Abstract
Voltage-gated potassium (Kv) channels are membrane proteins that open a selective pore upon membrane depolarization, allowing K+ ions to flow down their electrochemical gradient. In neurons, Kv channels play a key role in repolarizing the membrane potential during the falling phase of the action potential, often resulting in an after hyperpolarization. Opening of Kv channels results in a decrease of cellular excitability, whereas closing (or pharmacological block) has the opposite effect, increased excitability. We have developed a series of photosensitive blockers for Kv channels that enable reversible, optical regulation of potassium ion flow. Such molecules can be used for remote control of neuronal excitability using light as an on/off switch. Here we describe the design and electrophysiological characterization of photochromic blockers of ion channels. Our focus is on Kv channels but in principle, the techniques described here can be applied to other ion channels and signaling proteins.
Keywords: Azobenzene, Photoswitch, Photochromic ligand, Ion channel, Photopharmacology, Quaternary ammonium compounds, Electrophysiology
1 Introduction
Photochromic ligands (PCLs) are small, freely diffusing molecules that can be reversibly interconverted between two isomeric forms using different wavelengths of light (Fig. 1a). The two isomers bind their protein target with different affinities, making it possible to control binding events (and resulting signaling cascades) reversibly with light. The ligand can be an agonist, an antagonist, or an active site inhibitor, allowing one to turn the protein function on and off repeatedly with different wavelengths of light. Light as a stimulus has numerous advantages, including spatial and temporal precision, non-invasiveness, and orthogonality (most cells are not naturally light-responsive) (1, 2).
Fig. 1.
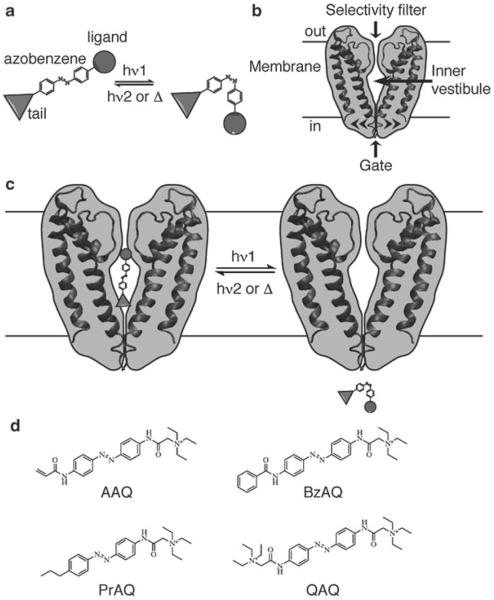
Photochromic ligands (PCLs) for K+ channels. (a) Schematic representation of a PCL containing a central photoisomerizable azobenzene core, a ligand head, and a tail. Isomerization between the elongated trans and the bent cis azobenzenes occurs upon irradiation with different wavelengths of light (hν1 and hν2). The cis isomer is of higher energy and can relax back to trans spontaneously in the dark (Δ). (b) Architectural features of K+ channels. K+ channels are tetrameric proteins but only two subunits are shown for clarity. The ion path has distinct elements: an external filter that is highly selective for K+ ions, a central vestibule, and an intracellular gate. Voltage-gated K+ channels have an additional voltage-sensing domain that opens the gate upon membrane depolarization (not depicted here). (c) Schematic view of a photochromic blocker for K+ channels that blocks the channel in the trans form (inner vestibule) and unbinds in the cis form. (d) Examples of PCLs for K+ channels: AAQ acrylamid azobenzene quaternary ammonium, BzAQ benzylamide azobenzene quaternary ammonium, PrAQ propyl azobenzene quaternary ammonium, and QAQ quaternary ammonium azobenzene quaternary ammonium
In an attempt to manipulate neuronal activity with light, we decided to engineer PCLs for K+ channels, a class of ion channels that are crucial for setting the resting membrane potential and shaping action potentials in neurons and other excitable cells. K+ channels are made of four subunits arranged around a central ion-conducting pore, which contains a selectivity filter, an inner vestibule, and an intracellular gate (Fig. 1b) (3). The inner vestibule is particularly important from a pharmacological point of view, as it constitutes the binding pocket for a class of molecules called open-channel blockers. Tetraethylammonium (TEA) and other quaternary ammonium (QA)-containing molecules prevent ion flow through the channel by sneaking into the inner vestibule after opening of the channel gate (4). Because of their permanent positive charge, most QAs are membrane impermeant and have to be brought inside the cell by artificial means (i.e., the patch pipette). In contrast, some hydrophobic QAs can penetrate cells by passively crossing the lipid bilayer and tend to be trapped inside cells for long periods of time (5, 6).
We have designed a small library of PCLs for K+ channels that have a fixed ligand head group (a QA), an azobenzene photoswitch linker, and a variable tail group (7–10). These PCLs are designed to bind to the inner vestibule of K+ channels in only one of the two configurations (Fig. 1c), light being used to toggle the ligand in and out of its binding pocket. The tail's chemical variability (see Fig. 1d for a few examples) allows for fine-tuning of the PCL's physicochemical, pharmacological, as well as photochemical properties. BzAQ for example is ten times more potent than AAQ, presumably due to better membrane permeation. PrAQ is a cis blocker (blocks predominantly in the cis configuration), which is an advantageous feature because the compound does not block the channel in the dark. QAQ does not cross the membrane and can be injected into cells through a micropipette to photosensitize a single cell and afford subcellular control of action potential propagation.
