ABSTRACT
Background: A primary goal of this study was to establish the serological mechanistic correlate of protection (mCoP) for an inactivated Enterovirus 71 (EV71) vaccine.Methods: We used the Prentice criterion framework and scaled logit model to explore the relationship between the neutralizing antibody (NTAb) and EV71-associated disease, and to build a protection curve for estimating the efficacy of EV71 vaccine. Data of NTAb at day 56 post-vaccination and the occurrence of EV71-associated disease during a 12-month follow-up period were collected from a phase 3 efficacy trial of EV71 vaccine in this study.Results: NTAb at day 56 post-vaccination in participants met the Prentice criterion framework. According to the protection curve, the antibody levels of 14.7, 27.8, 55.7, 129.0 and 459.4 (U/mL) were associated with 50%, 60%, 70%, 80% and 90% clinical protection rate, respectively. Vaccine efficacy predicted by the model was 81.5%, which was very similar to the actual vaccine efficacy of 80.4% (95% CI, 58.2, 90.8) observed in the phase 3 trial.Conclusions: NTAb titers post-vaccination can be validated as mCoP for evaluating the efficacy of an inactivated enterovirus 71 vaccine, with a titers of 14.7 (U/ml) as a surrogate associated with the protection of 50% against EV71-associated disease.
KEYWORDS: correlate of protection; Enterovirus 71 vaccine, Prentice criterion; surrogate endpoints; the scaled logit model; vaccine efficacy trial
Introduction
Enterovirus 71 (EV71) is a neurotropic human pathogen of the Picornaviridae family and one of the major causative agents of hand foot and mouth disease (HFMD). EV71 has been reported to be associated with severe diseases of the central nervous system in children less than 5 y old.1-3 In the past 10 y, outbreaks and epidemic caused by EV71 have occurred more frequently, which has emerged as a serious threat to public health, especially in the Asia-Pacific region.4-7 EV71 is now considered as the most dangerous neurotropic enterovirus of the post-polio era.8,9 Currently, 3 inactivated EV71 vaccines developed in China have demonstrated good efficacy and safety profile in the phase 3 clinical trials.10-12 These 3 EV71 vaccines are very likely to be the first available vaccines against EV71-associated disease in the world.
In two of the 3 trials,10,12 correlate of immunity againstEV71-associated disease was explored by applying a receiver operating characteristics (ROC) curve analysis in a subset of cohort selected by matching each case with 5 controls. The results suggested that antibody titers of 1:16–1:32 with the maximum Youden index might be a possible serologic marker for protection against EV71-associated disease. However, data in the subset of cohort using ROC curve analysis was limited and potentially biased by the selection of the controls. Therefore, the determination of surrogate endpoints is still uncertain for the protection of inactivated EV71 vaccines. When an immune marker is considered to be a reliable measure of protection against clinical endpoints, Phase 2 studies can be the definitive study for licensure with immune markers as outcomes.
In this study, we aimed to assess the relationship between NTAb titers post-vaccination and the occurrence of subsequent EV71-associated disease by fitting the Prentice framework13-16 and to estimate the mechanistic correlateof protection (mCoP)17 using the scaled logit model developed by Dunning18 based on the data collected from the whole cohort in a randomized, double-blind, placebo-control trial of inactivated EV71 vaccine.10
Results
Validation of NTAb titers day 56 post-vaccination as surrogate endpoint
The EV71 NTAb titers at day 56 post-vaccination and the occurrence of EV71 associated-disease in logistic regression model satisfied the 4 criterion (Tables 1-2). The incidence of EV71-associated disease was significantly lower in the vaccine group than that in the placebo group during 1 y of surveillance (P < 0.0001) (criterion I). The EV71 NTAb titers responses at day 56 post-vaccination were significantly different (P < 0.0001) between the vaccine group and the placebo group (criterion II). The regression models revealed that EV71 NTAb titers at day 56 post-vaccination was highly inversely related to EV71-associated incidence (P < 0.0001) (criterion III). The effect of vaccine group, however, changed from significant (P < 0.0001) to nonsignificant (P = 0.3937) after controlling for EV71 NTAb titers, indicating that the NTAb titers at day 56 post-vaccination accounted for most of the vaccine effect on the occurrence of EV71-associated disease (criterion IV).
