs are related to the unique RNA editing in plants.14-17
In general, PPR proteins have unique functions in RNA metabolism within the chloroplast and mitochondria. P-type PPR proteins are involved in diverse RNA metabolic processes, including cleavage, splicing, stabilization, and translation, while PLS-type PPR proteins mainly function in RNA editing.18 P-type PPR proteins mainly stabilize RNAs within organelles.19 During processing individual polycistronic transcripts, these proteins bind to the 5′ or 3′ termini of transcripts with a single open reading frame and function as barriers to prevent exonuclease-mediated transcript degradation.18 Additionally, they regulate splicing efficiency by directly associating with introns in chloroplasts and mitochondria.20-23 Therefore, PPR proteins are assumed to affect the folding of the introns of these transcripts or splicing efficiency via a mechanism similar to that used for transcript stabilization. For example, binding of the PPR5 protein to the chloroplast trnG intron might prevent endonuclease-mediated trnG intron cleavage. Both spliced and unspliced trnG transcripts accumulate in the ppr5 mutant, suggesting that PPR5 might stabilize trnG transcripts during splicing.24,25
In plant organelles, some transcribed RNAs require extra processing before maturation, such as cytosine (C)-to-uracil (U) RNA editing (i.e., pyrimidine exchange to convert a C to a U).26,27 RNA editing is a unique process that alters specific nucleotides in a given transcript during post-transcriptional modification, generating functionally diverse proteins or halting translation of certain pre-mRNAs. Editing is the predominant process that occurs in eukaryotic organelles.28 In flowering plants, RNA editing only occurs in chloroplasts and mitochondria, where specific C residues of certain transcripts are changed to U residues by a putative cytidine deamination mechanism.29 In Arabidopsis, 619 and 43 editing sites are present in the mitochondrion30 and chloroplast,31 respectively. Under certain circumstances, editing is essential to generate translational start and/or stop codons in a given transcript, or it may coordinate the regulation of certain proteins that function in these 2 unique organelles.32 Editing sites can be further classified into silent sites (no amino acid change after editing) and non-silent sites (amino acid change after editing); non-silent sites are predominant in both of these organelles in Arabidopsis.33 Compared to other plant species with mitochondria that have less editing, the edited nucleotides in the Arabidopsis mitochondrial RNA are more conserved among other plant species in which less editing occurs.28
In 2005, the first plant trans-factor involved in RNA editing was identified. A mutation in Arabidopsis CRR4 was found to result in defective RNA editing of chloroplast ndhD transcripts.34 In wild-type plants, functional RNA editing drives the conversion of ACG to AUG, which is used as the start codon for chloroplast NDHD translation; however, this conversion was defective in the crr4 mutant, and RNA editing activity was lost at this editing site. CRR4 encodes a PPR protein that serves as a site recognition factor, binding to a 25-bp region upstream and a 10-bp region downstream of the ndhD-1 site in vitro.32,35 Recently, several other PPRs have been found to have unique roles in sequence recognition around the editing sites of their targeted pre-mRNAs in chloroplasts (OTP82 to ndhB-9 and ndhG-1 sites and CRR22 to ndhB-7, ndhD-5, and rpoB-3 sites) and mitochondria (PpPPR_71 to ccmF-2 site),35-37 binding to RNA bases via their 2-helix structures.38 All of the site recognition PPRs involved in RNA editing belong to the PLS subfamily. Therefore, PLS-type PPR proteins are considered site recognition trans-factors for editing that act by directly binding to the surrounding regions of certain edited sites in both Arabidopsis mitochondrial and chloroplast transcripts.18 In contrast, only one P-type PPR protein, PPR596, has been found to be involved in organellar RNA editing.39 A PPR596 mutation has been demonstrated to decrease the editing efficiency of Arabidopsis mitochondrial rps3 transcripts; however, direct evidence of the involvement of PPR596 in editing remains to be elucidated. Therefore, the molecular mechanism by which P-type PPR proteins modulate the editing process in plant organelles must be further explored.
Here, we report the molecular mechanism of a mitochondria-localized P-type PPR protein, P-type PPR-modulating editing (PPME), in RNA editing. PPME mutations significantly disrupted typical growth and development, as occurs in most mitochondrial biogenesis mutants; further, PPME participated in editing activities at both the nad1-898 and 937 sites. Notably, it directly bound to regions up- and downstream (−20 to +10) of the nad1-898 editing site but did not bind to the upstream region of the nad1-937 editing site. The NAD1 protein is a component of mitochondrial NADH dehydrogenase (complex I), and mitochondrial complex I activity was greatly reduced in the homozygous ppme mutant. PPME is essential for the modulation of nad1-898 and nad1-937 editing efficiency and the direct coordination of mitochondrial activity. Our experimental data have revealed a unique role of an additional P-type PPR protein in modulating RNA editing within plant mitochondria.
Results
PPME is required for normal vegetative growth after post-embryonic development
The Arabidopsis SALK T-DNA insertional mutant collection was screened for mutants with defects in post-embryonic development and/or seed germination. One mutant had an abnormal phenotype in progenies segregated from SALK_019722 heterozygotes, and the homozygous mutant harbored a T-DNA insertion that disrupted the coding region of the Arabidopsis At3g18020 gene (Fig. 1A). This gene does not contain any intron; instead, it encodes an uncharacterized P-type PPR protein involved in mitochondrial RNA editing (see below). Therefore, we named it P-type PPR-Modulating Editing (PPME) protein. The homozygous ppme mutant, ppme-/-, is a null mutant with undetectable transcription, as demonstrated by RT-PCR (Fig. 1B). PPME is expressed during almost all growth and development stages (Fig. 1B), suggesting that it has a housekeeping role. Indeed, when heterozygous ppme (ppme+/−) seeds were germinated in soil, no ppme−/− mutants were detected in the ppme+/− self-pollinated F2 generation, for which the wild-type to ppme+/− progeny segregation ratio was 1:2 (Table 1). These results reflect defective embryonic and/or post-embryonic development in the ppme−/− mutant. Nevertheless, when ppme+/− F2 seeds were germinated on solid MS medium, small ppme-/- seedlings were recovered (Fig. 1C), but the growth of 14-day-old ppme−/− seedlings was significantly stunted (Fig. 1D). After continuous growth on solid MS medium for 21 days, these seedlings survived but exhibited significant dwarfing after transfer to soil (Fig. 1E).
Figure 1.
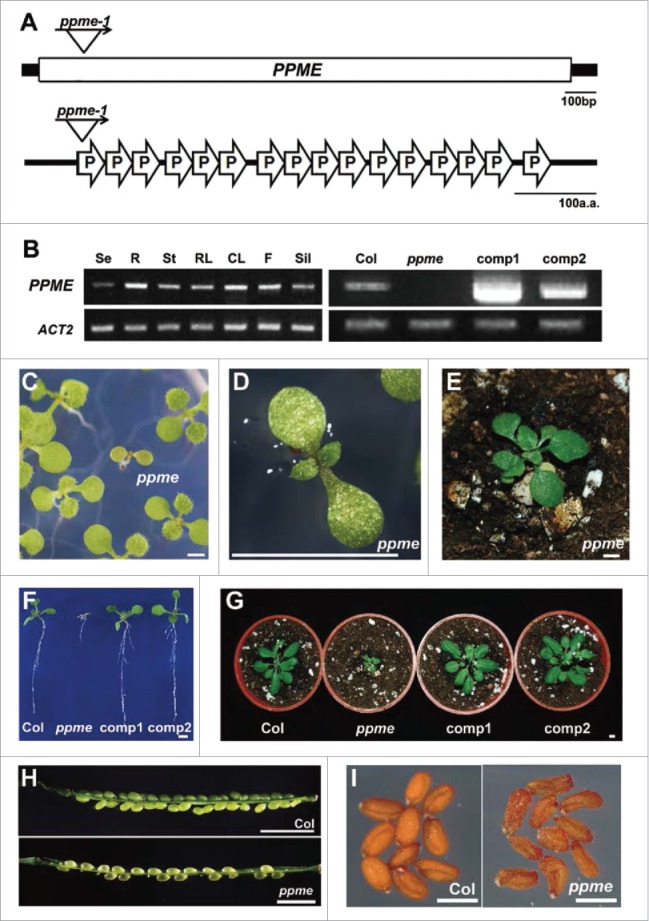
P-type pentatricopeptide repeat protein-modulating editing (PPME) protein is a P-type pentatricopeptide repeat (PPR) protein essential for normal Arabidopsis growth and development. (A) The PPME gene structure and its encoded P-type PPR protein harboring 16 PPR (35 amino acids) motifs is shown; ppme-1 was generated by T-DNA insertion in the coding region. (B) RT-PCR analysis of constitutive PPME expression, of a null homozygous ppme-1 mutant and of transformed PPME in T3 transgenic lines complemented with CaMV 35S promoter-driven (35S::PPME-GFP/ppme−/−, comp1) or PPME native promoter-driven (PPMEg-GFP/ppme−/−, comp2) PPME genomic fragments in a homozygous ppme-1 background. (C) Fourteen-day-old heterozygous ppme+/− germinated F2 seedlings grown on solid MS medium. (D) Fourteen-day-old homozygous ppme−/− seedlings with stunted vegetative growth. (E) Forty-day-old homozygous ppme−/− plants survived on solid MS medium for 21 d before being transferred to soil. (F-G) Fourteen-day-old seedlings (F) and 40-day-old (G) plants from wild-type, homozygous ppme−/−, comp1, and comp2 plants. (H-I) Siliques of homozygous ppme−/− showed an abortion phenotype (H), with shrunken seeds and reduced viability (I). Scale bars = 0.5 cm in C-G; 2 mm in H; and 500 μm in I.
