SUMMARY
We previously suggested that the discrepancy between the critical cerebral perfusion pressures (CPP) of 30 mmHg, obtained by increasing intracranial pressure (ICP), and 60 mmHg, obtained by decreasing arterial pressure, was due to pathological microvascular shunting at high ICP [1] and that the determination of the critical CPP by the static cerebral blood flow (CBF) autoregulation curve is not valid with intracranial hypertension. Here we demonstrated that critical CPP, measured by induced dynamic ICP reactivity (iPRx) and cerebrovascular reactivity (CVRx), accurately identifies the critical CPP in the hypertensive rat brain which differs from that obtained by the static autoregulation curve. Step changes in CPP from 70 to 50 and 30 mmHg were made by increasing ICP using an artificial cerebrospinal fluid reservoir connected to the cisterna magna. At each CPP, a transient 10-mmHg rise in arterial pressure was induced by bolus i.v. dopamine. iPRx and iCVRx were calculated as ΔICP/ΔMAP and as ΔCBF/ΔMAP, respectively. The critical CPP at high ICP, obtained by iPRx and iCVRx, is 50 mmHg, where compromised capillary flow, transition of blood flow to non-nutritive microvascular shunts, tissue hypoxia and BBB leakage begin to occur, which is higher than the 30 mmHg determined by static autoregulation.
Keywords: cerebral perfusion pressure (CPP), intracranial pressure (ICP), cerebral blood flow (CBF), CBF autoregulation, microvascular shunts (MVS), NADH, blood brain barrier (BBB), induced intracranial pressure reactivity (iPRx), induced cerebrovascular reactivity (iCVRx), rats
INTRODUCTION
Cerebrovascular autoregulation is the ability of the brain to maintain cerebral blood flow (CBF) constant with changes in cerebral perfusion pressure (CPP). The loss of autoregulation after severe cerebral insults is associated with the development of secondary brain injuries [2,3,4] frequently resulting in high intracranial pressure (ICP) which is one of the most serious secondary insults occurring after brain injury. Accurate determination of the critical CPP, i.e. a lowest CPP at which autoregulation is maintained, is important in the clinical management of high ICP. Historically, a critical CPP of 60 mmHg was determined by static autoregulation curves i.e., decreasing arterial pressure to lower CPP [5,6]. However, studies on different animal models showed that the critical CPP falls from 60 to 30 mmHg when CPP was manipulated by increasing ICP instead of decreasing arterial pressure [7,8,9,10]. The reason for this difference remained unexplained until we showed that the decrease in critical CPP was due to microvascular shunt (MVS) flow which occurred with high ICP but not when arterial pressure was used to lower CPP [1]. The increase in MVS was accompanied by tissue hypoxia, brain edema and BBB damage which began at a CPP of 50 mmHg, not 30 mmHg. Thus, static CBF autoregulation failed to identify the critical CPP at high ICP.
An alternative concept in assessing cerebral autoregulation is that of measuring of dynamic cerebrovascular reactivity (iCVRx) by induction of cerebrovascular response to a vasodilatory stimulus such as CO2, [11,12] and acetazolamide (Diamox) [13,14,15] or to a transient change in arterial pressure [4,16]. No or low cerebrovascular response indicates intact autoregulation and a large cerebrovascular response, reflected by a large iCVRx increase, indicates loss of autoregulation. A similar measurement can be obtained by the dynamic ICP response to an arterial pressure challenge which is induced ICP reactivity (iPRx) [2,3,17]. Both the iCVRx and iPRx responses are complex reflecting the status of cerebrovascular dilatation and compliance of the intracranial compartment, respectively. As with iCVRx, no or low response in intracranial pressure indicates intact autoregulation and a large response in ICP, reflected by increase of iPRx, indicates loss of autoregulation. Based on our earlier study showing that the historic method of evaluation of CBF autoregulation may not apply to the brain at high ICP, our aim was to determine whether iCVRx and iPRx more accurately identifies the critical CPP in the brain at high ICP.
