Abstract
Background
The candidate malaria vaccine RTS,S/AS01 is being evaluated in order to inform a decision regarding its inclusion in routine vaccination schedules.
Methods
We conducted 7 years of follow-up in children who had been randomly assigned, at 5 to 17 months of age, to receive three doses of either the RTS,S/AS01 vaccine or a rabies (control) vaccine. The end point was clinical malaria (temperature of ≥37.5°C and infection with Plasmodium falciparum of >2500 parasites per cubic millimeter). In an analysis that was not prespecified, the malaria exposure of each child was estimated with the use of information on the prevalence of malaria among residents within a 1-km radius of the child’s home. Vaccine efficacy was defined as 1 minus the hazard ratio or the incidence-rate ratio, multiplied by 100, in the RTS,S/AS01 group versus the control group.
Results
Over 7 years of follow-up, we identified 1002 episodes of clinical malaria among 223 children randomly assigned to the RTS,S/AS01 group and 992 episodes among 224 children randomly assigned to the control group. The vaccine efficacy, as assessed by negative binomial regression, was 4.4% (95% confidence interval [CI], −17.0 to 21.9; P = 0.66) in the intention-to-treat analysis and 7.0% (95% CI, −14.5 to 24.6; P = 0.52) in the per-protocol analysis. Vaccine efficacy waned over time (P = 0.006 for the interaction between vaccination and time), including negative efficacy during the fifth year among children with higher-than-average exposure to malaria parasites (intention-to-treat analysis: −43.5%; 95% CI, −100.3 to −2.8 [P = 0.03]; per-protocol analysis: −56.8%; 95% CI, −118.7 to −12.3 [P = 0.008]).
Conclusions
A three-dose vaccination with RTS,S/AS01 was initially protective against clinical malaria, but this result was offset by rebound in later years in areas with higher-than-average exposure to malaria parasites. (Funded by the PATH Malaria Vaccine Initiative and others; ClinicalTrials.gov number, NCT00872963.)
RTS,S/ASO1 is a malaria vaccine candidate that has undergone phase 3 evaluation across several sites in Africa that have varying intensities of malaria transmission. During more than 48 months of follow-up, immunization with the RTS,S/AS01 vaccine was estimated to be associated with rates of protection against clinical malaria of 36.3% (95% confidence interval [CI], 31.8 to 40.5) among children 5 to 17 months of age who had received a fourth dose and 28.3% (95% CI, 23.3 to 32.9) among those who had not received a fourth dose.1 The rates among young infants (6 to 12 weeks of age at the time of first vaccination) with more than 38 months of follow-up were 25.9% (95% CI, 19.9 to 31.5) among those who had received a fourth dose and 18.3% (95% CI, 11.7 to 24.4) among those who had not received a fourth dose.
The efficacy of vaccination with RTS,S/AS01 wanes over time.2 The potential for rebound in malaria cases (also referred to as “age shift”) as immunity wanes may lessen the public health usefulness of malaria vaccines. We present data from 7 years of follow-up to assess the possibility of a rebound.
Methods
Trial Design
We conducted this trial as part of a double-blind, randomized, controlled, phase 2 trial of the RTS,S/AS01 vaccine in children who were 5 to 17 months of age at the time of the first vaccination and who lived in Kilifi, Kenya, or in Korogwe, Tanzania.3 The original two-site trial was initiated in March 2007 and was completed in August 2008 in Korogwe and, after a site-specific extension, in November 2008 in Kilifi. Additional follow-up was then conducted in Kilifi until April 2011 (4-year efficacy results were published in 2013).2 A further extension trial was conducted until November 2014, at which time follow-up was discontinued. The data reported here are from the Kilifi site only. The full details of the conduct of the trial are provided in the protocol (including the statistical analysis plan), available with the full text of this article at NEJM.org.
The trial extension was designed by the academic authors, and employees of GlaxoSmithKline Biologicals provided review of the protocol. Until November 1, 2008, the sponsorship, monitoring, and data management were the responsibility of GlaxoSmithKline Biologicals; after that date, these aspects of the trial were the responsibility of the Kenya Medical Research Institute–Wellcome Trust Research Programme. Data were gathered by the academic team, and the analysis was conducted by the first, second, and last authors. The first draft of the manuscript was written by the first author and revised by the last author, with comments from all the authors. Safety reporting to the regulatory authorities was undertaken by GlaxoSmithKline Biologicals.
Participants
In early 2007, we recruited 447 healthy children who were 5 to 17 months of age. We conducted three extensions: from the end of 12 months of follow-up until November 2008, then until April 2011, and then finally for an additional 3 years. Written informed consent for the extension was obtained from the parents or guardians of all the children with the use of approved consent forms provided in Swahili or Giriama. Nonliterate parents indicated consent by using a thumbprint, and a signature was obtained from an independent literate witness. The original trial and its extensions were approved by the Kenya Medical Research Institute National Ethics Committee, the Western Institutional Review Board, and the Oxford Tropical Research Ethics Committee. This article is published with the permission of the Director of Kenya Medical Research Institute.