Table 1.
Genotyping of heterozygous ppme F2 generation. In total, 236 seeds harvested from heterozygous ppme plants were germinated in soil for 1 month. Genotyping revealed that 34% (80) and 66% (156) of the progeny were wild-type and heterozygous plants, respectively, and no homozygous ppme plants were found.
| Genotype | Wild-type | Heterozygous | Homozygous | P value* (2:1) |
|---|---|---|---|---|
| ppme heterozygous F2 | 80 | 156 | 0 | 0.853923 |
The p-value indicates that the segregation ratio was 2:1 (heterozygous to wild-type) according to the chi-square test.
To complement the defective growth of ppme−/−, CaMV 35S promoter-driven (comp1) and PPME native promoter-driven (comp2) PPME genomic fragments tagged with GFP were transformed into the ppme+/− mutant. Both constructs successfully rescued the ppme−/− phenotypes (Fig. 1F–G), and RT-PCR confirmed the presence of transformed PPME expression in complementation lines (Fig. 1B). Furthermore, at the reproductive stage, ppme−/− produced normal flowers with severely aborted siliques containing shriveled seeds, and the plants exhibited significantly reduced viability (Figs. 1H–I). Therefore, the stunted phenotype of ppme−/− was caused by the loss of functional PPME, which is essential for both post-embryonic development and vegetative and reproductive growth and development.
PPME encodes a mitochondrial P-type PPR protein responsible for mitochondrial nad1 transcript editing
PPME encodes a protein harboring 16 degenerate pentatricopeptide motifs, and it is classified as a P-type PPR protein (Fig. 1A, lower panel). In flowering plants, most PPRs are localized to the chloroplasts or mitochondria.12 Additionally, the growth-retarded phenotype of ppme−/− is similar to the phenotypes of several Arabidopsis mitochondria-localized PPR mutants with defective mitochondrial RNA processing, including slo1 and aef1/mpr25.40,41 Next, we investigated the subcellular localization of PPME. Complete colocalization of the PPME-GFP and Mito-tracker signals was observed in root hairs of stable, complementation lines containing GFP-tagged PPME genomic fragments in a ppme−/− background (Fig. 2). Similar results were obtained using TargetP prediction software (http://www.cbs.dtu.dk/services/TargetP/). Thus, we confirmed that PPME is a mitochondrial PPR protein that may be involved in regulating mitochondrial RNA metabolism.
Figure 2.
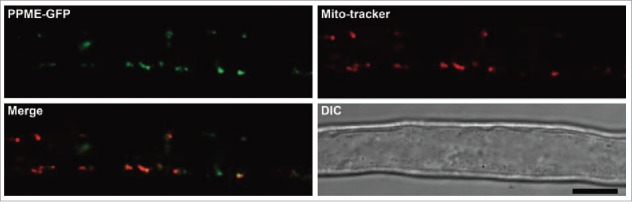
Specific localization of PPME in Arabidopsis plant mitochondria. Root hairs from 7-day-old PPMEg-GFP transgenic plants were treated with Mito-tracker to observe PPME subcellular localization. Co-localization of the GFP (green) and Mito-tracker signals (red) in elongating root hair cells, as observed by confocal microscopy. Scale bars = 10 μm.
Because most PPR proteins have been shown to function as regulators of organellar RNA metabolism,18 we first examined the effects of the PPME mutation on the splicing efficiency (Fig. S1) and abundance (Fig. S2) of all Arabidopsis mitochondrial transcripts. In general, real-time qRT-PCR revealed comparable splicing efficiencies for most mitochondrial transcripts between wild-type and CaMV 35S promoter-driven comp1 seedlings (Fig. S1A). The ppme−/− seedlings had a lower splicing efficiency for nad2 intron 1 transcripts, but this decreased efficiency was not as obvious compared with our previously characterized splicing mutants, mterf15 and slow growth 3.10,23 We examined the levels of spliced transcripts for individual mitochondrial exons and found that the nad2 exon1 to exon2 (nad2 exon1-2) transcript level was slightly reduced in ppme−/− seedlings compared with wild-type and comp1 seedlings (Fig. S1B). Then, we examined the abundances of individual mitochondrial transcripts in ppme−/−, wild-type, and comp1 seedlings. In ppme−/− seedlings, most transcripts were upregulated, as previously reported in other mitochondrial mutants,10,23 while the nad2a mRNA level was slightly downregulated (Fig. S2). Similar impairments in nad2 intron 1 splicing have been observed in several unrelated mutants with defects in mitochondrial RNA processing.20,42-44 Therefore, this phenomenon might simply be due to abnormal mitochondrial activity (see below) and may not contribute to the phenotypic defects observed in these mutants, including ppme−/− described in this study.
Next, we examined another aspect of RNA metabolism–the overall mitochondrial RNA editing profiles in wild-type, ppme−/−, and complemented seedlings. To identify mitochondrial editing sites, mitochondrial transcripts were extracted from all samples and converted to cDNA. The cDNA fragments complementary to editing sites were amplified using specific primer pairs described previously40 and were subjected to DNA sequencing. Surprisingly, among all examined editing sites, only the nad1-898 and nad1-937 sites, located at positions 898 and 937 of the mitochondrial NAD1 gene, respectively exhibited completely abolished and substantially reduced editing in ppme−/− seedlings (Fig. 3A). The reduced editing in these seedlings could be completely rescued by either CaMV 35S promoter-driven (comp1 in Fig. 3A) or PPME native promoter-driven constructs (comp2 in Fig. 3A). Both nad1-898 and nad1-937 are non-silent editing sites,33 leading to codon switches from CGG (Arg) to UGG (Trp) at nad1-898 and from CCU (Pro) to UCU (Ser) at nad1-937 after editing (Fig. 3A), resulting in amino acid changes in the NAD1 subunit of NADH dehydrogenase.
Figure 3.
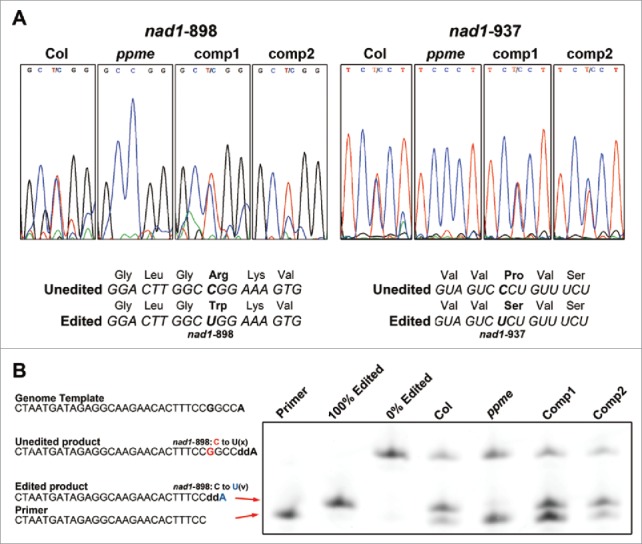
Defective editing at mitochondrial nad1-898 and nad1-937 RNA editing sites in homozygous ppme−/− seedlings. (A) Sequencing of cDNAs from 14-day-old wild-type, homozygous ppme−/−, and complemented seedlings for assessment of nad1-898 and nad1-937 RNA editing efficiencies in ppme−/− and complementation lines. The middle cytosine or thymine in each panel is the position of the nad1-898 or nad1-937 site, respectively. The lower panel depicts the predicted amino acid changes after nad1-898 and nad1-937 editing. In wild-type mitochondria, nad1-898 and nad1-937 RNA editing causes amino acid changes from Arg to Trp and from Pro to Ser, respectively, in the NAD1 protein after translation. The bold cytosine (C) and uracil (U) indicate the nad1-898 and nad1-937 editing sites, respectively. (B) Poisoned primer extension assay of the nad1-898 editing site. The edited products were terminated earlier than the unedited products by stopping the reaction with ddATP. The edited and unedited products were separated in a sequencing gel and visualized by detection of FAM fluorescence signals. The edited products are from wild-type and ppme−/− seedlings and seedlings from 2 complementation lines.
