Abstract
The first transgenic mouse of the TCL1 oncogene was described more than 15 years ago, and since then, the overexpression of the gene in T- and B-cells in vivo has been extensively studied to reveal the molecular details in the pathogenesis of some lymphocytic leukemias. This review discusses the main features of the original TCL1 models and the different lines of research successively developed with particular attention to genetically compound mice and the therapeutic applications in drug development.
Key words: Lck-TCL1, B-Cell, Eμ-TCL1, Chronic lymphocytic leukemia (CLL), Mouse models
INTRODUCTION
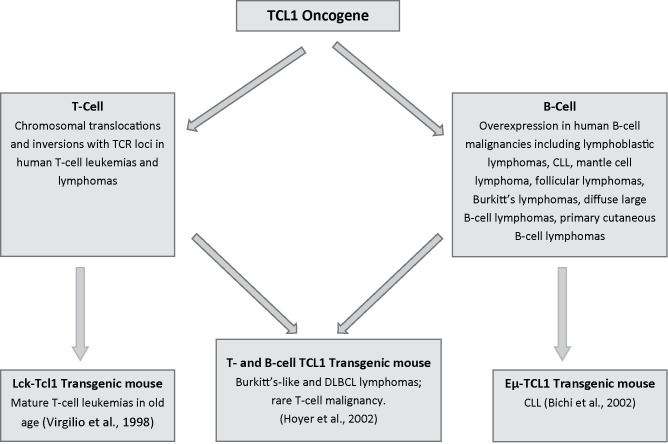
Twenty years ago, Giandomenico Russo and colleagues in our laboratory were studying the TCL1 locus on human chromosome region 14q32.1 that was frequently involved in chromosomal translocations and inversions with one of the T-cell receptor loci in human T-cell leukemias and lymphomas (1). They eventually identified a gene coding for a 1.3-kb transcript, expressed only in restricted subsets of cells within the lymphoid lineage while expressed at high levels in leukemic cells carrying specific translocations or inversions within the chromosome regions 14q11 and 14q32. The TCL1 gene, which is preferentially expressed early in T- and B-cell differentiation, was thus discovered (1). Later, TCL1 was showed to be part of a new gene family of proteins involved in T-cell proliferations and leukemias, including T chronic lymphocytic leukemia (T-CLL) [reviewed in (2)]. Then, Narducci et al. characterized the murine Tcl1 gene with the final aim to develop a mouse model of CLL, thus far the best way to decipher the role of the new oncogene in hematologic malignancies (3). The murine Tcl1 gene was shown to reside on mouse chromosome 12, in a region syntenic to human chromosome 14, and to express early in mouse embryonic development, as well as in fetal hematopoietic organs and in immature T- and B-cells. Finally, to show that transcriptional alteration of TCL1 was causally involved in the generation of T-cell malignant transformation, Virgilio et al. generated transgenic mice that carry the human cDNA for the TCL1 gene under the transcriptional control of the p56 (lck) promoter element (4). The lck-TCL1 transgenic mice developed mature T-cell leukemias after a long period of latency in old age, while younger mice presented preleukemic T-cell expansions expressing TCL1 (4). In contrast to human leukemias, whose phenotype is, predominantly, CD4+/CD8−, murine leukemias are CD4−/CD8+. Thus, the lck-TCL1 mouse, produced more than 15 years ago, was the first transgenic animal model to definitively prove the oncogenic role of TCL1 in human leukemia and the initiator of a long line of studies with mouse models of leukemia that is thriving now more than ever. Immediately after the demonstration that TCL1 overexpression causes mature T-cell proliferation in transgenic mice, expansion of TCL1 analysis in many different types of human hematopoietic malignancies revealed strong expression of the gene in almost all tumor cells of B-cell lineage (5), thus indicating an important role in B-cell proliferation. Narducci et al. (6) confirmed that, in B-cell neoplasia, TCL1 was expressed in the majority of the cases, including lymphoblastic lymphoma, chronic lymphocytic leukemia, mantle cell lymphoma, follicular lymphoma, Burkitt lymphoma, diffuse large B-cell lymphoma, and primary cutaneous B-cell lymphoma. Following these studies, times became mature to generate new models with TCL1 expressed also in B-cells. Hoyer et al. (7) generated a murine model in which a TCL1 transgene was expressed at similar levels in both B- and T-cells. These transgenic mice developed Burkitt-like lymphoma and diffuse large B-cell lymphoma at a very high penetrance beginning at 4 months of age. Only one mouse developed a T-cell malignancy at 15 months, consistent with a longer latency for transformation of T-cells by TCL1. These data demonstrated that, when overexpressed in both T- and B-cells, TCL1 predominantly yields mature B-cell lymphomas (7). What about the TCL1 overexpression in B-cells alone? The answer to this question was provided by the Eμ-TCL1 transgenic mouse (8) (Fig. 1).