MATERIAL AND METHODS
The animal protocol was approved by the Institutional Animal Care and Use Committee of the University of New Mexico Health Sciences Center and done in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
Surgery
The procedures used in this study have been previously described [1,18]. Briefly, acclimated male Sprague-Dawley rats (10 rats, Harlan Laboratories, Indianapolis, IN) weighing between 300-350 g were intubated and mechanically ventilated on 2% isoflurane / 30% oxygen / 70% nitrous oxide. Femoral venous and arterial catheters were inserted. Step changes in CPP from 70 to 50 and 30 mmHg were made by increasing ICP by vertical positioning of an artificial cerebrospinal fluid reservoir connected to the cisterna magna. The time spent at each level was 30 min, which was sufficient for stabilization of physiological variables and to make microvascular and physiological measurements.
Two Photon Laser Scanning Microscopy (2PLSM)
Using in-vivo 2PLSM through a cranial window over the rat parietal cortex we measured microvascular red blood cell flow (RBC) velocity and diameters (tetramethylrhodamine dextran), NADH autofluorescence (tissue oxygenation) and blood brain barrier (BBB) integrity by tetramethylrhodamine dextran extravasation. For 2PLSM, an Olympus BX51WI upright microscope and water-immersion LUMPlan FL/IR 20x/0.50W objective was used. Excitation (740 nm) was provided by a Prairie View Ultima multiphoton laser scan unit powered by a Millennia Prime 10 W diode laser source pumping a Tsunami Ti: sapphire laser (Spectra-Physics, Mountain View, CA). Blood plasma was labeled by i.v. injection of tetramethylrhodamine isothiocyanate dextran (155 kDa) in physiological saline (5% wt/vol). All microvessels in an imaging volume (500X500X300 μm) were scanned at each CPP, measuring the diameter and blood flow velocity in each vessel (3-20 μm Ø). Cortical Doppler flux (probe Ø = 0.8 mm), rectal and cranial temperatures, ICP, arterial pressure and arterial blood gases were monitored.
Cerebrovascular autoregulation
At each CPP, a transient 10-mmHg rise in mean arterial pressure (MAP) was induced by i.v. dopamine bolus. Induced intracranial pressure reactivity (iPRx) was calculated as the ratio of the change in ICP in response to a 10-mmHg MAP increase (iPRx=ΔICP/ΔMAP). Induced cerebrovascular reactivity (iCVRx) was defined as a ratio of the change in CBF with a 10-mmHg MAP change (iCVRx=ΔCBF/ΔMAP). Static CBF autoregulation curves were also assessed using cortical Doppler flux. Statistical analyses were done by Student's t-test or Kolgomorov-Smirnov test where appropriate. Significance level was preset to P<0.05. Data are presented as mean ± SEM.
RESULTS
In-vivo two-photon laser scanning microscopy
Arterial pressure; blood gases, electrolytes, hematocrit and pH; rectal and cranial temperatures were monitored and maintained within normal limits throughout the studies.
Cerebral microvascular flow
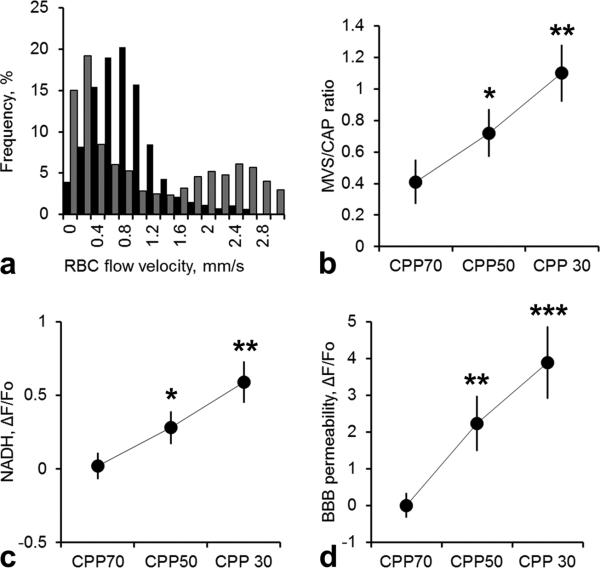
At a normal CPP of 70mmHg, microvascular RBC flow velocity in microvessels ranged from 0.14 to 3.15 mm/sec with a normal frequency distribution (Figure 1 a). Reduction of CPP by progressive increase in ICP caused a decrease in capillary flow (diameter of 3-8 μm and velocities <1mm/sec) and a transition from capillary to high-velocity non-nutritive microvascular shunt flow (MVS, 8-20 μm and velocities >1mm/sec), consistent with our previous report [19]. The proportion of MVS and capillary flow, expressed as the MVS/CAP ratio, showed that the transition from capillary flow to MVS flow begins to occur at CPP of 50 mmHg compared to baseline at CPP of 70 mmHg (Table 1, Figure 1 b, 0.72±0.15 vs. 0.41±0.14, respectively, n=10, p<0.05). The frequency distribution of microvessels flow velocities at a CPP of 50 mmHg became bimodal, reflecting compromised low-velocity capillary flow and high-velocity MVS flow (Figure 1 a).