To validate the sequencing results, the nad1-898 site, exhibiting complete loss editing deficiency, was analyzed using a more sensitive approach, the poisoned primer extension (PPE) assay.45 To determine the corresponding sizes of completely edited and non-edited controls, synthetic correct and mutated nucleotide sequences were used in the PPE assay (Fig. 3B). Accumulated edited products were detected in the mitochondria of wild-type seedlings and seedlings from 2 complemented lines; however, almost no edited products were detected in the mitochondria of ppme−/− seedlings. These results are consistent with the previous sequencing results. Taken together, these findings not only suggest that defects in both the nad1-898 and nad1-937 editing sites coordinately contribute to the ppme−/− phenotype but also imply that both the Arg-to-Trp and Pro-to-Ser amino acid conversions are critical for proper mitochondrial functioning during normal Arabidopsis growth and development.
PPME directly binds to sequences surrounding the nad1-898 editing site
Most PPRs are known as site recognition factors because of their abilities to bind to specific RNA targets. For example, Arabidopsis PLS-PPRs recognize and bind to corresponding editing sites, e.g., CRR4 directly binds to the region surrounding ndhD-1 to regulate the ndhD-1 RNA editing site.18,35 We showed that PPME influenced the editing efficiencies of both nad1-898 and nad1-937 and subsequently more closely examined this PPME-mediated regulation. First, to assess the RNA-binding capacity of PPME, a recombinant PPME protein N-terminally tagged with maltose-binding protein (MBP) and an in vitro transcribed region spanning -40 to +20 of the nad1-898 editing site were tested by RNA-electrophoretic mobility shift assay (EMSA). Nad1-898 was used as it exhibited completely disrupted editing in the PPME null mutant. MBP alone and the sequences surrounding atp9-83, which showed complete conversion from cytidine to uridine in both wild-type and ppme−/− seedlings in our editing screen, were used as the trans and cis negative controls, respectively, to validate the binding specificity. MBP alone had no detectable affinity for the nad1-898 or atp9-83 probe (Fig. 4A); however, MBP-tagged PPME exhibited dose-dependent binding to the nad1-898, but not to the atp9-83, probe. The affinity between PPME and nad1-898 was completely titrated by addition of competitive cold nad1-898 probe (“+” in the right panel of Fig. 4A). The EMSA result clearly demonstrated that PPME can bind the region surrounding the nad1-898 site, likely affecting RNA editing by this means.
Figure 4.
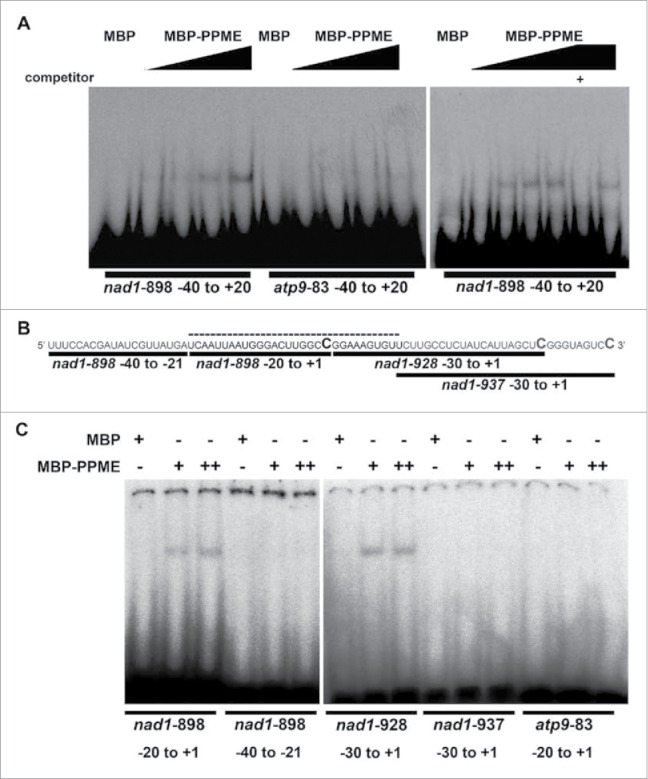
RNA-EMSA showing that recombinant PPME specifically binds to sequences surrounding the nad1-898 editing site. (A) MBP-tagged PPME recombinant proteins were co-incubated with nad1-898 probes or atp9-83 probes for sequences located up- and downstream of nad1-898 or atp9-83, respectively. The left panel shows the interaction between MBP-PPME and nad1-898 or atp9-83. The black triangles above each gel indicate the increasing concentrations of MBP-PPME in each gel. The right panel shows the binding between MBP-PPME and nad1-898, which was titrated by the exogenous addition of cold nad1-898 probe. +: cold competitor. (B) Nucleotide sequences of the probes specifically designed for EMSA. The RNA sequence (from 5′ to 3′) includes the region from the -40 nucleotide of nad1-898 to the +1 nucleotide of nad1-937. The bold C nucleotides indicate the corresponding nad1-898, nad1-928, and nad1-937 editing sites. The bold solid lines indicate the regions individually probed with specific probes, and the dotted line represents the putative nad1-898 cis-element recognized by PPME. 64. (C) RNA-EMSA revealed that among the 4 different probes, PPME specifically bound to only the nad1-898 −20 to +1 and nad1-928 -30 to +1 probes. However, the 20 nucleotides (putative cis-element for nad1-928) upstream of nad1-928 that overlapped with the nad1-937 −30 to +1 region did not exhibit PPME binding activity. The atp9-83 −20 to +1 probe was used as a cis-element negative control. The + and ++ symbols denote 200 nM MBP and 100 nM MBP-PPME or 200 nM MBP-PPME recombinant protein, respectively, and 200 pM probe was used for the all RNA-EMSAs.
Next, to validate the specificity of the cis-element for PPME binding to nad1-898 and other possible target sites, including nad1-937, 4 RNA probes specifically designed to cover and/or overlap the −25/+10 nucleotides in the areas surrounding the editing sites were used to examine affinity for PPME by RNA-EMSA (Fig. 4B). Among the 5 different probes used, including the negative control cis-element atp9-83 −20 to +1, only the nad-898 −20/+1 and nad−928 −30/+1 regions were bound by MBP-tagged PPME (Fig. 4C). However, the nad−937 −30/+1 probe partially overlapped with nad−928 −30/+1 but was not recognized by MBP-tagged PPME. The cis-elements required for trans-acting factor binding and RNA editing specificity have been reported to be located in regions including the −25/+10 nucleotides around the editing sites.27 In addition, a region upstream of nad1-937 near the downstream of nad1-898 is required for PPME binding. The reduced RNA editing efficiency at the nad1-937 site in the ppme mutant may be a secondary effect of the loss of interaction between PPME and nad1-898 in the downstream region. Therefore, this interaction may be critical for the binding of other trans-factors to these editing sites downstream of nad1-898. These results suggest that PPME functions as a promising editing factor that specifically binds to the typical cis-elements spanning the −20 to +10 nucleotides surrounding the nad1-898 site in vitro.
Recently, several studies have suggested that PPR proteins recognize their targets by screening their targeted transcripts for combination codes 46-48 In brief, pairing of the sixth amino acid residue in each PPR motif with the first amino acid residue in the following motif may result in tracking and binding to specific nucleotides located upstream of editing sites. The PPME protein contains 16 P-type PPR motifs; 12 thus, the combination of sixth amino acid residues (6 position) in motifs 1 to 15 and the first amino acid residues (1′ position) in motifs 2 to 16 were analyzed to determine the PPR code for the nad1-898 upstream sequence (Fig. S3A). However, PPME had a less conserved combination code (the residue in the sixth position was frequently threonine or asparagine).46 Another prediction software tool, TPRpred (http://toolkit.tuebingen.mpg.de/tprpred),49 revealed that PPME had 17 PPR motifs, and a less commonly predicted combination code for PPME binding was obtained (Fig. S3B). Additionally, aPPRove software (http://www.cs.colostate.edu/cstop/index.php) was used to analyze the conserved combination code for PPME, but none was detected. The current known PPR combination code might not be suitable for our PPME protein, and further extensive study of amino acid changes within the PPR domains of PPME may provide new insights into PPR combination code usage. Alternatively, PPME may interact with other PPR and/or DYW domain-containing proteins to perform RNA editing, as observed in other PPR family members.32 To test this hypothesis, we immunoprecipitated potential PPME-interacting proteins from 14-day-old seedlings complemented with GFP-tagged CaMV 35S promoter-driven or PPME native promoter-driven PPME genomic fragments (Table 2). Neither DYW-containing proteins nor PPR proteins were found. Compared with the well-characterized editing functions of PLS-type PPRs, the P-type PPR protein PPME may be a new site recognition trans-factor that modulates the RNA editing of nad1-898 in Arabidopsis mitochondria.
Table 2.
