Abstract
Purpose
For individuals with acute stroke, it is difficult to conduct an exercise test to assess peak oxygen consumption (peak Vo2). Therefore, the purpose of this study was to use a clinically feasible tool for assessing prestroke peak Vo2 using a nonexercise estimation equation to test whether estimated prestroke peak Vo2 was related to the functional outcome measures at discharge from the hospital in individuals after an acute stroke. We hypothesized that the estimated prestroke peak Vo2 would be significantly related to discharge Physical Performance Test (PPT), 6-minute walk test (6MWT), and lower extremity Fugl-Meyer (LEFM) assessment.
Methods
Estimated prestroke peak Vo2 was calculated using a previously validated prediction equation using the following variables: body mass index, age, sex, resting heart rate, and a self-reported measure of physical activity. Outcome measures were assessed 4 days after enrollment or immediately before discharge (whichever occurred first).
Results
Thirty-four participants (mean age = 56.0, SD = 12.6 years; 20 men) with acute stroke were enrolled within 48 hours of admission. For all individuals, mean estimated prestroke peak Vo2 was 27.3 (SD = 7.4) mL·kg−1·min−1 and had a weak, nonsignificant relationship with the PPT (r = 0.19; P = .28), 6MWT (r = 0.10; P = .56), and LEFM (r = 0.32; P = .06). However, when considering sex, women, but not men, had a significant relationship with LEFM (r = 0.73; P = .005) and moderate but nonsignificant relationship with PPT (r = 0.53; P = .06) and 6MWT (r = 0.47; P = .10).
Conclusions
Within 48 hours of stroke admission, we were able to administer a nonexercise equation to estimate prestroke peak Vo2. For the entire sample, functional measures conducted at discharge were not related to estimated prestroke peak Vo2. However, when considering sex, the relationship between prestroke Vo2 and the functional measures was strengthened.
Keywords: estimated peak Vo2, acute stroke, functional performance, nonexercise Vo2 estimate
INTRODUCTION
Individuals who have had a stroke are physically inactive, spend little time walking per day,1 and have poor cardiorespiratory (CR) fitness, or peak oxygen consumption (peak Vo2).2–5 Assessing peak Vo2 using a metabolic cart with gas analysis is considered the gold standard.6 Peak Vo2 may be an indicator of one’s ability to participate in varying levels of activity such as exercise and activities of daily living (ADLs), especially in older adults7,8 and individuals after stroke.2 However, peak exercise testing is difficult, if not impossible, to conduct during the acute stroke hospital stay, particularly with shorter lengths of stay, especially in the United States. In our previous work, we reported an average of 2.5 days for an acute stroke hospital stay.9 This, combined with ongoing medical diagnostic testing, patient fatigue, and neuromotor impairments, makes exercise testing difficult. Therefore, using nonexercise measurements, such as prediction equations, to estimate peak Vo2 could be a simple lowcost alternative to peak exercise testing for health care providers and especially physical therapists during the acute stroke hospital stay.
The American Heart Association published a scientific statement, The Importance of Cardiorespiratory Fitness (Vo2 max) in the United States,10 which highlights that CR fitness is recognized as an important marker of both functional ability and cardiovascular (CV) health. Furthermore, CR fitness is currently the only major risk factor that is not routinely or regularly assessed.10 Individuals who have had a stroke already have poor CR fitness in the acute stroke setting11 and our work has demonstrated that a high percentage of the acute hospital stay is spent being sedentary.9 Therefore, using a previously validated non-exercise estimation of peak Vo2 may be a feasible option for rehabilitation professionals to assess CR fitness during the acute hospital stay when exercise testing may not be available or easily conducted and may be used as a marker of functional ability.10
Jurca et al12 developed and validated a prediction equation to estimate peak Vo2 in a large population of community dwelling middle-aged adults. Their prediction equation was then crossvalidated in a targeted older adult population (60–80 years of age) with 83.3% of the sample having between 1 and 6 CV risk factors and 15.7% having no CV risk factors.13 The measured Vo2max (21.58 mL·kg−1·min−1) and predicted Vo2max (21.42 mL·kg−1·min−1) were similar in this group of older adults. This non-exercise prediction equation uses the following variables: sex, age, body mass index (BMI), resting heart rate (rHR), and a self-reported measure of physical activity. The equation would be simple and easy to use in the acute stroke setting and has clinical utility for estimating peak Vo2.
