ABSTRACT
Pediatric tuberculosis contributes significantly to the burden of TB disease worldwide. In order to achieve the goal of eliminating TB by 2050, an effective TB vaccine is urgently needed to prevent TB transmission in children. BCG vaccination can protect children from the severe types of TB such as TB meningitis and miliary TB, while its efficacy against pediatric pulmonary TB ranged from no protection to very high protection. In recent decades, multiple new vaccine candidates have been developed, and shown encouraging safety and immunogenicity in the preclinical experiments. However, the limited data on protective efficacy in infants evaluated by clinical trials has been disappointing, an example being MVA85A. To date, no vaccine has been shown to be clinically safer and more effective than the presently licensed BCG vaccine. Hence, before a new vaccine is developed with more promising efficacy, we must reconsider how to better use the current BCG vaccine to maximize its effectiveness in children.
KEYWORDS: BCG, children, immune response, tuberculosis, vaccination
Introduction
Tuberculosis (TB), caused by Mycobacterium tuberculosis complex (MTBC), is one of the top 10 causes of death among children worldwide.1 It is estimated that one million cases of TB occur among children at ages of less than 15 y globally each year, 75% of which emerge in the 22 high TB-burden countries.2 Although pediatric tuberculosis significantly contributes to the burden of disease, it has been neglected, when compared to other focuses of National TB Control Programmes (NTP) in most settings.3,4 The lower priority afforded to pediatric tuberculosis is mainly due to its lower infectivity. This is commonplace in most NTPs, despite the fact that TB is a major cause of childhood morbidity and mortality, especially in the developing countries with poor public-health infrastructure.2 Recently, pediatric tuberculosis has received greater attention, and in 2013 the World Health Organization (WHO) developed a road map aiming to achieve zero deaths due to childhood TB by 2025.5 In order to accomplish this goal, an effective TB vaccine is urgently needed to prevent TB transmission in children.6 At present, Mycobacterium bovis bacillus Calmette-Guerin (BCG) is the only licensed tuberculosis vaccine, which has been recommended by WHO for neonatal inoculation in the countries with a high TB prevalence.7 BCG vaccination can protect the children from severe types of TB such as TB meningitis and miliary TB, while its efficacy against pediatric pulmonary TB has ranged from no protection to very high protection (0–80%).8-10 Many new vaccines show promising results against M. tuberculosis infection in preclinical trials.8,11,12
In this review, the references were retrieved by searches of Pubmed, and website associated with TB vaccines including WHO, Aeras and ClinicalTrial.gov with key words: “tuberculosis vaccine,” or “tuberculosis vaccination” or “tuberculosis prevention,” or “BCG” and “children.” The search was limited to reports published from January, 2000 to May, 2015. More than 200 articles were found, while only studies reporting data on current preventive TB vaccine candidates for the pediatric population will be reviewed.
BCG
BCG is now the most widely used vaccine worldwide.13 As of 1974, the WHO Expanded Programme on Immunization recommends BCG should be given as soon as possible after birth in high TB-prevalence countries, with coverage in infants exceeding 80%.7 Although the efficacy of BCG in preventing the development of adult pulmonary TB is controversial, BCG vaccination clearly protects infants and children from tuberculosis meningitis and severe forms of disseminated TB.7 In a prospective community-based study from Turkey, child household contacts of smear-positive adult pulmonary TB cases with a BCG scar had a much lower risk of latent tuberculosis infection than those with no BCG scar.14 Similar findings were observed from an outbreak in a nursery in the UK, which showed a significant protective effect of BCG vaccination against M. tuberculosis infection among infants.15 A meta-analysis by Trunz et al demonstrated that the BCG vaccine had prevented an estimated 73% of TB meningitis and 77% of miliary disease in children from birth to 5 y of age.16 Considering the low cost at US$2–3 per dose, BCG vaccination is a highly cost-effective intervention against childhood tuberculosis.16
Several published papers from various sources, and from various global locations, have shown that the effectiveness of BCG vaccine among children varies notably.17-20 The variation of protection by BCG may be attributed to different types of BCG, to genetic differences between populations, and to cold-chain maintenance of BCG.18 In addition, another important issue affecting this variability is the exposure to environmental mycobacteria.20 A systematic review of 21 randomized controlled trials evaluating the protection by BCG revealed that BCG imparted greater protection in northern latitudes, which may be due to less exposure to non-tuberculous mycobacteria (NTM) faced by vaccine recipients in these areas.20 In another study from China, the researchers also found that the immune response to BCG vaccination varied according to the NTM exposure among neonates.21 Although the exact reason for these observations is currently unclear, it is hypothesized that prior exposure to NTM may produces antigens which may block the replication of BCG.22 Further studies are needed to illuminate the effects of NTM exposure on BCG efficacy, which may be helpful in the future, in the development of the candidate vaccines which can be free from the compromise of NTM exposure.
Epidemics of HIV/AIDS have increased the global prevalence of TB.23 Due to the low CD4+ T cells, HIV-infected infants are more prone to develop disseminated BCG disease following neonatal inoculation.24,25 Based on these findings, the WHO recommended that BCG vaccine, a live attenuated Mycobacterium bovis, should not be given to children diagnosed as HIV positive.26 Although this strategy is essential to reduce the emergency of BCG-related diseases, it may be difficult to implement, and is rarely employed. There is an urgent need to develop a non-live alternative bacterial vaccine suitable for preventing TB transmission among children living in HIV-epidemic regions.
New TB vaccine candidates
In recent studies, findings from basic research have focused on antigens which are immunodominate, essential for virulence, containing recognized T cell epitopes and to which T cell responses are protective in animal models.27,28 Based on these findings, many new TB vaccine candidates have been developed, many showing moderately increased efficacy or/and safety over BCG in preclinical trials (Fig. 1, Table 1).29 In terms of strategies of the candidates, vaccines can be divided into 3 groups, including live or killed recombinant mycobacteria, viral-vector and protein-adjuvanted vaccines.
Figure 1.
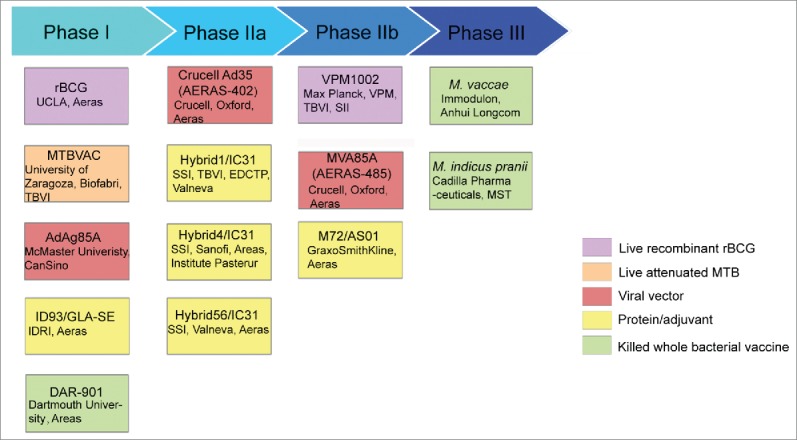
TB vaccine candidates in clinical trials in 2015. Based on the Tuberculosis Vaccines Pipeline and AREAS website.29
Table 1.
Preventive tuberculosis vaccine candidates in clinical trials.
| Namea | Composition | Classification | Strategy |
|---|---|---|---|
| rBCG30 | BCG overexpressing Ag85B | Recombinant BCG | Prime |
| VPM1002 | Recombinant BCG strain | Recombinant BCG | Prime |
| MTBVAC | Live-attenuated Mycobacterium tuberculosis | Attenuated M. tuberculosis | Prime |
| DAR-901 | M. obuense lysate | Inactivated mycobacterium | Prime-boost |
| Mycobacterium vaccae | M. vaccae lysate | Inactivated mycobacterium | Prime-Boost |
| MVA85A(AERAS-485) | Modified vaccinia virus Ankara expressing MTB antigen Ag85A | Viral vector | Prime-Boost |
| Crucell Ad35(AERAS-402) | Replication-deficient adenovirus 35 expressing MTB antigens 85A, 85B and TB10.4 | Viral vector | Prime-Boost |
| AdAg85A | Replication-deficient adenovirus 5 expressing Ag85A | Aerosol Viral vector | Prime-Boost |
| Hybrid 1/IC31 | Ag85B-ESAT6 fusion protein + IC31 adjuvant | Protein/adjuvant | Prime-Boost |
| Hybrid 4/IC31 | Ag85B-TB10.4 fusion protein + IC31 adjuvant | Protein/adjuvant | Prime-Boost |
| Hybrid 56/IC31 | Ag85B-ESAT6-Rv2660c fusion protein + IC31 adjuvant | Protein/adjuvant | Prime-Boost |
| M72/AS01 | Mtb39a-Mtb32a fusion protein + AS01 adjuvant | Protein/adjuvant | Prime-Boost |
| ID93/GLA-SE | Rv2608-Rv3619-Rv3620-Rv1813 fusion protein + GLA-SE adjuvant | Protein/adjuvant | Prime-Boost |
Based on the Tuberculosis Vaccines Pipeline and AREAS website.
