ABSTRACT
Vaccines and extended vaccination programs have had an extensive impact on morbidity and mortality rates due to infectious diseases. Because of the continuous and extensive use of vaccines in industrialized countries, many infectious diseases such as poliomyelitis, diphtheria and measles have been reduced to near-extinction. However, in recent years, many countries including the United States of America, the United Kingdom and Belgium, have been confronted with a resurgence of mumps and pertussis, despite high vaccination coverage for both vaccines. In this commentary, possible causes of this resurgence will be discussed, such as the occurrence of adapted microbes, failure to vaccinate and primary and secondary vaccine failure. Additional research of the immunological mechanisms is clearly needed to support the development of possible new and more immunogenic vaccines against mumps and pertussis. Meanwhile, extensive vaccination campaigns with both vaccines remain necessary.
Keywords: genotyping, mumps, outbreak, pertussis, primary vaccine failure, secondary vaccine failure, vaccination coverage
Introduction
Vaccines are very effective preventive tools, developed to protect humans and animals against disease. The public health importance of vaccination cannot be overestimated, since it is the only effective preventive measure, besides clean water and sanitation, which was able to substantially reduce morbidity and mortality of infectious diseases. Vaccines not only protect the immunized but thanks to the development of herd immunity at a population level, they can also influence the epidemiology of infectious diseases and respective causative microbial agents. Evidence for this is the absence or near elimination of some important infectious diseases such as smallpox, diphtheria, poliomyelitis, measles and rubella.
Large-scale vaccination campaigns against mumps and pertussis (whooping cough) have had a major impact on disease incidence in industrialized countries.1,2 But since a decade, countries with high vaccination coverage for measles-mumps-rubella (MMR) vaccine and pertussis-containing vaccines, including the United States of America, the United Kingdom and Belgium, are witnessing a resurgence in the number of cases of mumps and pertussis.3-7
In Belgium, the MMR vaccine was added to the childhood vaccination schedule at 15 months of age in 1985 when the combined measles-mumps-rubella (MMR) vaccine was introduced. In 1995, a second dose of MMR vaccine was added for boys and girls at the age of 10–11 y and substituted the rubella vaccination for girls.8 Despite achieving a high MMR vaccination coverage, Belgium faced an epidemic of mumps in a population of students in 2011, spreading from the Netherlands and then followed by a more extensive epidemic in the general population from 2012 until the end of 2013 (Fig. 1).4
Figure 1.
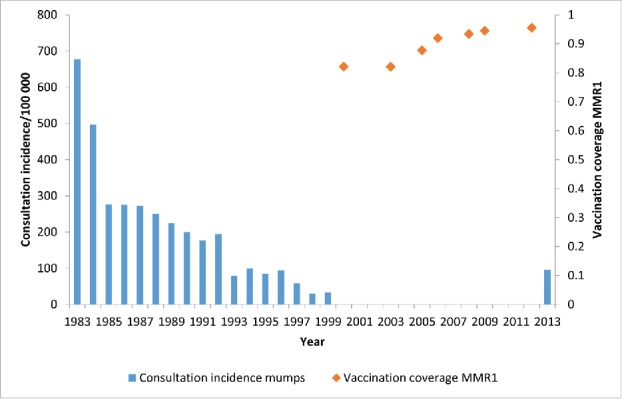
Consultation incidence for mumps and vaccination coverage with the first dose of the measles-mumps-rubella vaccine (MMR1) in Belgium. Source: Sentinel network of general practitioners (GPs) and weighted average of the vaccination coverage surveys (Scientific Institute of Public Health, Brussels, Belgium).