Figure 1.
Generation of the first three TCL1 transgenic mice.
THE Eμ-TCL1 TRANSGENIC MOUSE AND EARLY STUDIES
This animal model is characterized by exogenous overexpression of the human TCL1 oncogene under the control of the a V(H) promoter–Ig(H)-Eμ enhancer to target gene expression specifically to B-cells and results in the clonal expansion of CD5+/IgM+ B-cells (8,9). Between 12 and 18 months of age, virtually all the mice develop an overt leukemia and massive infiltrations of monoclonal CD5+ B-cells in both lymphoid and nonlymphoid organs, thus recapitulating the main features of human B CLL (or simply CLL). The Eμ-TCL1 transgenic mouse was the first of a long series of engineered mice to develop a CLL-like disease but one of the very few to closely resemble the real human disease (9–11). Since its generation 12 years ago, the transgenic mouse has been widely used in the study of CLL pathogenesis and therapy. In the first study to exploit this model for potential treatment of CLL, we transplanted mouse CLL cells into syngeneic mice that led to a reliable and consistent development of the disease in the recipient mice (12). This approach allowed us to verify the involvement of the Tcl1/Akt/mTOR biochemical pathway in CLL by testing the ability of a specific pharmacologic agent, rapamycin, to slow the leukemia. Previously, in fact, the link between Akt and Tcl1, a coactivator of Akt, was demonstrated in vitro (13,14). The rapamycin-treated mice survived some time longer than the untreated ones but eventually succumbed to the disease. It was also demonstrated that the transformed murine lymphocytes, besides Akt, expressed other relevant therapeutic targets, like Bcl-2, and an in vitro sensitivity to therapeutic agents relevant to the treatment of human CLL (15). The same authors demonstrated also the in vivo clinical activity of fludarabine, the classic CLL drug, in transgenic mice with active leukemia evidenced by early reduction in blood lymphocyte count, spleen size, and survival prolongation compared with control mice but, again, as in the case of rapamycin, an emergence of resistance put an end to the in vivo treatment (15).
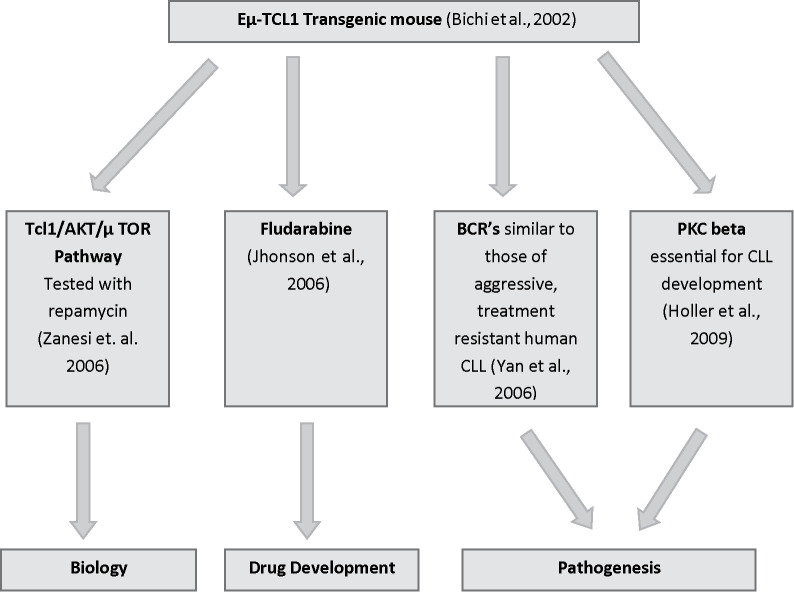
The B-cell receptors (BCRs) expressed in Eμ-TCL1 mice by lymphoid proliferation resembled those of aggressive, treatment-resistant human CLL (16). Protein kinase C β (PKCβ) is an essential signaling element of the BCR and has been shown to be overexpressed in human CLL (17). The laboratory of Alexander Egle used the TCL1 transgenic mouse to directly target PKCβ in the development of murine CLL (18). TCL1 overexpression did restore the CD5+ B-cell population that is absent in PKCβ-deficient mice. However, the compound PKCβ-deleted/TCL1 transgenic model did not develop a CLL disease, suggesting a role of PKCβ in the development of the malignancy and thus identifying a potential target for therapy (18) (Fig. 2).