Figure 1.
a) Normalized frequency histograms showing normal microvascular red blood cell flow (RBC) velocity distribution at CPP of 70 mmHg (■) and at CPP of 30 mmHg (■). Decrease of CPP by increasing ICP resulted in redistribution of microvascular flow: capillary flow became very slow while higher flow velocity microvascular shunt flow appeared suggesting a shift from capillaries to higher flow velocity and larger MVS. The vertical dashed line demarcates a velocity of 1.0mm/sec. b) Changes in microvascular shunt/capillary flow (MVS/CAP) ratio showing that decrease of CPP by increasing ICP resulted in the transition to MVS flow. c) Graph shows progression of tissue hypoxia reflected by NADH autofluorescence increase during reduction of CPP by ICP elevation. Data a presented as ΔF/Fo, where Fo is NADH at CPP = 70 mmHg. d) Graph illustrates the average of tetramethylrhodamine fluorescence in brain tissue (extravasation) reflecting progression of BBB degradation during reduction of CPP by ICP elevation. Data a presented as ΔF/Fo, where Fo is fluorescence at CPP = 70 mmHg. All data are presented as Mean±SEM, n=10,*=p<0.05, **=p<0.01, ***=p<0.01.
Table 1.
Monitored variables
| ICP, MAP and CPP, mmHg | Cortical Doppler flux, % of baseline | iPRx | iCVRx | MVS/CAP ratio | NADH, ΔF/Fo | BBB damage, ΔF/Fo |
|---|---|---|---|---|---|---|
| ICP 10 / MAP 80 / CPP 70 | 100.1 ± 9.3 | −0.03 ± 0.07 | −0.02 ± 0.09 | 0.41 ± 0.14 | 0.02 ± 0.09 | 0.01 ± 0.34 |
| ICP 30 / MAP 80 / CPP 50 | 98.3 ± 9.4 | 0.24 ± 0.09* | 0.31 ± 0.13* | 0.72 ± 0.15* | 0.28 ± 0.11* | 2.24 ± 075** |
| ICP 50 / MAP 80 / CPP 30 | 71.2 ± 10.5* | 0.33 ± 0.11** | 0.46 ± 0.14** | 1.1 ± 0.18** | 0.59 ± 0.14** | 3.89 ± 0.98*** |
ICP, intracranial pressure; MAP, mean arterial pressure; CPP, cerebral perfusion pressure; iPRx, induced intracranial pressure reactivity; iCVRx, induced cerebrovascular reactivity; MVS/CAP, microvascular shunt flow/capillary flow ratio; BBB, blood brain barrier. N= 10 rats, Mean ± SEM
=p<0.05
=p<0.01
=p<0.001. Data are compared with baseline CPP of 70 mmHg.
NADH
The decrease of CPP by elevation of ICP resulted in a marked rise in NADH autofluorescence to 0.28±0.11, P<0.05 at a CPP of 50 mmHg which increased further to 0.59±0.14, P<0.01 at a CPP of 30 mmHg from a baseline level at a CPP of 70 mmHg (ΔF/FO [CPP = 70 mmHg], Table 1, Figure 1 c). NADH is a sensitive indicator of tissue hypoxia. [19,20] suggesting that our results show the development of tissue hypoxia at a CPP of 50 mmHg.
Blood Brain Barrier Permeability (BBB)
A decrease in CPP by an increase in ICP resulted in an increase in BBB permeability as reflected by transcapillary dye extravasation and increased fluorescence of dye in the brain parenchyma. Average dye fluorescence in brain parenchyma, reflecting opening of the BBB, progressively increased to 2.24±0.75, P<0.01 and 3.89.1 ± 0.98, P<0.001, at CPP of 50 and 30 mmHg, respectively, compared to a baseline level at CPP of 70 mmHg (ΔF/FO [CPP = 70 mmHg], Table 1, Figure 1 d).