Procedures
In the initial trial, three doses of the RTS,S/AS01 vaccine or rabies (control) vaccine were administered, at baseline and at 1 and 2 months. No vaccines were administered during the extended follow-up phase. Participants were followed up by means of both weekly active surveillance and passive surveillance to identify clinical malaria cases. We obtained blood samples for the assessment of asymptomatic parasitemia at 8, 12, 15, 25, 38, and 49 months (as reported previously2) and at 65, 78, and 91 months after vaccination. The participants and clinicians who were involved in the follow-up were unaware of the trial-group assignments. The principal investigators became aware of the trial-group assignments after the end of the initial phase of the trial but did not take part in the clinical evaluation of participants.
Malaria-Parasite Exposure
Malaria transmission shows fine-scale geographic heterogeneity.4 We previously found that fine-scale variations in exposure could be predicted by estimation of the prevalence of malaria infection among children who reside within a 1-km radius of each participant as an exposure index. Furthermore, the accuracy of this exposure index is refined by weighting according to the inverse of the distance between homes within the 1-km radius and the index child. Hence, homes that are near to the index child contribute more important information than do those on the edge of the 1-km radius.5 In an analysis that was not prespecified, we used data from 870 children who were under active surveillance in the same trial area to determine exposure indexes and categorized the participants into low-exposure and high-exposure groups according to whether they were at or below the cohort mean or above the cohort mean, respectively.
Statistical Analysis
The primary end point was clinical malaria caused by Plasmodium falciparum (temperature of ≥37.5°C and P. falciparum parasitemia density of >2500 parasites per cubic millimeter). The intention-to-treat cohort included all the children who had undergone randomization. The per-protocol cohort included children who received three doses of vaccine according to the trial protocol and for whom surveillance data were available at any time from 2 weeks after receipt of the third dose. We censored data from children at 7 years of follow-up.
We used Cox proportional-hazard regression for analysis of the first malarial episode. Multiple episodes were analyzed by means of negative binomial regression and the Andersen–Gill extension of Cox regression, with clustering by participant. The first-episode analysis was used as the primary analysis after 8 months of follow-up,3 but longer-term follow-up showed lower efficacy when all episodes were considered.2 Vaccine efficacy was defined as 1 minus the hazard ratio or the incidence-rate ratio multiplied by 100 in the RTS,S/AS01 group versus the control group.
Adjustments were made for age, bed-net use, and malaria exposure. We plotted the incidence-rate ratios over time by first aggregating the data into 4-month groups and then calculating the incidence rates in each trial group; we then divided the incidence in the RTS,S/AS01 group by the incidence in the control group. A quadratic equation was then fitted to these values.
As previously reported,1 we calculated the cases averted in each year of follow-up by subtracting the measured incidence per person-year among participants in the RTS,S/AS01 group from the incidence per person-year among participants in the control group and then multiplying by 1000 to express the result as the number of cases averted per 1000 children vaccinated with RTS,S/AS01. We calculated cumulative cases by summing the cases averted up to and including each given year. We used bootstrapping methods to obtain 95% confidence intervals by taking the 2.5th and 97.5th percentiles of 1000 iterations. All the analyses were performed with the use of Stata software, version 13 (StataCorp).