Having a measure of CR fitness using an estimated peak Vo2, such as the Jurca equation, allows for investigating whether prestroke CR fitness is related to stroke recovery. There is evidence in animal models that suggests that exercise preconditioning protects against injury following myocardial ischemia14 and cerebral ischemia (midcerebral artery occlusion followed by reperfusion).15–18 In human studies19–21 physical activity before stroke may be neuroprotective and may reduce stroke-related impairment as measured by the Barthel Index19,21 and modified Rankin Score.19,20
The purpose of this study was to use a clinically feasible tool for assessing prestroke peak Vo2 using a nonexercise estimation equation to test whether estimated prestroke peak Vo2 was related to functional outcome measures at discharge from the hospital in individuals after an acute stroke. We hypothesized a priori that after controlling for lesion volume, the estimated prestroke peak Vo2 would be moderately and significantly related to the Physical Performance Test (PPT), our primary outcome measure, the 6-minute walk test (6MWT), and the lower extremity Fugl-Meyer assessment (LEFM) at discharge. A post hoc analysis was conducted to determine whether sex differences existed between estimated prestroke peak Vo2 and our outcome measures at discharge.
METHODS
Study Design
This study used a sample of convenience and included individuals admitted to KU Hospital with a diagnosis of acute stroke. The study was approved by the Human Subjects Committee (HSC) at KU Medical Center and institutionally approved written informed consent was obtained from all participants before the study enrollment.
Participants
Between the months of June 2012 and October 2013, 683 individuals admitted to KU Hospital with a diagnosis of acute stroke were screened for inclusion into the parent study, which has been published.9 Individuals were enrolled if they were between 20 and 80 years of age with a diagnosis of acute ischemic or hemorrhagic stroke. Individuals were excluded if: (1) consent was not possible without surrogate consent; (2) the individual was unable to provide written consent within 48 hours of admission to the stroke unit; (3) discharge from the hospital was to occur before baseline assessments; and (4) the individual was prescribed physician-ordered bed rest. Table 1 outlines demographic characteristics of study participants. Of the 683 individuals screened, 238 were ineligible because of the anticipation of discharge before the study team conducted baseline testing, 113 were ineligible due to physician ordered bed rest, 112 were ineligible because the time on the stroke unit exceeded 48 hours before the study team initiated consent, 94 did not meet the inclusion criterion of age, 67 were ineligible because of intubation or the inability to consent, and 21 individuals declined participation.
TABLE 1.
Participant Demographics
| Characteristics, n = 34 | Number or Group Mean (SD) |
|---|---|
| Men | 20 |
| Age, yrs | 56.0 (12.6) |
| Race/ethnicity | |
| Caucasian | 24 |
| African American | 5 |
| Hispanic | 3 |
| Native American | 1 |
| Asian | 1 |
| Diabetes | |
| None | 18 |
| Type 1 | 1 |
| Type 2 | 10 |
| Unknown | 5 |
| NIH Stroke Scale on Admission | 3.7 (4.4) |
| Discharge performance | |
| Physical Performance Test | 18.9 (11.2) |
| 6-min Walk Test, m | 199.7 (149.8) |
| Lower Extremity Fugl-Meyer Score | 27.4 (9.3) |
| Body Mass Index | 28.0 (5.2) |
Abbreviations: NIH, National Institutes of Health.
Outcome Measures
All functional performance outcome measures were assessed on either the fourth day after consent (day 4) or on the day of discharge from the hospital, whichever occurred first.9 A stroke neurologist administered the NIH Stroke Scale (NIHSS) on admission to the hospital. A radiologist (N.H.) calculated lesion volumes from structural magnetic resonance images performed on admission to the hospital using established standardized neuroimaging techniques for ischemic and hemorrhagic stroke.22,23 An ellipsoidal estimation function was used.23 In cases of multiple lesions, largest lesion volume was used and all volumes are reported in cm3.23
Estimated Peak Oxygen Consumption
We selected the previously established and validated equation by Jurca et al12 and Mailey et al13 based on data from our laboratory examining nonexercise estimated peak Vo2 and measured peak Vo2 in healthy adults (n = 110). The results of our unpublished data were similar to those of previous reports12,13 in which estimated peak Vo2 was strongly correlated to measured peak Vo2 (r = 0.88 P<.001). Based on these data, we chose to use this nonexercise equation to estimate prestroke peak Vo2 in patients with acute stroke during the hospital stay.