Live Recombinant Mycobacteria for primary immunization
rBCG30
In general, the rationale of live recombinant mycobacteria is to add certain genes to BCG, or to remove specific genes from the natural mycobacterial genome, which will create a new vaccine to directly replace BCG.28 The first recombinant BCG vaccine was rBCG30, developed at the University of California, Los Angeles.30 By overexpressing the M. tuberculosis protein Ag85B, the recombinant BCG stimulated a strong immune response to M. tuberculosis in guinea pig models.31 The animals immunized with rBCG30 survived significantly longer after challenge from a highly virulent strain of M. tuberculosis than those immunized with BCG.31 The Phase I clinical trial completed in 2011 demonstrated that rBCG30 was safe and immunogenic. Unfortunately, this vaccine is not being further developed while awaiting the development of the next generation of auxotrophic recombinant BCG strains which avoid the inclusion of antibiotic resistance genes.11
VPM1002
A promising substitute candidate with substantially greater protection than BCG in animal models is VPM1002, which is currently in a Phase II trial in newborn infants.11,32 A total of 3 organizations participated in the development ofVPM1002, including Vakzine Projekt Management GmbH, the Max Planck Institute for Infection Biology, and the Tuberculosis Vaccine Initiative (TBVI). The vaccine is now owned and being aggressively developed by the Serum Institute of India. The vaccine contains a new BCG strain expressing the listeriolysin (hly) from the bacterium Liusteria monocytogenes.33,34 The exogenous listeriolysin facilitates the perforation of the phagosome membrane, allowing the release of recombinant BCG antigens into the cytosol of host cells.35 Hence, the vaccine is able to stimulate the CD8 T cells via major histocompatibility complex (MHC) I presentation, and further activate both T helper (Th)1 and Th17 cytokine responses.35,36 In addition, an endogenous gene ureC, encoding urease C, has been deleted in VPM1002.35 Urease C is crucial for the hydrolysis of urea, resulting in the ammonia production and a basic environment in the milieu.32 Because hly has a stringent optimum pH of 5.5, inactivating urease C is necessary to producing the acidic pH environment for hly activity.32 In early animal testing, VPM1002 showed encouraging immunogenicity, safety and tolerability in comparison with BCG vaccine.36 Further clinical trials demonstrated that VPM1002 could induce multifunctional CD4 and CD8 T cell subsets.36 Recently, a Phase II trial has been completed in South Africa to evaluate the immunogenicity and safety of VPM1002 compared with the BCG vaccine in newborn infants, while the clinical trial data is not published till now (http://ClinicalTrials.gov identifier NCT01479972, Table 2). Of note, this vaccine is produced by standard fermentation methodology, which overcomes the very poor production yield and lot to lot variability associated with the standard pellicle method for the growth of BCG.
Table 2.
Clinical trials of current preventive tuberculosis vaccine candidates in children.
| Name | Identifiera | Objective | Locations | Status |
|---|---|---|---|---|
| VPM1002 | NCT01479972 | To evaluate the safety and immunogenicity of VPM1002 in comparison with BCG in newborn infants | South Africa | Completed |
| NCT02391415 | To evaluate the safety and immunogenicity of VPM1002 in comparison with BCG in HIV-exposed/-unexposed newborn infants | South Africa | Recruiting participants | |
| MVA85A | NCT00953927 | To evaluate the safety, immunogenicity and efficacy of MVA85A in BCG vaccinated infants without tuberculosis or HIV infection | South Africa | Completed |
| Crucell Ad35(AERAS-402) | NCT01198366 | To evaluate the safety and immunogenicity of AERAS-402 in BCG-vaccinated, HIV-uninfected infants without evidence of tuberculosis | Kenya, Mozambique, South Africa | Completed. |
| Hybrid 4/IC31 | NCT01861730 | To evaluate the safety and immunogenicityof Hybrid 4+IC31 in BCG-vaccinated infants | South Africa | Recruiting participants |
| NCT02075203 | To evaluate the safety, immunogenicity, and prevention of infection with Mycobacterium tuberculosis of Hybrid4/IC31 and BCG revaccination in healthy adolescents | South Africa | Recruiting participants | |
| M72/AS01 | NCT01098474 | To evaluate the safety and immunogenicity of M72/AS01in healthy infants | Gambia | Completed |
Referenced from the website of ClinicalTrials.gov.
MTBVAC
MTBVAC, developed at the University of Zaragoza, is the first live attenuated M. tuberculosis strain in Phase I clinical trial.37 In order to generate a both safer and more effective vaccine, phoP, which encodes the transcriptional regulator associated with the regulation of M. tuberculosis virulence, and fadD26, which is crucial to major mycobacterial surface virulence factors (PDIMs) of M. tuberculosis, were knocked out.37,38 In preclinical studies, the recombinant MTBVAC vaccine exhibited similar safety and biodistribution profiles, and superior protection in animal model as compared with Mycobacterium bovis BCG.39,40 Recently, a highly attenuated MTBVAC-based live vaccine was developed by Solans et al. through an additional gene inactivation generated in erp of MTBVAC.40 Although the virulence of the MTBVAC erp(-) strain was hyper-attenuated, the results from immunocompetent mice revealed that it did not compromise its protective efficacy profile as compared with BCG.40 These findings indicate that it can be used as a potential vaccine candidate for high-risk children with immune suppression.39
Mycobacterium vaccae
Mycobacterium vaccae, a saprophytic Mycobacterium containing numerous antigenic epitopes common to M. tuberculosis, has been used as an immunotherapeutic vaccine in combination with drug therapy.41,42 There are 3 available preparations of M. vaccae currently, including a heat killed product from Immodulon of U.K, a related heat killed strain developed by Dartmouth and recently identified to be M. obuense (a close relative of M. vaccae.) and a lysate vaccine from AnHui Zhifei Longcom of China.43 Interestingly, a recent clinical trail from Tanzania has demonstrated that the protective effectiveness of M. vaccae against TB was observed among HIV-infected and BCG-vaccinated adults with CD4 counts of not less than 200 cells/μl, suggesting that M. vaccae can be used as a preventive vaccine for TB.44 Further clinical studies on the usefulness of M. vaccae for preventing infant population from TB infection are warranted.
DAR-901
DAR-901, developed at Dartmouth University and Areas, is a whole-cell mycobacterial vaccine consisting of inactivated Mycobacterium obuense.45 Different from an earlier therapeutic TB vaccine candidate SRL-172, the primary component of which was also inactivated M. obuense, DAR-901 is grown broth rather than agar, a more scalable production method.45-47 Recently, a Phase I trial of DAR-901, is currently conducted in HIV negative and HIV positive adults previously vaccinated with BCG to assess the safety, tolerability, and immunogenicity of multiple doses of DAR-901 at different dose levels. Further clinical trials need to be performed to determine the role of DAR-901 in the prevention of TB infection among children.45
Viral-vector and protein-adjuvanted vaccines that boost bcg prime
There are several new subunit TB vaccine candidates in preclinical and clinical trials that are used to complement the immune response following priming with BCG in early infant.8 These candidates are based on dominant antigens that are expressed by metabolically active M. tuberculosis.11 Compared with BCG, all the adjuvanted protein vaccines which contain fusion proteins of one or more antigens showed similar or better efficacy to protect mice and guinea pigs against M. tuberculosis infection.11 Two types of products have been developed, including viral-vectored vaccines and adjuvanted subunit vaccines.