Results
Trial Participants
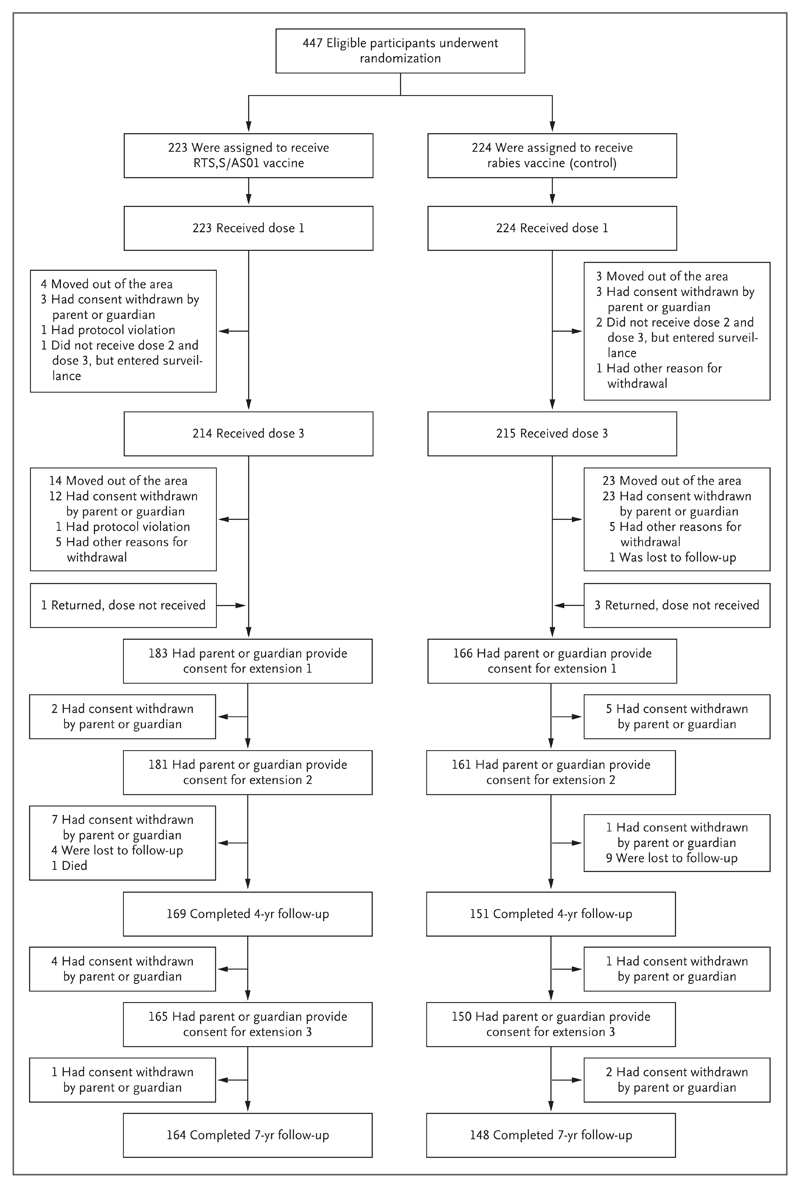
Of the 447 children enrolled in the original trial, 312 completed all three extensions of follow-up (164 participants in the RTS,S/AS01 group and 148 in the control group) (Fig. 1). We included all enrolled children in our analysis (i.e., 447 participants in the intention-to-treat cohort and 415 in the per-protocol cohort). All the participants who underwent randomization received at least one dose of vaccine. The characteristics of the RTS,S/AS01 group and the control group were similar at baseline (Table S1 in the Supplementary Appendix, available at NEJM.org). Participants who were lost to follow-up were significantly less likely to have bed nets and more likely to live farther away from the dispensary than were other participants. In addition, a nonsignificantly greater number of participants in the control group than in the RTS,S/AS01 group were lost to follow-up, and participants who were lost to follow-up had a nonsignificantly lower incidence of malaria (Table S2 in the Supplementary Appendix).
Figure 1 (facing page). Randomization and Follow-up of Trial Participants.
We conducted three extensions: from the end of 12 months of follow-up until November 2008, from then until April 2011, and finally for an additional 3 years. Other reasons for withdrawal included children missing vaccinations because of hospital admission (with contraindications to further vaccination), medical conditions not permitted by the protocol, and incomplete documentation regarding concomitant vaccinations.
Efficacy Against First Episode
In the intention-to-treat cohort, there were 150 cases of first episodes of clinical malaria among 223 participants in the RTS,S/AS01 group and 157 cases among 224 participants in the control group. In a Cox regression analysis, after adjustment for person-years of follow-up but no covariates, the vaccine efficacy against the first episode of clinical malaria was 27.0% (95% CI, 8.5 to 41.8; P = 0.006) (Table 1). Consistent results were seen in the per-protocol analysis.
Table 1. Vaccine Efficacy.*.
| Variable | RTS,S/AS01 Vaccine | Control | Efficacy (95% CI) | P Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of Participants | No. of Events | Person-Yr at Risk | Event Rate | No. of Participants | No. of Events | Person-Yr at Risk | Event Rate | |||
| Intention-to-treat cohort | ||||||||||
| First episode, Cox regression | ||||||||||
| >2500 parasites/mm3 | 223 | 150 | 668.0 | 0.22 | 224 | 157 | 501.6 | 0.31 | 27.0 (8.5 to 41.8) | 0.006 |
| >0 parasites/mm3 | 223 | 158 | 622.9 | 0.25 | 224 | 163 | 470.1 | 0.35 | 25.6 (7.2 to 40.3) | 0.009 |
| Multiple episodes, negative binomial regression | ||||||||||
| >2500 parasites/mm3 | 223 | 1002 | 1372.8 | 0.73 | 224 | 992 | 1282.5 | 0.77 | 4.4 (−17.0 to 21.9) | 0.66 |
| >0 parasites/mm3 | 223 | 1157 | 1372.8 | 0.84 | 224 | 1164 | 1282.5 | 0.91 | 4.7 (−15.3 to 21.3) | 0.62 |
| Per-protocol cohort | ||||||||||
| First episode, Cox regression | ||||||||||
| >2500 parasites/mm3 | 209 | 139 | 605.6 | 0.22 | 206 | 145 | 440.3 | 0.33 | 33.8 (16.4 to 47.6) | 0.001 |
| >0 parasites/mm3 | 209 | 144 | 567.8 | 0.25 | 206 | 151 | 412.5 | 0.37 | 34.1 (17.1 to 45.6) | <0.001 |
| Multiple episodes, negative binomial regression | ||||||||||
| >2500 parasites/mm3 | 209 | 889 | 1223.6 | 0.73 | 206 | 844 | 1109.2 | 0.76 | 7.0 (−14.5 to 24.6) | 0.52 |
| >0 parasites/mm3 | 209 | 1021 | 1223.6 | 0.83 | 206 | 990 | 1109.2 | 0.89 | 8.6 (−11.4 to 25.0) | 0.37 |
The control group received a rabies vaccine. All clinical malaria cases were detected by active case detection or passive case detection. Vaccine efficacy was defined as 1 minus the hazard ratio or the incidence-rate ratio, then multiplied by 100. P values were calculated with the use of a Cox proportional-hazards model for first episodes and negative binomial regression for multiple episodes.