Since 1998, pertussis cases have been increasing in Belgium compared to the previous years with a further significant increase since 2012 (Fig. 2).9,10 Originally, the whole cell pertussis vaccine was used worldwide for the vaccination of infants and toddlers up to the age of 2 y. Due to side effects such as collapse, high fever and persistent crying, pertussis vaccination was discontinued in several countries and lead to a flare-up of the disease in Japan, the United Kingdom and Sweden.11 This lead to the development of an acellular pertussis vaccine, which was systematically implemented as a primary vaccination in industrialized countries.2 In Belgium, acellular pertussis vaccine was introduced in 2001 and a booster dose was added for 6-year-olds in 2004. Responding to the local epidemiology, a booster vaccination in adolescents (14–15 years) was introduced in 2009.12 Finally, since 2013, pertussis vaccination of pregnant women is recommended.13
Figure 2.
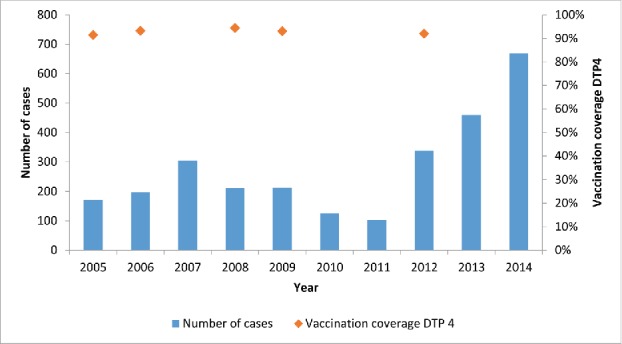
Number of cases of pertussis and vaccination coverage with the 4th doses of the diphtheria-tetanus-pertussis vaccine (DTP4) in Belgium. Source: Sentinel network of laboratories and weighted average of the vaccine coverage surveys (Scientific Institute of Public Health, Brussels, Belgium).
This article provides an overview of possible causes of the resurgence of mumps and pertussis, and the importance of in-depth and concerted research when, despite high vaccination coverage, outbreaks of vaccine-preventable diseases emerge.
Genetic modification of the mumps virus and pertussis bacterium
The current mumps and pertussis vaccines have been developed in the 1950–60s. However, mumps viruses and pertussis bacteria may have been subjected concurrently to a genetic evolution. Therefore, it is important to verify that the vaccines that are still in use adequately protect against the currently circulating pathogens. Because of antigenic variation, the structure or the regulatory functions of different antigens of a pathogen could have adjusted to such an extent that recognition by and interaction with our immune system may have changed. Also, up- or down-regulation of certain genes may have had an impact on the pathogenicity of a microbe.14
Mumps
The mumps virus is an RNA virus of the family of the Paramyxoviridae. The RNA codes for 9 proteins, each with its own function. The genotyping of the mumps virus is based on the Small Hydrophobic (SH) protein, a nonstructural protein and genetically the most variable one. Based on the SH-protein 12 different mumps viruses were detected up to now. In recent epidemics in Western countries the genotype G was mainly detected, while the mumps viruses used in the live attenuated mumps vaccines belong to genotype A (Jeryl Lynn) and to a lesser extent to genotype B (Urabe). However, antibodies against the SH protein have not yet been observed in human serum. It is, therefore, unlikely that antibodies against the SH protein play an important role in antibody-mediated virus neutralization. The two major proteins responsible for the attachment of the virus to the host cell and for the virus-to-cell and cell-to-cell-fusion are the fusion protein (F) and the hemagglutinin-neuraminidase protein (HN). Antibodies directed against these latter proteins are crucial to the development of protection against mumps. In particular, antibodies directed against the HN protein are essential for testing neutralizing activity against the mumps virus. The gene for the F- and the HN-protein, however, exhibits much less variation as compared to the SH-Gen.15 Rubin and colleagues showed that antibodies induced by immunization with the Jeryl Lynn strain were able to adequately neutralise 7 mumps viruses with different HN genotypes. Although there were differences in neutralization efficiency of sera from vaccinnees against the mumps virus isolates, this study showed that the mumps virus is serologically monotypic and the genetic evolution of the virus so far cannot explain the new epidemics of mumps.15,16
Pertussis
Bordetella pertussis is a small gram-negative pleomorphic bacterium. Research shows that over the last 60 y the population of B. pertussis bacteria genetically changed in a significant way. This may result in an adjustment of the transfer of information between the host and pathogen and/or the physiology of B. Pertussis over time.17 In particular, the occurrence of mutations that increase the pathogenicity of the pertussis bacteria (eg. ptxP3-allele which gives rise to higher production of pertussis toxin) or mutations in genes encoding for proteins against which vaccine-antibodies are produced (eg. pertactin), are strains that may play a role in the persistence and outbreak of pertussis.18 Antigenic diversity can both undermine the efficacy of the antibodies or have an effect on the induction of immunological memory. An increase in the production of pertussis toxin, may increase suppression of the innate and acquired immune system.14 If these new strains spread globally, this could contribute to a reduced efficiency of pertussis vaccines.17 In the Netherlands, it was shown that the increase in the number of pertussis cases coincides with the emergence of B. Pertussis containing the ptxP3-allele.17 In contrast to mumps, the genetic modification of B. Pertussis seems to play a role in the current epidemiology.