Table 1.
NTAb titers at day 56 post-vaccination and the incidence of EV71-associated disease in the per-protocol population (n=9654).
| Vaccine group | Placebo group | ||
|---|---|---|---|
| N | 4817 | 4837 | P value |
| GMT (95% CI) | 788.4 (749.9,828.8) | 14.6 (13.5,15.8) | <0.0001 |
| N (cases/1000) | 8 (1.7) | 41 (8.5) | <0.0001 |
EV71, enterovirus 71; NTAb, neutralizing antibody; GMT, geometric mean titers; CI, confidence interval; N, the number of EV71-associated disease
Table 2.
Estimates of parameters of logistic regression models used to evaluate risk of EV71-associated disease.
| Parameter |
β |
SE |
Wald |
P value |
OR (95% CI) |
|---|---|---|---|---|---|
| Logistic regression 1 | |||||
| Intercept | −3.125 | 0.473 | 43.678 | <0.0001 | |
| Vaccination status | −1.637 | 0.387 | 17.885 | <0.0001 | 0.195(0.091,0.416) |
| Logistic regression 2 | |||||
| Intercept | −4.349 | 0.570 | 58.269 | <0.0001 | |
| Vaccination status | 0.328 | 0.577 | 0.323 | 0.5699 | 1.388(0.448,4.297) |
| Day 56 antibody titers | −0.546 | 0.107 | 18.178 | <0.0001 | 0.633(0.513,0.781) |
SE, standard error; OR, odd ratio; CI, confidence interval.
Validation of the scaled logit model
The scaled logit model was fitted using the data of NTAb titers at day 56 post-vaccination and the occurrence of EV71-associated disease as described above. The protection curve estimated according to the NTAb titers post-vaccination and the probability of protection is shown in Figure 1, with a 95% confidence band. The variance of the probability of protection was greater at low titers than that at high titers. Individual protection curves fitted for the vaccine group and placebo group were well consistent with each other (P = 0.932), as shown in Figure 2, as well as no differences between the treatment groups was observed in the estimated exposure parameters (P = 0.925; Table S1). The parameter estimates (λ, α, β) of the per-protocol population are shown in Table 3, and the exposure parameter was estimated at 0.018 with a 95% CI of 0.00244 to 0.0292 (Table 3). The EV71 NTAb titers associated with 50%, 60%, 70%, 80% and 90% protection against the EV71-associated disease from the model was 14.7 (95%CI: 8.6,25.0), 27.8(95%CI: 15.5,49.9), 55.7(95%CI: 28.8,107.8),129.0(95%CI: 60.9,273.1), 459.4(95%CI: 184.9,1141.4) (U/mL), respectively (Table 4).
Figure 1.
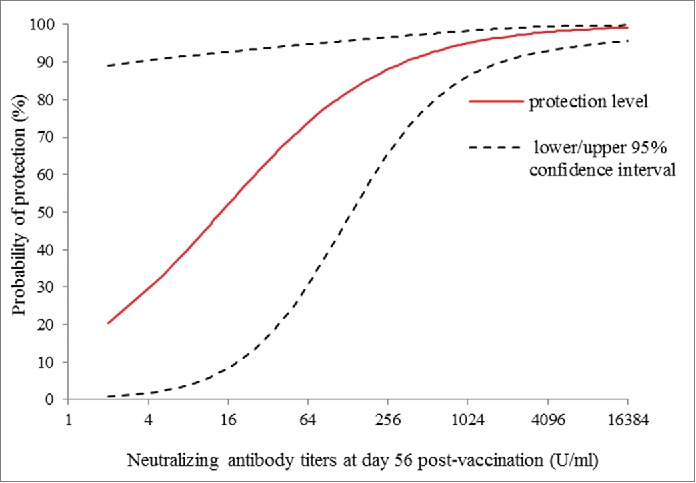
Estimated curve of protection against EV71-associated disease versus neutralizng antibody titers at day 56 post-vaccination, with the estimated curve (solid line) is charted with 95% confidence interval (dashed line) in per-protocal population.