Efficacy Against All Episodes
In the intention-to-treat cohort, 1002 episodes of malaria occurred in the RTS,S/AS01 group and 992 in the control group. The rate of loss to follow-up was higher in the control group than in the RTS,S/AS01 group, and after the inclusion of person-years of observation in the negative binomial model, the estimate of vaccine efficacy was 4.4% (95% CI, −17.0 to 21.9; P = 0.66) (Table 1). Similar results were seen in the per-protocol analysis.
Efficacy was consistently lower in the cohort with high exposure to malaria parasites than in the cohort with low exposure (Table 2). Efficacy against all episodes of clinical malaria was 16.6% (95% CI, −24.6 to 44.2) in the low-exposure cohort (P = 0.38) and −2.4% (95% CI, −26.1 to 16.8) in the high-exposure cohort (P = 0.82).
Table 2. Vaccine Efficacy against All Episodes, According to Malaria-Parasite Exposure and Year of Follow-up.*.
| Variable | All Participants | Low-Exposure Cohort | High-Exposure Cohort | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Participants | No. of Events | Efficacy (95% Cl) % |
P Value | No. of Participants | No. of Events | Efficacy (95% Cl) % |
P Value | No. of Participants | No. of Events | Efficacy (95% Cl) % |
P Value | |
| Intention-to-treat cohort | ||||||||||||
| All years | 1938 | 447 | 4.4 (−17.0 to 21.9) |
0.66 | 395 | 222 | 25.9 (−9.5 to 49.8) |
0.13 | 1543 | 225 | 1.9 (−20.6 to 20.3) |
0.85 |
| Year 1 | 225 | 447 | 35.9 (8.1 to 55.3) |
0.02 | 51 | 222 | 56.7 (−4.9 to 0.82) |
0.06 | 166 | 225 | 30.5 (−2.5 to 52.8) |
0.07 |
| Year 2 | 238 | 420 | 22.9 (−11.5 to 46.6) |
0.17 | 41 | 191 | 13.3 (−108.8 to 64.0) |
0.75 | 192 | 197 | 25.5 (−10.6 to 49.8) |
0.15 |
| Year 3 | 268 | 341 | 16.8 (−16.9 to 40.8) |
0.29 | 47 | 132 | 36.7 (−35.4 to 70.4) |
0.24 | 214 | 188 | 3.9 (−38.0 to 33.2) |
0.83 |
| Year 4 | 291 | 338 | 2.5 (−34.2 to 29.2) |
0.88 | 47 | 130 | 24.0 (−57.6 to 63.4) |
0.46 | 235 | 187 | −4.2 (−46.1 to 25.6) |
0.81 |
| Year 5 | 289 | 336 | −27.6 (−74.8 to 6.8) |
0.13 | 52 | 128 | 15.3 (−73.4 to 58.6) |
0.65 | 230 | 186 | −43.5 (−100.3 to −2.8) |
0.03 |
| Year 6 | 150 | 331 | −13.9 (−71.1 to 24.1) |
0.53 | 51 | 125 | 11.1 (−75.1 to 54.8) |
0.74 | 95 | 185 | −34.3 (−127.1 to 20.6) |
0.27 |
| Year 7 | 353 | 321 | 3.6 (−29.5 to 28.2) |
0.81 | 64 | 120 | 22.2 (−46.4 to 58.7) |
0.44 | 280 | 181 | −6.7 (−46.3 to 22.2) |
0.69 |
| Per-protocol cohort | ||||||||||||
| All years | 1733 | 415 | 7.0 (−14.5 to 24.6) |
0.49 | 331 | 200 | 15.6 (−23.6 to 44.2) |
0.38 | 1353 | 215 | −2.4 (−26.1 to 16.8) |
0.82 |
| Year 1 | 215 | 415 | 46.3 (21.4 to 62.8) |
0.001 | 46 | 200 | 57.5 (7.4 to 81.3) |
0.03 | 161 | 215 | 39.5 (10.1 to 59.7) |
0.01 |
| Year 2 | 223 | 376 | 25.2 (−19.4 to 52.5) |
0.23 | 35 | 184 | 39.2 (−38.3 to 72.8) |
0.24 | 183 | 192 | 31.2 (−0.9 to 53.0) |
0.06 |
| Year 3 | 250 | 309 | 22.2 (−16.8 to 48.3) |
0.23 | 43 | 126 | 61.9 (24.3 to 82.4) |
0.006 | 200 | 183 | −4.3 (−61.8 to 33.2) |
0.86 |
| Year 4 | 280 | 306 | −1.0 (−46.5 to 31.4) |
0.95 | 46 | 124 | 41.9 (−8.5 to 69.0) |
0.08 | 225 | 182 | −28.9 (−98.2 to 15.6) |
0.22 |
| Year 5 | 282 | 304 | −34.4 (−83.9 to 1.8) |
0.06 | 51 | 123 | −0.8 (−100.7 to 49.3) |
0.98 | 224 | 181 | −56.8 (−118.7 to −12.3) |
0.008 |
| Year 6 | 145 | 301 | −29.9 (−91.9 to 12.1) |
0.19 | 48 | 121 | −29.8 (−139.8 to 29.7) |
0.40 | 93 | 180 | −37.8 (−129.2 to 17.2) |
0.22 |
| Year 7 | 338 | 292 | 4.9 (−27.0 to 28.9) |
0.73 | 62 | 116 | 22.5 (−48.7 to 59.7) |
0.44 | 267 | 176 | −10.6 (−51.4 to 19.3) |
0.53 |
Vaccine efficacy against all episodes was estimated with the use of negative binominal regression. The intention-to-treat cohort included all the children who had undergone randomization. The per-protocol cohort included children who received three doses of vaccine according to the trial protocol and for whom surveillance data were available from 2 weeks after receipt of the third dose. The cohort with a low malaria-parasite exposure index had a distance-weighted local prevalence of malaria that was at or below the cohort mean, and the cohort with a high exposure index had a prevalence that was above the cohort mean.