In this chapter we describe the design, handling, and use of PCLs to control current through Kv channels. We are focusing on photochromic blockers for Kv channels, but the general strategy is applicable to different types of proteins, as already shown for ion channels (7–13), metabotropic receptors (14), and enzymes (15–18).
2 Materials
2.1 Cell Line
Human embryonic kidney (HEK293) cells are a popular choice among electrophysiologists due to multiple attributes: They are easy to grow and maintain, can be transfected with high efficiency, and produce high quantities of proteins. In addition, background Kv channel current from endogenous channels is small (19).
The Xenopus laevis oocyte is another classical cell of choice for electrophysiologists (20) but its opacity limits optical control and is therefore not recommended for studying light-regulation of ionic currents.
2.2 Kv Channel Clones
The Shaker K+ channel from Drosophila melanogaster was the first K+ channel to be cloned (21) and has since become an archetypical channel for biophysical and biochemical studies. It belongs to the Kv1 subfamily and shows rapid inactivation, due to the presence of a peptidic “ball and chain” N-terminus. The “ball and chain” (or N-type) inactivation can be removed by deleting amino acid residues 6–46 (Shaker-IR or inactivation removed) (22). Screening of PCLs for Kv channels was performed on Shaker-IR, since the presence of a “ball and chain” may interfere with PCL block (7, 8).
2.3 Chemicals
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless specified otherwise.
All photochromic blockers were synthesized as previously described (7).
2.4 Cell Culture
HEK293 medium: Dulbecco's Modified Eagle Medium containing high glucose, glutamine, and sodium pyruvate (D-MEM, Gibco) supplemented with 10% Fetal Bovine Serum (FBS Gibco 26140). Store at 4 °C.
Phosphate buffered saline (PBS), pH 7.4 (Gibco). Store at RT.
Trypsin 0.25% with EDTA (Gibco); 1 ml aliquots, store at −20 °C.
Poly l-lysine (10 mg/ml); 1 ml aliquots, store at −20 °C.
Glass coverslips, 12 mm diameter (Fisher).
Borate buffer: 50.1 mM boric acid, 26.5 mM sodium tetraborate. pH does not need to be adjusted. Filter-sterilize (0.2 μm) and store at 4 °C.
24-well disposable tissue culture plates with flat bottom (Nunc multidishes, Nunclon).
Nitric acid.
12.1 N Hydrochloric acid.
2.5 Transfection
HEPES-buffered saline solution (2×-HeBS): 274 mM NaCl, 10 mM KCl, 1.4 mM Na2HPO4×7H2O, 15 mM glucose, 42 mM HEPES free acid. Adjust pH to exactly 7.12 with NaOH. Filter-sterilize and store at 4 °C. Do not freeze. Make fresh every month.
2.5 M CaCl2. Filter-sterilize and store at 4 °C.
2.6 Recording Solutions
-
For whole-cell recordings.
External solution: 138 mM NaCl, 1.5 mM KCl, 1.2 mM MgCl2, 2.5 mM CaCl2, 5 mM HEPES free acid, and 10 mM glucose. pH is adjusted to 7.4 with NaOH. Store at 4 °C.
Internal solution: 10 mM NaCl, 135 mM K+ gluconate, 10 mM HEPES free acid, 2 mM MgCl2, 2 mM MgATP, 1 mM EGTA. pH is adjusted to 7.4 with KOH. Filter-sterilize solution (0.2 μm) and store 1 ml aliquots at −20 °C.
-
For inside-out patches.
Bath solution: 160 mM KCl, 0.5 mM MgCl2, 1 mM EGTA, 20 mM HEPES free acid. pH is adjusted to 7.4 with KOH. Store at 4 °C
Pipette solution: 150 mM NaCl, 10 mM KCl, 10 mM HEPES free acid, 1 mM MgCl2, 3 mM CaCl2. pH is adjusted to 7.4 with NaOH. Filter-sterilize solution (0.2 μm) and store 1 ml aliquots at −20 °C.
2.7 Equipment
The list of equipment here below is purely informative; other models are compatible too.
Spectrophotometer: SmartSpec Plus (Bio-Rad) or NanoDrop 1000 (Thermo scientific).
Pipette puller (Sutter P97).
Polisher-microforge (Narishige MF830).
Capillaries: Borosilicate thin wall with filament (Warner G150TF-3).
Syringe needle for filling micropipettes (Microfil 28 gauge MF28G, World Precision Instruments, Inc.).
Faraday cage located in a dark room or covered with a black curtain (see Note 1).
Air table.
Amplifier Axopatch 200A (Molecular Devices).
Digitizer: Digidata 1,200 interface (Molecular Devices).
Recording/analysis software: pClamp software (Molecular Devices).
Microscope: Preferentially inverted.
Objectives: 20 or 40×, with high UV light transmission (Nikon Plan Fluor series).
Filter cube for GFP fluorescence (GFP-3035B Semrock).
Total reflector mirror (Omega opticals XF125).
Light source: There are four main possibilities: (1) monochromator (polychrome V, Till photonics, see Note 2); (2) Lambda DG4 (Sutter Instruments); (3) Xenon lamp (175 W or higher) in combination with a filter wheel controlled by a Lambda 10–2 (Sutter Instruments) and narrow band-pass filters (380BP10 and 500BP5); and (4) high-power microscope-LED with corresponding dichroic beam splitters (Prizmatix). All light sources can be controlled by pClamp and provide light intensities compatible with PCL photoswitching (see Note 3). LEDs are less flexible in terms of action spectrum (need to buy a new LED for any new wavelength needed) but are definitely a cheap alternative.