Figure 2.

Estimated curves of protection against EV71-associated disease vs. neutralizng antibody titers at day 56 post-vaccination in vaccine (solid line) and placebo group (dashed line) in per-protocal population.
Table 3.
Estimates of parameters of the scaled logit model by using procedure NLMIXED in the per-protocol population.
| Paramter | Estimate | Standard Error | DF | P value | Lower | Upper |
|---|---|---|---|---|---|---|
| λ | 0.0158 | 0.00682 | 9654 | 0.0205 | 0.00244 | 0.0292 |
| α | −1.835 | 1.944 | 9654 | 0.345 | −5.645 | 1.976 |
| β | 0.692 | 0.266 | 9654 | 0.0092 | 0.171 | 1.212 |
Table 4.
Estimated day 56 antibody titers needed to provide various levels of protection.
| Probability of Protection | Antibody Titers Level (U/ml) (95%CI) |
|---|---|
| 50% | 14.7 (8.6,25.0) |
| 60% | 27.8 (15.5,49.9) |
| 70% | 55.7 (28.8,107.8) |
| 80% | 129.0 (60.9,273.1) |
| 90% | 459.4 (184.9,1141.4) |
CI, confidence interval.
Then, we used the NTAb titers post-vaccination in the per-protocol population with the parameters estimated above to predict the efficacy of EV71 vaccine by applying the following formation:
A predicted vaccine efficacy of 81.5% was found based on the EV71 NTAb titers post-vaccination, which was very similar with the actual vaccine efficacy observed in the trial (80.4%, 95% CI: 58.2, 90.8).10
Discussion
An immune correlate of protection is an immunological assay (either humoral or cellular) that reliably predicts the level of vaccine efficacy to prevent a clinically meaningful endpoint.19 A mCoP is of great importance because it can be used as a surrogate endpoint for vaccine efficacy. Immunological vaccine trials are much less costly and much less time-consuming than large-scale field efficacy trials. Besides, A mCoP could also provide a convenient way to evaluate the immunological level of a population which is essential for the controlling of prevalence.20 For these reasons, determining surrogate endpoints is one of the 14 Grand Challenges of Global Health of the National Institutes of Health (NIH) and the Bill and Melinda Gates Foundation.21
We addressed the objective of this study to assess NTAb titers post-vaccination as the mCoP against EV71-associated disease and estimate the protective levels for a range of mCoPs. The concept of a mCoP is the same as a valid surrogate endpoint in the clinical trials statistical literature.22 The analysis of the Prentice framework showed that NTAb titers post-vaccination met statistically Prentice criteria I-IV for a valid surrogate endpoint. As Prentice acknowledged,13 the criterion are based on statistical associations and the requirement that the surrogate endpoints explains all the effect of the vaccination on the clinical endpoint is restrictive. Nevertheless, studies in animal models, populations and vaccine clinical trials have demonstrated that neutralizing antibody responses played a critical role in controlling EV71-associated disease,23-25 and protection against EV71 is mainly dependent on humoral-mediated immunity.26 As a result, NTAb titers at day 56 post-vaccination could be used as surrogate of protection to predict the occurrence of EV71-associated disease for a year period.
In this study, the scaled logit model was used to quantify the relationship between NTAb titers at day 56 post-vaccination and EV71-associated disease, which revealed that the level of protection varied as a continuous function of NTAb titers at day 56 post-vaccination. It can be seen from Figure 1 that the variance of the probability of protection is greater at low assay than at high, which is consistent with the principle of the scaled logit model.18 The model separates the effect of NTAb titers on the occurrence of disease from exposure factors independent of NTAb titers, 2 possible reasons may be suggested for this. Firstly, infectious disease occurrence depends upon the immunity of a given population as well as their exposure to circulating viruses. At low antibody titers, the exposure parameter λ has more influence on the occurrence of disease than at high antibody titers. Secondly, low assay values tend to be measured with relative less precision than high values, thus tending to increase the variance of the parameter estimates at lower values.
