Abstract
Previous research has demonstrated that Parkinson’s disease patients have an increased susceptibility to response conflict. Here, we investigate whether Parkinson patients have a similar sensitivity to interference from observed movements. Ten patients and ten controls performed horizontal and vertical arm movements, while watching a video of a person performing similar movements or a moving dot. Movements were performed in the same plane (congruent) and orthogonal to the observed movement (incongruent). The off-axis variance of movements was our index of interference. While patients tended to exhibit more off-axis variability than controls, both groups demonstrated similar congruence effects, with greater variance in incongruent conditions. These results indicate that increased susceptibility to interference in Parkinson’s disease does not extend to interference from observed movements.
Keywords: Parkinson’s disease, mirror neuron system, action observation, response selection, interference
Introduction
In conflict tasks, such as the Eriksen flanker task and the Stroop task, patients with Parkinson’s disease (PD) demonstrate an enhanced susceptibility to interference, induced by simultaneous activation of conflicting motor responses (Henik, Singh, Beckley, & Rafal, 1993; Praamstra, Stegeman, Cools, & Horstink, 1998; Wylie, Stout, & Bashore, 2005). The present study investigates whether PD patients also have an increased sensitivity to interference from observed movements, which could be mediated by the mirror neuron system (Rizzolatti & Craighero, 2004).
The mirror neuron system is a collection of brain areas supporting the performance of voluntary actions, but also involved in the representation of the actions of others. Mirror neurons were first described in the ventral premotor cortex of non-human primates (Gallese, Fadiga, Fogassi, & Rizzolatti, 1996) but other brain regions (e.g., dorsal premotor cortex, inferior portions of the parietal lobe) have similar response characteristics (Buccino, Binkofski, Fink, et al., 2001; Cisek & Kalaska, 2004; Fogassi et al., 2005). Given the properties of the mirror neuron system, action observation may influence voluntary actions by setting up a conflict between observed and intended actions. In fact, measurable interference effects have been reported in healthy young subjects. The performance of repeated horizontal arm movements synchronized to an observed vertical arm movement has been found more variable than when the observed movements are in a plane congruent to the performed movements (Kilner, Paulignan, Blakemore, 2003; Stanley, Gowen, & Miall, 2007). This congruence effect depends on the provenance of the observed actions. It is maintained if the participants observe a moving dot that represents another’s hand movement, but not if they observe a dot controlled by computer (Stanley et al., 2007), or the moving arm of a robot (Kilner et al., 2003). This finding means that interference from observed movements, mediated by the mirror neuron system, is dependent on the movement being perceived as performed by a human agent. By capitalizing on this agency modulation, it should be possible to assess the integrity of the mirror neuron system, within an individual participant.
Perhaps also related to the mirror neuron system is an observation that sequential dependencies can be similar for performed and observed movements. For instance, hand movements towards a target are slowed down when that target appears at a location that was just visited, regardless of whether the first movement was actually performed by the actor or just observed (Welsh et al., 2005).
In traditional conflict tasks, interference effects largely arise from competition between the action associated with task-relevant information and prepotent response tendencies associated with distracters. Thus, in the well-known Stroop task, subjects have to name the colour in which a colour name is printed. Interference is induced by incongruent print colour and colour name and leads to slower and more error-prone naming responses compared to a congruent condition (MacLeod, 1991). In the flanker task a task-relevant letter instructing for one response is surrounded by task-irrelevant flanking letters that instruct for a different response or have no response assigned (Eriksen & Eriksen 1974). Interference in this task has been found to correlate with covert activation of the response associated with the flankers (Coles, Gratton, Bashore, Eriksen, & Donchin, 1985; Coles, Smid, Scheffers, & Otten, 1995). In accordance with the response competition account of interference effects in conflict tasks, enhanced susceptibility to interference in PD has been attributed to inadequate suppression of prepotent responses, indicative of a response selection deficit (Henik et al., 1993; Praamstra et al., 1998; Wylie et al., 2005, 2009; Seiss & Praamstra, 2004). Against this backdrop, the present study asked whether individuals with PD have an altered susceptibility to interference from observed actions onto their performed action.