Figure 2.
Early studies and main fields of investigation.
THE TCL1 MODEL OF CLL TAKES OFF
A similar approach to counteract the onset of CLL in Eμ-TCL1 mice, but targeting a different pathway, has been adopted by Wu et al. (19). Signaling of the Wnt/Fzd pathway is known to play a key role in development, tissue-specific stem cell maintenance, and tumorigenesis (20), particularly through stabilization of β-catenin, possibly affecting B-cell differentiation (21). To determine whether this pathway is involved in B-cell leukemogenesis, the authors examined the expression of Wnt/Fzd pathway genes in TCL1 transgenic mice and found that, in the course of leukemogenesis, the expression of different genes, and most dramatically Fzd6, is progressively upregulated in the transformed CD5+ B-cells, as are protein levels of β-catenin. Interestingly, elimination of Fzd6 expression by crossing into Fzd6 −/− mice significantly delayed the onset of CLL in the compound animals. These findings suggest that self-renewal signals mediated by Wnt/Fzd, enlisted during B-cell development, may be pathologically reactivated in the neoplastic transformation of mature B-cells (19). Like rapamycin, silvestrol is another natural product that has been tested for its effects in TCL1 transgenic mice (22). A plant-based agent with a unique chemical structure, silvestrol produces an unusual pattern of cytotoxicity that suggests activity in leukemia and selectivity for B-cells (23). In human CLL cells, silvestrol LC50 (concentration lethal to 50%) is 6.9 nM at 72 h, while in vivo, causes significant B-cell reduction in TCL1 mice and other models identifying itself as a potential therapeutic agent for CLL and other B-cell leukemias (22). Immune-suppressive mechanisms that are important in human cancers have been largely ignored in preclinical mouse models. In an effort to fill in this vacuum, John Gribben’s laboratory examined the T-cell function in the Eμ-TCL1 transgenic model (24). With development of leukemia, the mice also developed functional T-cell defects and alteration of gene and protein expression closely resembling changes previously seen in CLL human patients. Moreover, infusion of CLL cells into young, healthy TCL1 mice induced defects comparable to those seen in animals with established leukemia, showing a causal relationship between CLL and T-cell defects. Altered pathways involved regulation of actin remodeling, dysfunctional immunological T-cell synapse formation, and T-cell signaling, which was reversed by the immune-modulatory drug lenalidomide. These results defined the Eμ-TCL1 transgenic mice a versatile model to investigate both the molecular mechanisms of cancer-induced immune suppression and immunotherapeutic repair strategies (24).
A hallmark of nearly every malignancy, epigenetic investigations include gain or loss of DNA methylation (25). Different types of cancer-related genes can be affected by changes in DNA methylation, and because these changes are reversible, they are being extensively investigated as potential targets for therapy. Chen et al. (26) used the TCL1 mouse to determine the timing and patterns of aberrant DNA methylation and to investigate the underlying mechanisms. Murine CLL cells were shown to recapitulate epigenetic alterations seen in the human counterpart. Aberrant methylation of promoter sequences was observed as early as 3 months of age in these animals, well before disease onset. Binding sites for the transcription factor FOXD3 were among the promoter regions abnormally methylated. It was demonstrated that loss of Foxd3 expression, due to an NF-κB p50/p50:HDAC1 repressor complex, occurs in TCL1-positive B-cells before methylation. Thus, specific transcriptional repression is an early event leading to epigenetic silencing of target genes in CLL. The results provide strong rationale for the development of strategies to target NF-κB elements in CLL (26).