Failure to vaccinate
To interrupt virus circulation in a population, a high vaccination coverage (> 90–95%) is needed and for measles and mumps estimated at 92–94%. This phenomenon, known as herd immunity, makes elimination of certain pathogens possible. It is important to reach this high vaccination coverage not only once, but to maintain it year after year. When this high vaccination coverage is not reached, the term ‘failure to vaccinate’ is used because of the failure to reach those within a population in a satisfactory manner with well-functioning vaccines.19
Mumps
For the first dose of the MMR-vaccine, a vaccination coverage of > 95% of the infants was reached in Belgium. For the second dose (10–13 y in Belgium) a vaccination coverage of 93% was reached in the region of Flanders.20 But in order to interrupt the circulation of the mumps virus, a vaccination coverage of > 95% is necessary for both doses. Proof of vaccination of a second dose does not necessarily imply that the first dose was also given in infancy which can be problematic for the current generation of adolescents. In Flanders, Belgium, for example, altough a vaccination coverage of 93% was reached for the second dose, only 86% had written proof for both doses.20 This may partly be because of the fact that at the start of the MMR vaccination program, comprising the current generation of young adults and students, the vaccination coverage did not reach > 90%. In addition, the importance of providing an accurate registration of the vaccination in centralized databases has become apparent only during the last 5–10 y. It is much more difficult to obtain accurate data in adolescents on childhood vaccinations, compared to when coverage is measured at 18–24 months of age, shortly after finishing the primary vaccination schedule.20 In Belgium, the region of Flanders makes use of an electronic recording system for vaccinations since 2006 and in the region of Wallonia since 2015, which facilitates the monitoring of vaccination coverage.
Pertussis
In Belgium, the vaccination of all administered doses is consistently high (> 92%) and should be sufficient to prevent the circulation of B.pertussis.
Despite the fact that mumps vaccination coverage is not yet optimal, failure to vaccinate seems not to be the main reason of the current epidemics of mumps and pertussis.
Vaccine failure
Vaccine failure occurs when the disease sought to be avoided occurs in a person who was vaccinated correctly. Within a population, one can also measure the degree of vaccine failure by measuring the efficacy and effectiveness of a vaccine.21
The efficacy of a vaccine is measured in randomized clinical trials under controlled conditions during which a comparison is made of the occurrence of the disease in a group of vaccinated and unvaccinated individuals. In the study cohort, the proportionate reduction in attack rate (AR) among unvaccinated and vaccinated persons is calculated. Efficacy is the ‘best case’ scenario of the protective effect of a vaccine and is determined before the commercialization of a vaccine.21 The efficacy is mainly determined by the degree of primary vaccine failure or inability to develop an immune response after vaccination.
This is in contrast to the effectiveness of a vaccine, where the protective effect of a vaccine, or the possibility of a vaccine to prevent disease is measured in the general population in real-life conditions.21 Several factors determine the effectiveness, including the amount of primary and secondary vaccine failure, the vaccination coverage achieved within a population and the structural problems related to vaccination including the preservation of the cold chain. Secondary vaccine failure occurs when, after an initial good immune response, protection by vaccination decreases over time and people become susceptible again to the infectious disease.