MVA85A(AERAS-485)
MVA85A is a modified vaccinia virus Ankara (MVA) expressing the major secreted antigen Ag85A (MVA85A, AERAS-485) of M. tuberculosis.48 With the support from Aeras, the Oxford-Emergent Tuberculosis Consortium developed this virally vectored TB vaccine. As a heterologous boost for BCG, MVA85A moderately improved BCG-induced protective efficacy against M. tuberculosis challenge in animal models,49-52 which was predominantly attributable to better induction of CD4 and CD8 T cell responses, as well as antigen-specific Th1 and Th17 cells responsible for protection against M. tuberculosis.53 Several clinical trials have demonstrated that MVA85A appears to be safe and well tolerated.53,54 However, an underpowered Phase II trial of MVA85A in adults infected with HIV revealed that MVA85A showed no trend in efficacy against M. tuberculosis infection or disease.55 Similar results were observed in another Phase IIb trial of MVA85A in infants conducted in South Africa. Healthy infants aged 4 to 6 months who had been previously inoculated with BCG shortly after birth received a dose of MVA85A or a placebo between 4 and 6 months of age. In the follow-up period, the incidence of TB between the experimental and placebo groups was not different. The further vaccine efficacy analysis revealed that the low protective efficacy of 17.3% with no significance to placebo indicated that a single dose of MVA85A was unable to confer significant protection against tuberculosis disease or M. tuberculosis infection in infants (http://ClinicalTrials.gov identifier: NCT00953927).56 Further work is underway with the vaccine being used either by the aerosol route or given as a heterologous boost to an adenoviral vector prime.57
Crucell Ad35(AERAS-402)
CrucellAd35 (AERAS-402), developed by Crucell, is a replication-deficient adenovirus vector that produces 3 natural M. tuberculosis antigens 85A, 85B and TB10.4.58 The one-piece fusion polyprotein containing 3 antigens could be expressed upon immunization because the vector seroprevalence, and levels of neutralizing antibody titers, to Ad35 are relatively low in people living in developing countries.59 In mouse models and monkey, the Crucell Ad35 (Aeras-402) has been shown to elicit robust CD4 and CD8 T cell responses, producing multiple cytokines and other immune effector molecules.58 Studies in adults revealed that Crucell Ad35 (Aeras-402) was safe and immunogenic in healthy adults previously vaccinated with BCG and with no previous Mycobacterium tuberculosis infection.60 Multiparameter flow cytometric assays demonstrated that the vaccine could induce a robust CD8 T cell response as well as a polyfunctional CD4 T cell response after BCG priming.60 Another clinical Phase IIb trial with the planned recruitment of over 400 infants revealed that AERAS-402 has an acceptable safety profile in infants; however the polyfunctional T cell responses were lower than those previously measured with this vaccine in adults (http://ClinicalTrials.gov identifier: NCT01198366).61 Therefore the trial was stopped after the first 400 subjects were enrolled and did not move on to the efficacy phase.
AdAg85A
Similar to MVA85A, AdAg85A consists of a replication-deficient serotype 5 adenoviral vector containing the natural M. tuberculosis antigen 85A.62 It has been developed by McMaster University. Primary data showed thatAdAg85A provided promising protection against TB infection in mice when priming as booster vaccine for BCG when administered intranasally.62,63 Compared with intramuscular injection, intranasal administration induced stronger CD4 and CD8 T cell responses.63,64 More recently, a literature from Mu et al. reported that a new intranasally bivalent adenovirus-vectored vaccine expressing both Ag85A and TB10.4 antigen conferred a significantly improved level of protection against M. tuberculosis challenge comparable to Ag85A alone or BCG immunization.65 In a Phase I clinical trial evaluating safety and immunogenicity of AdAg85A administered intramuscularly, the vaccine was found to be safe and well tolerated.66 Although the recombinant Ad5 vaccine has shown good safety profile, the prevalence of neutralizing antibody titers against Ad5 was up to 90% in sub-Saharan Africa, which may limit the usefulness of this vaccine.66 Concern also remains on the increased rate of HIV acquisition seen in the HIV STEP trial, and the use of intramuscular adenoviruses in areas with high HIV rates is unlikely to be acceptable.
Hybrid 1/IC31
With backing from Statens Serum Institut (SSI), TBVI, and the European & Developing Countries Clinical Trials Partnership (EDCTP), a recombinant subunit vaccine named Hybrid 1/IC31(H1/IC31) was developed. It contains the hybrid protein of Antigen 85B (Ag85B) and Early Secretory Antigenic Target 6 (ESAT6), and is adjuvanted with IC31, an adjuvant system combining an antibacterial peptide (KLK) and a synthetic Toll-like receptor 9 agonist (ODN1a).67,68 Numerous studies have demonstrated that the fusion subunit vaccine was safe in HIV-infected adults with a CD4 Lymphocyte count greater than 350 cells/mm3, and no serious adverse reactions associated with the vaccine were observed.69 In addition, H1/IC31 resulted in a robust CD4 T cell response, as well as the secretion of IFN-γ.68,70,71 These strong responses persisted over 2.5 y of follow-up in BCG-naïve volunteers.70 However, because ESAT6 was also the most important antigen used in the diagnosis of latent TB, the inclusion of ESAT6 in the vaccine may increase the risk of interference with the ESAT-6-based diagnostic assay. A recent study found that 17% of the participants administered with a high dose of H1/IC31 showed positive test results with Quantiferon Gold.70
Hybrid 4/IC31
Hybrid 4/IC31 (H4/IC31), originally developed by SSI and now under development by Sanofi Pasteur, is a subunit vaccine that consists of a recombinant fusion protein of Ag85B and TB10.4, and the adjuvant IC31.72 Similar to H1/IC31, it provided promising safety and tolerability, while H4/IC31 could avoid the interference with the result of IFN-γ release assay (IGRA).72,73 When administered as priming or booster vaccine, H4/IC31 showed moderate protective efficacy against pulmonary TB in mice and guinea pigs.72,73 The inoculation of H4/IC31 as a booster for BCG in the mouse model could elicit the multifunctional CD4 T cells, which was associated with higher expression of IFN-γ, TNF-α and IL-2.73 A Phase II trial sponsored by Sanofi Pasteur, Aeras and the HIV Vaccine Trials Network to evaluate its safety and immunogenicity in BCG vaccinated health infants is currently in progress (http://ClinicalTrials.gov identifier: NCT01861730).
Hybrid 56/IC31
Hybrid 56/IC31 (H56/IC31), designed by the Statens Serum Institut (SSI) in Denmark, is an immunogenic fusion protein containing Ag85B, ESAT6 and the latency-associated protein Rv2660c, as well as the adjuvant IC31.74 In BCG-vaccinated non-human primate models, H56/IC31 has been shown to be well-tolerated and immunogenic.74 Moreover the vaccine showed excellent protective effectiveness against TB reactivation after animals were given with BCG vaccine.74 This booster vaccine is currently undergoing Phase I/IIa clinical trials to evaluate its safety and immunogenicity in HIV-negative, BCG vaccinated volunteers with/without latent TB. Unfortunately, no evaluation results have been reported on the efficacy of this vaccine to protect children against TB infection.
M72/AS01
Developed by GlaxoSmithKline, the M72/AS01 vaccine is a recombinant vaccine comprising Mtb39a and Mtb32a antigens, which are only expressed in M. tuberculosis and BCG rather than in other mycobacteria.75 AS01 is an adjuvant consisting of immunostimulants MPL and Quillaja saponaria fraction 1 (QS21) combined with liposomes, which induced humoral andTh1 cellular responses.76 A clinical trial in 110 volunteers completed in Belgium found that M72/AS01 was clinically well tolerated and induced high magnitude and persistent cell-mediated and humoral immune responses.76 In addition, there was no report of serious adverse events related to the vaccination.76,77 A Phase IIa trial from South Africa was completed in 45 M. tuberculosis infected or uninfected adults, which demonstrated that M72/AS01 elicited a novel T cell responses different from Th1 and Th17 responses.12 Although the exact function of these novel T cell populations was unknown, these cells may mediate the inflammation induced by Th1 and Th17.12 Another Phase II trial performed in South Africa found that M72/AS01 showed clinically acceptable safety and immunogenicity profile in the adolescents aged 13˜17 y.78 In a Phase II study, the assessment for the safety and immunogenicity of M72/AS01 has been completed in Gambia, which showed that M72/AS01 was acceptably tolerated with no vaccine-related serious adverse events reported in infants (http://ClinicalTrials.gov identifier: NCT01098474).79 A Phase 2b proof of concept efficacy study of the vaccine is underway in 3500 latently infected young adults in 3 countries in Africa, with results likely available in 2018.
ID93/GLA-SE
ID93, developed by the Infectious Disease Research Institute (IDRI) in Seattle, is a fusion of 4 M. tuberculosis proteins, including Rv2608, Rv3619, Rv1813 and Rv3620.Rv2608, Rv3619 and Rv1813 confer the virulence of M. tuberculosis, while Rv3620 is associated with the latent growth of M. tuberculosis.80 Combined with the TLR adjuvant glucopyranosyl lipid adjuvant-stable emulsion (GLA-SE), ID93/GLA-SE induced polyfunctional CD4 Th1cell responses characterized by secretion of antigen-specific IFN-γ, TNF and IL-2in a mouse model.80,81 In addition, boosting BCG-vaccinated guinea pigs with ID93/GLA-SE leaded to reduced pathology and fewer bacilli within the lungs, and prevented the death of animals challenged with virulent M. tuberculosis.81 This vaccine activated CD4 and CD8 T cell responses in BCG-vaccinated or TB-exposed human peripheral blood mononuclear cells.80 In a recent preclinical publication, it was shown that the use of ID93/GLA-SE vaccine may result in cross-protection against M. leprae infection.82 A Phase I clinical trial to evaluate the safety, tolerability and immunogenicity of the vaccine in healthy adults is currently in progress (http://ClinicalTrials.gov identifier: NCT02508376).