Like traditional conflict tasks, action observation tasks might reveal increased susceptibility to interference in PD patients. Functional imaging studies have found action observation-related activity in fronto-parietal structures including the ventral premotor cortex and inferior parietal lobule (Buccino et al., 2001). In addition, covert activation of the primary motor cortex has been established by transcranial magnetic stimulation (Fadiga, Craighero, & Olivier, 2005). At each of these levels, observation-related neural activity might interfere with the generation of a voluntary movement, as a result of degraded selectivity or loss of segregation between different basal ganglia-thalamocortical circuits (cf. Pessiglione et al., 2005). Note that this scenario implicitly assumes functional integrity of the mirror neuron system. However, it cannot be ruled out that the pathology of Parkinson’s disease compromises the mirror neuron system itself, given that basal ganglia-thalamocortical loops include circuits passing through the ventral premotor cortex and the inferior parietal lobule (Hoover & Strick, 1993; Clower, Dum, & Strick, 2005). If the mirror neuron system itself is affected in PD, there may not be action observation-related activity potent enough to interfere with intended actions. Thus, normal or enhanced agency-modulated interference would suggest an intact mirror neuron system in individuals with PD, while reduced interference would suggest dysfunction of the mirror neuron system.
Methods
Participants
Ten patients with PD (age: 60 ± 9 yrs; mean ± SD) and ten neurologically unimpaired control participants (age: 60 ± 7 yrs) volunteered for this experiment. All participants provided written informed consent, and all procedures were approved by the South Birmingham Research Ethic Committee and comply with the principles outlined in the Declaration of Helsinki.
Individuals in the PD group were tested after a minimum of 12 hr medication withdrawal. Their UPDRS motor score tested off-medication was: 35 ± 4; mean ± SD. All participants were able to perform the task. Selected patients were predominantly akinetic-rigid with absent or minimal tremor. Tremor was present in only 3 patients and, typical for PD rest tremor, suppressed during movement. See Table 1 for further description of the PD group demographics.
Table 1. Demographic data of PD patients.
| Patient | Sex | Age (years) | Disease Duration (years) | Side of onset (Left/Right) | UPDRS (off-med) | Rest tremor | Medication |
|---|---|---|---|---|---|---|---|
| 1 | M | 67 | 8 | L | 42 | 0 | L-dopa 800 mg Entacapone 800 mg |
| 2 | M | 60 | 6 | R | 35 | 0 | L-dopa 800 mg |
| 3 | M | 64 | 9 | R | 33 | 0 | L-dopa 400 mg Entacapone 600 mg Ropinirole 21 mg Selegiline 10 mg |
| 4 | M | 50 | 6 | L | 35 | 0 | L-dopa 600 mg Pramipexole 1 mg |
| 5 | F | 64 | 4 | R | 38 | 1 | L-dopa 600 mg |
| 6 | M | 42 | 6 | R | 35 | 3 | L-dopa 750 mg Pramipexole 3 mg |
| 7 | M | 70 | 10 | L | 40 | 0 | L-dopa 1000 mg Entacapone 1000 mg Pramipexole 2.25 mg |
| 8 | M | 68 | 1 | R | 30 | 3 | None |
| 9 | M | 58 | 5 | L | 36 | 0 | L-dopa 450 mg Entacapone 600 mg Selegiline 10 mg |
| 10 | M | 58 | 4 | R | 29 | 0 | L-dopa 400 mg Selegiline 10 mg |
|
Mean (SD) |
60.1 (8.7) |
5.9 (2.6) |
35.3 (4.1) |
||||
UPDRS, Unified Parkinson’s Disease Rating Scale; SD, Standard Deviation; Med, Medication. Rest tremor refers to the UPDRS rest tremor score of the right hand. None of the patients had an action tremor.