Mumps
The proportion of persons who are not protected after a first dose of the MMR vaccine is estimated at an average of 5% for all mumps components currently used.22 After the administration of a second dose, the immune response amounts up to 100%. But both research and the occurrence of mumps epidemics in populations who were vaccinated twice demonstrated that antibodies decrease over time and the risk to develop mumps increases with prolonged time since vaccination.23,24 This phenomenon occurs with all mumps vaccines used worldwide, so that in many countries where mumps vaccines have been used since the eighties are confronted with major epidemics of mumps. Typically, these epidemics occur among young adults, mostly students, in contrast to the epidemics in the pre-vaccine era which mainly affected primary school children (4–10 years).22 Moreover, it is increasingly apparent that mumps occurs despite a properly applied 2-dose policy. Studies have shown that after mumps vaccination cellular immunity is often longer present than the antibodies.23,25 Finally, there is limited evidence that with the waning of antibodies, also memory B cells disappear and the long-term protection can not be guaranteed anymore.26 These studies indicate that mumps vaccines appear to be less immunogenic than initially assumed. This implies that the primary immune response is able to eliminate mumps, but that the protection lasts less long than originally thought.
Pertussis
The first pertussis vaccines used were killed, whole-cell vaccines (wP). The immunogenicity and efficacy of these wP-vaccines varied as was demonstrated in a study comparing 13 different acellular and whole-cell pertussis vaccines.27 Because of the side effects such as high fever and persistent crying, many countries replaced the wP-vaccine with an acellular pertussis vaccine (aP) with fewer side effects. Initial studies showed that vaccine-elicited antibodies were immunogenic, but antibody titers could also vary from vaccine to vaccine.27,28 Compared to aP-vaccines, wP-vaccines and natural infection will induce an immune response in which the cellular immunity is more important while aP-vaccines induce a mixed Th1/Th2 immune response.27,29,30 Although the exact nature of the immune responses after wP and aP vaccination remain unclear, several studies indicate that the protection against pertussis after administration of aP-vaccines wanes more quickly than was previously assumed. This is an indication for “waning immunity” which appears to be more pronounced compared with the well-functioning wP-vaccines.28
Both for mumps and pertussis current vaccines seem less immunogenic than assumed based on the first studies. One of the important difficulties in evaluating both mumps and pertussis is the lack of correlate of protection against these diseases. Which biomarkers can be used and are related to protection? Are antibodies sufficient as a measure of protection? And if so, which antibodies should be determined? There is an important difference between the total amount of antibodies and neutralizing antibodies. An ELISA assay measures all antibodies developed after vaccination but may not specifically measure neutralizing antibodies and can give an overestimation of protection. Neutralizing antibodies are a better indication of protection, but this can only be determined by the labor-intensive plaque reduction neutralization (PRN) test. It is not feasible to carry out the PRN for routine application. And what about the long-term protection? Is it sufficient to measure antibodies, or should we look for underlying immunological mechanisms such as central memory cells?31 Both for mumps and pertussis, the development of antibodies is crucial in the protection against disease and infection. But which antigens are essential? What is the role of the cellular immune system? Especially for pertussis the Th1 response seems to play a significant role.32 Additional research of the immunological mechanisms involved will hopefully provide insights in the development of possible new and more immunogenic vaccines against mumps and pertussis.
One last thought: in the pre-vaccine era lifelong protection following disease was most probably also maintained by natural boosting through exposure to wild-type pathogens during cyclic epidemics of infectious diseases. Immunization nor natural infection provides lifelong protection against pertussis and in future the need to implement booster vaccinations throughout life for both mumps and pertussis might be necessary.33,34
Conclusions
After more than 30 y of systematic use of vaccines and vaccination, the imperfections of the program become apparent. Current mumps and pertussis epidemics are probably due to several factors. But the long-term immunogenicity of the 2 vaccines, in particular the underlying immunological mechanisms such as the immunological memory, probably play a major role in the development of the current epidemics.