Potential PPME-interacting candidate proteins from the in vivo immunoprecipitation experiment.
| Gene ID | Protein description |
|---|---|
| AT4G37910 | mtHsc70-1 mitochondrial heat shock protein 70-1 |
| AT1G55490 | CPN60B, LEN1 chaperonin 60 β |
| AT3G13470 | TCP-1/cpn60 chaperonin family protein |
| AT5G56500 | TCP-1/cpn60 chaperonin family protein |
| AT2G33210 | HSP60-2 heat shock protein 60-2 |
| AT3G23990 | HSP60, HSP60-3B heat shock protein 60 |
| AT5G09590 | MTHSC70-2, HSC70-5 mitochondrial HSO70 2 |
| AT5G44120 | CRA1, ATCRA1, CRU1 RmlC-like cupin superfamily protein |
| AT2G28000 | CPN60A, CH-CPN60A, SLP chaperonin-60 α |
| AT2G20580 | RPN1A, ATRPN1A 26S proteasome regulatory subunit S2 1A |
| AT5G20630 | GLP3, GLP3A, GLP3B, ATGER3, GER3 germin 3 |
| AT3G27280 | ATPHB4, PHB4 prohibitin 4 |
| AT5G40770 | ATPHB3, PHB3 prohibitin 3 |
| AT5G14300 | ATPHB5, PHB5 prohibitin 5 |
Mitochondrial complex I activity is reduced in homozygous ppme mutant
The stunted growth of ppme−/− is similar to that of a mutant with defective trans-splicing of mitochondrial NAD1.43 NAD1 encodes the NAD1 component of NADH dehydrogenase, which is essential for functional complex I activity in the mitochondria.50 Loss of function of NAD1 has been shown to cause disassembly of mitochondrial complex I in one PPR mutant, otp43.20 Further, a mutation of PPME resulted in the failed editing of both mitochondrial nad1-898 and nad1-937 and no change in the Arg300 or Pro313 amino acid in the NAD1 protein. Nevertheless, an amino acid comparison revealed that a Trp residue resulting from the change from Arg300 to Trp300 in nad1-898 but not a Ser residue from Pro313 to Ser313 in nad1-937 after editing is highly conserved in humans and in most plant mitochondrial NAD1 proteins (Fig. 5A and Fig. S4). We next examined the activity of mitochondrial complex I in ppme−/− seedlings. Crude mitochondrial protein extracts were separated using native PAGE, and then NADH dehydrogenase activity was examined.10 Interestingly, complex I activity was markedly reduced in ppme−/− seedlings, and this defect could be fully rescued by either 35S::PPME-GFP (comp1) or genomic PPMEg-GFP (comp2) constructs (Fig. 5B). These results also demonstrate the critical influences of conserved Trp300 and likely of divergent Ser313 in NAD1 on Arabidopsis mitochondrial complex I activity. Briefly, our findings suggest that abnormal developmental growth of ppme−/− is caused by decreased complex I activity resulting from loss of RNA editing of mitochondrial NAD1 transcripts.
Figure 5.
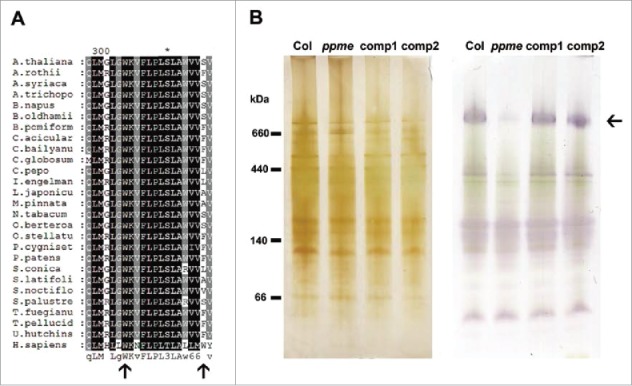
Accurate nad1-898 and nad1-937 editing plays an important role in Arabidopsis mitochondrial complex I activity. (A) Comparison of the amino acid identities of NAD1 C-termini from different organisms shows that the conserved edited form of nad1-898 has an amino acid change to Trp and that the less-conserved edited form of nad1-937 has an amino acid change to Ser. The black arrows from the left to right indicate the amino acids translated from the nad1-898 and nad1-937 editing sites, respectively. (B) The significant reduction in mitochondrial complex I activity in the ppme mutant was restored in the complementation lines. Crude total mitochondrial protein extracts from 14-day-old seedlings were separated by native PAGE. The activity was visualized as a purple-blue color resulting from interaction of the substrate (NADH) with the electron acceptor (nitroblue tetrazolium). The left panel shows the total protein profiles, which were determined by silver staining, and the right panel shows the parallel native PAGE staining for assessment of mitochondrial complex I activity. The black arrow shows mitochondrial complex I.
Discussion
RNA editing is crucial for organellar RNA metabolism in plants, and it commonly involves post-transcriptional substitution of C with U. This process regulates organellar gene expression by modifying the coding information of genes, for example, by generating start codons (ACG sites) and/or stop codons, which changes conserved amino acid codons.51 Over 600 editing sites have been identified in transcripts in the plastid and mitochondrion, and approximately 200 PPR proteins may modulate the editing efficiencies at these sites.52 Nevertheless, the precise roles of these PPR proteins and their molecular mechanisms remain unclear. For example, an editing enzyme has not yet been identified in plants. In this study, we characterized the function of a P-subfamily PPR protein, PPME, which lacks the typical RNA editing E and DYW domains present in PLS-subfamily PPRs (Fig. 1). Interestingly, PPME seemingly has distinct molecular functions in RNA editing, as it directly binds to sequences surrounding nad1-898 and is responsible for editing at both the nad1-898 and nad1-937 sites. Our results show that PPME is a unique RNA-binding protein that modulates RNA editing in Arabidopsis mitochondria.
Dysfunctional editing of both nad1-898 and nad1-937 contributes to retarded growth of homozygous ppme−/− seedlings
We showed that PPME has distinct molecular functions in RNA editing at both the nad1-898 and nad1-937 sites. The impaired editing efficiency in homozygous ppme−/− seedlings resulted in significantly decreased mitochondrial complex I activity and consequently in growth and developmental defects. Our results indicate that PPME is a unique RNA-binding protein that modulates RNA editing in Arabidopsis mitochondria. However, the precise underlying molecular mechanism remains unknown. Recently,55 mutated maize EMP5 (EMPTY PERICARP5) was found to lack the E+ and DYW domains while maintaining its substrate specificity and editing function; however, its editing efficiency was reduced. The E+ and DYW domains of some PPR proteins may be necessary but not sufficient for RNA editing efficiency and function, respectively. Another PPR protein, CRR4, lacks the C-terminal DYW domain and physiologically interacts with the DYW domain-containing protein DYW1 to regulate editing at the ndhD-1 site.32 PPME has only 16 degenerate PPR motifs with RNA-binding activity and lacks the E and DYW domains found in most PLS-type PPR proteins. Thus, it may act as a trans-recognition factor that recognizes sequences surrounding the nad1-898 site and recruits an unknown editing enzyme and/or complex to function as editing machinery. However, no obvious PPME-interacting PPR proteins, such as DYW domain-containing proteins or possible editing enzymes, were detected in our immunoprecipitation results (Table 2). Notably, these results might have been affected by weak and/or transient binding between PPME and its editing partners.
Most characterized plant P-type PPR proteins help to regulate organellar RNA splicing and RNA stability by protecting their target RNAs from endonuclease or exonuclease attack.18 We found that the mitochondrial nad2a mRNA level was decreased because of the lower splicing efficiency of mitochondrial nad2 intron 1 (Figs. S1 and S2), which suggests that PPME may be involved in RNA splicing. However, similar impairments in nad2 intron 1 splicing, which occurred via an unknown mechanism, have been observed in several unrelated mitochondrial RNA processing-defective mutants. For example, disruption of nad2 pre-mRNA splicing is caused by many unrelated complex I defective mutants, such as those in genes encoding PPRs, including OTP43, 20 BIR6, 42 and MTSF1,44 and those in maturases, including nMAT143 and nMAT2. 56 Haïli et al. (2013) have suggested that variation in nad2 pre-mRNA splicing might be a result of the pleiotropic effect of disrupted mitochondrial complex I activity. Additionally, we observed only a slight reduction in the mRNA level of spliced nad2 exon1-2 in ppme−/− seedlings, although the level of nad2a was reduced compared to that in wild-type seedlings (Figs. S1B and S2). Interestingly, the Arabidopsis rug3 mutant has a more severe nad2 splicing defect than the ppme mutant, but complex I assembly is not affected.57 The results of this study further suggest that a slight reduction in the mature nad2a transcript level might not markedly contribute to defective complex I activity in ppme−/− seedlings.