In particular, the protection curves provide information on the degree of protection conferred for a range of immune response thresholds. For example, a neutralizing antibody titers of 14.7, 27.8, 55.7, 129.0(U/ml) corresponds to estimated protection level of 50%, 60%, 70% and 80%, respectively. Jozef Nauta21 proposed that an obvious definition of a protection threshold in protection curves is the antibody value for which the predicted probability of protection is 50% in the book of statistics in clinical vaccine trials. The hemagglutination inhibition (HI) titers of ≥40 defined as “surrogate of protection” is use to evaluate the efficacy of influenza vaccines, which corresponds to 50% protection from infection.27 However, there have been no criterion for which level should be used as the surrogate endpoint at present. The risk of disease could be highly influenced by the probability of exposure to an infectious contact and the prevalence of infection. Therefore, it is necessary to appropriately improve the protection level in high-risk population or high prevalence areas. The titers of 14.7 (U/mL) corresponds to estimated protection level of 50% against EV71-associated disease in our study. If the goal is to protect children against EV71 infection, it makes much more sense from a public health perspective to target 80% or 90% protection rather than 50%. Targeting a high level of protection against the risk of infection can reduce EV71 circulation in young children and provide the indirect effects, which has important public health implications both for the vaccines and for EV71-associated disease control in general.
The estimated protection curves were similar for vaccine and placebo groups, suggesting that the relationship between NATb titers and protection is independent of vaccination status. In the case, vaccine efficacy predicted from NTAb titers at day 56 post-vaccination and parameter estimates was 81.5%, which was consistent with the observed vaccine efficacy. The analysis presented here further support NATb titers post-vaccination as an ideal mCoP. Nevertheless, future research is needed to examine the predictive capability of the model in the context of vaccine clinical trials similar to the one in this study.
Zhu et al.10 assessed exploratory correlates of immunity using the ROC curve analysis in the inactivated EV71 vaccine clinical trial. The results indicated that the cutoff 1:16 with the maximum Youden index could be thought of as surrogate of protection for it could provide the clearest distinction between cases and controls, which equaled to standardized NTAb titers15.1 (U/mL) by dividing the tested original NTAb titer to the NTAb titer of the reference serum and multiplied by the assigned potency of the reference serum (N12: 1000U).28 The NTAb titers 15.1 (U/ml) was consistent with the NTAb titers corresponding to protection level of 50% in this study. If the NTAb titers corresponding to protection level of 50% is used the surrogate of protection, the results obtained by the ROC curve will be consistent with the results of this study. Therefore, the titers of 14.7 (U/mL) corresponding to protection level of 50% may be used for future EV71 vaccine development. The Youden index gives the same weight to both sensitivity and specificity, which separates the protected and non-protected populations. However, the rates of infectious disease among individuals with low titers may be strongly associated with the chance of exposure and disease prevalence, and individuals who were not infected are a mixture of individuals who were protected and those who were unprotected but also unexposed. Therefore, false negatives are likely to occur and sensitivity should determine the cut-off value. However, taking into account these issues, the scaled logit model is proposed for involving exposure factors independent of antibody level and providing the calculation of antibody titer-specific rates of disease.
Another issue that should be addressed is the difference between a population correlates and individual correlates of protection. Here, we are interested in population correlates of protection based on efficacy and immunological results in an efficacy population. A population degree of protection was derived across different levels of mCoP using the scaled logit model. Such population correlates of protection is useful in aspects of controlling infectious diseases and evaluating vaccine efficacy.29 This is because in the individual situation, a subject with antibody titers above the protective threshold of the population will occasionally develop disease due to natural variation and exposure intensity.
We recognize that there are limitations to the use of these data. First, reported EV71-associated disease is relative small in just one year follow-up period. 49 cases is unlikely to be enough to obtain a reliable, precise estimate of the relationship between NTAb titers at day 56 post-vaccination and long-term protection. Second, any generalization from the results obtained in this study to other formulation EV71 vaccines with different characteristics used in other counties or regions must be done carefully in the future. Ideally, a correlates of protection should be equally useful for multiple and/or different settings. Meta-analysis is suitable for evaluating an correlates of protection that is predictive of vaccine efficacy in different settings.14 A protective concentration of 0.35 g/ml for invasive pneumococcal disease adopted by the World Health Organization was estimated by utilizing 3 double blind controlled efficacy trials in a meta-analysis.30
In conclusion, the analyses above support the assumption that NTAb titers at day 56 post-vaccination may serve as a correlate of protection for evaluating the efficacy of an inactivated Enterovirus 71 vaccine in children aged 6–35 months. These data also indicate that a cutoff titers of 14.7 (U/mL) would predict a 50% protective level for a year period.