Apparatus and Stimuli
Participants stood 1.8m away from a 60 × 60 cm projected image, centered 1.5m above the ground, on a white screen directly facing them. Stimuli were projected onto this screen using an Optoma EzPro 735 DLP projector connected to a PC using the Psychophysics Toolbox (Brainard, 1997; Pelli, 1997) in MATLAB. The stimulus was comprised of a video image of the movements of an actor making vertical or horizontal whole arm movements with the arm straight and with extended index finger of the right hand. During the recording of this video, the actor’s fingertip position was also captured with a motion tracker and processed off-line to create a moving dot stimulus, which had the same apparent kinematics as the actor’s movements. Stimulus movements (i.e. fingertip or dot) spanned approx. 50cm. The image of the actor was presented at his actual size and elevation; the dot motion had the same spatial parameters (amplitude, speed, position on screen). The dot and the finger tip subtended approximately 0.3 degrees at the participant’s eye. Each cycle of the action took 1 second, corresponding to a movement speed of ~ 1 m/s. The relatively slow movement speed helped to ensure that bradykinesia did not adversely affect the performance of patients. This was motivated by the consideration that significant delays between observed and executed movements, in the PD group, would undermine the comparison of congruency effects between the groups. Conceivably, a significant phase lag between observed and executed movements would dilute the interference effect.
Throughout each trial, the participant’s index finger position was recorded with a single sensor FASTRAK electromagnetic motion tracking system (Polhemus Inc., Burlington, VT). Sensor position was recorded at 120 Hz with about 1 mm spatial resolution. Only data from the horizontal x-dimension and the vertical z-dimension were used in the analysis, ignoring anterior-posterior motion in the y-dimension that mainly reflected the arc of the hand around the shoulder. The plane of instructed movement is referred to as dominant plane; the orthogonal plane as the error plane. The FASTRAK transmitter was located 90cm directly in front of the participant, 80cm above the ground.
Procedure
Each participant performed horizontal or vertical movements with their right arm, in phase with the stimulus displayed on the video screen. In one condition (congruent condition) participants performed the same movements as they observed (e.g. both horizontal or both vertical). In a second condition (incongruent condition) they made movements in the plane perpendicular to the observed movements (e.g., horizontal motion while observing vertical motion, or vice versa).
Each trial began with a verbal instruction which was followed by a visual presentation of an arrow to indicate both the starting position in which the participant should hold their arm, and the initial movement direction for the forthcoming trial. The arrow always pointed to the right (on horizontal trials) or upwards (on vertical trials) and was presented at the center of the screen. After the preparatory arrow stimulus was presented for 3s, the video stimulus (either human or dot) was presented for 30s.
The experiment started after the performance of four instructional trials. During these instruction trials, participants were given verbal feedback about the size (too large or too small) and their speed of movement (too fast or too slow). These trials included two vertical movements and two horizontal movements, all made during the presentation of the congruent visual stimuli (two dots trials and two human trials). Each participant then completed 40 trials during the testing session which took about one hour. Participants took breaks after every eight trials, with additional breaks provided upon request. The experiment included five repetitions of each of eight conditions and trial order was counterbalanced across participants.
Analysis
Fingertip position data were filtered and segmented into single movement segments (e.g. a movement from extreme left to extreme right made up one segment and the return movement another segment). The extremes of each movement segment were eliminated from the analysis by restricting the analysis to the part where movement velocity was greater than 5% of the peak velocity. To quantify interference, the standard deviation of fingertip position within the error axis for each movement was calculated. A single within-subject mean score for each condition was calculated across all trials of that condition. These values were analyzed using a 2 × 2 × 2 × 2 (Group × Congruence × Stimulus × Direction) repeated-measures analysis of variance, with group serving as the sole between subjects factor.