CLL was once considered a disease characterized by an accumulation of noncycling B-cells, but studies based on heavy water labeling challenged this notion (27). Thomas Kipps and colleagues examined leukemia cell turnover in Eμ-TCL1 transgenic mice and found that their leukemia cells not only had higher proportions of proliferating cells but also of apoptotic cells than did nonleukemic lymphocytes (28). Then, they crossed TCL1-Tg with BAFF-Tg mice, which express high levels of CD257. The derived compound mice, TCL1/BAFF, developed CLL-like disease at a significantly younger age and had more rapid disease progression and shorter survival than TCL1 animals. Leukemic cells of double transgenic mice had similar proportions of proliferating cells, but fewer proportions of apoptotic cells, than did the CLL cells of TCL1 mice. In addition, leukemia cells from double transgenic or TCL1 mice produced more aggressive disease when transferred into BAFF mice than into wild-type animals. Neutralization of CD257 resulted in rapid reduction in circulating CLL cells. The results indicate that leukemic cells of TCL1 mice undergo high levels of spontaneous apoptosis that is offset by relatively high rates of cell proliferation, which might allow for acquisition of mutations that, in turn, contribute to the evolution of the disease (28). The ζ-chain-associated protein kinase 70 (ZAP70) is a well-known prognostic factor in human CLL. RhoH is a hematopoietic-specific member of the Rho GTPase family that functions as a regulator of thymocyte development and T-cell receptor signaling by facilitating localization of ZAP70 to the immunological synapse (29). Sanchez-Aguilera et al. (30) investigated the function of RhoH in B-cell lineage. The BCR signaling was intact in Rhoh-KO mice. Because RhoH interacts with ZAP70, the mRNA levels of RhoH in primary human CLL cells were analyzed and showed a twofold higher RhoH expression compared with normal B-cells. RhoH expression in CLL positively correlated with the protein levels of ZAP70. By crossing TCL1 transgenic mice with Rhoh −/− animals, the accumulation of CD5+/IgM+ leukemic cells in peripheral blood and the leukemic burden in the peritoneal cavity, bone marrow, and spleen of TCL1-Tg/Rhoh-KO mice were significantly delayed when compared with their TCL1 counterparts. Phosphorylation of AKT and ERK in response to BCR stimulation was also notably decreased in the double mutant splenocytes. Overall, the data suggest that RhoH has a function in the progression of murine CLL (30).
RECENT DEVELOPMENTS
As shown in Figure 2, the Eμ-TCL1 transgenic mouse model has been used by many different research groups approximately along three main lines of investigation: a) biology, with attention to basic mechanisms of molecular biology and genetics; b) pathogenesis, to elucidate the mechanisms of CLL onset; and c) drug development, focused on testing novel therapeutic molecules. Here, we briefly summarize the achievements of the last 5 years along these lines of research.
Biology
An ever more detailed picture of interactions of TCL1 with other genes and different pathways has been derived from the crosses of the TCL1 mouse with other transgenic and knockout models. At least seven compound mice, involving four knockouts, Hs1 −/−, p53 −/−, Id4 +/−, and Tir8 −/− (31–34), respectively, and three transgenic, the dominant-negative RAG1, APRIL-TACI, and ROR1 (35–37), showed phenotypes characterized by earlier onset of the disease and reduced survival compared to the single TCL1 transgenic mouse. Two other compound animals, TCL1/mif −/− and TCL1/CD44 −/− (38,39), on the other hand, displayed a later onset of CLL and longer survival. Therefore, from this rich panel of mutant combinations, we deduce that five genes, the intracellular protein hematopoietic cell-specific Lyn substrate-1 (HS1), the recombinant-activating gene 1 (RAG1), p53, through the p53/miR15a/16-1/Mcl-1 axis, the inhibitor of DNA-binding protein 4 (ID4), and TIR8, behave like tumor suppressors against the TCL1-induced leukemia. On the contrary, the other four genes, the macrophage migration inhibitory factor (MIF), a proliferation-inducing ligand (APRIL), the receptor tyrosine kinase-like orphan receptor (ROR1), and the cell-surface glycoprotein CD44 work to potentiate the oncogenic effects of TCL1. We generated a more complex compound mouse (40) based on the Sleeping Beauty transposon-mediated mutagenesis (41). This mouse genetic system had the aim to identify oncogenes that cooperate with TCL1 and, through analysis of transposon common insertion sites, revealed that four out of seven genes identified were elements of the NF-κB pathway, thus confirming the importance of the cooperation between TCL1 and NF-κB in CLL (40). We also generated a transgenic mouse using a construct containing the 3′ and 5′ UTRs of TCL1 (full length) in B-cells (Eμ-TCL1FL). This model revealed accumulation of leukemic CD5+/CD23+ B-cells and increased levels of Akt phosphorylation, but overall, the phenotype was milder than in the classical TCL1 mouse (42). From proteomic experiments to identify Tcl1-interacting partners, we found that Tcl1 protein physically interacts with de novo DNA methyltransferases Dnmt3A and Dnmt3B and, accordingly, B-cells from TCL1 transgenic mice showed a significant decrease in DNA methylation compared with WT controls (43). Finally, TCL1 transgenic mice were also studied to compare the interleukin-10 (IL-10) competence and immunosuppressive function of CLL cells and regulatory B-cells. Malignant IL-10 competent CLL cells were found to exhibit regulatory functions comparable to normal regulatory B-cells that may contribute to the immunosuppression observed in TCL1 mice as well as in CLL patients (44).