Materials and methods
Study design
We collected data from a randomized, double-blind, placebo-controlled phase 3 trial in children aged 6–35 months in China, which was described previously.10 Briefly, a total of 10245 healthy infants and young children aged 6–35 months were randomized to receive inactivated EV71 vaccine or placebo in a 1:1 ratio. Blood samples from all participants were obtained at day 56 (28 d after second dose) and NTAb titers against EV71 was measured with a modified cytopathogenic effect assay, and EV71-associated disease was identified through active surveillance for one year.
In this study, we evaluated mCoP based on the per-protocol population (n = 9654), which consisted of all eligible participants who completed the 2-dose regimen and with available EV71 NTAb titers in serum post-vaccination (4817 in the vaccine group vs 4837 in the placebo group). Participants with sero-positivity at baseline were not screened out from the original trial, and sero-positivity is defined as pre-vaccination NTAb titers≥1:8. The proportion of participants with sero-positivity and geometric mean titers did not differ significantly between placebo and vaccine groups (28.9% vs 28.8%; 9.70(9.31, 10.11) vs 9.56(9.18, 9.96)). Surveillance lasted for 12 months from March, 2012 to March, 2013, during which time a total of 49 EV71-associated cases (8 in the vaccine group vs 41 in the placebo group) was confirmed, including 33 HFMD and 16 EV71-associated other disease.
Measurement of EV71 neutralizing antibody
Serum samples of 3 mL were collected from subjects on day 56 (day 28 after second dose) for determination of EV7 NTAb titers. Serums samples were stored at a temperature of −20°C or below before tested. A modified cytopathogenic effect method (CPE method) was used to measure EV71 NTAb titers.31 In brief, samples were 2-fold serially diluted from 1:8 to 1:16384 and NTAb titers were defined as the highest dilution capable of inhibiting 50% of the CPEs. Antibody titers lower than 1:8 was assigned values of 1:4 for calculation. 1219 serum samples from immunogenicity cohort collected on day 56 had been detected by National Institutes for Food and Drug Control (NIFDC),10 and remaining 8435 serum samples were measured by Beijing Vigoo Biological Ltd later using the same method. The tested original NTAb titers from NIFDC and Beijing Vigoo were standardized according to the NTAb titers (U/mL) of the reference serum (N12: 1000U). The standardization method could convert the tested NTAb titers from different laboratories into standardized NTAb titers (U/mL). This method was adapted from the calibration methods applied for polio previously.28,32
Statistical methods
According to the Prentice criterion proposed by Qin et al,13,14 4 items were applied for the validation of EV71 NTAb titers post-vaccination as an immunological surrogate:
Protection against the clinical endpoint is significantly related to having received the vaccine, which is determined using a Chi-squared test in which the dependent variable is EV71-associated disease rate and the independent variable is inactivated EV71 vaccine and placebo group.
The surrogate endpoint is significantly related to the vaccination status, which is examined using a t-test in which the dependent variable is the log-transformed NTAb titers at day 56 post-vaccination and the independent variable is inactivated EV71 vaccine and placebo group.
The surrogate endpoint is significantly related to protection against the clinical endpoint, which demonstrates that the log-transformed NTAb titers at day 56 post-vaccination is significantly related to EV71-associated disease by fitting a logistic regression.
The full effect of the vaccine on the frequency of the clinical endpoint is explained by the surrogate endpoint. The relationship is examined by fitting the regression models with log-transformed NTAb titers at day 56 post-vaccination and vaccine group as independent variables and EV71-associated disease as the dependent variable. If the effect of the vaccine group variable changes from significant to nonsignificant after controlling for antibody titers, the criterion has been met.)