Waning in Vaccine Efficacy
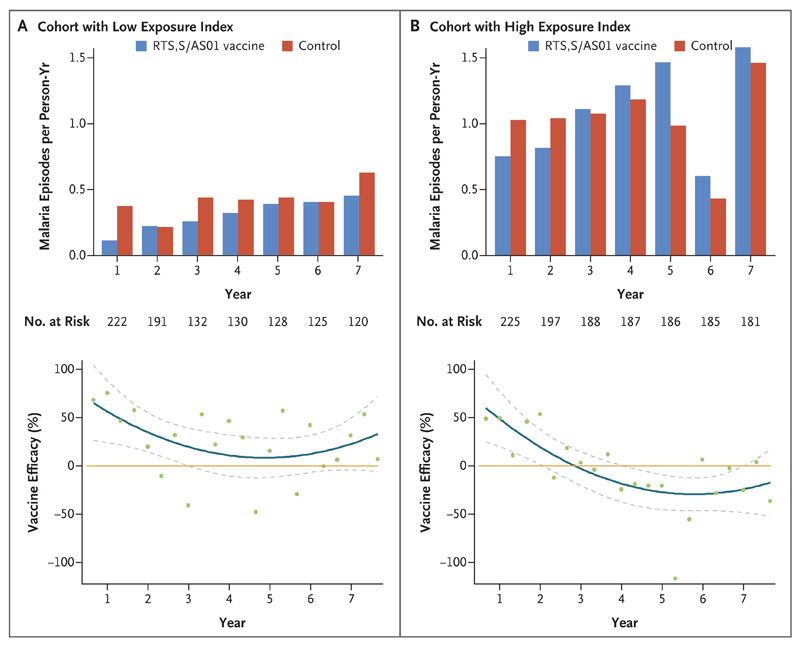
There was a significant interaction between receipt of the RTS,S/AS01 vaccine and follow-up time in the adjusted negative binomial regression model (incidence-rate ratio, 1.07; 95% CI, 1.02 to 1.14; P = 0.006) and in the Andersen–Gill extension of the Cox regression analysis (hazard ratio, 1.22; 95% CI, 1.02 to 1.45; P = 0.03). These interaction terms indicate that the efficacy of vaccination changed significantly over time. This finding can be quantified further by an examination of efficacy in individual years. In the intention-to-treat analysis, vaccine efficacy declined from 35.9% (95% CI, 8.1 to 55.3; P = 0.02) in the first year to 3.6% (95% CI, −29.5 to 28.2; P = 0.81) in the seventh year (Table 2 and Fig. 2). In year 5, negative efficacy was observed. This finding was of marginal significance overall (−34.4%; 95% CI, −83.9 to 1.8; P = 0.06) in the per-protocol analysis, but most of the negative efficacy was seen in the high-exposure cohort. At year 5, negative efficacy was not significant in the low-exposure cohort (−0.8%; 95% CI, −100.7 to 49.3; P = 0.98) but was significant in the high-exposure cohort (−56.8%; 95% CI, −118.7 to −12.3, P = 0.008).
Figure 2. Malaria Incidence and Vaccine Efficacy, According to Malaria Exposure and Trial Group in the Intention-to-Treat Cohort.
Panel A shows the incidence of malaria in the cohort with a low exposure index (distance-weighted local prevalence of malaria at or below the cohort mean), and Panel B the incidence in the cohort with a high exposure index (distance-weighted local prevalence of malaria above the cohort mean). The top graphs show the incidence of malaria in the RTS,S/AS01 group and the control group according to year of follow-up. The bottom graphs show the estimates of vaccine efficacy, aggregated in 4-month windows, on the basis of the calculation of 1 minus the incidence-rate ratio times 100, with the incidence-rate ratio calculated as the incidence of malaria in the RTS,S/AS01 group divided by the incidence in the control group. The orange line indicates 0% efficacy, and the blue line indicates smoothed estimates of efficacy over time. The dashed lines indicate 95% confidence intervals, and green dots point estimates of efficacy.
Waning of vaccine efficacy was more rapid in the cohort with high exposure to malaria parasites than in the cohort with low exposure. A three-way interaction among vaccination, time, and malaria-parasite exposure resulted in an incidence-rate ratio of 1.23 (95% CI, 1.07 to 1.42; P = 0.004) in the negative binomial analysis. This three-way interaction indicated significant variation in the rate of decline, with more rapidly declining efficacy observed with increasing exposure index.