Potential strategy with bcg vaccination against tuberculosis in children
The development of novel vaccines against TB has shown tremendous growth in the past decade.11 Many of the vaccine candidates mentioned above have already entered, or will enter into clinical trials among infants.8 Although the preclinical experiments of these vaccine candidates such as MVA85A are always encouraging in safety and immunogenicity, the protective efficacy evaluated by clinical trials in humans may be disappointing.56 The conflicting results between preclinical and clinical trials indicate that our knowledge on the interplay between human host and M. tuberculosis pathogen is still limited. Previous development of TB vaccines has focused on achieving cell-mediated immunity by inducing Th1 cytokines (including IFN-γ, IL-2, and TNF-α) expression by CD4 or CD8 T cells.83 However, a clinical study in South Africa infants found that there was no correlation between the magnitude of expression of Th1 cytokines and protection against TB disease.83 These results highlight that the field should look beyond Th1 cytokines as primary indicators of immunogenicity and correlates of vaccine-induced protection. Thus, in conjunction with the progression of further TB vaccine trials, we should reconsider how to make better use of the current BCG to yield its full effectiveness as an alternative in children.84
An appropriate time for BCG vaccination
Due to the high risk of disseminated BCG disease after vaccination in HIV-infected infants, HIV infection is a relative contraindication to BCG vaccination in infants.85,86 Since infant HIV status is usually unknown at birth, it is relatively dangerous to use BCG to immunize neonates of HIV positive mothers living in regions of high HIV endemicity. Recently, Tchakoute et al. performed a study to determine whether administering the delayed BCG vaccination altered BCG-specific T-cell responses.87 Their findings revealed that the levels of polyfunctional T cells and IFN-γ produced by CD4 T cells were higher in infants giving vaccination at 14 weeks of life compared with those giving vaccination at birth.87 Hence, delayed BCG vaccination could be used as a safer alternative to vaccination at birth for HIV-infected infants or infants in the HIV-prevalent region.88 Concerns about this approach have been raised, however, as in some studies BCG has lowered all-cause mortality.89
In addition to HIV-infected infants, a recent study by Kagina et al. demonstrated that delaying BCG vaccination from birth to 10 weeks of age in HIV-unexposed infants resulted in higher frequencies of BCG-specific, polyfunctional CD4 T cells at 1 y of age.90 In contrast, Burl and his colleagues found that delaying BCG vaccination from birth to 18 weeks of age led to decreased IFN-γ and IL-17 production in the delayed vaccinated group.91 They hypothesized that the decrease might be attributed to the exposure to NTM prior to BCG vaccination, conferring the induction of Tregs, which would reduce the immune response to BCG vaccination.91 The findings from several other publications supported this hypothesis, that the increased efficacy of BCG vaccination was observed in locations farther from equator, where the infants suffer from lower exposure to NTMs.22 This conflicting data provides us several competing factors to keep in mind during development and testing. First, all studies to date were all based on the measurement of immunologic responses. Although the production of BCG-specific T cell responses may be used as a crucial mediator of protection in TB, it is unknown whether there is any resulting difference in clinical outcomes. Second, considering the different prevalence of NTM worldwide, the vaccination time after birth in different regions may be different. Another issue needed to be considered is the actual adherence of the parents to the vaccine schedule and guidelines. Numerous reports have shown that adherence to vaccination can be poor among rural or migrating populations. Hence, it is necessary to balance the dilemma that exists between delayed time and worsened vaccine uptake.
Aside from the aforementioned considerations, the interaction between BCG and other vaccines is another potential concern affecting the immunogenicity and protective efficacy. Children suffer a high frequency and severity of microbial infection leading to millions of deaths worldwide. Many children have more than 9 infections in their first year of life; thereby the need for combined vaccines has been endorsed as a feasible solution to improve the compliance of vaccination for this high-risk population. BCG is usually co-inoculated with other vaccines such as those for hepatitis B in the regions with high TB prevalence at the neonatal stage. Because of the potential interactions between live vaccines or immunological interference, there may be loss of protective efficacy andthe induction of adverse reactions by BCG vaccination. Unfortunately, the data on the interaction between BCG and other vaccines is limited, and further experiments on BCG-vaccine interactions if the timing of BCG vaccination is moved will be essential for future clinical BCG studies.
Revaccination of BCG
BCG revaccination used to be an integral part of many national tuberculosis programmes to maintain the protective efficacy of primary BCG vaccination in the tuberculin-negative school children.92 To date, little data on the efficacy of BCG revaccination is available.92-94 The first study to evaluate its efficacy was in Karonga District of Malawi.93 The researchers found that both primary vaccination and revaccination protected children and adults against leprosy, while neither the first vaccination nor revaccination showed protection against TB.93 Similarly, a cluster-randomized trial from Brazil revealed that the efficacy of BCG revaccination was 9%, suggesting that the revaccination given to children aged 7–14 y in this study did not provide substantial additional protection.94 Based on this and other data, WHO recommend not revaccination BCG in children.95 An important consideration herein is that both studies have enrolled children with one BCG scar. The detailed infection background of these participants, including infection by NTM or latent TB, was unknown. Several clinical trials have proved that both M. tuberculosis and NTM infection have diverse effects on BCG efficacy against M. tuberculosis.22,96 Thus, future studies aiming to evaluate the protective effectiveness of revaccination may wish o enroll children clearly documented to have received primary BCG vaccination, and who also have a negative PPD result to prevent the potential interference of M. tuberculosis and NTM infection.
Another important consideration raised by these 2 studies is the relative short follow-up period to observe the protective efficacy of revaccination. With the extended 4 y of follow-up and the additional cases accrued, the previous study in Brazil revealed that the overall vaccine efficacy was 12% as compared with9% for the 5-year follow up,97 indicating that the revaccination with BCG provided the additional protective efficacy against TB, and this efficacy varied with distance from equator, ranging from 1% of Manaus (with short distance from equator) to 19% of Salvador (with long distance from equator).97 This difference further strengthened the earlier hypothesis that BCG vaccination offers higher efficacy in low NTM prevalence. Taken together, revaccination with BCG may have some available protective effectiveness in some certain settings. A large cohort study would be required to assess the efficacy of BCG revaccination given to adolescent children, and to explore the factors influencing the protection against TB of BCG revaccination. An ongoing randomized trial in South Africa is examining BCG revaccination in IGRA-negative school age adolescents to study whether BCG revaccination has potential effect on TB infection rather than TB disease (http://ClinicalTrials.gov identifier: NCT02075203).
Conclusion
Pediatric tuberculosis contributes significantly to the burden of TB disease worldwide.1 Thanks to a deeper understanding of the host–pathogen relationship, impressive strides have been made in TB vaccine development in the past decades. Several TB vaccine candidates have already entered, or will enter into clinical trials among infants. Unfortunately, although the preclinical experiments of these vaccine candidates such as MVA85A are encouraging in safety and immunogenicity, the protective efficacy evaluated by clinical trials in the infants may be disappointing. The conflicting results between preclinical and clinical trials indicate that the complexity of the protective immune response induced by M. tuberculosis is currently beyond our knowledge, and the vaccine containing antigens that induce simple Th1 cell-mediated immune responses may have unsatisfactory protective efficacy against TB. Hence, future vaccine strategies may need to be focus on more variable parts of the M. tuberculosis genome and structure, rather than the conserved T-cell epitopes. To date, no vaccine has been shown to be safer and more effective than BCG vaccine. Hence, before the appearance of a new vaccine with more promising efficacy, we should reconsider how to make better use of the current BCG to yield its full effectiveness in children. Delaying BCG vaccination may be a safer alternative to vaccination at birth for HIV-infected infants or in HIV-prevalent region. A large cohort group study would be required to help us to generate the appropriate strategies for use of BCG vaccine in children.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgment
We thank Dr. Thomas G. Evans from AERAS for his constructive and valuable comments.
Funding
This study was supported by National Key Project (2012ZX10004701).