Results
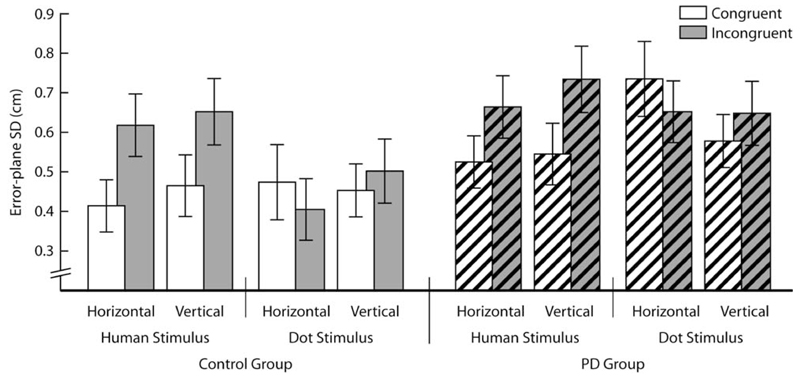
Movement accuracy was significantly influenced by the congruence of the observed movement with respect to the performed action (F(1,18)=12.8, p<0.01, η2=0.42; Figures 1 and 2). Specifically, the standard deviation of movement in the error plane was greater for the incongruent condition than for the congruent condition. As seen previously, this effect was modulated by the type of the stimulus, with a stronger congruence effect for the human actor than for moving dots (F(1,18)=12.0, p<0.01, η2=0.40). The congruence effect was also slightly stronger for horizontal than vertical movements (F(1,18)=5.3, p<0.05, η2=0.23). These results are in line with previous work and confirm that our stimuli and test protocol were effective in eliciting interference from observed movement.
Figure 1.
Error-plane variability in each task. The variability of actions increased when they were performed while observing movements in an orthogonal direction. This effect was greater for observation of human movements than for observation of moving dots, despite the portrayal of equivalent kinematics in both stimuli. While PD patients (hashed bars) were more variable overall, no interaction involving group approached significance.
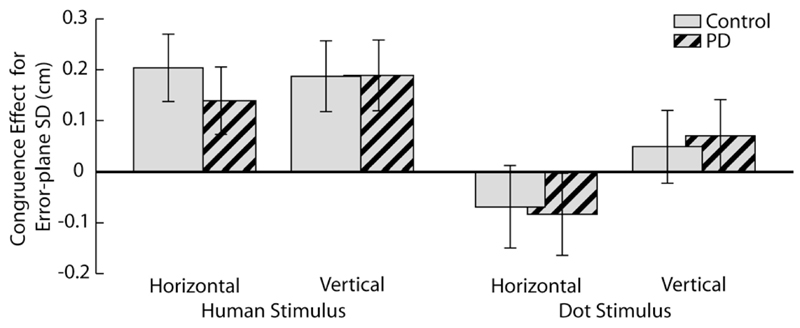
Figure 2.
Congruence effect in error-plane variability in each task. The variability of actions was typically increased when they were performed while observing movements in an orthogonal direction (indicated by bars being above zero). This effect was significantly greater than zero when observing a human actor, but was not different from zero when observing a moving dot, despite the portrayal of equivalent kinematics in both stimuli. These effects were present to a similar extent in the control and PD groups.
PD patients tended to have greater standard deviations for movement in the error axis than controls, as expressed in a borderline significant effect of Group (F(1,18)=4.1, p=0.058, η2=0.19). Crucially, this was not specific to the incongruent condition, as evidenced by the Congruence × Group interaction which did not approach significance (F(1,18)=0.09, η2=0.01). Neither were there other significant interactions involving Group.
The presence of an interference effect modulated by agency (human action vs. moving dot) is critical for an interpretation of action observation interference effects as mediated by the mirror neuron system. Note that we interpret a normal or enhanced interference effect from observed movement in PD as evidence for an intact mirror neuron system (see Introduction). In order for this conclusion not to rely too heavily on a statistical null result (i.e., the lack of a difference between the groups), we ensured that a subtle group difference did not fall victim to type II error. Thus, we evaluated whether the modulation of the congruency effect by stimulus agency, seen across both groups, was also significant within the PD group alone. This repeated measures ANOVA confirmed that interference was greater when observing human movement than when observing dot movement (Congruence × Stimulus interaction F(1,9)=5.4, p<0.05, η2=0.34). Hence our methods are sensitive to agency-modulated congruency effects, but do not show a difference between the PD and control group.
Discussion
The findings of this investigation are relevant, first, to the pathophysiology of PD patients’ susceptibility to interference and, second, to the question whether the mirror neuron system may be affected in PD. We discuss both in turn.