After obtaining consent from acute stroke participants, we recorded the necessary information for the Jurca equation.12 The Jurca equation uses the following variables to estimate prestroke peak Vo2: age, sex, BMI, rHR, and a constant variable associated with the participants’ self-reported physical activity level before being admitted to KU Hospital for acute stroke. The self-reported physical activity score is separated into 5 categories (levels 1–5) with a constant variable assigned to each. Each level considers a different duration, frequency, and intensity of activity performed in a typical week. The information regarding participants’ activity levels shown in Table 2. The Jurca prediction equation is as follows, estimated metabolic equivalents = ([Sex, F = 0; M = 1 × 2.77] − [Age in years × 0.10] − [BMI × 0.17] − [rHR × 0.03] + [Physical Activity Score × 1.00] + 18.07).12 Estimated metabolic equivalents is multiplied by 3.5 mL·kg−1·min−1 (1 metabolic equivalent = 3.5 mL·kg−1·min−1)6 to obtain estimated prestroke peak Vo2.
TABLE 2.
Self-Reported Physical Activity Scores for Prediction Equation
| Physical Activity Score, n = 34 | Number | Constant |
|---|---|---|
| Level 1 | 6 | 0.00 |
| Level 2 | 19 | 0.32 |
| Level 3 | 5 | 1.06 |
| Level 4 | 0 | 1.76 |
| Level 5 | 5 | 3.03 |
Level 1: Primarily sedentary; Level 2: 5 days or more per week of light physical activity for 10 minutes or more at a time; Level 3: Moderate aerobic exercise for 20 to 60 minutes per week; Level 4: Moderate aerobic exercise for 1 to 3 hours per week; Level 5: Moderate aerobic exercise for over 3 hours per week.
Although the Jurca equation has not been validated in the acute stroke population, it has been previously validated in older adults with multiple CV risk factors.13 We did not expect BMI to change within 1 to 2 days after stroke. Physical activity was assessed as “activities performed in a typical week before the patient underwent stroke.” However, rHR could be influenced after a stroke. Therefore, we wanted to ensure that the rHR at the time of stroke was appropriate to use. To our knowledge, there is no information in the literature regarding whether rHR has similar values before and after stroke. We examined the medical record of a small subset of the cohort who had rHR captured in the medical record before their stroke. In these individuals (n = 19), rHR was not significantly different before and after stroke (before stroke: mean = 76.4, SD = 10.8 bpm; after stroke: mean = 76.4, SD = 13.8 bpm; P = .983).
Physical Performance Test
The PPT has been identified as a useful clinical tool and has demonstrated high interrater reliability and concurrent validity for measuring performance of ADLs in older adults and individuals after a stroke.24–26 To measure performance of ADLs, we previously used the 9-item version of the PPT in older adults with and without Alzheimer disease27 and on the acute stroke unit.9 The PPT includes tasks that individuals complete on a daily basis such as writing a sentence, simulated eating, lifting a book onto a shelf above shoulder height, putting on and removing a jacket, picking up an item from the floor, walking 50 feet, turning in a circle, rising from a chair 5 times without the use of the upper extremities, and a progressive Rhomberg test of balance (standing with feet side-by-side, semi-tandem, and full tandem).27,28
Six-Minute Walk Test
The 6MWT is a reliable and valid tool for assessing walking endurance in individuals after stroke.29–35 The 6MWT was performed with minimal distractions on a 14-m section of the walking track outside the participants’ room on the stroke unit. A 14-m ribbon was secured to the floor and orange cones were placed at both ends. A stopwatch was used to record time and standardized verbal cues were given every minute according to previously published guidelines.36 All participants wore a gait belt during the test for safety. If the tester needed to provide greater than minimal physical assistance with walking or if the participant asked to stop and needed to sit down to rest, the testing was terminated and the distance walked in that time was recorded. Participants were allowed to use an assistive device (eg, walker or cane) if needed during the test. If a participant was unable to ambulate with or without an assistive device, a score of 0 m was recorded.