References
- [1].Swaminathan S, Rekha B. Pediatric tuberculosis: global overview and challenges. Clin Infect Dis 2010; 50(Suppl 3):S184-94; PMID:20397947; http://dx.doi.org/ 10.1086/651490 [DOI] [PubMed] [Google Scholar]
- [2].Sandgren A, Cuevas LE, Dara M, Gie RP, Grzemska M, Hawkridge A, Hesseling AC, Kampmann B, Lienhardt C, Manissero D, et al.. Childhood tuberculosis: progress requires an advocacy strategy now. Eur Respir J 2012; 40:294-7; PMID:22337859; http://dx.doi.org/ 10.1183/09031936.00187711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Marais BJ, Pai M. New approaches and emerging technologies in the diagnosis of childhood tuberculosis. Paediatr Respir Rev 2007; 8:124-33; PMID:17574156; http://dx.doi.org/ 10.1016/j.prrv.2007.04.002 [DOI] [PubMed] [Google Scholar]
- [4].Marais BJ, Schaaf HS. Childhood tuberculosis: an emerging and previously neglected problem. Infect Dis Clin North Am 2010; 24:727-49; PMID:20674801; http://dx.doi.org/ 10.1016/j.idc.2010.04.004 [DOI] [PubMed] [Google Scholar]
- [5].Acosta CD, Rusovich V, Harries AD, Ahmedov S, van den Boom M, Dara M. A new roadmap for childhood tuberculosis. Lancet Glob Health 2014; 2:e15-7; PMID:25104625; http://dx.doi.org/ 10.1016/S2214-109X(13)70153-0 [DOI] [PubMed] [Google Scholar]
- [6].Hamzaoui A, Yaalaoui S, Tritar Cherif F, Slim Saidi L, Berraies A. Childhood tuberculosis: a concern of the modern world. Eur Respir Rev 2014; 23:278-91; PMID:25176964; http://dx.doi.org/ 10.1183/09059180.00005314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Guidance for National Tuberculosis Programmes on the management of tuberculosis in children. Chapter 1: introduction and diagnosis of tuberculosis in children. Int J Tuberc Lung Dis 2006; 10:1091-7; PMID:17044200 [PubMed] [Google Scholar]
- [8].Kaufmann SH, Hussey G, Lambert PH. New vaccines for tuberculosis. Lancet 2010; 375:2110-9; PMID:20488515; http://dx.doi.org/ 10.1016/S0140-6736(10)60393-5 [DOI] [PubMed] [Google Scholar]
- [9].Chawla S, Garg D, Jain RB, Khanna P, Choudhary S, Sahoo S, Singh I. Tuberculosis vaccine: time to look into future. Hum Vaccin Immunother 2014; 10:420-2; PMID:24231233; http://dx.doi.org/ 10.4161/hv.27108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Svenson S, Kallenius G, Pawlowski A, Hamasur B. Towards new tuberculosis vaccines. Hum Vaccin 2010; 6:309-17; PMID:20372087; http://dx.doi.org/ 10.4161/hv.6.4.10711 [DOI] [PubMed] [Google Scholar]
- [11].Kaufmann SH. Tuberculosis vaccine development: strength lies in tenacity. Trends Immunol 2012; 33:373-9; PMID:22560865; http://dx.doi.org/ 10.1016/j.it.2012.03.004 [DOI] [PubMed] [Google Scholar]
- [12].Principi N, Esposito S. The present and future of tuberculosis vaccinations. Tuberculosis (Edinb) 2015; 95:6-13; PMID:25458613; http://dx.doi.org/ 10.1016/j.tube.2014.10.004 [DOI] [PubMed] [Google Scholar]
- [13].Hong CY, Wang F, Gui J, Liu XL. [Characteristics of pncA gene in multidrug-resistant Mycobacterium tuberculosis isolates and its correlation with drug resistance to pyrazinamide]. Zhonghua Yu Fang Yi Xue Za Zhi 2012; 46:436-9; PMID:22883731 [PubMed] [Google Scholar]
- [14].Soysal A, Millington KA, Bakir M, Dosanjh D, Aslan Y, Deeks JJ, Efe S, Staveley I, Ewer K, Lalvani A. Effect of BCG vaccination on risk of Mycobacterium tuberculosis infection in children with household tuberculosis contact: a prospective community-based study. Lancet 2005; 366:1443-51; PMID:16243089; http://dx.doi.org/ 10.1016/S0140-6736(05)67534-4 [DOI] [PubMed] [Google Scholar]
- [15].Eriksen J, Chow JY, Mellis V, Whipp B, Walters S, Abrahamson E, Abubakar I. Protective effect of BCG vaccination in a nursery outbreak in 2009: time to reconsider the vaccination threshold? Thorax 2010; 65:1067-71; PMID:21030395; http://dx.doi.org/ 10.1136/thx.2010.140186 [DOI] [PubMed] [Google Scholar]
- [16].Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet 2006; 367:1173-80; PMID:16616560; http://dx.doi.org/ 10.1016/S0140-6736(06)68507-3 [DOI] [PubMed] [Google Scholar]
- [17].Rodrigues LC, Diwan VK, Wheeler JG. Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: a meta-analysis. Int J Epidemiol 1993; 22:1154-8; PMID:8144299; http://dx.doi.org/ 10.1093/ije/22.6.1154 [DOI] [PubMed] [Google Scholar]
- [18].Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 1995; 346:1339-45; PMID:7475776; http://dx.doi.org/ 10.1016/S0140-6736(95)92348-9 [DOI] [PubMed] [Google Scholar]
- [19].Colditz GA, Berkey CS, Mosteller F, Brewer TF, Wilson ME, Burdick E, Fineberg HV. The efficacy of bacillus Calmette-Guerin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics 1995; 96:29-35; PMID:7596718 [PubMed] [Google Scholar]
- [20].Mangtani P, Abubakar I, Ariti C, Beynon R, Pimpin L, Fine PE, Rodrigues LC, Smith PG, Lipman M, Whiting PF, et al.. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis 2014; 58:470-80; PMID:24336911; http://dx.doi.org/ 10.1093/cid/cit790 [DOI] [PubMed] [Google Scholar]
- [21].Pang Y, Kang W, Zhao A, Liu G, Du W, Xu M, Wang G, Zhao Y, Zheng S. The effect of bacille Calmette-Guerin vaccination at birth on immune response in China. Vaccine 2015; 33:209-13; PMID:25454854; http://dx.doi.org/ 10.1016/j.vaccine.2014.10.030 [DOI] [PubMed] [Google Scholar]
- [22].Poyntz HC, Stylianou E, Griffiths KL, Marsay L, Checkley AM, McShane H. Non-tuberculous mycobacteria have diverse effects on BCG efficacy against Mycobacterium tuberculosis. Tuberculosis (Edinb) 2014; 94:226-37; PMID:24572168; http://dx.doi.org/ 10.1016/j.tube.2013.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Harries AD, Zachariah R, Corbett EL, Lawn SD, Santos-Filho ET, Chimzizi R, Harrington M, Maher D, Williams BG, De Cock KM. The HIV-associated tuberculosis epidemic–when will we act? Lancet 2010; 375:1906-19; PMID:20488516; http://dx.doi.org/ 10.1016/S0140-6736(10)60409-6 [DOI] [PubMed] [Google Scholar]
- [24].Azzopardi P, Bennett CM, Graham SM, Duke T. Bacille Calmette-Guerin vaccine-related disease in HIV-infected children: a systematic review. Int J Tuberc Lung Dis 2009; 13:1331-44; PMID:19861003 [PubMed] [Google Scholar]
- [25].Hesseling AC, Marais BJ, Gie RP, Schaaf HS, Fine PE, Godfrey-Faussett P, Beyers N. The risk of disseminated Bacille Calmette-Guerin (BCG) disease in HIV-infected children. Vaccine 2007; 25:14-8; PMID:16959383; http://dx.doi.org/ 10.1016/j.vaccine.2006.07.020 [DOI] [PubMed] [Google Scholar]
- [26].World Health Organization. Revised BCG vaccination guidelines for infants at risk for HIV infection. Wkly Epidemiol Rec 2007; 82:193-6; PMID:17526121 [PubMed] [Google Scholar]
- [27].Kaufmann SH. Fact and fiction in tuberculosis vaccine research: 10 years later. Lancet Infect Dis 2011; 11:633-40; PMID:21798463; http://dx.doi.org/ 10.1016/S1473-3099(11)70146-3 [DOI] [PubMed] [Google Scholar]
- [28].Kupferschmidt K. Infectious disease. Taking a new shot at a TB vaccine. Science 2011; 334:1488-90; PMID:22174226; http://dx.doi.org/ 10.1126/science.334.6062.1488 [DOI] [PubMed] [Google Scholar]
- [29].Beresford B, Sadoff JC. Update on research and development pipeline: tuberculosis vaccines. Clin Infect Dis 2010; 50(Suppl 3):S178-83; PMID:20397946; http://dx.doi.org/ 10.1086/651489 [DOI] [PubMed] [Google Scholar]