Optic fiber (UV/Vis quartz fiber) connecting the light source to the microscope.
Power meter (Newport 840-C).
Recording chamber for 12 mm coverslips (Warner RC25).
Recording chamber for inside-out patches (Warner RC28).
Micromanipulator (Sutter MP285).
Perfusion system (Warner VC-8).
3 Methods
3.1 PCL Design
The general design of a PCL can be considered as the structural sum of two or three components: a head (ligand), a photoisomerizable core (photoswitch), and an optional tail (Fig. 1a).
Ligand: The ligand can be an agonist (11, 13), a competitive antagonist (14, 23), a pore blocker (7–10, 12), a substrate (16), an inhibitor (15, 17, 18), or even an allosteric modulator, depending on the protein target and the experimental use.
Photoswitch: Among the photosensitive groups available, azobenzene has been predominantly used (7–18, 23) due to its advantageous chemical and photochemical properties. Indeed, azobenzene is a small, relatively simple molecule, easy to synthesize and derivatize. The two isomers have significantly different absorption spectra, making it possible to accumulate up to 95% of one isomer under appropriate light conditions (see Note 3). Azobenzenes can be switched back and forth quickly numerous times (no photobleaching) with particular wavelengths of light, typically 360–380 for converting to cis (see Note 4) and 460–500 nm for switching back to trans. The cis isomer also converts back to the low-energy trans form slowly in the dark, which implies that in darkness azobenzenes exist almost exclusively in the trans form. Importantly, trans to cis photoisomerization can affect the docking of the PCL into the receptor-binding pocket in two ways: through a dramatic change in geometry (planar to bent) and a slight increase in polarity (~3 Debye).
Position for photoswitch attachment: For the ligand, check published structure–activity relationship or crystallographic structures when available to identify positions where a chemical extension is well tolerated by the receptor. Concerning the photoswitch, the para position on the azobenzene is probably best suited to maximize geometrical change upon photoisomerization, although ortho- and meta-substituted analogues have also been successfully used (11, 23).
Linker: The linker between the photoswitch and the ligand should be as short and as rigid as possible, to maximize the deformation endured by the molecule during photoisomerization, and therefore the dynamic range of affinities between the two isomers. Methylene and amide bonds are good starting choices. Care should be taken with substituants in para position of the azobenzene as their chemical nature can drastically alter the photochemical properties of the photoswitch (this is also true for meta and ortho positions). Indeed, azobenzenes that are para-substituted with an electron-donating group (i.e., amino) usually relax back to trans much more rapidly, which may impair accumulation of the cis analogue, and are rather used as fluorophore or quencher (e.g., DABCYL (24)).
Tail: In theory, addition of a tail will maximize geometrical change between trans and cis forms, which may result in a greater shift in affinity upon light isomerization. In practice however, experiment will be necessary to reveal if a tail is required as for K+ channels (7) or not tolerated as for glutamate receptors (13). Interestingly, the tail structure may also dictate whether the molecule is active in cis or in trans (7, 10) (see Note 5). One should therefore consider making a series of PCLs with (and without) various tails, as it may be hard to predict PCL pharmacological profiles on a new system.
3.2 Cell Culture
Coverslip acid wash: Incubate coverslips in nitric acid overnight. Remove nitric acid and incubate in hydrochloric acid another night. Wash extensively with water. Wash with 100% ethanol. Store coverslips in 100% ethanol.
Coverslip coating: The day before transfection, flame coverslips and place one coverslip in each well of the culture dish. Add 500 μl of poly-l-lysine (100 μg μl−1 dissolved in borate buffer) in each well.
Cell plating: Wash poly-l-lysine-treated coverslips four times with 500 μl of water. Trypsinize and plate HEK293 cells at a density of 20–30 thousands cells per coverslip (500 μl of medium per well). Give them enough time to settle back down before transfecting (5–12 h).
Transfection: Cells are transfected with Kv channel cDNA (0.5–2 μg) using the calcium–phosphate precipitation method, which is a very efficient and low-cost means to introduce DNA into cells (25). Co-transfection with a fluorescent reporter (eGFP) is usually required (0.05–0.1 μg of DNA) except if the channel is fused to a fluorescent reporter, or if it is contained with GFP in a mammalian bicistronic expression vector (e.g., pIRES).
Prepare the precipitates: For each well put 11 μl of 2×-HeBS in a sterile 1.5 ml tube. In another tube mix the DNAs (0.5–2 μg for Kv + 0.05–0.1 μg for eGFP) with CaCl2 2.5 M (1.1 μl) and add water to a final volume of 11 μl. Add slowly the DNA-CaCl2 mix to the HeBS solution and mix gently (no vortex). Let sit for 20 min. Add the transfection mix to the well (22 μl per well) in dropwise fashion, and shake the plate gently. Place in the incubator overnight (37 °C, 7% CO2). Replace media the next day with 500 μl of fresh HEK293 medium. Electrophysiological recordings can start as soon as 12 h after transfection, depending on ion channel expression level.