Fugl-Meyer Assessment
The FMA is a commonly used tool to measure motor performance and has been shown to be a valid and reliable tool in individuals after stroke.37 We collected data using only the lower extremity motor portion (LEFM), which was scored on a scale of 0 to 34, with 34 indicating no motor impairment.9
Sample Size Justification and Statistical Analysis
We calculated sample size for the parent study9 and determined that we needed 35 participants. We allowed for a small attrition rate of 10% and therefore, planned to enroll a total of 38 individuals.
Data analysis was performed using SPSS software Version 20.0 (SPSS Inc, Chicago, IL) for Windows. We report data as mean (standard deviation) where appropriate. All significance levels were set to a 0.05 alpha-level, with 95% confidence intervals (CI). A partial correlation was performed to determine the relationship of estimated prestroke peak Vo2 and functional outcomes (PPT, 6MWT, and LEFM) while controlling for lesion volume. We chose to be conservative in our analyses and controlled for initial lesion volume, a continuous variable, because of its potential influence on motor performance. Additionally, to examine whether there might be sex differences in estimated prestroke peak Vo2 and our selected outcome measures, partial correlations were performed.
RESULTS
We enrolled 38 participants with a total of 34 individuals completing the study (Table 1). Mean time between study enrollment and performance of discharge assessments was 2.5 days.
Most participants reported engaging in light physical activity prestroke (Table 2). Mean estimated prestroke peak Vo2 was 27.3 (7.4) mL·kg−1·min−1 and is consistent with that of previous studies using measured peak Vo2.5,11 This supports previous evidence that after stroke, patients had lower CR fitness prestroke for their age, using ACSM categories. Table 3 demonstrates estimated prestroke peak Vo2 and fitness level for sex and age (Figure 1).
TABLE 3.
Estimated Peak Vo2 Levels Using a Nonexercise Estimation
| Age Group, yrs |
n | Group Mean (SD), mL·kg−1·min−1 |
ASCM Fitness Category |
|---|---|---|---|
| All | 34 | 27.3 (7.4) | |
| 26–35 | 2 | 25.3 (4.9) | Very poor |
| 36–45 | 5 | 26.3 (7.0) | Poor |
| 46–55 | 9 | 33.2 (9.4) | Average |
| 56–65 | 9 | 24.9 (6.2) | Poor |
| 65+ | 9 | 24.7 (4.1) | Below average |
| Men | 20 | 31.3 (6.6) | |
| 26–35 | 0 | ||
| 36–45 | 3 | 31.0 (2.0) | Average |
| 46–55 | 6 | 38.7 (5.7) | Good |
| 56–65 | 4 | 30.4 (2.3) | Average |
| 65+ | 7 | 25.5 (3.3) | Average |
| Women | 14 | 21.6 (4.1) | |
| 26–35 | 2 | 25.3 (4.9) | Very poor |
| 36–45 | 2 | 19.2 (4.9) | Very poor |
| 46–55 | 3 | 22.3 (0.9) | Poor |
| 56–65 | 5 | 20.5 (4.2) | Poor |
| 65+ | 2 | 22.1 (7.3) | Average |
Abbreviations: Vo2, oxygen consumption; ACSM, American College of Sports Medicine.
Fig. 1.
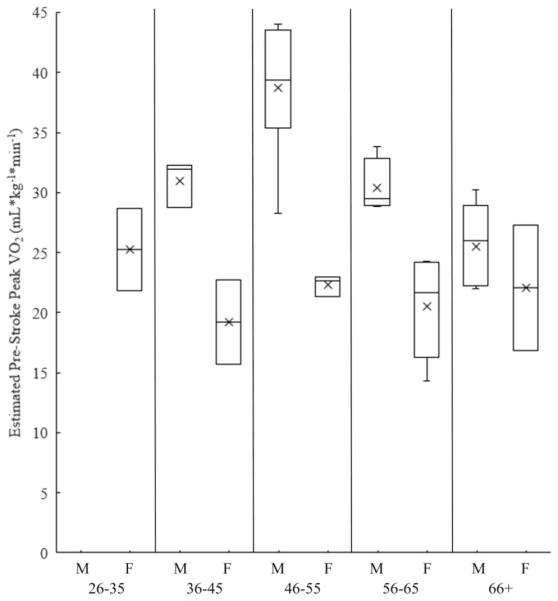
Box plot of sex and age differences in estimated prestroke peak Vo2.