Estimated Cases of Malaria Averted
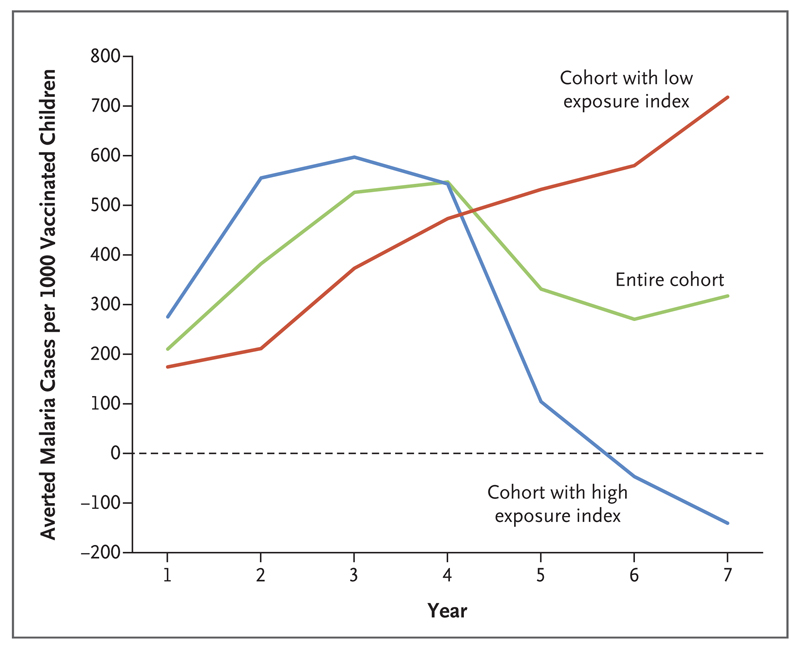
In the intention-to-treat analysis, the overall estimated number of clinical malaria cases averted over a period of 7 years was 317 cases (95% CI, −357 to 973) per 1000 children vaccinated with RTS,S/AS01, with wide confidence intervals that overlapped zero. In the low-exposure cohort, cases continued to be averted throughout the follow-up period, to a total of 718 cases (95% CI, 4 to 1404) per 1000 participants in the RTS,S/AS01 group. However, in the high-exposure cohort, there were negative cases averted in later years (i.e., there were more cumulative cases among participants in the RTS,S/AS01 group than among those in the control group), which more than offset the cases that were averted in earlier years, leading to −141 cases (95% CI, −1210 to 906) averted per 1000 participants (Fig. 3). The findings regarding averted cases were based on incidence rates rather than absolute numbers of cases per participant and hence were adjusted for person-years at risk (Table 2).
Figure 3. Malaria Cases Averted during Follow-up in the Intention-to-Treat Population.
Shown are the cumulative numbers of malaria cases averted, according to year of follow-up and exposure index of the cohort. We calculated cases averted in each year by subtracting the measured incidence per person-year among participants in the RTS,S/AS01 group from the incidence per person-year among participants in the control group and then multiplying by 1000 to express the result as the number of cases averted per 1000 children vaccinated with RTS,S/AS01. We calculated cumulative cases by summing the cases averted up to and including the year under consideration.
Cross-Sectional Analysis
The prevalence of asymptomatic P. falciparum parasitemia was lower in the RTS,S/AS01 group than in the control group at all cross-sectional surveys before the fourth year. Thereafter, the prevalences were similar in the two groups (Table S3 in the Supplementary Appendix).
Safety
There were no significant differences between the RTS,S/AS01 group and the control group in the percentage of children reporting one or more serious adverse events (17.9% [95% CI, 13.1 to 23.6] and 25.4% [95% CI, 19.9 to 31.7], respectively) (Table S4 in the Supplementary Appendix). No cases of meningitis were reported. A total of 15 cases of severe malaria were identified during follow-up: 5 cases in the RTS,S/AS01 group and 10 in the control group. In the control group, all the episodes of severe malaria occurred before 2.7 years of follow-up, whereas in the RTS,S/AS01 group, all the cases of severe malaria were observed after 2.7 years of follow-up (Fig. S1 in the Supplementary Appendix). All cases of severe malaria resolved without long-term sequelae.
Discussion
We found that RTS,S/AS01 provided protective efficacy in the first year after vaccination but that the efficacy subsequently waned. Efficacy was close to zero in the fourth year and may have been negative in the fifth year. The larger phase 3 trial of the RTS,S/AS01 vaccine showed efficacy estimates of 28.3% (95% CI, 23.3 to 32.9) against all malaria episodes over a median of 4 years of follow-up in the group that received three doses of the RTS,S/AS01 vaccine, as compared with the control group.1 The data set from the phase 2 trial presented here includes fewer participants than the phase 3 trial did (447 vs. 8923 participants) with a longer duration of follow up (7 years vs. 4 years).
There was a trend toward negative efficacy in the fifth year in the whole cohort, with a significant result in the subgroup of children who had a high malaria-exposure index, as compared with those with a low exposure index (Table 2). Only 312 of the 447 participants who underwent randomization completed follow-up, which may have introduced a risk of bias. There were indications that participants who were lost to followup lived farther from the dispensary and were less likely to have bed nets than participants who completed the trial (Table S2 in the Supplementary Appendix). Participants who were lost to follow-up were also more likely to be in the control group and to have a lower risk of malaria episodes, but these findings were not significant, which suggests a low risk of bias in the primary analysis.6 Furthermore, our analysis was exploratory among the two subgroup cohorts and hence is prone to type I error because of multiple comparisons. However, the negative efficacy during the fifth year fits an overall trend (Fig. 2B, bottom graph), and the variation in efficacy over time and according to malaria-parasite exposure is supported by a significant interaction between time and exposure in the determination of vaccine efficacy.