3.3 Preparation of PCL Stock Solutions
After synthesis, PCLs are purified as trifluoroacetate or formate salts (7). Compounds are kept with desiccant in the dark at −20 °C to prevent decomposition. Compounds are dissolved either in DMSO or in H2O to a final concentration usually ≥200 mM. QAQ is dissolved in water. More lipophilic PCLs like AAQ, BzAQ, or PrAQ are dissolved in dry DMSO. In the case of QAQ, we found that the trifluoroacetate salt was less soluble than the formate salt (20 mM vs. 200 mM in water). Stock solutions are aliquoted and kept with desiccant at −20 or −80 °C. Concentration can be checked by UV–vis spectrometry. For all the PCLs described in this chapter the molar extinction coefficient at 360 nm is ε360 = 29,000 M−1 cm−1. Azobenzenes are very resistant to photobleaching; it is not required to work in the dark when preparing solutions. Working concentrations are usually below 1 mM, which ensures that DMSO content is below 1% during electrophysiological experiments.
3.4 Whole-Cell Recording
The whole-cell configuration (Fig. 2) is a classically used configuration in electrophysiology and is the first step for a pharmacological characterization of a new PCL. The direct control of the potential across the cell membrane allows insight into the molecular interactions between the PCL and the different conformational states of an ion channel. The protocols described here below are deliberately succinct and written for people familiar with electrophysiological techniques. For an introduction to electrophysiological techniques see ref. 26.
Fig. 2.
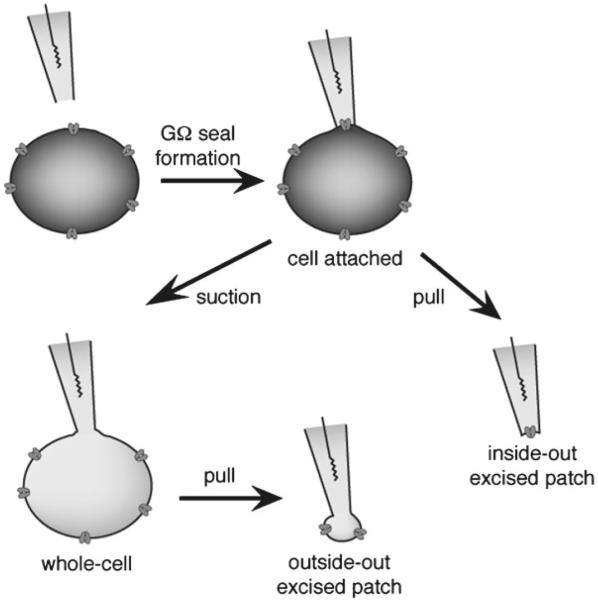
Different patch-clamp configurations. In a first step, the patch pipette is moved to the surface of the cell membrane and suction is applied to achieve a GΩ seal between the glass pipette and the membrane. This patch configuration is called cell-attached and is used to monitor very high current resolution through single channels. The cell content is intact, making it tricky to determine which ions contribute to the current since the ion concentrations inside the cell are unknown. Cell-attached is also an intermediate step towards getting whole-cell or excised-patch configurations. Inside-out, excised-patch configuration is achieved by pulling the pipette away from the cell. A small piece of membrane will stay attached to the pipette, with the cytosolic part facing the bath solution, allowing perfusion of drugs that normally have a cytosolic mode of action. Whole-cell recording is achieved from cell-attached mode after giving gentle suction to break the piece of membrane at the end of the tip. It is used to measure the average current through the entire cell while controlling ion concentrations on both sides of the membrane. Outside-out, excised patch is obtained from whole-cell recording by pulling the pipette away from the cell. The cell membrane will break and will reform as a convex bleb, leaving a little piece of membrane at the end of the pipette tip, with the external side facing the bath. Single-channel recording can be achieved, with the advantage of having control over ionic concentrations of both sides of the membrane. For electrophysiological characterization of Kv channels photochromic blockers, whole-cell and inside-out configurations have been used
There are three main strategies for applying a PCL onto cells:
3.4.1 Perfusion
Various concentrations of PCL can be perfused onto a cell while Kv current is measured in the whole-cell configuration. Current is monitored under 380 and 500 nm light to determine the PCL dose–response relationship and the kinetics of membrane permeation. An obvious disadvantage of this strategy is the significant amount of PCLs required, especially since permeation through the lipid bilayer can be slow. It is therefore recommended to perfuse PCLs only when compounds are available in large quantities (tens of mg). In cases where compounds are limited, see Subheadings 3.4.2 and 3.4.3.
Step-by-step protocol:
Prepare perfusion lines with various PCL concentrations. The potency of our PCLs for Kv channels usually falls between 10 and 1,000 μM.
Place a 12 mm coverslip in the recording chamber.
Fill the patch pipette with internal solution, place it into the pipette holder and apply positive pressure. Bring the pipette into the solution of the recording chamber; resistance should be 3–5 MΩ.
Select a transfected cell with the appropriate fluorescence filter cube (classically GFP).
Once a cell is selected, switch from the fluorescence filter cube to the full mirror position (required for photoswitching).
Bring the patch pipette close to the cell while monitoring the pipette resistance with the pClamp software. Stop when the resistance increases by 0.2 MΩ or when a dimple can be seen on the cell surface.
Remove positive pressure and start applying gentle suction. Pipette resistance should progressively increase.
Once GΩ seal is formed, apply a holding potential of −70 mV.
Give brief suctions to break the piece of membrane at the tip of the pipette (Fig. 2). Monitor capacitive currents and resistance through pClamp.