PPME is required for editing of the nad1-898 site and is critical for mitochondrial complex I function
PPR proteins participate in organellar RNA metabolism by directly binding to RNA,18 and our EMSA results revealed that PPME exhibits markedly stronger binding to the editing target nad1-898 than to nad1-928, nad1-937, or atp9-83 (Fig. 4). Therefore, the nad1-898 editing site is a primary target of PPME. However, we cannot exclude other possible causes of the nad1-898 editing deficiency in homozygous ppme−/− seedlings. First, we assessed whether defective nad1-898 editing is caused by dysfunctional nad1 RNA processing. We found that the efficiencies at the editing sites near nad1-898 were either unaffected or reduced but that some editing activity remained (unlike nad1-898 in the ppme mutant) (Fig. S5A). Although the nad1-937 editing efficiency was markedly reduced, no PPME-binding activity was detected at region spanning −30 upstream of the nad1-937 editing site by EMSA (the last bold C in Fig. 4B). Therefore, the decreased nad1-937 editing efficiency may have been caused by loss of interaction between PPME and the regions surrounding nad1-898, which is located upstream of nad1-937. Alternatively, this interaction might be crucial for the recruitment of other trans-factors responsible for nad1-937 editing. Second, to exclude indirect effects of PPME on nad1-898 RNA editing due to reduced complex I activity, we further examined nad1-898 editing efficiency in our previous complex I mutants, slo1 and slo323,40 (Fig. S5B). Editing at the nad1-898 site was not altered in either of these mutants. Thus, the nad1-898 site is a promising target of PPME; nevertheless, the proper editing of both nad1-898 and nad1-937 is crucial for proper mitochondrial complex I function and the development and growth of Arabidopsis seedlings.
NAD1 is a critical component of mitochondrial complex I and is responsible for modulating NADH dehydrogenase activity in metazoans. 50 The ppme−/− phenotype features stunted growth at the seedling stage, similarly to other nad1 RNA processing-defective mutants. 58 For example, mutants with defective nad splicing, including tang2, otp439, mterf15, and slo3, exhibit reduced complex I activity.10,23,59 In contrast to other complex I mutants, only ppme−/− exhibited failed nad1-898 RNA editing and a change in a single conserved amino acid within NAD1. This amino acid change in the NAD1 subunit resulting from failed editing has not yet been investigated. Recently, a 4175G>A mutation in human MTND1, a homolog of plant NAD1, has been identified that causes defective complex I assembly.60 It converts the codon from being translated as Trp to a stop codon, which halts translation and may cause complex I disassembly. These authors have suggested that the conserved Trp residue plays an important role in human mitochondrial complex I functionality. Notably, the same conserved Trp is present within nad1-898, the editing of which is in turn affected by PPME mutations. Therefore, this conserved Trp may be crucial for mitochondrial activity in both plant and human cells. Compared with Trp300, the Ser313 residue in nad1-937 is more divergent among different species. All of these results suggest that the complex I defect in the ppme mutant may be largely caused by failed Arg300-to-Trp300 conversion.
A few studies have shown that amino acid changes in mitochondrial respiratory subunits caused by PPR-mediated RNA editing may affect all mitochondrial activities; in addition, the PLS-type PPR proteins and their RNA editing activities are the best characterized. Recently, a maize PLS-type PPR protein, EMP7, has been found to be responsible for CcmFN transcript editing.61 The CcmFN protein promotes maturation of mitochondrial cytochrome c. In the null emp7 mutant, C-to-U editing of the ccmFN transcript at position 1553 is completely lost. As a result, amino acid conversion from Ser to Phe does not occur and the cytochrome c protein level decreases, causing mitochondrial complex III disassembly. Considering that no P-type PPR proteins have RNA editing functions or cause amino acid substitutions in the mitochondrial respiratory complex I subunit, PPME-mediated NAD transcript editing may be an exceptional case.
The mechanisms underlying the recognition of RNA targets by PPME appear to differ from those underlying the recognition of targets by most PLS-type PPR proteins (Fig. S3). Because of the limited number of studies examining the roles of P-type PPR proteins in RNA editing, it remains unclear how these proteins participate in the editing of mitochondrial transcripts. Interestingly, PPR596, a previously identified P-type PPR protein, has been shown to partially affect RNA editing.39 Although the relationship between PPR596 and its editing target is unclear, the site affected by PPR596 showed partial editing in wild-type seedlings, similar to the PPME-mediated nad1-898 and nad1-937 editing demonstrated in this study. It is possible that a certain subgroup of P-type PPR proteins may recognize their editing targets through a different mechanism than that used by PLS-type PPR proteins and that these proteins are specifically responsible for the partial editing efficiencies at these sites.
Nuclear-encoded PPR proteins belong to one of the largest protein families and mainly participate in plant organellar RNA metabolic processes, such as RNA splicing, stabilization, processing, translation, and editing. PLS-type PPR proteins are mainly involved in post-transcriptional RNA editing. Nevertheless, this study provides substantial evidence that a unique P-type PPR protein, PPME, may be a novel editing factor that functions by binding to a typical cis-element to modulate both nad1-898 and 937 RNA editing to contribute to mitochondrial complex I activity. This study of PPME is a novel exploration of the molecular mechanisms of P-type PPR proteins in plant organellar RNA editing.
Materials and methods
Plant materials and growth conditions
The ppme mutant (salk_019722) was obtained from the Arabidopsis Biological Resource Center (Ohio State University, USA). Seeds were surface sterilized and placed on half-strength Murashige and Skoog medium (Duchefa Biocheme, The Netherlands) containing 1% sucrose and 0.7% phytoagar (Duchefa), with subsequent stratification for 3 d at 4°C. Then, they were grown inside of a growth chamber under a 16-h light/8-h dark cycle at 22°C. After 21-days of germination on MS medium, ppme homozygous seedlings were transferred to soil for further growth.
Complementation of the homozygous ppme−/− mutant
The coding region and 4.3-kb genomic fragments of PPME were amplified using the following primers: 5′-BamHI-ATGTTCTTCGTCACTCGTCTGCG-3′, 5′-BamHI-CTATCCTGAGGTTGCAGGGTTTG-3′, 5′-BamHI-TTTGCCAGCAAAAATTTCACAG-3′, and 5′-BamHI-TCCTGAGGTTGCAGGGTTTG-3′. Phusion polymerase (Finnzymes) was used for amplification, and the amplicons were ligated into a pPZP221 vector harboring a C-terminal GFP sequence followed by the nopaline synthase (NOS) terminator. An Agrobacterium tumefaciens strain, GV3101, was used for transformation with the floral dip method.62 T1 seeds from transformed ppme heterozygous plants were harvested and sprayed onto 1/2 MS medium with 125 mg/ml gentamycin. Plants with a ppme homozygous background that were transformed with constructs were selected. T3 homozygous transgenic plants were used in further experiments.
Subcellular localization of PPME
PPME cellular localization was evaluated in root hair cells from homozygous PPMEg-GFP transgenic plants with a ppme homozygous background. Seven-day-old seedlings were stained with MitoTracker Orange (Molecular 587 Probes, Eugene, OR, USA) and observed using a confocal microscope, with excitation at 488 nm/543 nm and BP505-530/BP560-615 IR detection filters (510 META, Zeiss).
Mitochondrial RNA splicing and stability
Total RNA was isolated from 0.01-g 14-day-old wild-type, homozygous ppme−/−, and complemented seedlings with a Qiagen RNeasy Mini Kit (QIAGEN). Then, cDNA was synthesized with M-MLV reverse transcriptase (Invitrogen) and random hexamer primers as previously described.10 The RNA splicing efficiencies of mitochondrial genes were evaluated using 2 strategies. Primers targeting either the exon-exon or exon-intron junctions of individual introns in mitochondrial genes were used for amplification. Then, the amplicons for these junctions were detected using Power SYBR Green Supermix (Applied Biosystems) and an ABI Prism 7000 sequence detection system (Applied Biosystems). The spliced/unspliced ratio was calculated from the ratio of the quantity of exon-exon junctions to the quantity of exon-intron junctions, as previously described.10 The spliced products were further amplified by RT-PCR with the primer sets targeting the exon-exon junctions; PCR was performed for 35 cycles to saturation, and any unspliced products were detected. Mitochondrial RNA stability was evaluated using primer sets as previously described.9 All quantifications were normalized to 4 housekeeping genes: YSL8, RPL5B, UBC, and TUB6.
Mitochondrial RNA editing efficiency
All known editing sites in the coding regions of mitochondrial genes were amplified from cDNA generated from 14-day-old seedlings using previously described methods of a splicing experiment and the corresponding primer sets.40 The resulting amplicons harboring editing sites were further sequenced to determine the editing efficiency at each site. For example, the primers nad1-F3:TCTTTCCAGGAGGTTGGCCG and nad1-R3: AGAGGGCAATTCCTCGCACA were used to generate amplicons harboring nad1-898. Further sequencing was performed with the nad1-F3 primer. PPE assay was performed as previously described.63 The primer 5′-FAM-labeled CTAATGATAGAGGCAAGAACACTTTCC-3′ was used to assess the editing efficiency of nad1-898.