Cerebrovascular autoregulation assessment
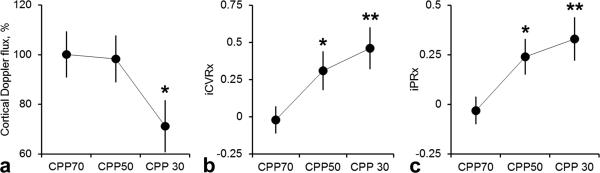
Static CBF autoregulation curves, obtained by the cortical surface Doppler probe with an increase in ICP, indicated that the loss of autoregulation occurred at CPP of 30 mmHg (Table 1, Figure 2 a, 71.2 ± 10.5% from baseline, P<0.05). At CPP of 50 mmHg, cortical Doppler flux was not different from baseline CPP of 70 mmHg (98.3±9.4 vs. 100.1±9.3, respectively) which contradicts physiological the 2PLSM data, where detrimental effects begin to occur at CPP of 50 mmHg (Table 1, Figure 1 b-c).
Figure 2.
a) Static autoregulation curve of cortical Doppler flux show preserved autoregulation with an increase of ICP at CPP 70 and 50 mmHg and impaired autoregulation at 30 mmHg. b) Negative values of induced cerebrovascular reactivity (iCVRx) at physiological CPP of 70 mmHg indicated normal cerebrovascular reactivity. After ICP elevation, a rise in iCVRx indicated impairment of cerebrovascular reactivity at CPP of 50 mmHg. c) At normal CPP of 70 mmHg, induced intracranial pressure reactivity (iPRx) has zero or negative values, indicating preserved intracranial pressure reactivity. In contrast, when CPP was decreased by ICP increase, an increase in PRx indicated impaired intracranial pressure reactivity at CPP of 50 mmHg. All data are presented as Mean±SEM, n=10,*=p<0.05, **=p<0.01.
Dynamic iCVRx
At a normal CPP of 70 mmHg, the acute transient arterial pressure challenge of 10 mmHg caused either a decrease or no change in CBF (CVRx = −0.02 ± 0.05), reflecting intact cerebrovascular reactivity (Table 1, Figure 2 b). When CPP was decreased to 50 and 30 mmHg by ICP elevation, arterial pressure challenge induced transient rise of CBF, reflected by significant increase in iCVRx to 0.11±0.13, p<0.05 and 0.26±0.14, p<0.01, respectively (Table 1, Figure 2 b).
Dynamic iPRx
At a normal CPP of 70 mmHg, arterial pressure challenge induced no change in ICP (iPRx=−0.03±0.07), reflecting intact intracranial pressure reactivity (Table 1, Figure 2 c). When CPP was decreased to 50 and 30 mmHg by ICP elevation, arterial pressure challenge caused transient increase of ICP, reflected by significant iPRx rise to 0.09±0.16, p<0.05 and 0.26±0.14, p<0.01, respectively (Table 1, Figure 2 c). Thus, both, iPRx and iCVRx reflect impaired cerebrovascular autoregulation beginning at a CPP of 50 mmHg in the brain at high ICP.
DISCUSSION
The determination of critical CPP using static CBF autoregulation by passively decreasing arterial pressure is inaccurate in the brain at high ICP likely due to microvascular shunting as we have suggested. The Doppler probe averages high velocity non-nutritive microvascular shunt flow and low velocity compromised capillary flow over a larger tissue volume and therefore is unable to detect a bimodal microvascular flow distribution at high ICP. This observation also applies to the injured brain with intracranial hypertension, where we have shown MVS flow [21] and possibly in other cerebrovascular accidents where MVS flow might appear due to an increase in ICP.