Once whole-cell mode is obtained, allow pipette solution to diffuse in the cell for 2–3 min while maintaining a −70 mV holding potential across the membrane.
Start recording Kv channel current using protocol described in Fig. 3a (looped at 1 Hz).
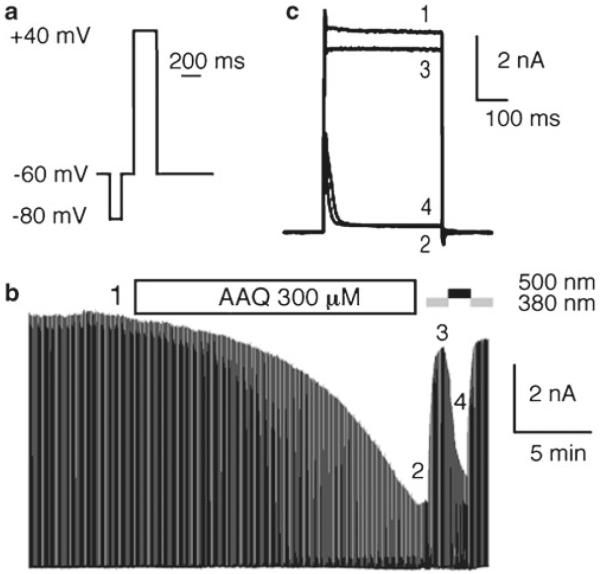
Perfuse bath solution while running the protocol loop. Because charged PCLs must cross the cell membrane before they bind to Kv channels, ion channel block can take a few of minutes to reach steady state (3–15 min in our case, depending on PCL and concentration, see Fig. 3b). Also, because PCLs are trapped inside cells for a long period of time (typically hours), perfusion can be switched to PCL-free external solution after steady-state value is reached (this saves compound). Figure 3c shows a superimposition of Kv current before perfusion and after perfusion under both wavelengths of light. Finally, because the compound cannot be washed quickly, it is recommended to start with low concentration and increase concentration until effective dose is determined.
Switch on 380 nm light until current is stable.
Switch to 500 nm light until current is stable.
Fig. 3.
Example of whole-cell characterization: perfusion. (a) Depolarization protocol used to monitor Shaker-IR current. The total protocol length is 1 s. A 100 ms pre-pulse to −80 mV is used to reset Kv channels to their basal state and to correct for eventual leak current. A 200 ms jump to +40 mV is used to open Kv channels. (b) Perfusion of 300 μM AAQ on a cell expressing Shaker-IR. Kv current was monitored using the depolarization protocol shown in (a) looped at 1 Hz. (c) Superimposition of Shaker-IR currents elicited before AAQ perfusion (1) and after AAQ perfusion in the dark (2) and under 380 (3) and 500 (4) nm light, at time points shown in (b). Capacitive currents have been cut or removed from (b) and (c) for clarity
3.4.2 Pretreatment
Lipophilic quaternary ammonium can permeate cellular membranes and be trapped inside cells for a prolonged period of time (5, 7, 27). In our case, we found that photosensitivity persisted for several hours after treatment (7). It is therefore possible to pretreat the cell with a certain concentration of PCL, and subsequently record current in whole-cell mode. This strategy has the advantage using much less PCL.
Step-by-step protocol:
Remove HEK medium and replace with 500 μl of external solution (Subheading 2.6, step 1) containing PCL (typically between 10 and 1,000 μM). Incubate for 15 min in the cell incubator (dark, 37 °C).
Rinse the cells by placing the coverslip into a 35 mm cell-culture dish containing PCL-free external solution for 2–3 min at room temperature.
Place coverslip in recording chamber, obtain a GΩ seal, and go to whole-cell mode as described in Subheading 3.4.1.
Record Kv channel current using protocol described in Fig. 3a (looped at 1 Hz) while switching between 380 and 500 nm light.
3.4.3 Loading Through the Patch Pipette
The PCLs we have developed are internal blockers for Kv channels. Hence they can be loaded directly into cells via the patch pipette in whole-cell mode. This strategy, like the PCL pretreatment (Subheading 3.4.2), uses very little compound.
Step-by-step protocol:
Dilute the PCL into internal solution (typically 10–1,000 μM, see Subheading 2.5, step 1). The solution must be filtered before inclusion in the patch pipette to remove non-soluble particles. This can be done by placing a syringe filter 0.2 μm) between the syringe and the syringe needle Microfil.
Place a 12 mm coverslip in the recording chamber.
Obtain a GΩ seal and go to whole-cell mode as described in Subheading 3.4.1.
Wait 3–4 min for the PCL to equilibrate in the cytoplasm.
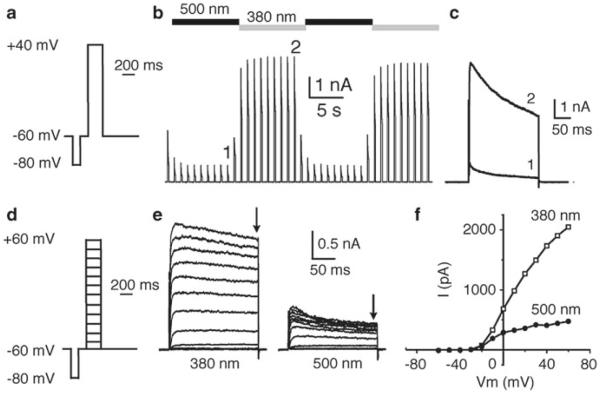
Record Kv channel current using protocol described in Fig. 4a (looped at 1 Hz) while switching between 380 and 500 nm light (Fig. 4b, c).