- [30].Horwitz MA, Harth G, Dillon BJ, Maslesa-Galic S. Recombinant bacillus calmette-guerin (BCG) vaccines expressing the Mycobacterium tuberculosis 30-kDa major secretory protein induce greater protective immunity against tuberculosis than conventional BCG vaccines in a highly susceptible animal model. Proc Natl Acad Sci U S A 2000; 97:13853-8; PMID:11095745; http://dx.doi.org/ 10.1073/pnas.250480397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Horwitz MA, Harth G. A new vaccine against tuberculosis affords greater survival after challenge than the current vaccine in the guinea pig model of pulmonary tuberculosis. Infect Immun 2003; 71:1672-9; PMID:12654780; http://dx.doi.org/ 10.1128/IAI.71.4.1672-1679.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Grode L, Seiler P, Baumann S, Hess J, Brinkmann V, Nasser Eddine A, Mann P, Goosmann C, Bandermann S, Smith D, et al.. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guerin mutants that secrete listeriolysin. J Clin Invest 2005; 115:2472-9; PMID:16110326; http://dx.doi.org/ 10.1172/JCI24617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Grode L, Ganoza CA, Brohm C, Weiner J 3rd, Eisele B, Kaufmann SH. Safety and immunogenicity of the recombinant BCG vaccine VPM1002 in a phase 1 open-label randomized clinical trial. Vaccine 2013; 31:1340-8; PMID:23290835; http://dx.doi.org/ 10.1016/j.vaccine.2012.12.053 [DOI] [PubMed] [Google Scholar]
- [34].Dussurget O, Pizarro-Cerda J, Cossart P. Molecular determinants of Listeria monocytogenes virulence. Annu Rev Microbiol 2004; 58:587-610; PMID:15487949; http://dx.doi.org/ 10.1146/annurev.micro.57.030502.090934 [DOI] [PubMed] [Google Scholar]
- [35].Desel C, Dorhoi A, Bandermann S, Grode L, Eisele B, Kaufmann SH. Recombinant BCG DeltaureC hly+ induces superior protection over parental BCG by stimulating a balanced combination of type 1 and type 17 cytokine responses. J Infect Dis 2011; 204:1573-84; PMID:21933877; http://dx.doi.org/ 10.1093/infdis/jir592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Farinacci M, Weber S, Kaufmann SH. The recombinant tuberculosis vaccine rBCG DeltaureC::hly(+) induces apoptotic vesicles for improved priming of CD4(+) and CD8(+) T cells. Vaccine 2012; 30:7608-14; PMID:23088886; http://dx.doi.org/ 10.1016/j.vaccine.2012.10.031 [DOI] [PubMed] [Google Scholar]
- [37].Arbues A, Aguilo JI, Gonzalo-Asensio J, Marinova D, Uranga S, Puentes E, et al.. Construction, characterization and preclinical evaluation of MTBVAC, the first live-attenuated M. tuberculosis-based vaccine to enter clinical trials. Vaccine 2013; 31:4867-73; PMID:23965219; http://dx.doi.org/ 10.1016/j.vaccine.2013.07.051 [DOI] [PubMed] [Google Scholar]
- [38].Nambiar JK, Pinto R, Aguilo JI, Takatsu K, Martin C, Britton WJ, Triccas JA. Protective immunity afforded by attenuated, PhoP-deficient Mycobacterium tuberculosis is associated with sustained generation of CD4+ T-cell memory. Eur J Immunol 2012; 42:385-92; PMID:22105536; http://dx.doi.org/ 10.1002/eji.201141903 [DOI] [PubMed] [Google Scholar]
- [39].Kaufmann SH, Gengenbacher M. Recombinant live vaccine candidates against tuberculosis. Curr Opin Biotechnol 2012; 23:900-7; PMID:22483201; http://dx.doi.org/ 10.1016/j.copbio.2012.03.007 [DOI] [PubMed] [Google Scholar]
- [40].Solans L, Uranga S, Aguilo N, Arnal C, Gomez AB, Monzon M, Badiola JJ, Gicquel B, Martin C. Hyper-attenuated MTBVAC erp mutant protects against tuberculosis in mice. Vaccine 2014; 32:5192-7; PMID:25066740; http://dx.doi.org/ 10.1016/j.vaccine.2014.07.047 [DOI] [PubMed] [Google Scholar]
- [41].Stanford J, Stanford C, Grange J. Immunotherapy with Mycobacterium vaccae in the treatment of tuberculosis. Front Biosci 2004; 9:1701-19; PMID:14977580; http://dx.doi.org/ 10.2741/1292 [DOI] [PubMed] [Google Scholar]
- [42].Dlugovitzky D, Fiorenza G, Farroni M, Bogue C, Stanford C, Stanford J. Immunological consequences of three doses of heat-killed Mycobacterium vaccae in the immunotherapy of tuberculosis. Respir Med 2006; 100:1079-87; PMID:16278080; http://dx.doi.org/ 10.1016/j.rmed.2005.09.026 [DOI] [PubMed] [Google Scholar]
- [43].Groschel MI, Prabowo SA, Cardona PJ, Stanford JL, van der Werf TS. Therapeutic vaccines for tuberculosis–a systematic review. Vaccine 2014; 32:3162-8; PMID:24726245; http://dx.doi.org/ 10.1016/j.vaccine.2014.03.047 [DOI] [PubMed] [Google Scholar]
- [44].Lahey T, Arbeit RD, Bakari M, Horsburgh CR, Matee M, Waddell R, Mtei L, Vuola JM, Pallangyo K, von Reyn CF. Immunogenicity of a protective whole cell mycobacterial vaccine in HIV-infected adults: a phase III study in Tanzania. Vaccine 2010; 28:7652-8; PMID:20875492; http://dx.doi.org/ 10.1016/j.vaccine.2010.09.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Whole Mycobacteria Cell Vaccines for Tuberculosis Summary Group . Developing whole mycobacteria cell vaccines for tuberculosis: Workshop proceedings, Max Planck Institute for Infection Biology, Berlin, Germany, July 9, 2014. Vaccine 2015; 33:3047-55; PMID:25882170; http://dx.doi.org/ 10.1016/j.vaccine.2015.03.056 [DOI] [PubMed] [Google Scholar]
- [46].Johnson JL, Kamya RM, Okwera A, Loughlin AM, Nyole S, Hom DL, Wallis RS, Hirsch CS, Wolski K, Foulds J, et al.. Randomized controlled trial of Mycobacterium vaccae immunotherapy in non-human immunodeficiency virus-infected ugandan adults with newly diagnosed pulmonary tuberculosis. The Uganda-Case Western Reserve University Research Collaboration. J Infect Dis 2000; 181:1304-12; PMID:10753731; http://dx.doi.org/ 10.1086/315393 [DOI] [PubMed] [Google Scholar]
- [47].da Costa C, Walker B, Bonavia A. Tuberculosis vaccines–state of the art, and novel approaches to vaccine development. Int J Infect Dis 2015; 32:5-12; PMID:25809749; http://dx.doi.org/ 10.1016/j.ijid.2014.11.026 [DOI] [PubMed] [Google Scholar]
- [48].McShane H, Pathan AA, Sander CR, Keating SM, Gilbert SC, Huygen K, Fletcher HA, Hill AV. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat Med 2004; 10:1240-4; PMID:15502839; http://dx.doi.org/ 10.1038/nm1128 [DOI] [PubMed] [Google Scholar]
- [49].Vordermeier HM, Villarreal-Ramos B, Cockle PJ, McAulay M, Rhodes SG, Thacker T, Gilbert SC, McShane H, Hill AV, Xing Z, et al.. Viral booster vaccines improve Mycobacterium bovis BCG-induced protection against bovine tuberculosis. Infect Immun 2009; 77:3364-73; PMID:19487476; http://dx.doi.org/ 10.1128/IAI.00287-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Whelan KT, Pathan AA, Sander CR, Fletcher HA, Poulton I, Alder NC, Hill AV, McShane H. Safety and immunogenicity of boosting BCG vaccinated subjects with BCG: comparison with boosting with a new TB vaccine, MVA85A. PLoS One 2009; 4:e5934; PMID:19529780; http://dx.doi.org/ 10.1371/journal.pone.0005934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Goonetilleke NP, McShane H, Hannan CM, Anderson RJ, Brookes RH, Hill AV. Enhanced immunogenicity and protective efficacy against Mycobacterium tuberculosis of bacille Calmette-Guerin vaccine using mucosal administration and boosting with a recombinant modified vaccinia virus Ankara. J Immunol 2003; 171:1602-9; PMID:12874255; http://dx.doi.org/ 10.4049/jimmunol.171.3.1602 [DOI] [PubMed] [Google Scholar]
- [52].Williams A, Goonetilleke NP, McShane H, Clark SO, Hatch G, Gilbert SC, Hill AV. Boosting with poxviruses enhances Mycobacterium bovis BCG efficacy against tuberculosis in guinea pigs. Infect Immun 2005; 73:3814-6; PMID:15908420; http://dx.doi.org/ 10.1128/IAI.73.6.3814-3816.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Scriba TJ, Tameris M, Mansoor N, Smit E, van der Merwe L, Mauff K, Hughes EJ, Moyo S, Brittain N, Lawrie A, et al.. Dose-finding study of the novel tuberculosis vaccine, MVA85A, in healthy BCG-vaccinated infants. J Infect Dis 2011; 203:1832-43; PMID:21606542; http://dx.doi.org/ 10.1093/infdis/jir195 [DOI] [PubMed] [Google Scholar]