In spite of the imperfections of these vaccines, both mumps and pertussis vaccine play an important role in the prevention of both the disease and complications following exposure to the wild mumps virus or pertussis bacterium. Therefore, extensive vaccination campaigns with both vaccines remain necessary.
Disclosure of potential conflicts of interest
CV was and is sub and principal investigator for several clinical studies for which the university received a research grant from GlaxoSmithKline Biologicals, Merck, Sanofi Pasteur MSD, Sanofi Pasteur, Novartis, Genticel. She received no personal payments from vaccine manufacturers. MS has no conflicts of interest.
Acknowledgments
The authors wish to thank Viviane Van Casteren and Gaëtan Muyldermans of the Scientific Institute of Public Health in Belgium for providing the mumps and pertussis surveillance data.
References
- [1].Rubin S, Plotkin S. Mumps Vaccine, In: Plotkin S, Orenstein W, Offit PA, eds. Vaccines. Philadelphia: Saunders, 2013:419-46 [Google Scholar]
- [2].Edwards KM, Decker MD. Pertussis Vaccine, In: Plotkin S, Orenstein W, Offit PA, eds. Vaccines. Philadelphia: Saunders, 2013:447-92 [Google Scholar]
- [3].Amirthalingam G, Gupta S, Campbell H. Pertussis immunisation and control in England and Wales, 1957 to 2012: a historical review. Euro Surveill 2013; 18: pii:20587; PMID:24084340; http://dx.doi.org/ 10.2807/1560-7917.ES2013.18.38.20587 [DOI] [PubMed] [Google Scholar]
- [4].Braeye T, Linina I, De RR, Hutse V, Wauters M, Cox P, Mak R. Mumps increase in Flanders, Belgium, 2012–2013: results from temporary mandatory notification and a cohort study among university students. Vaccine 2014; 32:4393-8; PMID:24973734; http://dx.doi.org/ 10.1016/j.vaccine.2014.06.069 [DOI] [PubMed] [Google Scholar]
- [5].Clark TA, Messonnier NE, Hadler SC. Pertussis control: time for something new? Trends Microbiol 2012; 20:211-3; PMID:22494804; http://dx.doi.org/ 10.1016/j.tim.2012.03.003 [DOI] [PubMed] [Google Scholar]
- [6].Gabutti G, Azzari C, Bonanni P, Prato R, Tozzi A E, Zanetti A, Zuccotti G. Pertussis: Current perspectives on epidemiology and prevention. Hum Vaccin Immunother 2015; 11(1):108-17; PMID:25483523; http://dx.doi.org/ 10.4161/hv.34364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zamir CS, Schroeder H, Shoob H, Abramson N, Zentner G. Characteristics of a large mumps outbreak: Clinical severity, complications and association with vaccination status of mumps outbreak cases. Hum Vaccin Immunother 2015; 11:1413-7; PMID:25874726; http://dx.doi.org/ 10.1080/21645515.2015.1021522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Burgmeijer R, Hoppenbrouwers K, Hahné S. Bof (parotitis epidemica), In: Burgmeijer R, Hoppenbrouwers K, Van Gompel F, eds. Handboek Vaccinaties. Assen: Van Gorcum; 2013:3-12 [Google Scholar]
- [9].Mahieu L, De SK, Van den Branden D, Boeckx H, Mahieu H, Wojciechowski M. Epidemiology of pertussis in children of Flanders Belgium: can healthcare professionals be involved in the infection? Acta Clin Belg 2014; 69: 104-10; PMID:24724749; http://dx.doi.org/ 10.1179/0001551214Z.00000000032 [DOI] [PubMed] [Google Scholar]
- [10].Pierard D. Bordetella Pertussis. Report national reference centre 2013; 2014, 1-8. [Google Scholar]