Because our PCLs are charged molecules, their binding to Kv channels is expected to be sensitive to membrane potential. Influence of membrane potential on PCL block and unblock can be analyzed using an I/V protocol (Fig. 4d–f).
Fig. 4.
Example of whole-cell characterization: loading through the patch pipette. (a) Depolarization protocol used to monitor Shaker-IR current. The total protocol length is 1 s. A 100 ms pre-pulse to −80 mV is used to reset Kv channels to their basal state and to correct for eventual leak current. A 200 ms jump to +40 mV is used to open Kv channels. (b) Shaker-IR current elicited using the protocol described in (a). 100 μM QAQ was included in the patch pipette. The protocol was looped at 1 Hz and light was switched every 10 s between 500 and 380 nm. QAQ blocks Shaker-IR current in the trans configuration and unblocks it in the cis configuration. (c) Superimposition of Shaker-IR currents elicited under 500 and 380 nm light, at time points 1 and 2 shown in (b), showing block and unblock of QAQ, respectively. (d) Depolarization protocol used to monitor Shaker-IR current at different membrane potentials (I/V protocol). The total protocol length is 1 s. A 100 ms pre-pulse to −80 mV is used to reset Kv channels to their basal state and to correct for eventual leak current. A 200 ms jump from −60 mV to values between −50 and +60 mV is used to open Kv channels. (e) Current through Shaker-IR channels elicited using the I/V protocol described in (d) and under 380 (left) or 500 nm (right) light irradiation, using 100 μM QAQ in the patch pipette. (f) Steady-state current (at the end of the depolarization, shown by the arrows in (e)) is plotted as function of membrane potential (I/V relationship of block and unblock). Capacitive currents have been cut or removed from (b) to (e) for clarity
3.5 Inside-Out Recording
The inside-out configuration (Fig. 2) is used to perfuse drugs to the cytosolic part of ion channels and to study how cytosolic events affect ion channel function. Because our PCLs are internal blockers of Kv channels, the inside-out configuration is used to characterize the IC50 (concentration that blocks half of the Kv channel current) of blockers independently of their partition coefficient into the cell membrane.
Step-by-step protocol:
Prepare all perfusion lines with various PCL concentrations. Ideally seven solutions are needed for a full IC50 determination, one at the expected IC50, three higher, and three lower. All solutions should be filtered to prevent small debris from breaking the patch.
Break the 12 mm coverslip in multiple little pieces (a few mm in diameter) and place one piece into a small recording chamber. The volume of the chamber should be as small as possible, typically 100 μl.
Obtain a GΩ seal as described in Subheading 3.4.1, using pipette and bath solutions adequate for inside-out patches (see Subheading 2.6, step 2).
Once GΩ seal is formed, apply a positive voltage of +70 mV to set the membrane potential to −70 mV (see Note 6).
Pull the pipette away from the cell as quickly as possible, using the coarse manipulator.
At this stage it is possible that a closed vesicle has formed instead of an open membrane patch (see Note 7). An I/V protocol (similar to Fig. 4d, but see Note 6) can be used to distinguish between a vesicle (no current) and an inside-out patch (typical I/V dependency, like in Fig. 4e, f). Once inside-out patches are obtained, they are very stable over tens of minutes.
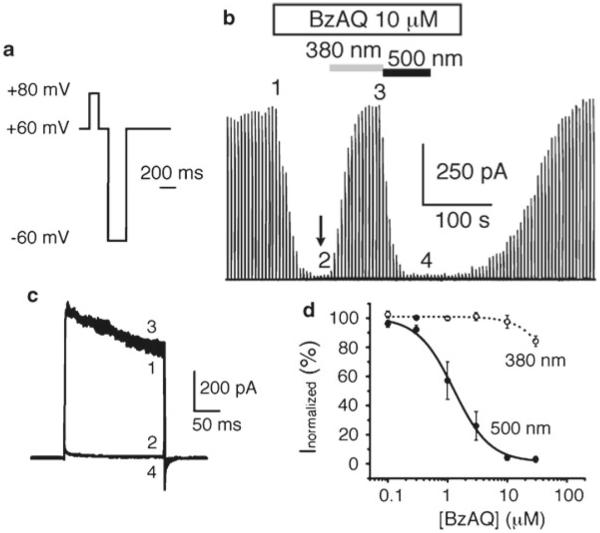
Record Kv channel current using protocol described in Fig. 5a (looped at 1 Hz).
Start perfusing bath solution and wait until Kv current is stable (see number 1 in Fig. 5b).
Switch from bath solution to PCL-containing solution, starting from low-concentrated solution. Figure 5b shows Kv current during perfusion of a 10 μM BzAQ solution onto an inside-out patch, which affords full ion channel block.
Wait until current is stable and stop the flow (see arrow in Fig. 5b). It is necessary to stop perfusion before switching between different wavelengths of light because photoisomerization rate may be slower than flow rate. Because the chamber has a tiny volume, PCL concentration is theoretically uniform in the entire chamber.
Switch on 380 nm light until current is stable (number 3 in Fig. 5b).
Switch to 500 nm light until current is stable (number 4 in Fig. 5b).
Turn off the light and wash out PCL by perfusing bath solution.