The summation of the described variation in efficacy over time since vaccination and according to malaria exposure led to undetectable efficacy over a period of 7 years in the cohort we studied (i.e., the confidence intervals for the estimates of efficacy and numbers of cases averted included zero). We note that the absolute number of malaria cases was in fact slightly higher in the RTS,S/AS01 group than in the control group (Table 1). The calculated vaccine efficacies and cases averted were marginally (and nonsignificantly) positive after correction for fewer person-years of observation among persons who received the control vaccine than among those who received RTS,S/AS01. The confidence intervals for cases averted are wide in our analysis. This uncertainty reflects the limited sample size in our trial, combined with high frequencies of clinical episodes: any uncertainty in estimates of relative efficacy therefore translates to greater uncertainty in the estimates of absolute numbers of cases averted.
We recorded a clinical malaria rate of 0.76 cases per person-year of observation among participants in the control group who were under conditions of active surveillance. In the phase 3 trial, at the site where the highest transmission was recorded (Siaya in western Kenya), a clinical malaria rate of 3.31 cases per person-year was recorded under conditions of passive surveillance.7 Therefore, our “higher-than-average transmission” cohort within the Junju geographic area in Kilifi may be equivalent to a moderate intensity of transmission in the wider African context,8 and hence our results may not be generalizable to areas with higher intensities of transmission.
The potential for malaria rebound has been suggested before as a possibility after pre-erythrocytic vaccination9 and may also be a concern with regard to insecticide-treated bed nets in the context of insecticide resistance.10 Malaria rebound has been observed in randomized trials involving children after the withdrawal of weekly malaria chemoprophylaxis.11–13 In contrast, studies of intermittent preventive treatment with antimalarial agents have not shown a rebound.14,15
Malaria rebound may occur because the RTS,S/AS01 vaccine protects against malaria sporozoites but does not induce clinical immunity against blood-stage parasites. We and others have previously found lower levels of antibodies against blood-stage parasites in children who have been immunized with the RTS,S/AS01 vaccine than in those given the control vaccine.9,16 The reduced exposure to blood-stage parasites among persons who have received the RTS,S/AS01 vaccine may lead to a slower acquisition of immunity to blood-stage parasites, leading to an increase in episodes of clinical malaria in later life. This effect may be less marked in geographic regions where children are only occasionally exposed to parasites, in which immunity is more slowly acquired, and hence we did not see any evidence of rebound in our low-exposure cohort.
A large phase 3 trial of RTS,S/AS01 showed an efficacy estimate of 28% against all malaria episodes over a median of 4 years of follow-up in the group that received three doses of the RTS,S/AS01 vaccine, as compared with the control group.1 Efficacy against clinical malaria was higher among children who received a fourth dose than among those who did not (36% vs. 28%).1 Extended follow-up is currently being undertaken at three sites from the phase 3 trial and includes some participants who are receiving a fourth dose, which will provide further information on outcomes in year 5 and beyond.
In conclusion, RTS,S/AS01 vaccination showed evidence of 35.9% efficacy in the first year after vaccination, but efficacy fell to 2.5% in the fourth year. The cohort with a high exposure index had a partial rebound in clinical malaria cases during the fifth year. This result eroded the benefits that were seen in early years, such that over a period of 7 years, vaccine efficacy was estimated at 4.4%, a rate that was substantially lower than that seen over short-term follow up.3 In areas with a high intensity of malaria-parasite transmission, some of the early gains in averting the malaria burden can be lost in later years owing to a waning in vaccine efficacy. A larger phase 3 trial has been conducted across a range of transmission conditions and with additional vaccine doses. It will be essential to monitor efficacy in longer-term follow-up for year 5 and beyond to accurately measure the benefit and potential risk of vaccination with the RTS,S/AS01 vaccine.
Supplementary Material
Acknowledgments
Supported by grants from the PATH Malaria Vaccine Initiative, GlaxoSmithKline Biologicals, and the Wellcome Trust.