PPME recombinant protein expression
For expression of the PPME recombinant protein, the N-terminal transit peptide of PPME was excluded. The primers 5′ EcoRI-GATAGAGCTTACTGGAGAAGACGGATACACAGTATC-3′ and 5′-HindIII-CTAATGATGATGATGATGATGTCCTGAGGTTGCAGGGTTTG-3′ were used to amplify the PPME coding region without the N-terminal transit peptide, which was then ligated into a pMAL-cRI vector. Both the PPME construct and pMAL-cRI empty vector were further transformed into the E. coli BL21 CodonPlus strain (Novagen). The recombinant proteins were expressed at 28°C after 4-hr induction with 1 mM IPTG and were then further purified using Dextrin Sepharose High-Performance Chromatography Resin (GE).
Electrophoretic mobility shift assay (EMSA)
The following primer sets were used to synthesize nad1-898 and atp9-83 probes, respectively, via in vitro transcription using T7 RNA polymerase (Promega): 5′-TAATACGACTCACTATAGGGAGACATTTCCACGATATCGTTATG-3′ and 5′-AGAGGCAAGAACACTTTCCGGCCAAGTCC-3′; and 5′-TAATACGACTCACTATAGGGAGACGGTGCAAAATCAATAG-3′ and 5′-TACCGATAGCAGCTCCCGCTGAAGC-3′. Further, the following synthetic RNA oligos were used as probes to examine the regions surrounding nad1-898: nad1-898 -20 to +1: 5′-UCAAUUAAUGGGACUUGGCC-3′; nad1-898 -21 to -40: 5′-UUUCCACGAUAUCGUUAUGA-3′; nad1-928 -30 to +1: 5′-GGAAAGUGUUCUUGCCUCUAUCAUUAGCUC-3′; nad1-937 -30 to +1: 5′-UCUUGCCUCUAUCAUUAGCUCGGGUAGUCC-3′; and atp9-83 -20 to +1: 5′-GGAGCUGCUACAAUUGCUUC-3′. The probes were further labeled with γ-32P ATP for subsequent EMSA. Probes (2.5 nM) were incubated with different concentrations of MBP-PPME recombinant protein (0, 0.75, 1.5 and 3 μM) at 25°C for 30 min in 20 μl buffer containing 20 mM Tris-HCl, pH 7.5, 180 mM NaCl, 2 mM DTT, 1.7μg/ml BSA, 0.5 mM EDTA, 8.3% glycerol, and 20 µg/ml heparin. The reaction mixtures were finally separated on a 6.6% TBE acrylamide gel.
To evaluate the influence of PPME on nad1-898 editing efficiency, 200 nM MBP, 200 pM of each probe and either 100 nM or 200 nM MBP-PPME recombinant protein were applied as described above.
Isolation of crude mitochondria and mitochondrial complex I activity assay
Crude mitochondria were obtained and native electrophoresis was performed as described previously.10 Crude mitochondria were extracted from fresh tissues (200 mg) from 14-day-old seedlings in 2 ml extraction buffer (75 mM MOPS-KOH, pH 7.6, 0.6 M sucrose, 4 mM EDTA, 0.2% polyvinylpyrrolidone 40, 8 mM cysteine, and 0.2% bovine serum albumin) on ice. The lysates were centrifuged at 13000 g for 5 min (twice); then, the supernatants were centrifuged at 22,000 g for 20 min. The pellets were resuspended in buffer containing 10 mM MOPS-KOH, pH 7.2, with 0.3 M sucrose, after which electrophoresis was immediately performed. Crude mitochondria were washed with distilled water, resuspended in buffer (50 mM NaCl, 50 mM imidazole/HCl, 2 mM 6-aminohexanoic acid, and 1 mM EDTA, pH 7.0) and solubilized by addition of Lauryl-β-D-maltoside (DDM) (10%) at a DDM/protein ratio of 2.5 (w/w). After centrifugation at 100,000 g for 15 min, 10% glycerol and 0.02% Ponceau S were added to the supernatants, and they were then subjected to 4–16% native PAGE with anode buffer (25 mM imidazole/HCl, pH 7.0) and cathode buffer (50 mM Tricine, 7.5 mM imidazole, pH 7.0, 0.01% DDM and 0.05% Sodium deoxycholate (DOC) for 2 hr at 100 V. The native gels were washed 3 times for 5 min each and incubated with 0.1 M Tris buffer, pH 7.4, with 1 mM nitroblue tetrazolium and 0.14 mM NADH. The reaction was stopped by exposure to a solution containing 40% methanol and 10% acetic acid until a dark blue signal appeared on the gel.
Accession numbers: PPME: at3g18020; ppme-1: salk_019722
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Ms. Mei-Jane Fang for providing assistance with qPCR (DNA Analysis Core Laboratory, IPMB, Academia Sinica) and Dr. Wen-Dar Lin for analyzing the sequencing data for RNA editing in silico (Bioinformatics Core Laboratory, IPMB, Academia Sinica).
Funding
This work was supported by research grants from Academia Sinica (Taiwan) and the Ministry of Science and Technology (MOST 104-2321-B-001 -008 and 103-2321-B-001 -029 - Taiwan) to G.-Y. Jauh.
References
- 1.Andersson SGE, Zomorodipour A, Andersson JO, Sicheritz-Ponten T, Alsmark UCM, Podowski RM, Naslund AK, Eriksson AS, Winkler HH, Kurland CG. The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature 1998; 396:133-40; PMID:9823893; http://dx.doi.org/ 10.1038/24094 [DOI] [PubMed] [Google Scholar]
- 2.Gray MW, Burger G, Lang BF. Mitochondrial evolution. Science 1999; 283: 1476-81; PMID:10066161; http://dx.doi.org/ 10.1126/science.283.5407.1476 [DOI] [PubMed] [Google Scholar]
- 3.Burger G, Gray MW, Franz Lang B. Mitochondrial genomes: anything goes. Trends in Genetics 2003; 19: 709-16; PMID:14642752; http://dx.doi.org/ 10.1016/j.tig.2003.10.012 [DOI] [PubMed] [Google Scholar]
- 4.Murcha MW, Wang Y, Narsai R, Whelan J. The plant mitochondrial protein import apparatus - the differences make it interesting. Biochim Biophys Acta 2014; 1840: 1233-45; PMID:24080405; http://dx.doi.org/ 10.1016/j.bbagen.2013.09.026 [DOI] [PubMed] [Google Scholar]
- 5.Liere K, Weihe A, Borner T. The transcription machineries of plant mitochondria and chloroplasts: composition, function, and regulation. J Plant Physiol 2011; 168: 1345-60; PMID:21316793; http://dx.doi.org/ 10.1016/j.jplph.2011.01.005 [DOI] [PubMed] [Google Scholar]
- 6.Binder S, Brennicke A. Gene expression in plant mitochondria: transcriptional and post-transcriptional control. Philos Trans R Soc Lond B Biol Sci 2003; 358: 181-8; discussion 8-9; PMID:12594926; http://dx.doi.org/ 10.1098/rstb.2002.1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammani K, Giege P. RNA metabolism in plant mitochondria. Trends Plant Sci 2014; 19: 380-9; PMID:24462302; http://dx.doi.org/ 10.1016/j.tplants.2013.12.008 [DOI] [PubMed] [Google Scholar]
- 8.de Longevialle AF, Small ID, Lurin C. Nuclearly encoded splicing factors implicated in RNA splicing in higher plant organelles. Mol Plant 2010; 3: 691-705; PMID:20603383; http://dx.doi.org/ 10.1093/mp/ssq025 [DOI] [PubMed] [Google Scholar]
- 9.Kim M, Lee U, Small I, des Francs-Small CC, Vierling E. Mutations in an Arabidopsis mitochondrial transcription termination factor-related protein enhance thermotolerance in the absence of the major molecular chaperone HSP101. Plant Cell 2012; 24: 3349-65; PMID:22942382; http://dx.doi.org/ 10.1105/tpc.112.101006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu YW, Wang HJ, Hsieh MH, Hsieh HL, Jauh GY. Arabidopsis mTERF15 Is required for mitochondrial nad2 Intron 3 splicing and functional complex I activity. PLoS One 2014; 9:e112360; PMID:25402171; http://dx.doi.org/ 10.1371/journal.pone.0112360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manna S. An overview of pentatricopeptide repeat proteins and their applications. Biochimie 2015; 113: 93-9; PMID:25882680; http://dx.doi.org/ 10.1016/j.biochi.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 12.Lurin C, Andres C, Aubourg S, Bellaoui M, Bitton F, Bruyere C, Caboche M, Debast C, Gualberto J, Hoffmann B, et al.. Genome-wide analysis of Arabidopsis pentatricopeptide repeat proteins reveals their essential role in organelle biogenesis. Plant Cell 2004; 16: 2089-103; PMID:15269332; http://dx.doi.org/ 10.1105/tpc.104.022236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Small I, Peeters N. The PPR motif- a TPR-related motif prevalent in plant organellar proteins. Trends Biochem Sci 2000; 25: 46-7; PMID:10664580; http://dx.doi.org/ 10.1016/S0968-0004(99)01520-0 [DOI] [PubMed] [Google Scholar]