Repeat steps 8–13 using a higher PCL concentration.
Kv currents can be superimposed to show the extent of block and unblock under the different wavelengths of light (Fig. 5c). The normalized steady-state current can be then plotted as a function of PCL concentration, to determine the IC50 for both wavelengths of light (Fig. 5d).
Fig. 5.
Example of inside-out characterization using Shaker-IR and BzAQ. (a) Command protocol used to open Kv channels in the inside-out configuration (see Note 6). (b) In inside-out configuration, Kv current is inward (from the bath to the pipette) but is displayed here as outward. The white bar indicates perfusion of 10 μM BzAQ onto the patch. Arrow indicates when the perfusion is stopped. 380 and 500 nm light irradiations are shown. (c) Superimposition of Shaker-IR currents elicited before BzAQ perfusion and during perfusion in the dark and under 380 and 500 nm light illumination, at time points 1, 2, 3, and 4 shown in (b). (d) Dose–response of BzAQ block under 380 and 500 nm light. Data points were fitted using the equation: y = ((A1 − A2)/(1 + ((x/IC50)n))) + A2, where A1 is the maximal current (without PCL), A2 is the current under maximal block, and n is the Hill coefficient. Capacitive currents have been cut or removed from (b) and (c) for clarity
4 Notes
Temperature inside the faraday cage can increase substantially when the cage is covered with a curtain. It is recommended to cut a vent in the top of the cage.
Polychrome V (Till Photonics) is by default not equipped with a shutter. Till Photonics can add a shutter to the polychrome upon request. It is also possible to use 700 nm light as “dark” since azobenzenes usually do not absorb light beyond 550 nm.
The photoisomerization process of azobenzenes is extremely rapid (ps) and population changes can be achieved on a sub-millisecond time scale. Both kinetics and extent of photo-switching are directly correlated to light intensity. Therefore, the more the better: A powerful light source will enable fast photoswitching and maximal photoconversion. We usually measure light intensity through a 20 or 40× objective by hand-placing the power meter at the focal area. Depending on the configuration of the rig (light source, microscope objective, optic fiber, etc.) and the wavelength of light (380 or 500 nm), light output lies between 0.3 and 5 mW/cm2, which is equivalent to 0.5–12.6 × 1015 photons/s/cm2. When measured through a 20× objective and normalized to the focal area at the specimen plane (estimated to be 300 μm in diameter), light output is between 4 and 70 mW/mm2, which is equivalent to 7–176 × 1015 photons/s/cm2. Converting radiometric unit (mW) to photometric unit (photons/s) can be done using the following equation: 1 W = λ × 5.03 × 1015 photons/s, with λ expressed in nm.
380 nm is a near-UV wavelength of light. It is usually not damaging to tissues. If any concern arises, 400 nm visible light can be used as an alternative, although accumulation of cis may be weaker. Tuning of the spectral properties of azobenzene can also be considered (10, 28).
Cis binders are attractive because in the dark only the “inactive” trans form exists (thermodynamically stable); binding to the protein occurs only upon light exposure and the systems returns spontaneously to the baseline in the dark (7, 10).
In inside-out configuration, the membrane surface that was originally intracellular is now exposed to bath solution (see Fig. 2). By definition, the patch-clamp command voltage is positive if it increases the potential inside the pipette. As a consequence, a positive command in inside-out mode will depolarize the cell membrane.
Vesicles can form inside-out patches when briefly exposed to air, though in our hands this method has a low success rate.
Acknowledgments
We are grateful to Matthew R. Banghart (Harvard Medical School), Michael Kienzler, and Dirk Trauner (University of Munich) for the design and synthesis of PCLs described in this chapter, and to Christopher Davenport for helpful comments and suggestions.
References
- 1.Kramer RH, Fortin D, Trauner D. New photochemical tools for controlling neuronal activity. Curr Opin Neurobiol. 2009;19:544–552. doi: 10.1016/j.conb.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorostiza P, Isacoff EY. Optical switches and triggers for the manipulation of ion channels and pores. Mol Biosyst. 2007;3:686–704. doi: 10.1039/b710287a. [DOI] [PubMed] [Google Scholar]
- 3.Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, Mackinnon R. The structure of the potassium channel: molecular basis of K + conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 4.Hille B. Ion channels of excitable membranes. 3rd edn Sinauer Associates Inc; Sunderland, MA: 2001. p. 814. [Google Scholar]
- 5.Wang GK, Quan C, Vladimirov M, Mok WM, Thalhammer JG. Quaternary ammonium derivative of lidocaine as a long-acting local anesthetic. Anesthesiology. 1995;83:1293–1301. doi: 10.1097/00000542-199512000-00020. [DOI] [PubMed] [Google Scholar]