- [11].Gangarosa EJ, Galazka AM, Wolfe CR, Phillips LM, Gangarosa RE, Miller E, Chen RT. Impact of anti-vaccine movements on pertussis control: the untold story. Lancet 1998; 351:356-61; PMID:9652634; http://dx.doi.org/ 10.1016/S0140-6736(97)04334-1 [DOI] [PubMed] [Google Scholar]
- [12].Burgmeijer R, Hoppenbrouwers K. Kinkhoest (pertussis), In: Burgmeijer R, Hoppenbrouwers K, Van Gompel F, eds. Handboek vaccinaties. Assen: Van Gorcum; 2013: 246-68 [Google Scholar]
- [13].Laenen J, Roelants M, Devlieger R, Vandermeulen C. Influenza and pertussis vaccination coverage in pregnant women. Vaccine 2015; 33:2125-31; PMID:25796339; http://dx.doi.org/ 10.1016/j.vaccine.2015.03.020 [DOI] [PubMed] [Google Scholar]
- [14].Mooi FR, Van Der Maas NA, de Melker HE. Pertussis resurgence: waning immunity and pathogen adaptation - two sides of the same coin. Epidemiol Infect 2014; 142:685-94; PMID:23406868; http://dx.doi.org/ 10.1017/S0950268813000071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rubin SA, Link MA, Sauder CJ, Zhang C, Ngo L, Rima BK, Duprex WP. Recent mumps outbreaks in vaccinated populations: no evidence of immune escape. J Virol 2012; 86:615-20; PMID:22072778; http://dx.doi.org/ 10.1128/JVI.06125-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Rubin SA, Qi L, Audet SA, Sullivan B, Carbone KM, Bellini WJ, Rota PA, Sirota L, Beeler J. Antibody induced by immunization with the Jeryl Lynn mumps vaccine strain effectively neutralizes a heterologous wild-type mumps virus associated with a large outbreak. J Infect Dis 2008; 198:508-15; PMID:18558869; http://dx.doi.org/ 10.1086/590115 [DOI] [PubMed] [Google Scholar]
- [17].Bart MJ, Harris SR, Advani A, Arakawa Y, Bottero D, Bouchez V, Cassiday PK, Chiang CS, Dalby T, Fry NK, et al.. Global population structure and evolution of Bordetella pertussis and their relationship with vaccination. MBio 2014; 5:e01074; PMID:24757216; http://dx.doi.org/ 10.1128/mBio.01074-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Barkoff AM, Mertsola J, Guillot S, Guiso N, Berbers G, He Q. Appearance of Bordetella pertussis strains not expressing the vaccine antigen pertactin in Finland. Clin Vaccine Immunol 2012; 19:1703-4; PMID:22914363; http://dx.doi.org/ 10.1128/CVI.00367-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fine PEM, Mulholland K. Community Immunity, In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. Philadelphia: Saunders, 2013: Chapter 71 [Google Scholar]
- [20].Braeckman T, Lernout T, Top G, Paeps A, Roelants M, Hoppenbrouwers K, Van DP, Theeten H. Assessing vaccination coverage in infants, survey studies versus the Flemish immunisation register: achieving the best of both worlds. Vaccine 2014; 32:345-9; PMID:24269616; http://dx.doi.org/ 10.1016/j.vaccine.2013.11.041 [DOI] [PubMed] [Google Scholar]
- [21].Weinberg GA, Szilagyi PG. Vaccine epidemiology: efficacy, effectiveness, and the translational research roadmap. J Infect Dis 2010; 201:1607-10; http://dx.doi.org/ 10.1086/652404 [DOI] [PubMed] [Google Scholar]
- [22].Rubin SA, Vandermeulen C. Mumps virus, In: Samal S, ed. The biology of Paramyxoviruses. Caister Academic Press, 2011:5-36 [Google Scholar]