- 14.Okuda K, Myouga F, Motohashi R, Shinozaki K, Shikanai T. Conserved domain structure of pentatricopeptide repeat proteins involved in chloroplast RNA editing. Proc Natl Acad Sci U S A 2007; 104: 8178-83; PMID:17483454; http://dx.doi.org/ 10.1073/pnas.0700865104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okuda K, Chateigner-Boutin A-L, Nakamura T, Delannoy E, Sugita M, Myouga F, Motohashi R, Shinozaki K, Small I, Shikanaia T. Pentatricopeptide repeat proteins with the DYW motif have distinct molecular functions in RNA editing and RNA cleavage in Arabidopsis chloroplasts. Plant Cell 2009; 21: 146-56; PMID:19182104; http://dx.doi.org/ 10.1105/tpc.108.064667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chateigner-Boutin A-L, des Francs-Small CC, Fujii S, Okuda K, Tanz SK, Small I. The E domains of pentatricopeptide repeat proteins from different organelles are not functionally equivalent for RNA editing. Plant J 2013; 74: 935-45; PMID:23521509; http://dx.doi.org/ 10.1111/tpj.12180 [DOI] [PubMed] [Google Scholar]
- 17.Schallenberg-Ruedinger M, Lenz H, Polsakiewicz M, Gott JM, Knoop V. A survey of PPR proteins identifies DYW domains like those of land plant RNA editing factors in diverse eukaryotes. RNA Biol 2013; 10: 1549-56; PMID:23899506; http://dx.doi.org/ 10.4161/rna.25755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barkan A, Small I. Pentatricopeptide repeat proteins in plants. Annu Rev Plant Biol 2014; 65: 415-42; PMID:24471833; http://dx.doi.org/ 10.1146/annurev-arplant-050213-040159 [DOI] [PubMed] [Google Scholar]
- 19.Binder S, Stoll K, Stoll B. P-class pentatricopeptide repeat proteins are required for efficient 5′ end formation of plant mitochondrial transcripts. RNA Biol 2013; 10: 1511-9; PMID:24184847; http://dx.doi.org/ 10.4161/rna.26129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Longevialle AF, Meyer EH, Andres C, Taylor NL, Lurin C, Millar AH, Small ID. The pentatricopeptide repeat gene OTP43 is required for trans-splicing of the mitochondrial nad1 Intron 1 in Arabidopsis thaliana. Plant Cell 2007; 19: 3256-65; PMID:17965268; http://dx.doi.org/ 10.1105/tpc.107.054841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khrouchtchova A, Monde R-A, Barkan A. A short PPR protein required for the splicing of specific group II introns in angiosperm chloroplasts. RNA 2012; 18: 1197-209; PMID:22495966; http://dx.doi.org/ 10.1261/rna.032623.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmitz-Linneweber C, Williams-Carrier RE, Williams-Voelker PM, Kroeger TS, Vichas A, Barkan A. A pentatricopeptide repeat protein facilitates the trans-splicing of the maize chloroplast rps12 pre-mRNA. Plant Cell 2006; 18: 2650-63; PMID:17041147; http://dx.doi.org/ 10.1105/tpc.106.046110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsieh WY, Liao JC, Chang C, Harrison T, Boucher C, Hsieh MH. The SLOW GROWTH3 pentatricopeptide repeat protein is required for the splicing of mitochondrial NADH Dehydrogenase Subunit7 intron 2 in Arabidopsis. Plant Physiol 2015; 168: 490-501; PMID:25888618; http://dx.doi.org/ 10.1104/pp.15.00354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beick S, Schmitz-Linneweber C, Williams-Carrier R, Jensen B, Barkan A. The pentatricopeptide repeat protein PPR5 stabilizes a specific tRNA precursor in maize chloroplasts. Mol Cell Biol 2008; 28: 5337-47; PMID:18591259; http://dx.doi.org/ 10.1128/MCB.00563-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams-Carrier R, Kroeger T, Barkan A. Sequence-specific binding of a chloroplast pentatricopeptide repeat protein to its native group II intron ligand. RNA 2008; 14: 1930-41; PMID:18669444; http://dx.doi.org/ 10.1261/rna.1077708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yagi Y, Tachikawa M, Noguchi H, Satoh S, Obokata J, Nakamura T. Pentatricopeptide repeat proteins involved in plant organellar RNA editing. RNA Biol 2013; 10: 1419-25; PMID:23669716; http://dx.doi.org/ 10.4161/rna.24908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chateigner-Boutin A-L, Small I. Plant RNA editing. RNA Biol 2014; 7: 213-9; PMID:20473038; http://dx.doi.org/24274753 10.4161/rna.7.2.11343 [DOI] [PubMed] [Google Scholar]
- 28.Takenaka M, Zehrmann A, Verbitskiy D, Hartel B, Brennicke A. RNA editing in plants and its evolution. Annu Rev Genet 2013; 47: 335-52; PMID:24274753; http://dx.doi.org/ 10.1146/annurev-genet-111212-133519 [DOI] [PubMed] [Google Scholar]
- 29.Yu W, Schuster W. Evidence for a site-specific cytidine deamination reaction involved in C to U RNA editing of plant-mitochondria. J Biol Chem 1995; 270: 18227-33; PMID:7629140; http://dx.doi.org/ 10.1074/jbc.270.31.18227 [DOI] [PubMed] [Google Scholar]
- 30.Bentolila S, Oh J, Hanson MR, Bukowski R. Comprehensive high-resolution analysis of the role of an Arabidopsis gene family in RNA editing. PLoS Genet 2013; 9: e1003584; PMID:23818871; http://dx.doi.org/ 10.1371/journal.pgen.1003584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruwe H, Castandet B, Schmitz-Linneweber C, Stern DB. Arabidopsis chloroplast quantitative editotype. FEBS Lett 2013; 587: 1429-33; PMID:23523919; http://dx.doi.org/ 10.1016/j.febslet.2013.03.022 [DOI] [PubMed] [Google Scholar]
- 32.Boussardon C, Salone V, Avon A, Berthome R, Hammani K, Okuda K, Shikanai T, Small I, Lurin C. Two interacting proteins are necessary for the editing of the NdhD-1 site in Arabidopsis plastids. Plant Cell 2012; 24: 3684-94; PMID:23001034; http://dx.doi.org/ 10.1105/tpc.112.099507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bentolila S, Heller WP, Sun T, Babina AM, Friso G, van Wijk KJ, Hanson MR. RIP1, a member of an Arabidopsis protein family, interacts with the protein RARE1 and broadly affects RNA editing. Proc Natl Acad Sci U S A 2012; 109: E1453-61; PMID:22566615; http://dx.doi.org/ 10.1073/pnas.1121465109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kotera E, Tasaka M, Shikanai T. A pentatricopeptide repeat protein is essential for RNA editing in chloroplasts. Nature 2005; 433: 326-30; PMID:15662426; http://dx.doi.org/ 10.1038/nature03229 [DOI] [PubMed] [Google Scholar]
- 35.Okuda K, Nakamura T, Sugita M, Shimizu T, Shikanai T. A pentatricopeptide repeat protein is a site recognition factor in chloroplast RNA editing. J Biol Chem 2006; 281: 37661-7; PMID:17015439; http://dx.doi.org/ 10.1074/jbc.M608184200 [DOI] [PubMed] [Google Scholar]
- 36.Tasaki E, Hattori M, Sugita M. The moss pentatricopeptide repeat protein with a DYW domain is responsible for RNA editing of mitochondrial ccmFc transcript. Plant J 2010; 62: 560-70; PMID:20163555; http://dx.doi.org/ 10.1111/j.1365-313X.2010.04175.x [DOI] [PubMed] [Google Scholar]
- 37.Okuda K, Shikanai T. A pentatricopeptide repeat protein acts as a site-specificity factor at multiple RNA editing sites with unrelated cis-acting elements in plastids. Nucleic Acids Res 2012; 40: 5052-64; PMID:22362750; http://dx.doi.org/ 10.1093/nar/gks164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yin P, Li Q, Yan C, Liu Y, Liu J, Yu F, Wang Z, Long J, He J, Wang HW, et al.. Structural basis for the modular recognition of single-stranded RNA by PPR proteins. Nature 2013; 504: 168-71; PMID:24162847; http://dx.doi.org/ 10.1038/nature12651 [DOI] [PubMed] [Google Scholar]
- 39.Doniwa Y, Ueda M, Ueta M, Wada A, Kadowaki K, Tsutsumi N. The involvement of a PPR protein of the P subfamily in partial RNA editing of an Arabidopsis mitochondrial transcript. Gene 2010; 454: 39-46; PMID:20117193; http://dx.doi.org/ 10.1016/j.gene.2010.01.008 [DOI] [PubMed] [Google Scholar]