Pathogenesis
Hofbauer et al. (45) investigated the composition of the T-cell compartment and observed a skewing of T-cell subsets from naive to antigen-experienced memory T-cells in TCL1 mice, analogous to human CLL. Samson Jacob’s research group, studying the truncated form of protein tyrosine phosphatase receptor type O (PTPROt), a tumor suppressor downregulated in CLL, found that a 60% decrease in PTPROt expression occurs in TCL1 mice at 7 weeks of age, and TCL1 can repress the PTPROt promoter by altering activating protein-1 (AP-1) element expression (46). Kriss et al. (47) described a novel mechanism of leukemic progression in these mice based on the activation of the endoplasmic reticulum stress response by TCL1. To elucidate host–tumor interactions, Mittal et al. (48) showed that TCL1 mice display a high percentage of leukemic cells from the lymph node microenvironment with expression of key BCR and NF-κB molecules. Another pivotal step in mouse CLL B-cell activation and proliferation is the reciprocal stimulation between CLL cells and resident mesenchymal stromal cells like follicular dendritic cells (49).
Drug Development
Suljagic et al. (50) investigated the inhibition of BCR signaling with the selective Syk inhibitor fostamatinib disodium (R788) in TCL1 mice that resulted in reduced proliferation and survival of malignant B-cells prolonging survival of treated mice. Among the p53-independent apoptosis inducers, actinomycin D was identified, tested in TCL1 mice and reported to be more effective than fludarabine, probably since both prosurvival genes, BCL2 and MCL1, are targeted by the drug (51). Another way to target the BCR signaling pathway in mouse CLL has been through the irreversible inhibition of Bruton’s tyrosine kinase by ibrutinib, an orally bioavailable kinase inhibitor that has shown outstanding activity in human CLL. TCL1-induced leukemia was in fact delayed by ibrutinib administration (52). Finally, the same group of researchers, in John Byrd’s laboratory, targeted another pivotal pathway to support CLL, the PI3K/AKT, with a novel molecule, the OSU-T315, whose AKT pathway disruption in CLL cell membranes of TCL1 mice prolongs their survival (53).
CONCLUSIONS
The almost complete disease penetrance in the Eμ-TCL1 transgenic mice, likely due to a strong oncogenic function of the human TCL1 gene, which affects multiple pathways, including coactivation of AKT, activation of the NF-κB pathway, and inhibition of the activity of DNA methyltransferases DNMT3A and DNMT3B, is a unique case among all existing mouse models of CLL (11). Since the first publication in 2002 (8), the Eμ-TCL1 transgenic mouse model of CLL has been put under the lenses of different fields in biomedicine, and several lines of research have come out among the most promising to pursue in the battle to know and tame this leukemia, such as compound genetic models, epigenetics, microenvironment, and three main biochemical pathways: BCR, AKT, and NF-κB. The deployment of this model has tremendously and logarithmically enriched the knowledge of the oncogenic activity of TCL1 in the context of the in vivo studies of CLL. As of our writing, several more investigations are going on with this model, some in the most cutting-edge fields of medicine.
ACKNOWLEDGMENTS
This project was supported by the NIH/NCI CA15319 grant. We apologize to colleagues whose works, although important and relevant, may not have been cited for lack of space.