- [23].Jokinen S, Osterlund P, Julkunen I, Davidkin I. Cellular immunity to mumps virus in young adults 21 years after measles-mumps-rubella vaccination. J Infect Dis 2007; 196:861-7; PMID:17703416; http://dx.doi.org/ 10.1086/521029 [DOI] [PubMed] [Google Scholar]
- [24].Vandermeulen C, Roelants M, Vermoere M, Roseeuw K, Goubau P, Hoppenbrouwers K. Outbreak of mumps in a vaccinated child population: a question of vaccine failure? Vaccine 2004; 22:2713-6; PMID:15246601; http://dx.doi.org/ 10.1016/j.vaccine.2004.02.001 [DOI] [PubMed] [Google Scholar]
- [25].Vandermeulen C, Clement F, Roelants M, Van DP, Hoppenbrouwers K, Leroux-Roels G. Evaluation of cellular immunity to mumps in vaccinated individuals with or without circulating antibodies up to 16 years after their last vaccination. J Infect Dis 2009; 199:1457-60; http://dx.doi.org/ 10.1086/598482 [DOI] [PubMed] [Google Scholar]
- [26].Vandermeulen C, Verhoye L, Vaidya S, Clement F, Brown KE, Hoppenbrouwers K, Leroux-Roels G. Detection of mumps virus-specific memory B cells by transfer of peripheral blood mononuclear cells into immune-deficient mice. Immunology 2010; 131:33-9; PMID:20586811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Edwards KM, Berbers GA. Immune responses to pertussis vaccines and disease. J Infect Dis 2014; 209 Suppl 1:S10-S15; http://dx.doi.org/ 10.1093/infdis/jit560 [DOI] [PubMed] [Google Scholar]
- [28].WHO SAGE pertussis working group Background paper, In: SAGE, ed. 2014 [Google Scholar]
- [29].Cassone A, Ausiello CM, Urbani F, Lande R, Giuliano M, La SA, Piscitelli A, Salmaso S.. Cell-mediated and antibody responses to Bordetella pertussis antigens in children vaccinated with acellular or whole-cell pertussis vaccines. The Progetto Pertosse-CMI Working Group. Arch Pediatr Adolesc Med 1997; 151:283-9; PMID:9080938; http://dx.doi.org/ 10.1001/archpedi.1997.02170400069013 [DOI] [PubMed] [Google Scholar]
- [30].Ross PJ, Sutton CE, Higgins S, Allen AC, Walsh K, Misiak A, Lavelle EC, McLoughlin RM, Mills KH. Relative contribution of Th1 and Th17 cells in adaptive immunity to Bordetella pertussis: towards the rational design of an improved acellular pertussis vaccine. PLoS Pathog 2013; 9:e1003264; PMID:23592988; http://dx.doi.org/ 10.1371/journal.ppat.1003264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol 2010; 17:1055-65; PMID:20463105; http://dx.doi.org/ 10.1128/CVI.00131-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Plotkin SA. Complex correlates of protection after vaccination. Clin Infect Dis 2013; 56:1458-65; PMID:23386629; http://dx.doi.org/ 10.1093/cid/cit048 [DOI] [PubMed] [Google Scholar]
- [33].Ogbuanu IU, Kutty PK, Hudson JM, Blog D, Abedi GR, Goodell S, Lawler J, McLean HQ, Pollock L, Rausch-Phung E, et al.. Impact of a third dose of measles-mumps-rubella vaccine on a mumps outbreak. Pediatrics 2012; 130:e1567-74; PMID:23129075; http://dx.doi.org/ 10.1542/peds.2012-0177 [DOI] [PubMed] [Google Scholar]
- [34].Zepp F, Heininger U, Mertsola J, Bernatowska E, Guiso N, Roord J, Tozzi AE, Van Damme P. Rationale for pertussis booster vaccination throughout life in Europe. Lancet Infect Dis 2011; 11:557-70; PMID:21600850; http://dx.doi.org/ 10.1016/S1473-3099(11)70007-X [DOI] [PubMed] [Google Scholar]