A scaled logit model for immunological correlates of protection
Then, a scaled logit model was used to fit the relationship between EV71 NTAb titers at day 56 post-vaccination and the occurrence of EV71-associated disease in the one-year surveillance.18 In the model, the probability of disease is the probability that the subject is susceptible multiplied by the probability that susceptible individuals develop disease and is modeled as
Where ti represents a subject's log-transferred EV71 NTAb titers, Yi=1 represents a laboratory-confirmed EV71-associated disease, and λ represents the probability of a susceptible individual suffered from EV71-associated disease, which may be interpreted as an estimate of exposure. π(t) represent the probability that an individual with titers t is protected and will be modeled as 2-parameter inverse logit function, with α and β denoting the location and the scale parameters of interest, respectively. That is, π(t) = .Parameters λ, α and β may be estimated by standard maximum likelihood methods. The scaled logit model is established by running NLMIXED procedure in SAS software (version 9.1). EV71 NTAb titers with 95% confidence intervals corresponding to different levels of protection was calculated by applying parameters α and β base on the bootstrap sampling. The consistency of the exposure parameters and protection curves was examined with Wald-type t test when individual models were fitted for vaccine and placebo group. Additionally, the model may be applied to immunogenicity data to obtain an estimate of vaccine efficacy, as
Supplementary Material
Abbreviations
- EV71
Enterovirus71
- mCoP
mechanistic correlate of protection
- NTAb
neutralizing antibody
- HFMD
hand foot and mouth
- ROC
receiver operating characteristics
- CPE method
modified cytopathogenic effect method
- NIFDC
National Institutes for Food and Drug Control
- NIH
National Institutes of Health
- GMT
geometric mean titers
- CI
confidence interval
- SE
standard error
- OR
odd ratio
Clinical trials registration
Disclosure of potential conflict of interest
No potential conflicts of interest were disclosed
Funding
This work was supported by the China 12–5 National Major Infectious Disease Programs (2012ZX10004-703 and 2012ZX10002-001) and Beijing Vigoo Biological.
References
- [1].Kung YA, Hung CT, Liu YC, Shih SR. Update on the development of enterovirus 71 vaccines. Expert Opin Biol Ther 2014; 14:1455-64; PMID:24989170; http://dx.doi.org/ 10.1517/14712598.2014.935330 [DOI] [PubMed] [Google Scholar]
- [2].McMinn PC. Enterovirus vaccines for an emerging cause of brain-stem encephalitis. N Engl J Med 2014; 370:792-4; PMID:24571750; http://dx.doi.org/ 10.1056/NEJMp1400601 [DOI] [PubMed] [Google Scholar]
- [3].Ooi MH, Wong SC, Lewthwaite P, Cardosa MJ, Solomon T. Clinical features, diagnosis, and management of enterovirus 71. Lancet Neurol 2010; 9:1097-105; PMID:20965438; http://dx.doi.org/ 10.1016/S1474-4422(10)70209-X [DOI] [PubMed] [Google Scholar]
- [4].Solomon T, Lewthwaite P, Perera D, Cardosa MJ, McMinn P, Ooi MH. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect Dis 2010; 10:778-90; PMID:20961813; http://dx.doi.org/ 10.1016/S1473-3099(10)70194-8 [DOI] [PubMed] [Google Scholar]
- [5].Yip CC, Lau SK, Woo PC, Yuen KY. Human enterovirus 71 epidemics: what's next? Emerg Health Threats J 2013; 6:19780; PMID:24119538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chong P, Liu CC, Chow YH, Chou AH, Klein M. Review of enterovirus 71 vaccines. Clin Infect Dis 2015; 60:797-803; PMID:25352588; http://dx.doi.org/ 10.1093/cid/ciu852 [DOI] [PubMed] [Google Scholar]