- 40.Sung TY, Tseng CC, Hsieh MH. The SLO1 PPR protein is required for RNA editing at multiple sites with similar upstream sequences in Arabidopsis mitochondria. Plant J 2010; 63: 499-511; PMID:20497377; http://dx.doi.org/ 10.1111/j.1365-313X.2010.04258.x [DOI] [PubMed] [Google Scholar]
- 41.Yap A, Kindgren P, Colas des Francs-Small C, Kazama T, Tanz SK, Toriyama K, Small I. AEF1/MPR25 is implicated in RNA editing of plastid atpF and mitochondrial nad5, and also promotes atpF splicing in Arabidopsis and rice. Plant J 2015; 81: 661-9; PMID:25585673; http://dx.doi.org/ 10.1111/tpj.12756 [DOI] [PubMed] [Google Scholar]
- 42.Koprivova A, des Francs-Small CC, Calder G, Mugford ST, Tanz S, Lee BR, Zechmann B, Small I, Kopriva S. Identification of a pentatricopeptide repeat protein implicated in splicing of intron 1 of mitochondrial nad7 transcripts. J Biol Chem 2010; 285: 32192-9; PMID:20682769; http://dx.doi.org/ 10.1074/jbc.M110.147603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keren I, Tal L, des Francs-Small CC, Araujo WL, Shevtsov S, Shaya F, Fernie AR, Small I, Ostersetzer-Biran O. nMAT1, a nuclear-encoded maturase involved in the trans-splicing of nad1 intron 1, is essential for mitochondrial complex I assembly and function. Plant J 2012; 71: 413-26; PMID:22429648; http://dx.doi.org/ 10.1111/j.1365-313x.2012.04998.x. [DOI] [PubMed] [Google Scholar]
- 44.Haili N, Arnal N, Quadrado M, Amiar S, Tcherkez G, Dahan J, Briozzo P, Colas des Francs-Small C, Vrielynck N, Mireau H. The pentatricopeptide repeat MTSF1 protein stabilizes the nad4 mRNA in Arabidopsis mitochondria. Nucleic Acids Res 2013; 41: 6650-63; PMID:23658225; http://dx.doi.org/ 10.1093/nar/gkt337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peeters NM, Hanson MR. Transcript abundance supercedes editing efficiency as a factor in developmental variation of chloroplast gene expression. RNA 2002; 8: 497-511; PMID:11991643; http://dx.doi.org/ 10.1017/S1355838202029424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barkan A, Rojas M, Fujii S, Yap A, Chong YS, Bond CS, Small I. A combinatorial amino acid code for RNA recognition by pentatricopeptide repeat proteins. PLoS Genet 2012; 8: e1002910; PMID:22916040; http://dx.doi.org/ 10.1371/journal.pgen.1002910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kobayashi K, Kawabata M, Hisano K, Kazama T, Matsuoka K, Sugita M, Nakamura T. Identification and characterization of the RNA binding surface of the pentatricopeptide repeat protein. Nucleic Acids Res 2012; 40: 2712-23; PMID:22127869; http://dx.doi.org/ 10.1093/nar/gkr1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takenaka M, Zehrmann A, Brennicke A, Graichen K. Improved computational target site prediction for pentatricopeptide repeat RNA editing factors. PLoS One 2013; 8: e65343; PMID:23762347; http://dx.doi.org/ 10.1371/journal.pone.0065343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karpenahalli MR, Lupas AN, Soeding J. TPRpred: a tool for prediction of TPR-, PPR- and SELI-like repeats from protein sequences. BMC Bioinformatics 2007; 8: 2; PMID:17199898; http://dx.doi.org/ 10.1186/1471-2105-8-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Babot M, Birch A, Labarbuta P, Galkin A. Characterisation of the active/de-active transition of mitochondrial complex I. Biochim Biophys Acta 2014; 1837: 1083-92; PMID:24569053; http://dx.doi.org/ 10.1016/j.bbabio.2014.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fujii S, Small I. The evolution of RNA editing and pentatricopeptide repeat genes. New Phytol 2011; 191: 37-47; PMID:21557747; http://dx.doi.org/ 10.1111/j.1469-8137.2011.03746.x [DOI] [PubMed] [Google Scholar]
- 52.Shikanai T. RNA editing in plants: machinery and flexibility of site recognition. Biochim Biophys Acta 2015; 1847: 779-85; PMID:25585161; http://dx.doi.org/ 10.1016/j.bbabio.2014.12.010 [DOI] [PubMed] [Google Scholar]
- 53.Salone V, Rudinger M, Polsakiewicz M, Hoffmann B, Groth-Malonek M, Szurek B, Small I, Knoop V, Lurin C. A hypothesis on the identification of the editing enzyme in plant organelles. FEBS Lett 2007; 581: 4132-8; PMID:17707818; http://dx.doi.org/ 10.1016/j.febslet.2007.07.075 [DOI] [PubMed] [Google Scholar]
- 54.Nakamura T, Sugita M. A conserved DYW domain of the pentatricopeptide repeat protein possesses a novel endoribonuclease activity. FEBS Lett 2008; 582: 4163-8; PMID:19041647; http://dx.doi.org/ 10.1016/j.febslet.2008.11.017 [DOI] [PubMed] [Google Scholar]
- 55.Liu Y-J, Xiu Z-H, Meeley R, Tan B-C. Empty Pericarp5 encodes a Pentatricopeptide Repeat protein that Is required for mitochondrial RNA editing and seed development in Maize. Plant Cell 2013; 25: 868-83; PMID:23463776; http://dx.doi.org/ 10.1105/tpc.112.106781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keren I, Bezawork-Geleta A, Kolton M, Maayan I, Belausov E, Levy M, Mett A, Gidoni D, Shaya F, Ostersetzer-Biran O. AtnMat2, a nuclear-encoded maturase required for splicing of group-II introns in Arabidopsis mitochondria. RNA 2009; 15: 2299-311; PMID:19946041; http://dx.doi.org/ 10.1261/rna.1776409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuhn K, Carrie C, Giraud E, Wang Y, Meyer EH, Narsai R, des Francs-Small CC, Zhang B, Murcha MW, Whelan J. The RCC1 family protein RUG3 is required for splicing of nad2 and complex I biogenesis in mitochondria of Arabidopsis thaliana. Plant J 2011; 67: 1067-80; PMID:21623974; http://dx.doi.org/ 10.1111/j.1365-313X.2011.04658.x [DOI] [PubMed] [Google Scholar]
- 58.Solotoff V, Moseler R, Schulte U. Two pentatricopeptide repeat domain proteins are required for the synthesis of respiratory complex I. Curr Genet 2015; 61: 19-29; PMID:25108509; http://dx.doi.org/ 10.1007/s00294-014-0441-2 [DOI] [PubMed] [Google Scholar]
- 59.des Francs-Small CC, de Longevialle AF, Li Y, Lowe E, Tanz SK, Smith C, Bevan MW, Small I. The pentatricopeptide repeat proteins TANG2 and ORGANELLE TRANSCRIPT PROCESSING439 are involved in the splicing of the multipartite nad5 transcript encoding a subunit of mitochondrial complex I. Plant Physiol 2014; 165: 1409-16; PMID:24958715; http://dx.doi.org/ 10.1104/pp.114.244616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gorman GS, Blakely EL, Hornig-Do H-T, Tuppen HAL, Greaves LC, He L, Baker A, Falkous G, Newman J, Trenell MI, et al.. Novel MTND1 mutations cause isolated exercise intolerance, complex I deficiency and increased assembly factor expression. Clin Sci 2015; 128: 895-904; PMID:25626417; http://dx.doi.org/ 10.1042/CS20140705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun F, Wang X, Bonnard G, Shen Y, Xiu Z, Li X, Gao D, Zhang Z, Tan BC. Empty pericarp7 encodes a mitochondrial E-subgroup pentatricopeptide repeat protein that is required for ccmF editing, mitochondrial function and seed development in maize. Plant J 2015; 84: 283-95; PMID:26303363; http://dx.doi.org/ 10.1111/tpj.12993 [DOI] [PubMed] [Google Scholar]
- 62.Zhang XR, Henriques R, Lin SS, Niu QW, Chua NH. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat Protoc 2006; 1: 641-6; PMID:17406292; http://dx.doi.org/ 10.1038/nprot.2006.97 [DOI] [PubMed] [Google Scholar]
- 63.Chateigner-Boutin AL, Small I. A rapid high-throughput method for the detection and quantification of RNA editing based on high-resolution melting of amplicons. Nucleic Acids Res 2007; 35: e114; PMID:17726051; http://dx.doi.org/ 10.1093/nar/gkm640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shikanai T. RNA editing in plant organelles: machinery, physiological function and evolution. Cell Mol Life Sci 2006; 63: 698-708; PMID:16465445; http://dx.doi.org/ 10.1007/s00018-005-5449-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