REFERENCES
- 1. Virgilio L, Narducci MG, Isobe M, Billips LG, Cooper MD, Croce CM, et al. Identification of the TCL1 gene involved in T-cell malignancies. Proc Natl Acad Sci USA 1994; 91(26):12530–12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pekarsky Y, Hallas C, Croce CM. The role of TCL1 in human T-cell leukemia. Oncogene 2001; 20(40):5638–56343. [DOI] [PubMed] [Google Scholar]
- 3. Narducci MG, Virgilio L, Engiles JB, Buchberg AM, Billips L, Facchiano A, et al. The murine Tcl1 oncogene: Embryonic and lymphoid cell expression. Oncogene 1997; 15(8):919–926. [DOI] [PubMed] [Google Scholar]
- 4. Virgilio L, Lazzeri C, Bichi R, Nibu K, Narducci MG, Russo G, et al. Deregulated expression of TCL1 causes T cell leukemia in mice. Proc Natl Acad Sci USA 1998; 95(7): 3885–3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Takizawa J, Suzuki R, Kuroda H, Utsunomiya A, Kagami Y, Joh T, et al. Expression of the TCL1 gene at 14q32 in B-cell malignancies but not in adult T-cell leukemia. Jpn J Cancer Res 1998; 89(7):712–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Narducci MG, Pescarmona E, Lazzeri C, Signoretti S, Lavinia AM, Remotti D, et al. Regulation of TCL1 expression in B- and T-cell lymphomas and reactive lymphoid tissues. Cancer Res 2000; 60(8):2095–2100. [PubMed] [Google Scholar]
- 7. Hoyer KK, French SW, Turner DE, Nguyen MT, Renard M, Malone CS, et al. Dysregulated TCL1 promotes multiple classes of mature B cell lymphoma. Proc Natl Acad Sci USA 2002; 99(22):14392–14397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bichi R, Shinton SA, Martin ES, Koval A, Calin GA, Cesari R, et al. Human chronic lymphocytic leukemia modeled in mouse by targeted TCL1 expression. Proc Natl Acad Sci USA 2002; 99(10):6955–6960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zanesi N, Balatti V, Bottoni A, Croce CM, Pekarsky Y. Novel insights in molecular mechanisms of CLL. Curr Pharm Des 2012; 18(23):3363–3372. [DOI] [PubMed] [Google Scholar]
- 10. Pekarsky Y, Zanesi N, Croce CM. Molecular basis of CLL. Sem Cancer Biol 2010; 20(6):370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Simonetti G, Bertilaccio MT, Ghia P, Klein U. Mouse models in the study of chronic lymphocytic leukemia pathogenesis and therapy. Blood 2014; 124(7):1010–1019. [DOI] [PubMed] [Google Scholar]
- 12. Zanesi N, Aqeilan R, Drusco A, Kaou M, Sevignani C, Costinean S, et al. Effect of rapamycin on mouse chronic lymphocytic leukemia and the development of nonhematopoietic malignancies in Emu-TCL1 transgenic mice. Cancer Res 2006; 66(2):915–920. [DOI] [PubMed] [Google Scholar]
- 13. Pekarsky Y, Koval A, Hallas C, Bichi R, Tresini M, Malstrom S, et al. Tcl1 enhances Akt kinase activity and mediates its nuclear translocation. Proc Natl Acad Sci USA 2000; 97(7):3028–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Laine J, Kunstle G, Obata T, Sha M, Noguchi M. The protooncogene TCL1 is an Akt kinase coactivator. Mol Cell 2000; 6(2):395–407. [DOI] [PubMed] [Google Scholar]
- 15. Johnson AJ, Lucas DM, Muthusamy N, Smith LL, Edwards RB, De Lay MD, et al. Characterization of the TCL-1 transgenic mouse as a preclinical drug development tool for human chronic lymphocytic leukemia. Blood 2006; 108(4):1334–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yan XJ, Albesiano E, Zanesi N, Yancopoulos S, Sawyer A, Romano E, et al. B cell receptors in TCL1 transgenic mice resemble those of aggressive, treatment-resistant human chronic lymphocytic leukemia. Proc Natl Acad Sci USA 2006; 103(31):11713–11718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abrams ST, Lakum T, Lin K, Jones GM, Treweeke AT, Farahani M, et al. B-cell receptor signaling in chronic lymphocytic leukemia cells is regulated by overexpressed active protein kinase CbetaII. Blood 2007; 109(3):1193–1201. [DOI] [PubMed] [Google Scholar]
- 18. Holler C, Pinon JD, Denk U, Heyder C, Hofbauer S, Greil R, et al. PKCbeta is essential for the development of chronic lymphocytic leukemia in the TCL1 transgenic mouse model: Validation of PKCbeta as a therapeutic target in chronic lymphocytic leukemia. Blood 2009; 113(12):2791–2794. [DOI] [PubMed] [Google Scholar]