- [7].Xing W, Liao Q, Viboud C, Zhang J, Sun J, Wu JT, Chang Z, Liu F, Fang VJ, Zheng Y,et al. Hand, foot, and mouth disease in China, 2008–12: an epidemiological study. Lancet Infect Dis 2014; 14:308-18; PMID:24485991; http://dx.doi.org/ 10.1016/S1473-3099(13)70342-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].McMinn P, Lindsay K, Perera D, Chan HM, Chan KP, Cardosa MJ. Phylogenetic analysis of enterovirus 71 strains isolated during linked epidemics in Malaysia Singapore,and Western Australia. J Virol 2001; 75:7732-8; PMID:11462047; http://dx.doi.org/ 10.1128/JVI.75.16.7732-7738.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lee MS, Chang LY. Development of enterovirus 71 vaccines. Expert Rev Vaccines 2010; 9:149-56; PMID:20109026; http://dx.doi.org/ 10.1586/erv.09.152 [DOI] [PubMed] [Google Scholar]
- [10].Zhu FC, Meng FY, Li JX, Li XL, Mao QY, Tao H, Zhang YT, Yao X, Chu K, Chen QH,et al. Efficacy, safety, and immunology of an inactivated alum-adjuvant enterovirus 71 vaccine in children in China: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2013; 381:2024-32; PMID:23726161; http://dx.doi.org/ 10.1016/S0140-6736(13)61049-1 [DOI] [PubMed] [Google Scholar]
- [11].Li R, Liu L, Mo Z, Wang X, Xia J, Liang Z, Zhang Y, Li Y, Mao Q, Wang J, et al.. An inactivated enterovirus 71 vaccine in healthy children. N Engl J Med 2014; 370:829-37; PMID:24571755; http://dx.doi.org/ 10.1056/NEJMoa1303224 [DOI] [PubMed] [Google Scholar]
- [12].Zhu F, Xu W, Xia J, Liang Z, Liu Y, Zhang X, Tan X, Wang L, Mao Q, Wu J, et al.. Efficacy, safety, and immunogenicity of an enterovirus 71 vaccine in China. N Engl J Med 2014; 370:818-28; PMID:24571754; http://dx.doi.org/ 10.1056/NEJMoa1304923 [DOI] [PubMed] [Google Scholar]
- [13].Prentice RL. Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med 1989; 8:431-40; PMID:2727467; http://dx.doi.org/ 10.1002/sim.4780080407 [DOI] [PubMed] [Google Scholar]
- [14].Qin L, Gilbert PB, Corey L, McElrath MJ, Self SG. A framework for assessing immunological correlates of protection in vaccine. J Infect Dis 2007; 196:1304-12; PMID:17922394; http://dx.doi.org/ 10.1086/522428 [DOI] [PubMed] [Google Scholar]
- [15].Golber PB, Qin L, Self SG. Evaluating a surrogate endpoint at three levels, with application to vaccine. Stat Med 2008; 27:4758-78; PMID:17979212; http://dx.doi.org/ 10.1002/sim.3122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kohberger RC, Jemiolo D, Noriega F. Prediction of pertussis vaccine efficacy using a correlates of protection model. Vaccine 2008; 26:3516-21; PMID:18495303; http://dx.doi.org/ 10.1016/j.vaccine.2008.04.016 [DOI] [PubMed] [Google Scholar]
- [17].Plotkin SA, Gilbert PB. Nomenclature for Immune Correlates of Protection After Vaccination. Clinical Infectious Disease 2012; 54:1615-7; http://dx.doi.org/ 10.1093/cid/cis238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dunning AJ. A model for immunological correlates of protection. Stat Med 2006; 25:1485-97; PMID:16158409; http://dx.doi.org/ 10.1002/sim.2282 [DOI] [PubMed] [Google Scholar]
- [19].Sadoff JC, Wittes J. Correlates, surrogates, and vaccines. J Infect Dis 2007; 196:1279-81; PMID:17922389; http://dx.doi.org/ 10.1086/522432 [DOI] [PubMed] [Google Scholar]
- [20].Djomo PN, Thomas SL, Fine PEM. Correlates of vaccine-induced protection: methods and implications. World Health Organization 2013:1-49. http://www.who.int/vaccines-documents/ [Google Scholar]