We thank the parents and guardians of the participants and the village and district authorities for their cooperation; the members of the data and safety monitoring board (Dr. Malcolm Molyneux, chair) and the local safety monitor, Dr. Jay Berkley in Kilifi; Janet Musembi, Omar Ngoto, Ester Kache, and Steven Chakaya (trial clinicians); and all the community field assistants who conducted active surveillance in the field.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org
Contributor Information
Ally Olotu, Kenya Medical Research Institute (KEMRI)–Wellcome Trust Programme, Kilifi, Kenya; Ifakara Health Institute, Bagamoyo, Tanzania
Gregory Fegan, Kenya Medical Research Institute (KEMRI)–Wellcome Trust Programme, Kilifi, Kenya; Nuffield Department of Medicine, University of Oxford, Oxford, United Kingdom
Juliana Wambua, Kenya Medical Research Institute (KEMRI)–Wellcome Trust Programme, Kilifi, Kenya
George Nyangweso, Kenya Medical Research Institute (KEMRI)–Wellcome Trust Programme, Kilifi, Kenya
Amanda Leach, GlaxoSmithKline Vaccines, Wavre, Belgium
Marc Lievens, GlaxoSmithKline Vaccines, Wavre, Belgium
David C. Kaslow, PATH, Seattle
Patricia Njuguna, Kenya Medical Research Institute (KEMRI)–Wellcome Trust Programme, Kilifi, Kenya
Kevin Marsh, Kenya Medical Research Institute (KEMRI)–Wellcome Trust Programme, Kilifi, Kenya; Nuffield Department of Medicine, University of Oxford, Oxford, United Kingdom
Philip Bejon, Kenya Medical Research Institute (KEMRI)–Wellcome Trust Programme, Kilifi, Kenya; Nuffield Department of Medicine, University of Oxford, Oxford, United Kingdom
References
- 1.RTS,S Clinical Trials Partnership. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet. 2015;386:31–45. doi: 10.1016/S0140-6736(15)60721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olotu A, Fegan G, Wambua J, et al. Four-year efficacy of RTS,S/AS01E and its interaction with malaria exposure. N Engl J Med. 2013;368:1111–20. doi: 10.1056/NEJMoa1207564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bejon P, Lusingu J, Olotu A, et al. Efficacy of RTS,S/AS01E vaccine against malaria in children 5 to 17 months of age. N Engl J Med. 2008;359:2521–32. doi: 10.1056/NEJMoa0807381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bejon P, Williams TN, Nyundo C, et al. A micro-epidemiological analysis of febrile malaria in Coastal Kenya showing hotspots within hotspots. Elife. 2014;3:e02130. doi: 10.7554/eLife.02130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olotu A, Fegan G, Wambua J, et al. Estimating individual exposure to malaria using local prevalence of malaria infection in the field. PLoS One. 2012;7(3):e32929. doi: 10.1371/journal.pone.0032929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akl EA, Briel M, You JJ, et al. Potential impact on estimated treatment effects of information lost to follow-up in randomised controlled trials (LOST-IT): systematic review. BMJ. 2012;344:e2809. doi: 10.1136/bmj.e2809. [DOI] [PubMed] [Google Scholar]
- 7.RTS,S Clinical Trials Partnership. Efficacy and safety of the RTS,S/AS01 malaria vaccine during 18 months after vaccination: a phase 3 randomized, controlled trial in children and young infants at 11 African sites. PLoS Med. 2014;11(7):e1001685. doi: 10.1371/journal.pmed.1001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noor AM, Kinyoki DK, Mundia CW, et al. The changing risk of Plasmodium falciparum malaria infection in Africa: 2000-10: a spatial and temporal analysis of transmission intensity. Lancet. 2014;383:1739–47. doi: 10.1016/S0140-6736(13)62566-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bejon P, Cook J, Bergmann-Leitner E, et al. Effect of the pre-erythrocytic candidate malaria vaccine RTS,S/AS01E on blood stage immunity in young children. J Infect Dis. 2011;204:9–18. doi: 10.1093/infdis/jir222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trape JF, Tall A, Diagne N, et al. Malaria morbidity and pyrethroid resistance after the introduction of insecticide-treated bednets and artemisinin-based combination therapies: a longitudinal study. Lancet Infect Dis. 2011;11:925–32. doi: 10.1016/S1473-3099(11)70194-3. [DOI] [PubMed] [Google Scholar]
- 11.Menendez C, Kahigwa E, Hirt R, et al. Randomised placebo-controlled trial of iron supplementation and malaria chemoprophylaxis for prevention of severe anaemia and malaria in Tanzanian infants. Lancet. 1997;350:844–50. doi: 10.1016/S0140-6736(97)04229-3. [DOI] [PubMed] [Google Scholar]
- 12.Oyediran AB, Topley E, Osunkoya BO, et al. Severe morbidity among children in a trial malaria chemoprophylaxis with pyrimethamine or chloroquine in Ibarapa, Nigeria. Afr J Med Med Sci. 1993;22:55–63. [PubMed] [Google Scholar]
- 13.Senn N, Rarau P, Stanisic DI, et al. Intermittent preventive treatment for malaria in Papua New Guinean infants exposed to Plasmodium falciparum and P. vivax: a randomized controlled trial. PLoS Med. 2012;9(3):e1001195. doi: 10.1371/journal.pmed.1001195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grobusch MP, Gabor JJ, Aponte JJ, et al. No rebound of morbidity following intermittent preventive sulfadoxine-pyrimethamine treatment of malaria in infants in Gabon. J Infect Dis. 2009;200:1658–61. doi: 10.1086/647990. [DOI] [PubMed] [Google Scholar]
- 15.Odhiambo FO, Hamel MJ, Williamson J, et al. Intermittent preventive treatment in infants for the prevention of malaria in rural western Kenya: a randomized, double-blind placebo-controlled trial. PLoS One. 2010;5(4):e10016. doi: 10.1371/journal.pone.0010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campo JJ, Aponte JJ, Skinner J, et al. RTS,S vaccination is associated with serologic evidence of decreased exposure to Plasmodium falciparum liver- and blood-stage parasites. Mol Cell Proteomics. 2015;14:519–31. doi: 10.1074/mcp.M114.044677. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.