- 19. Wu QL, Zierold C, Ranheim EA. Dysregulation of Frizzled 6 is a critical component of B-cell leukemogenesis in a mouse model of chronic lymphocytic leukemia. Blood 2009; 113(13):3031–3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta 2003; 1653(1):1–24. [DOI] [PubMed] [Google Scholar]
- 21. Reya T, O’Riordan M, Okamura R, Devaney E, Willert K, Nusse R, et al. Wnt signaling regulates B lymphocyte proliferation through a LEF-1 dependent mechanism. Immunity 2000; 13(1):15–24. [DOI] [PubMed] [Google Scholar]
- 22. Lucas DM, Edwards RB, Lozanski G, West DA, Shin JD, Vargo MA, et al. The novel plant-derived agent silvestrol has B-cell selective activity in chronic lymphocytic leukemia and acute lymphoblastic leukemia in vitro and in vivo. Blood 2009; 113(19):4656–4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, et al. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J Natl Cancer Inst 1991; 83(11):757–766. [DOI] [PubMed] [Google Scholar]
- 24. Gorgun G, Ramsay AG, Holderried TA, Zahrieh D, Le Dieu R, Liu F, et al. E(mu)-TCL1 mice represent a model for immunotherapeutic reversal of chronic lymphocytic leukemia-induced T-cell dysfunction. Proc Natl Acad Sci USA 2009; 106(15):6250–6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jones PA, Baylin SB. The epigenomics of cancer. Cell 2007; 128(4):683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen SS, Raval A, Johnson AJ, Hertlein E, Liu TH, Jin VX, et al. Epigenetic changes during disease progression in a murine model of human chronic lymphocytic leukemia. Proc Natl Acad Sci USA 2009; 106(32):13433–13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Messmer BT, Messmer D, Allen SL, Kolitz JE, Kudalkar P, Cesar D, et al. In vivo measurements document the dynamic cellular kinetics of chronic lymphocytic leukemia B cells. J Clin Invest 2005; 115(3):755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Enzler T, Kater AP, Zhang W, Widhopf GF 2nd, Chuang HY, Lee J, et al. Chronic lymphocytic leukemia of Emu-TCL1 transgenic mice undergoes rapid cell turnover that can be offset by extrinsic CD257 to accelerate disease progression. Blood 2009; 114(20):4469–4476. [DOI] [PubMed] [Google Scholar]
- 29. Gu Y, Chae HD, Siefring JE, Jasti AC, Hildeman DA, Williams DA. RhoHGTPase recruits and activates Zap70 required for T cell receptor signaling and thymocyte development. Nat Immunol 2006; 7(11):1182–1190. [DOI] [PubMed] [Google Scholar]
- 30. Sanchez-Aguilera A, Rattmann I, Drew DZ, Muller LU, Summey V, Lucas DM, et al. Involvement of RhoHGTPase in the development of B-cell chronic lymphocytic leukemia. Leukemia 2010; 24(1):97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Scielzo C, Bertilaccio MT, Simonetti G, Dagklis A, ten Hacken E, Fazi C, et al. HS1 has a central role in the trafficking and homing of leukemic B cells. Blood 2010;116(18):3537–3546. [DOI] [PubMed] [Google Scholar]
- 32. Liu J, Chen G, Feng L, Zhang W, Pelicano H, Wang F, et al. Loss of p53 and altered miR15-a/16-1short right arrowMCL-1 pathway in CLL: Insights from TCL1-Tg:p53(-/-) mouse model and primary human leukemia cells. Leukemia 2014; 28(1):118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen SS, Claus R, Lucas DM, Yu L, Qian J, Ruppert AS, et al. Silencing of the inhibitor of DNA binding protein 4 (ID4) contributes to the pathogenesis of mouse and human CLL. Blood 2011; 117(3):862–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bertilaccio MT, Simonetti G, Dagklis A, Rocchi M, Rodriguez TV, Apollonio B, et al. Lack of TIR8/SIGIRR triggers progression of chronic lymphocytic leukemia in mouse models. Blood 2011; 118(3):660–669. [DOI] [PubMed] [Google Scholar]
- 35. Nganga VK, Palmer VL, Naushad H, Kassmeier MD, Anderson DK, Perry GA, et al. Accelerated progression of chronic lymphocytic leukemia in Emu-TCL1 mice expressing catalytically inactive RAG1. Blood 2013; 121(19):3855–3866, S1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lascano V, Guadagnoli M, Schot JG, Luijks DM, Guikema JE, Cameron K, et al. Chronic lymphocytic leukemia disease progression is accelerated by APRIL-TACI interaction in the TCL1 transgenic mouse model. Blood 2013; 122(24):3960–3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Widhopf GF 2nd, Cui B, Ghia EM, Chen L, Messer K, Shen Z, et al. ROR1 can interact with TCL1 and enhance leukemogenesis in Emu-TCL1 transgenic mice. Proc Natl Acad Sci USA 2014; 111(2):793–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reinart N, Nguyen PH, Boucas J, Rosen N, Kvasnicka HM, Heukamp L, et al. Delayed development of chronic lymphocytic leukemia in the absence of macrophage migration inhibitory factor. Blood 2013; 121(5):812–821. [DOI] [PubMed] [Google Scholar]