- [21].Nauta J. Statistics in clinical vaccine trials Vol. 8, Correlates of Protection. Heidelberg (Berlin: ): Springer; 2011. 107p. [Google Scholar]
- [22].Gilbert PB, Gabriel EE, Miao XP, Li XM, Su SC, Parrino J, Chan IS. Fold rise in antibody titers by measured by glycoprotein-based enzyme-linked immunosorbent assay is an excellent correlate of protection for a herpes zoster vaccine, demonstrated via the vaccine efficacy curve. J Infect Dis 2014; 210:1573-81; PMID:24823623; http://dx.doi.org/ 10.1093/infdis/jiu279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wu CN, Lin YC, Fann C, Liao NS, Shih SR, Ho MS. Protection against lethal enterovirus 71 infection in newborn mice by passive immunization with subunit VP1 vaccines and inactivated virus. Vaccine 2001; 20:895-904; PMID:11738755; http://dx.doi.org/ 10.1016/S0264-410X(01)00385-1 [DOI] [PubMed] [Google Scholar]
- [24].Foo DG, Alonso S, Chow VT, Poh CL. Passive protection against lethal enterovirus 71 infection in newborn mice by neutralizing antibodies elicited by a synthetic peptide. Microbes Infect 2007; 9:1299-306; PMID:17890123; http://dx.doi.org/ 10.1016/j.micinf.2007.06.002 [DOI] [PubMed] [Google Scholar]
- [25].Chang LY, King CC, Hsu KH, Ning HC, Tsao KC, Li CC, Huang YC, Shih SR, Chiou ST, Chen PY, et al.. Risk factors of enterovirus 71 infection and associated hand, foot, and mouth disease/herpangina in children during an epidemic in Taiwan. Pediatrics 2002; 109:e88; PMID:12042582; http://dx.doi.org/ 10.1542/peds.109.6.e88 [DOI] [PubMed] [Google Scholar]
- [26].Wang SM, Liu CC. Update of enterovirus 71 infection: epidemiology, pathogenesis and vaccine. Expert Rev Anti Infect Ther 2014; 12:447-56; PMID:24579906; http://dx.doi.org/ 10.1586/14787210.2014.895666 [DOI] [PubMed] [Google Scholar]
- [27].Cox RJ. Correlates of protection to influenza virus, where do we go from here?. Hum Vaccin Immunother 2013; 9:405-8; PMID:23291930; http://dx.doi.org/ 10.4161/hv.22908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chen YJ, Meng FY, Mao Q, Li JX, Wang H, Liang ZL, Zhang YT, Gao F, Chen QH, Hu Y, et al.. Clinical evaluation for batch consistency of an inactivated enterovirus 71 vaccine in a large-scale phase 3 clinical trial. Hum Vaccin Immunother 2014; 10:1366-72; PMID:24633366; http://dx.doi.org/ 10.4161/hv.28397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Black S, Nicolay U, Vesikari T, Knuf M, Del Giudice G, Della Cioppa G, Tsai T, Clemens R, Rappuoli R. Hemagglutination inhibition antibody titers as a correlate of protection for inactivated influenza vaccines in children. Pediatr Infect Dis J 2011; 30:1081-5; PMID:21983214; http://dx.doi.org/ 10.1097/INF.0b013e318236-7662 [DOI] [PubMed] [Google Scholar]
- [30].Siber GR, Chang I, Baker S, Fernsten P, O'Brien KL, Santosham M, Klugman KP, Madhi SA, Paradiso P, Kohberger R. Estimating the protective concentration of anti-pneumococcal capsular polysccharide antibodies. Vaccine 2007; 25:3816-26; PMID:17368878; http://dx.doi.org/ 10.1016/j.vaccine.2007.01.119 [DOI] [PubMed] [Google Scholar]
- [31].Liang Z, Mao Q, Gao Q, Li X, Dong C, Yu X, Yao X, Li F, Yin W, Li Q, et al. Establishing China's national standards of antigen content and neutralizing antibody responses for evaluation of enterovirus 71 (EV71) vaccines. Vaccine 2011; 29:9668-74; PMID:22015395; http://dx.doi.org/ 10.1016/j.vaccine.2011.10.018 [DOI] [PubMed] [Google Scholar]
- [32].World Health Organization Manual for the virological investigation of poliomyelitis World Health Organization 1997. http://WHO/EPI/CD5/POLIO/90.1. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