The dynamic CBF autoregulation test, utilizing the induced reactivity of intracranial pressure (iPRx) and cerebral microvasculature (iCVRx) to the transient 10 mmHg rise of arterial pressure, is a fast and reliable method for assessing critical CPP in hypertensive brain. The critical CPP of 50 mmHg, obtained by dynamic iPRx and iCVRx, is in agreement with concurrently acquired in-vivo 2PLSM data showing that compromised capillary flow, transition of blood flow to nonnutritive microvascular shunts, tissue hypoxia and BBB leakage begin to occur at CPP of 50 mmHg. High ICP-induced increase of cortical water content, reported in our earlier study [1], was also observed at CPP of 50 mmHg. Thus, the results of our study show that in the brain at high ICP, the critical CPP of 30 mmHg reported by the static CBF autoregulation curve is erroneous.
The dynamic iPRx described here is similar to the PRx thoroughly described by Czosnyka et al where spontaneous changes in MAP are correlated with ICP in a moving average to manage the patients at an optimal CPP which appears to improve outcome [22,23,24]. However, there is a caveat in the interpretation of the PRx and CVRx concept in that the failing brain will also show reduced PRx or CVRx or iPRx or iCVRx, which could be misleading.
In conclusion, the static CBF autoregulation curve does not accurately identify the critical CPP in the brain at high ICP which can be determined by dynamic iPRx and iCVRx.
ACKNOWLEDGEMENTS
The work was supported by the National Institutes of Health: NS061216 and CoBRE 8P30GM103400, Dedicated Health Research Funds from the University of New Mexico School of Medicine and the American Heart Association grant 12BGIA11730011.
Footnotes
CONFLICT OF INTEREST STATEMENT We declare that we have no conflict of interest.
REFERENCES
- 1.Bragin DE, Bush RC, Muller WS, Nemoto EM. High intracranial pressure effects on cerebral cortical microvascular flow in rats. J Neurotrauma. 2011;28:775–785. doi: 10.1089/neu.2010.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aries MJ, Czosnyka M, Budohoski KP, Kolias AG, Radolovich DK, et al. Continuous Monitoring of Cerebrovascular Reactivity Using Pulse Waveform of Intracranial Pressure. Neurocrit Care. 2012 doi: 10.1007/s12028-012-9687-z. [DOI] [PubMed] [Google Scholar]
- 3.Hlatky R, Valadka AB, Robertson CS. Analysis of dynamic autoregulation assessed by the cuff deflation method. Neurocrit Care. 2006;4:127–132. doi: 10.1385/NCC:4:2:127. [DOI] [PubMed] [Google Scholar]
- 4.Panerai RB. Assessment of cerebral pressure autoregulation in humans--a review of measurement methods. Physiol Meas. 1998;19:305–338. doi: 10.1088/0967-3334/19/3/001. [DOI] [PubMed] [Google Scholar]
- 5.Harper AM. The inter-relationship between aPco-2 and blood pressure in the regulation of blood flow through the cerebral cortex. Acta Neurol Scand Suppl. 1965;14:94–103. doi: 10.1111/j.1600-0404.1965.tb01964.x. [DOI] [PubMed] [Google Scholar]
- 6.Rapela CE, Green HD. Autoregulation of Canine Cerebral Blood Flow. Circ Res. 1964;15(SUPPL):205–212. [PubMed] [Google Scholar]
- 7.Grubb RL, Jr., Raichle ME, Phelps ME, Ratcheson RA. Effects of increased intracranial pressure on cerebral blood volume, blood flow, and oxygen utilization in monkeys. J Neurosurg. 1975;43:385–398. doi: 10.3171/jns.1975.43.4.0385. [DOI] [PubMed] [Google Scholar]
- 8.Hauerberg J, Juhler M. Cerebral blood flow autoregulation in acute intracranial hypertension. J Cereb Blood Flow Metab. 1994;14:519–525. doi: 10.1038/jcbfm.1994.64. [DOI] [PubMed] [Google Scholar]
- 9.Johnston IH, Rowan JO, Harper AM, Jennett WB. Raised intracranial pressure and cerebral blood flow. I. Cisterna magna infusion in primates. J Neurol Neurosurg Psychiatry. 1972;35:285–296. doi: 10.1136/jnnp.35.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller JD, Stanek A, Langfitt TW. Concepts of cerebral perfusion pressure and vascular compression during intracranial hypertension. Prog Brain Res. 1972;35:411–432. doi: 10.1016/S0079-6123(08)60102-8. [DOI] [PubMed] [Google Scholar]