- [54].Pathan AA, Minassian AM, Sander CR, Rowland R, Porter DW, Poulton ID, Hill AV, Fletcher HA, McShane H. Effect of vaccine dose on the safety and immunogenicity of a candidate TB vaccine, MVA85A, in BCG vaccinated UK adults. Vaccine 2012; 30:5616-24; PMID:22789508; http://dx.doi.org/ 10.1016/j.vaccine.2012.06.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ndiaye BP, Thienemann F, Ota M, Landry BS, Camara M, Dieye S, Dieye TN, Esmail H, Goliath R, Huygen K, et al.. Safety, immunogenicity, and efficacy of the candidate tuberculosis vaccine MVA85A in healthy adults infected with HIV-1: a randomised, placebo-controlled, phase 2 trial. Lancet Respir Med 2015; 3:190-200; PMID:25726088; http://dx.doi.org/ 10.1016/S2213-2600(15)00037-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, Shea JE, McClain JB, Hussey GD, Hanekom WA, et al.. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet 2013; 381:1021-8; PMID:23391465; http://dx.doi.org/ 10.1016/S0140-6736(13)60177-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Satti I, Meyer J, Harris SA, Manjaly Thomas ZR, Griffiths K, Antrobus RD, Rowland R, Ramon RL, Smith M, Sheehan S, et al.. Safety and immunogenicity of a candidate tuberculosis vaccine MVA85A delivered by aerosol in BCG-vaccinated healthy adults: a phase 1, double-blind, randomised controlled trial. Lancet Infect Dis 2014; 14:939-46; PMID:25151225; http://dx.doi.org/ 10.1016/S1473-3099(14)70845-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Radosevic K, Wieland CW, Rodriguez A, Weverling GJ, Mintardjo R, Gillissen G, Vogels R, Skeiky YA, Hone DM, Sadoff JC, et al.. Protective immune responses to a recombinant adenovirus type 35 tuberculosis vaccine in two mouse strains: CD4 and CD8 T-cell epitope mapping and role of gamma interferon. Infect Immun 2007; 75:4105-15; PMID:17526747; http://dx.doi.org/ 10.1128/IAI.00004-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Vogels R, Zuijdgeest D, van Rijnsoever R, Hartkoorn E, Damen I, de Bethune MP, Kostense S, Penders G, Helmus N, Koudstaal W, et al.. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J Virol 2003; 77:8263-71; PMID:12857895; http://dx.doi.org/ 10.1128/JVI.77.15.8263-8271.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Abel B, Tameris M, Mansoor N, Gelderbloem S, Hughes J, Abrahams D, Makhethe L, Erasmus M, de Kock M, van der Merwe L, et al.. The novel tuberculosis vaccine, AERAS-402, induces robust and polyfunctional CD4+ and CD8+ T cells in adults. Am J Respir Crit Care Med 2010; 181:1407-17; PMID:20167847; http://dx.doi.org/ 10.1164/rccm.200910-1484OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Tameris M, Hokey DA, Nduba V, Sacarlal J, Laher F, Kiringa G, Gondo K, Lazarus EM, Gray GE, Nachman S,et al. A double-blind, randomised, placebo-controlled, dose-finding trial of the novel tuberculosis vaccine AERAS-402, an adenovirus-vectored fusion protein, in healthy, BCG-vaccinated infants. Vaccine 2015; 33:2944-54; PMID:25936724; http://dx.doi.org/ 10.1016/j.vaccine.2015.03.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Wang J, Thorson L, Stokes RW, Santosuosso M, Huygen K, Zganiacz A, Hitt M, Xing Z. Single mucosal, but not parenteral, immunization with recombinant adenoviral-based vaccine provides potent protection from pulmonary tuberculosis. J Immunol 2004; 173:6357-65; PMID:15528375; http://dx.doi.org/ 10.4049/jimmunol.173.10.6357 [DOI] [PubMed] [Google Scholar]
- [63].Santosuosso M, McCormick S, Zhang X, Zganiacz A, Xing Z. Intranasal boosting with an adenovirus-vectored vaccine markedly enhances protection by parenteral Mycobacterium bovis BCG immunization against pulmonary tuberculosis. Infect Immun 2006; 74:4634-43; PMID:16861651; http://dx.doi.org/ 10.1128/IAI.00517-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Santosuosso M, Zhang X, McCormick S, Wang J, Hitt M, Xing Z. Mechanisms of mucosal and parenteral tuberculosis vaccinations: adenoviral-based mucosal immunization preferentially elicits sustained accumulation of immune protective CD4 and CD8 T cells within the airway lumen. J Immunol 2005; 174:7986-94; PMID:15944305; http://dx.doi.org/ 10.4049/jimmunol.174.12.7986 [DOI] [PubMed] [Google Scholar]
- [65].Mu J, Jeyanathan M, Small CL, Zhang X, Roediger E, Feng X, Chong D, Gauldie J, Xing Z. Immunization with a bivalent adenovirus-vectored tuberculosis vaccine provides markedly improved protection over its monovalent counterpart against pulmonary tuberculosis. Mol Ther 2009; 17:1093-100; PMID:19319120; http://dx.doi.org/ 10.1038/mt.2009.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Montagnani C, Chiappini E, Galli L, de Martino M. Vaccine against tuberculosis: what's new? BMC Infect Dis 2014; 14 Suppl 1:S2; PMID:24564340; http://dx.doi.org/ 10.1186/1471-2334-14-S1-S2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Olafsdottir TA, Lingnau K, Nagy E, Jonsdottir I. IC31, a two-component novel adjuvant mixed with a conjugate vaccine enhances protective immunity against pneumococcal disease in neonatal mice. Scand J Immunol 2009; 69:194-202; PMID:19281531; http://dx.doi.org/ 10.1111/j.1365-3083.2008.02225.x [DOI] [PubMed] [Google Scholar]
- [68].van Dissel JT, Soonawala D, Joosten SA, Prins C, Arend SM, Bang P, Tingskov PN, Lingnau K, Nouta J, Hoff ST, et al.. Ag85B-ESAT-6 adjuvanted with IC31(R) promotes strong and long-lived Mycobacterium tuberculosis specific T cell responses in volunteers with previous BCG vaccination or tuberculosis infection. Vaccine 2011; 29:2100-9; PMID:21256189; http://dx.doi.org/ 10.1016/j.vaccine.2010.12.135 [DOI] [PubMed] [Google Scholar]
- [69].Reither K, Katsoulis L, Beattie T, Gardiner N, Lenz N, Said K, Mfinanga E, Pohl C, Fielding KL, Jeffery H, et al.. Safety and immunogenicity of H1/IC31(R), an adjuvanted TB subunit vaccine, in HIV-infected adults with CD4+ lymphocyte counts greater than 350 cells/mm3: a phase II, multi-centre, double-blind, randomized, placebo-controlled trial. PLoS One 2014; 9:e114602; PMID:25490675; http://dx.doi.org/ 10.1371/journal.pone.0114602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].van Dissel JT, Arend SM, Prins C, Bang P, Tingskov PN, Lingnau K, Nouta J, Klein MR, Rosenkrands I, Ottenhoff TH, et al.. Ag85B-ESAT-6 adjuvanted with IC31 promotes strong and long-lived Mycobacterium tuberculosis specific T cell responses in naive human volunteers. Vaccine 2010; 28:3571-81; PMID:20226890; http://dx.doi.org/ 10.1016/j.vaccine.2010.02.094 [DOI] [PubMed] [Google Scholar]
- [71].Ottenhoff TH, Doherty TM, van Dissel JT, Bang P, Lingnau K, Kromann I, Andersen P. First in humans: a new molecularly defined vaccine shows excellent safety and strong induction of long-lived Mycobacterium tuberculosis-specific Th1-cell like responses. Hum Vaccin 2010; 6:1007-15; PMID:21178394; http://dx.doi.org/ 10.4161/hv.6.12.13143 [DOI] [PubMed] [Google Scholar]
- [72].Skeiky YA, Dietrich J, Lasco TM, Stagliano K, Dheenadhayalan V, Goetz MA, Cantarero L, Basaraba RJ, Bang P, Kromann I, et al.. Non-clinical efficacy and safety of HyVac4:IC31 vaccine administered in a BCG prime-boost regimen. Vaccine 2010; 28:1084-93; PMID:19896449; http://dx.doi.org/ 10.1016/j.vaccine.2009.10.114 [DOI] [PubMed] [Google Scholar]