- 39. Fedorchenko O, Stiefelhagen M, Peer-Zada AA, Barthel R, Mayer P, Eckei L, et al. CD44 regulates the apoptotic response and promotes disease development in chronic lymphocytic leukemia. Blood 2013; 121(20):4126–4136. [DOI] [PubMed] [Google Scholar]
- 40. Zanesi N, Balatti V, Riordan J, Burch A, Rizzotto L, Palamarchuk A, et al. A Sleeping Beauty screen reveals NF-kB activation in CLL mouse model. Blood 2013; 121(21):4355–4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dupuy AJ, Akagi K, Largaespada DA, Copeland NG, Jenkins NA. Mammalian mutagenesis using a highly mobile somatic Sleeping Beauty transposon system. Nature 2005; 436(7048):221–226. [DOI] [PubMed] [Google Scholar]
- 42. Efanov A, Zanesi N, Nazaryan N, Santanam U, Palamarchuk A, Croce CM, et al. CD5+CD23+ leukemic cell populations in TCL1 transgenic mice show significantly increased proliferation and Akt phosphorylation. Leukemia 2010; 24(5):970–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Palamarchuk A, Yan PS, Zanesi N, Wang L, Rodrigues B, Murphy M, et al. Tcl1 protein functions as an inhibitor of de novo DNA methylation in B-cell chronic lymphocytic leukemia (CLL). Proc Natl Acad Sci USA 2012; 109(7):2555–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. DiLillo DJ, Weinberg JB, Yoshizaki A, Horikawa M, Bryant JM, Iwata Y, et al. Chronic lymphocytic leukemia and regulatory B cells share IL-10 competence and immunosuppressive function. Leukemia 2013; 27(1):170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hofbauer JP, Heyder C, Denk U, Kocher T, Holler C, Trapin D, et al. Development of CLL in the TCL1 transgenic mouse model is associated with severe skewing of the T-cell compartment homologous to human CLL. Leukemia 2011; 25(9):1452–1458. [DOI] [PubMed] [Google Scholar]
- 46. Motiwala T, Zanesi N, Datta J, Roy S, Kutay H, Checovich AM, et al. AP-1 elements and TCL1 protein regulate expression of the gene encoding protein tyrosine phosphatase PTPROt in leukemia. Blood 2011; 118(23):6132–6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kriss CL, Pinilla-Ibarz JA, Mailloux AW, Powers JJ, Tang CH, Kang CW, et al. Overexpression of TCL1 activates the endoplasmic reticulum stress response: A novel mechanism of leukemic progression in mice. Blood 2012; 120(5):1027–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mittal AK, Chaturvedi NK, Rai KJ, Gilling-Cutucache CE, Nordgren TM, Moragues M, et al. Chronic lymphocytic leukemia cells in a lymph node microenvironment depict molecular signature associated with an aggressive disease. Mol Med 2014; 20(1):290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Heinig K, Gatjen M, Grau M, Stache V, Anagnostopoulos I, Gerlach K, et al. Access to follicular dendritic cells is a pivotal step in murine chronic lymphocytic leukemia B cell activation and proliferation. Cancer Discov 2014. [DOI] [PubMed] [Google Scholar]
- 50. Suljagic M, Longo PG, Bennardo S, Perlas E, Leone G, Laurenti L, et al. The Syk inhibitor fostamatinib disodium (R788) inhibits tumor growth in the Emu-TCL1 transgenic mouse model of CLL by blocking antigen-dependent B-cell receptor signaling. Blood 2010; 116(23):4894–4905. [DOI] [PubMed] [Google Scholar]
- 51. Merkel O, Wacht N, Sifft E, Melchardt T, Hamacher F, Kocher T, et al. Actinomycin D induces p53-independent cell death and prolongs survival in high-risk chronic lymphocytic leukemia. Leukemia 2012; 26(12):2508–2516. [DOI] [PubMed] [Google Scholar]
- 52. Woyach JA, Bojnik E, Ruppert AS, Stefanovski MR, Goettl VM, Smucker KA, et al. Bruton’s tyrosine kinase (BTK) function is important to the development and expansion of chronic lymphocytic leukemia (CLL). Blood 2014; 123(8):1207–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Liu TM, Ling Y, Woyach JA, Beckwith K, Yeh YY, Hertlein E, et al. OSU-T315: A novel targeted therapeutic that antagonizes AKT membrane localization and activation of chronic lymphocytic leukemia cells. Blood 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]