- 11.Lepur D, Kutlesa M, Barsic B. Prospective observational cohort study of cerebrovascular CO2 reactivity in patients with inflammatory CNS diseases. Eur J Clin Microbiol Infect Dis. 2011;30:989–996. doi: 10.1007/s10096-011-1184-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spano VR, Mandell DM, Poublanc J, Sam K, Battisti-Charbonney A, et al. CO2 blood oxygen level-dependent MR mapping of cerebrovascular reserve in a clinical population: safety, tolerability, and technical feasibility. Radiology. 2013;266:592–598. doi: 10.1148/radiol.12112795. [DOI] [PubMed] [Google Scholar]
- 13.Nemoto EM, Yonas H, Pindzola RR, Kuwabara H, Sashin D, et al. PET OEF reactivity for hemodynamic compromise in occlusive vascular disease. J Neuroimaging. 2007;17:54–60. doi: 10.1111/j.1552-6569.2006.00080.x. [DOI] [PubMed] [Google Scholar]
- 14.Przybylski GJ, Yonas H, Smith HA. Reduced stroke risk in patients with compromised cerebral blood flow reactivity treated with superficial temporal artery to distal middle cerebral artery bypass surgery. J Stroke Cerebrovasc Dis. 1998;7:302–309. doi: 10.1016/s1052-3057(98)80047-5. [DOI] [PubMed] [Google Scholar]
- 15.Uchino K, Lin R, Zaidi SF, Kuwabara H, Sashin D, et al. Increased Cerebral Oxygen Metabolism and Ischemic Stress in Subjects with Metabolic Syndrome-Associated Risk Factors: Preliminary Observations. Transl Stroke Res. 2010;1:178–183. doi: 10.1007/s12975-010-0028-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenthal G, Sanchez-Mejia RO, Phan N, Hemphill JC, 3rd, Martin C, et al. Incorporating a parenchymal thermal diffusion cerebral blood flow probe in bedside assessment of cerebral autoregulation and vasoreactivity in patients with severe traumatic brain injury. J Neurosurg. 2011;114:62–70. doi: 10.3171/2010.6.JNS091360. [DOI] [PubMed] [Google Scholar]
- 17.Budohoski KP, Czosnyka M, Smielewski P, Varsos GV, Kasprowicz M, et al. Cerebral autoregulation after subarachnoid hemorrhage: comparison of three methods. J Cereb Blood Flow Metab. 2013;33:449–456. doi: 10.1038/jcbfm.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bragin DE, Bush RC, Nemoto EM. Effect of cerebral perfusion pressure on cerebral cortical microvascular shunting at high intracranial pressure in rats. Stroke. 2013;44:177–181. doi: 10.1161/STROKEAHA.112.668293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chance B, Cohen P, Jobsis F, Schoener B. Intracellular oxidation-reduction states in vivo. Science. 1962;137:499–508. doi: 10.1126/science.137.3529.499. [DOI] [PubMed] [Google Scholar]
- 20.Takano T, Tian GF, Peng W, Lou N, Lovatt D, et al. Cortical spreading depression causes and coincides with tissue hypoxia. Nat Neurosci. 2007;10:754–762. doi: 10.1038/nn1902. [DOI] [PubMed] [Google Scholar]
- 21.Bragin DE, Statom G, Nemoto EM. Microvascular Shunt Flow after Traumatic Brain Injury with Intracranial Hypertension in Rats. Journal of Neurotrauma. 2012;29:A–22. [Google Scholar]
- 22.Czosnyka M, Hutchinson PJ, Balestreri M, Hiler M, Smielewski P, et al. Monitoring and interpretation of intracranial pressure after head injury. Acta Neurochir Suppl. 2006;96:114–118. doi: 10.1007/3-211-30714-1_26. [DOI] [PubMed] [Google Scholar]
- 23.Czosnyka M, Pickard JD. Monitoring and interpretation of intracranial pressure. J Neurol Neurosurg Psychiatry. 2004;75:813–821. doi: 10.1136/jnnp.2003.033126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Czosnyka M, Smielewski P, Kirkpatrick P, Laing RJ, Menon D, et al. Continuous assessment of the cerebral vasomotor reactivity in head injury. Neurosurgery. 1997;41:11–17. doi: 10.1097/00006123-199707000-00005. discussion 17-19. [DOI] [PubMed] [Google Scholar]