The now well-established vulnerability of PD patients to interference in traditional conflict tasks is commonly explained in terms of a response selection deficit (Seiss & Praamstra, 2004; Wylie et al., 2005, 2009), based on the view that the opponent action of direct and indirect striato-pallidal projections implements a mechanism for the selection and suppression of competing actions (Mink, 1996). For the present study, we hypothesized that the mirror neuron system might mediate similarly enhanced interference effects driven by competition between neural activation resulting from action observation and voluntary response activation, both represented within the motor loop of the basal ganglia-thalamocortical circuitry. The results showed neither enhanced nor reduced interference. The normal magnitude of interference effects cannot be attributed to small group size or patients not being sufficiently affected. In fact, patients showed marked impairment on the UPDRS motor score and demonstrated a borderline significant increase of movement variability in the error plane across the board. Hence the normal size interference effects are likely due to the nature of the task. In traditional conflict tasks, the information associated with competing responses is presented very briefly, while selection takes place under time pressure, biased to induce initial capture of the incorrect response. In our task, by contrast, observed movements were presented for the entire duration of a trial, guiding ongoing movement in their timing if not in their direction. This interpretation indicates that the sensitivity to interference in PD selectively affects processes involved in the selection of movements rather than those underlying the guidance of ongoing movement, which converges with existing evidence for preserved on-line movement guidance in PD (Desmurget et al., 2004; Vaillancourt, Slifkin, & Newell, 2001).
We turn now to the second question concerning the integrity of the mirror neuron system in PD. A recent investigation used transcranial magnetic stimulation (TMS) to probe motor cortical excitability during movement observation (Tremblay, Leonard, & Tremblay, 2008), demonstrating an attenuated modulation in PD patients compared to controls. Our data, by contrast, suggest that movement observation engages an essentially intact mirror neuron system. A significantly affected mirror neuron system would have compromised the representation of observed movement in the observer’s motor system, thus reducing the potential for interference with voluntary movements. In fact, PD patients exhibited a normal interference effect between observed and executed actions, with greater variability of their actions when observing an incongruent stimulus. Crucially, like the control group, the PD group was more susceptible to interference when observing a human actor than when viewing the moving dot stimuli. Taken together, the normal magnitude and agency-modulated congruence effects provide strong evidence that the PD patients we tested had an intact mirror neuron system. The difference with the study by Tremblay and co-workers may be related to the fact that in our investigation participants needed information from the observed movement to execute the movements required by the task. This interpretation is in line with PD patients’ normal modulation of motor cortical excitability during action imitation (Tremblay et al., 2008).
In summary, the present data demonstrate normal performance of PD patients in a well-established movement observation interference task with congruency effects of normal magnitude and normal modulation of the effect by agency. The modulation of the congruency effect by agency provides relatively strong support for the assumption that the interference effect was mediated by the mirror neuron system, although this is not directly demonstrated. The results do not rule out that movements, whether of human or non-human agency, are still a potentially important source of interference with voluntary movements in Parkinson’s disease. Sudden movements have a strong tendency to grab attention and interrupt ongoing action, which might be relevant (e.g., contribute to fall risks) in less controlled environments than the movement laboratory.
Acknowledgements
The authors acknowledge the financial support of the Wellcome Trust.