- 6.Taglialatela M, Vandongen AM, Drewe JA, Joho RH, Brown AM, Kirsch GE. Patterns of internal and external tetraethylammonium block in four homologous K + channels. Mol Pharmacol. 1991;40:299–307. [PubMed] [Google Scholar]
- 7.Banghart MR, Mourot A, Fortin DL, Yao JZ, Kramer RH, Trauner D. Photochromic blockers of voltage-gated potassium channels. Angew Chem Int Ed. 2009;48:9097–9101. doi: 10.1002/anie.200904504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fortin D, Banghart M, Dunn TW, Borges K, Wagenaar DA, Gaudry Q, Karakossian MH, Otis TS, Kristan WB, Trauner D, Kramer RH. Photochemical control of endogenous ion channels and cellular excitability. Nat Methods. 2008;5:331–338. doi: 10.1038/nmeth.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mourot A, Fehrentz T, Lefeuvre Y, Smith C, Herold C, Dalkara D, Nagy F, Trauner D, Kramer R. Rapid optical control of nociception with an ion channel photoswitch. Nat Methods. 9:396–402. doi: 10.1038/nmeth.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mourot A, Kienzler MA, Banghart MR, Fehrentz T, Huber FME, Stein M, Kramer RH, Trauner D. Tuning photochromic ion channel blockers. ACS Chem Neurosci. 2011;2:536–543. doi: 10.1021/cn200037p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartels E, Wassermann NH, Erlanger BF. Photochromic activators of the acetylcholine receptor. Proc Natl Acad Sci U S A. 1971;68:1820–1823. doi: 10.1073/pnas.68.8.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lester HA, Krouse ME, Nass MM, Wassermann NH, Erlanger BF. Light-activated drug confirms a mechanism of ion channel blockade. Nature. 1979;280:509–510. doi: 10.1038/280509a0. [DOI] [PubMed] [Google Scholar]
- 13.Volgraf M, Gorostiza P, Szobota S, Helix MR, Isacoff EY, Trauner D. Reversibly caged glutamate: a photochromic agonist of ionotropic glutamate receptors. J Am Chem Soc. 2007;129:260–261. doi: 10.1021/ja067269o. [DOI] [PubMed] [Google Scholar]
- 14.Nargeot J, Lester HA, Birdsall NJ, Stockton J, Wassermann NH, Erlanger BF. A photoisomerizable muscarinic antagonist. Studies of binding and of conductance relaxations in frog heart. J Gen Physiol. 1982;79:657–678. doi: 10.1085/jgp.79.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaufman H, Vratsanos SM, Erlanger BF. Photoregulation of an enzymic process by means of a light-sensitive ligand. Science. 1968;162:1487–1489. doi: 10.1126/science.162.3861.1487. [DOI] [PubMed] [Google Scholar]
- 16.Wainberg MA, Erlanger BF. Investigation of the active center of trypsin using photochromic substrates. Biochemistry. 1971;10:3816–3819. doi: 10.1021/bi00797a002. [DOI] [PubMed] [Google Scholar]
- 17.Westmark PR, Kelly JP, Smith BD. Photoregulation of enzyme activity. Photochromic, transition-state-analogue inhibitors of cysteine and serine proteases. J Am Chem Soc. 1993;115(9):3416–3419. [Google Scholar]
- 18.Zhang Y, Erdmann F, Fischer G. Augmented photoswitching modulates immune signaling. Nat Chem Biol. 2009;5:724–726. doi: 10.1038/nchembio.214. [DOI] [PubMed] [Google Scholar]
- 19.Jiang B, Sun X, Cao K, Wang R. Endogenous Kv channels in human embryonic kidney (HEK-293) cells. Mol Cell Biochem. 2002;238:69–79. doi: 10.1023/a:1019907104763. [DOI] [PubMed] [Google Scholar]
- 20.Smart T, Krishek B. Xenopus oocyte microinjection and Ion-channel expression. In: Boulton AA, Baker GB, Walz W, editors. Patch clamp applications and protocols, neuromethods. Vol. 26. Humana Press; Totowa, NJ: 1995. pp. 1–47. [Google Scholar]
- 21.Papazian DM, Schwarz TL, Tempel BL, Jan YN, Jan LY. Cloning of genomic and complementary DNA from shaker, a putative potassium channel gene from drosophila. Science. 1987;237:749–753. doi: 10.1126/science.2441470. [DOI] [PubMed] [Google Scholar]
- 22.Hoshi T, Zagotta WN, Aldrich RW. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science. 1990;250:533–538. doi: 10.1126/science.2122519. [DOI] [PubMed] [Google Scholar]
- 23.Krouse ME, Lester HA, Wassermann NH, Erlanger BF. Rates and equilibria for a photoisomerizable antagonist at the acetylcholine receptor of electrophorus electroplaques. J Gen Physiol. 1985;86:235–256. doi: 10.1085/jgp.86.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen CT, Wagner H, Still WC. Fluorescent, sequence-selective peptide detection by synthetic small molecules. Science. 1998;279:851–853. doi: 10.1126/science.279.5352.851. [DOI] [PubMed] [Google Scholar]
- 25.Kingston RE, Chen CA, Okayama H. Calcium phosphate transfection. Curr Protoc Cell Biol. 2003;Chapter 20(Unit 20.3) doi: 10.1002/0471143030.cb2003s19. [DOI] [PubMed] [Google Scholar]
- 26.Molleman A. Patch clamping: an introductory guide to patch clamp electrophysiology. 1st edn Wiley; New York: 2002. p. 186. [Google Scholar]
- 27.Kirsch GE, Taglialatela M, Brown AM. Internal and external TEA block in single cloned K + channels. Am J Physiol. 1991;261:C583–C590. doi: 10.1152/ajpcell.1991.261.4.C583. [DOI] [PubMed] [Google Scholar]
- 28.Sadovski O, Beharry AA, Zhang F, Woolley GA. Spectral tuning of azobenzene photo-switches for biological applications. Angew Chem Int Ed. 2009;48:1484–1486. doi: 10.1002/anie.200805013. [DOI] [PubMed] [Google Scholar]