- [73].Billeskov R, Elvang TT, Andersen PL, Dietrich J. The HyVac4 subunit vaccine efficiently boosts BCG-primed anti-mycobacterial protective immunity. PLoS One 2012; 7:e39909; PMID:22768165; http://dx.doi.org/ 10.1371/journal.pone.0039909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Lin PL, Dietrich J, Tan E, Abalos RM, Burgos J, Bigbee C, Bigbee M, Milk L, Gideon HP, Rodgers M, et al.. The multistage vaccine H56 boosts the effects of BCG to protect cynomolgus macaques against active tuberculosis and reactivation of latent Mycobacterium tuberculosis infection. J Clin Invest 2012; 122:303-14; PMID:22133873; http://dx.doi.org/ 10.1172/JCI46252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Skeiky YA, Lodes MJ, Guderian JA, Mohamath R, Bement T, Alderson MR, Reed SG. Cloning, expression, and immunological evaluation of two putative secreted serine protease antigens of Mycobacterium tuberculosis. Infect Immun 1999; 67:3998-4007; PMID:10417166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Leroux-Roels I, Forgus S, De Boever F, Clement F, Demoitie MA, Mettens P, Moris P, Ledent E, Leroux-Roels G, Ofori-Anyinam O, et al.. Improved CD4(+) T cell responses to Mycobacterium tuberculosis in PPD-negative adults by M72/AS01 as compared to the M72/AS02 and Mtb72F/AS02 tuberculosis candidate vaccine formulations: a randomized trial. Vaccine 2013; 31:2196-206; PMID; http://dx.doi.org/ 10.1016/j.vaccine.2012.05.035 [DOI] [PubMed] [Google Scholar]
- [77].Day CL, Tameris M, Mansoor N, van Rooyen M, de Kock M, Geldenhuys H, Erasmus M, Makhethe L, Hughes EJ, Gelderbloem S, et al.. Induction and regulation of T-cell immunity by the novel tuberculosis vaccine M72/AS01 in South African adults. Am J Respir Crit Care Med 2013; 188:492-502; PMID:23306546; http://dx.doi.org/ 10.1164/rccm.201208-1385OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Penn-Nicholson A, Geldenhuys H, Burny W, van der Most R, Day CL, Jongert E, Moris P, Hatherill M, Ofori-Anyinam O, Hanekom W, et al.. Safety and immunogenicity of candidate vaccine M72/AS01 in adolescents in a TB endemic setting. Vaccine 2015; PMID:26072017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Idoko OT, Owolabi OA, Owiafe PK, Moris P, Odutola A, Bollaerts A, Ogundare E, Jongert E, Demoitié MA, Ofori-Anyinam O, et al.. Safety and immunogenicity of the M72/AS01 candidate tuberculosis vaccine when given as a booster to BCG in Gambian infants: an open-label randomized controlled trial. Tuberculosis (Edinb) 2014; 94:564-78; PMID:25305000 [DOI] [PubMed] [Google Scholar]
- [80].Bertholet S, Ireton GC, Ordway DJ, Windish HP, Pine SO, Kahn M, Phan T, Orme IM, Vedvick TS, Baldwin SL, et al.. A defined tuberculosis vaccine candidate boosts BCG and protects against multidrug-resistant Mycobacterium tuberculosis. Sci Transl Med 2010; 2:53ra74; PMID:20944089; http://dx.doi.org/ 10.1126/scitranslmed.3001094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Baldwin SL, Bertholet S, Reese VA, Ching LK, Reed SG, Coler RN. The importance of adjuvant formulation in the development of a tuberculosis vaccine. J Immunol 2012; 188:2189-97; PMID:22291184; http://dx.doi.org/ 10.4049/jimmunol.1102696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Duthie MS, Coler RN, Laurance JD, Sampaio LH, Oliveira RM, Sousa AL, Stefani MM, Maeda Y, Matsuoka M, Makino M, et al.. Protection against Mycobacterium leprae infection by the ID83/GLA-SE and ID93/GLA-SE vaccines developed for tuberculosis. Infect Immun 2014; 82:3979-85; PMID:25024362; http://dx.doi.org/ 10.1128/IAI.02145-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Kagina BM, Abel B, Scriba TJ, Hughes EJ, Keyser A, Soares A, Gamieldien H, Sidibana M, Hatherill M, Gelderbloem S, et al.. Specific T cell frequency and cytokine expression profile do not correlate with protection against tuberculosis after bacillus Calmette-Guerin vaccination of newborns. Am J Respir Crit Care Med 2010; 182:1073-9; PMID:20558627; http://dx.doi.org/ 10.1164/rccm.201003-0334OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Dye C. Making wider use of the world's most widely used vaccine: Bacille Calmette-Guerin revaccination reconsidered. J R Soc Interface 2013; 10:20130365; PMID:23904584; http://dx.doi.org/ 10.1098/rsif.2013.0365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].BCG vaccine . WHO position paper. Wkly Epidemiol Rec 2004; 79:27-38; PMID:14768305 [PubMed] [Google Scholar]
- [86].Meeting of the immunization Strategic Advisory Group of Experts, April 2007–conclusions and recommendations. Wkly Epidemiol Rec 2007; 82:181-93; PMID:17526120 [PubMed] [Google Scholar]
- [87].Tchakoute CT, Hesseling AC, Kidzeru EB, Gamieldien H, Passmore JA, Jones CE, Gray CM, Sodora DL, Jaspan HB. Delaying BCG vaccination until 8 weeks of age results in robust BCG-specific T-cell responses in HIV-exposed infants. J Infect Dis 2015; 211:338-46; PMID:25108027; http://dx.doi.org/ 10.1093/infdis/jiu434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Kay AW, Blish CA. Delayed BCG vaccination–time to take a shot. J Infect Dis 2015; 211:335-7; PMID:25108029; http://dx.doi.org/ 10.1093/infdis/jiu435 [DOI] [PubMed] [Google Scholar]
- [89].Blakney AK, Tchakoute CT, Hesseling AC, Kidzeru EB, Jones CE, Passmore JA, Sodora DL, Gray CM, Jaspan HB.. Delayed BCG vaccination results in minimal alterations in T cell immunogenicity of acellular pertussis and tetanus immunizations in HIV-exposed infants. Vaccine 2015; 33:4782-9; PMID:26259542; http://dx.doi.org/ 10.1016/j.vaccine.2015.07.096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Kagina BM, Abel B, Bowmaker M, Scriba TJ, Gelderbloem S, Smit E, Erasmus M, Nene N, Walzl G, Black G, et al.. Delaying BCG vaccination from birth to 10 weeks of age may result in an enhanced memory CD4 T cell response. Vaccine 2009; 27:5488-95; PMID:19616494; http://dx.doi.org/ 10.1016/j.vaccine.2009.06.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Burl S, Adetifa UJ, Cox M, Touray E, Ota MO, Marchant A, Whittle H, McShane H, Rowland-Jones SL, Flanagan KL. Delaying bacillus Calmette-Guerin vaccination from birth to 4 1/2 months of age reduces postvaccination Th1 and IL-17 responses but leads to comparable mycobacterial responses at 9 months of age. J Immunol 2010; 185:2620-8; PMID:20644160; http://dx.doi.org/ 10.4049/jimmunol.1000552 [DOI] [PubMed] [Google Scholar]
- [92].Tala-Heikkila MM, Tuominen JE, Tala EO. Bacillus Calmette-Guerin revaccination questionable with low tuberculosis incidence. Am J Respir Crit Care Med 1998; 157:1324-7; PMID:9563757; http://dx.doi.org/ 10.1164/ajrccm.157.4.9706037 [DOI] [PubMed] [Google Scholar]
- [93].Randomised controlled trial of single BCG, repeated BCG, or combined BCG and killed Mycobacterium leprae vaccine for prevention of leprosy and tuberculosis in Malawi. Karonga Prevention Trial Group. Lancet 1996; 348:17-24; PMID:8691924; http://dx.doi.org/ 10.1016/S0140-6736(96)02166-6 [DOI] [PubMed] [Google Scholar]
- [94].Rodrigues LC, Pereira SM, Cunha SS, Genser B, Ichihara MY, de Brito SC, Hijjar MA, Dourado I, Cruz AA, Sant'Anna C, et al.. Effect of BCG revaccination on incidence of tuberculosis in school-aged children in Brazil: the BCG-REVAC cluster-randomised trial. Lancet 2005; 366:1290-5; PMID:16214599; http://dx.doi.org/ 10.1016/S0140-6736(05)67145-0 [DOI] [PubMed] [Google Scholar]
- [95].World Health Organization Guidance for national tuberculosis programmes on the management of tuberculosis in children. 2006; WHO/HTM/TB/2006.371. [PubMed] [Google Scholar]
- [96].Trial of BCG vaccines in south India for tuberculosis prevention: first report–Tuberculosis Prevention Trial. Bull World Health Organ 1979; 57:819-27; PMID:396057 [PMC free article] [PubMed] [Google Scholar]
- [97].Barreto ML, Pereira SM, Pilger D, Cruz AA, Cunha SS, Sant'Anna C, Ichihara MY, Genser B, Rodrigues LC. Evidence of an effect of BCG revaccination on incidence of tuberculosis in school-aged children in Brazil: second report of the BCG-REVAC cluster-randomised trial. Vaccine 2011; 29:4875-7; PMID:21616115; http://dx.doi.org/ 10.1016/j.vaccine.2011.05.023 [DOI] [PubMed] [Google Scholar]