References
- Brainard DH. The Psychophysics Toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, et al. Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. European Journal of Neuroscience. 2001;13:400–404. [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Neural correlates of mental rehearsal in dorsal premotor cortex. Nature. 2004;431:993–996. doi: 10.1038/nature03005. [DOI] [PubMed] [Google Scholar]
- Clower DM, Dum RP, Strick PL. Basal ganglia and cerebellar inputs to ‘AIP’. Cerebral Cortex. 2005;15:913–20. doi: 10.1093/cercor/bhh190. [DOI] [PubMed] [Google Scholar]
- Coles MG, Gratton G, Bashore TR, Eriksen CW, Donchin E. A psychophysiological investigation of the continuous flow model of human information processing. Journal of Experimental Psychology: Human Perception and Performance. 1985;11:529–553. doi: 10.1037//0096-1523.11.5.529. [DOI] [PubMed] [Google Scholar]
- Coles MGH, Smid HGOM, Scheffers MK, Otten LJ. Mental chronometry and the study of human information processing. In: Rugg MD, Coles MGH, editors. Electrophysiology of mind: event related brain potentials and cognition. Oxford: Oxford University Press; 1995. pp. 86–131. [Google Scholar]
- Desmurget M, Gaveau V, Vindras P, Turner RS, Broussolle E, Thobois S. On-line motor control in patients with Parkinson’s disease. Brain. 2004;127:1755–73. doi: 10.1093/brain/awh206. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon identification of a target letter in a nonsearch task. Perception & Psychophysics. 1974;16:143–149. [Google Scholar]
- Fadiga L, Craighero L, Olivier E. Human motor cortex excitability during the perception of others’ action. Current Opinion in Neurobiology. 2005;15:213–8. doi: 10.1016/j.conb.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersi F, Rizzolatti G. Parietal lobe: From action organization to intention understanding. Science. 2005;308:662–667. doi: 10.1126/science.1106138. [DOI] [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119:593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- Henik A, Singh J, Beckley DJ, Rafal RD. Disinhibition of automatic word reading in Parkinson’s disease. Cortex. 1993;29:589–599. doi: 10.1016/s0010-9452(13)80283-3. [DOI] [PubMed] [Google Scholar]
- Hoover JE, Strick PL. Multiple output channels in the basal ganglia. Science. 1993;259:819–21. doi: 10.1126/science.7679223. [DOI] [PubMed] [Google Scholar]
- Kilner JM, Paulignan Y, Blakemore SJ. An interference effect of observed biological movement on action. Current Biology. 2003;13:522–525. doi: 10.1016/s0960-9822(03)00165-9. [DOI] [PubMed] [Google Scholar]
- MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychological Bulletin. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Progress in Neurobiology. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spatial Vision. 1997;10:437–442. [PubMed] [Google Scholar]
- Pessiglione M, Czernecki V, Pillon B, Dubois B, Schüpbach M, Agid Y, Tremblay L. An effect of dopamine depletion on decision-making: the temporal coupling of deliberation and execution. Journal of Cognitive Neuroscience. 2005;17:1886–96. doi: 10.1162/089892905775008661. [DOI] [PubMed] [Google Scholar]
- Praamstra P, Stegeman DF, Cools AR, Horstink MWIM. Reliance on external cues for movement initiation in Parkinson’s disease - Evidence from movement-related potentials. Brain. 1998;121:167–177. doi: 10.1093/brain/121.1.167. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annual Review of Neuroscience. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Seiss E, Praamstra P. The basal ganglia and inhibitory mechanisms in response selection: evidence from subliminal priming of motor responses in Parkinson’s disease. Brain. 2004;127:330–339. doi: 10.1093/brain/awh043. [DOI] [PubMed] [Google Scholar]
- Stanley J, Gowen E, Miall RC. Effects of agency on movement interference during observation of a moving dot stimulus. Journal of Experimental Psychology: Human Perception and Performance. 2007;33:915–926. doi: 10.1037/0096-1523.33.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay F, Leonard G, Tremblay L. Corticomotor facilitation associated with observation and imagery of hand actions is impaired in Parkinson’s disease. Experimental Brain Research. 2008;185:249–257. doi: 10.1007/s00221-007-1150-6. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Slifkin AB, Newell KM. Visual control of isometric force in Parkinson’s disease. Neuropsychologia. 2001;39:1410–1418. doi: 10.1016/s0028-3932(01)00061-6. [DOI] [PubMed] [Google Scholar]
- Welsh TN, Elliott D, Anson JG, Dhillon V, Weeks DJ, Lyons JL, Chua R. Does Joe influence Fred’s action? Inhibition of return across different nervous systems. Neuroscience Letters. 2005;385:99–104. doi: 10.1016/j.neulet.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Wylie SA, Stout JC, Bashore TR. Activation of conflicting responses in Parkinson’s disease: evidence for degrading and facilitating effects on response time. Neuropsychologia. 2005;43:1033–1043. doi: 10.1016/j.neuropsychologia.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Wylie SA, van den Wildenberg WP, Ridderinkhof KR, Bashore TR, Powel VD, et al. The effect of Parkinson’s disease on interference control during action selection. Neuropsychologia. 2009;47:145–157. doi: 10.1016/j.neuropsychologia.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]