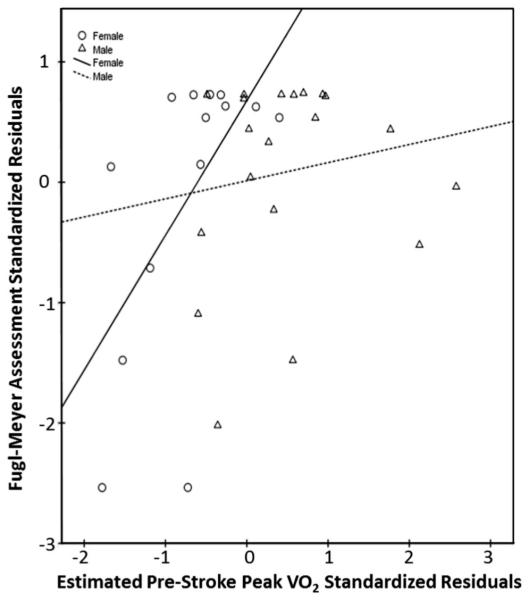
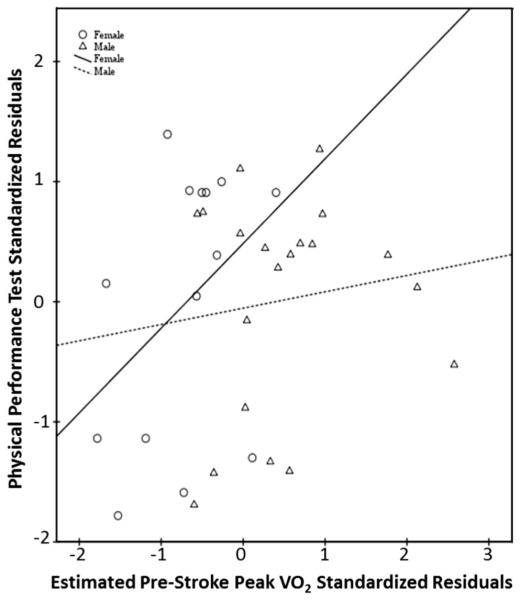
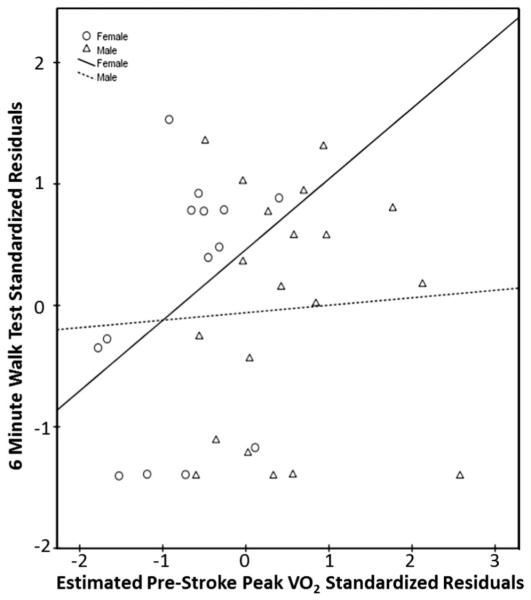
Estimated prestroke peak Vo2 was weakly, but not significantly, related to discharge outcome scores for the PPT (r = 0.19, P = .28, CI −0.14 to 0.48), 6MWT distance (r = 0.10, P = .56, CI −0.24 to 0.45), and LEFM scores (r = 0.32, P = .06, CI −0.03 to 0.61) when controlling for initial lesion volume. When separating data by sex and controlling for initial lesion volume, estimated prestroke peak Vo2 in men did not have any significant relationships with functional measures (PPT: r = 0.13, P = .60, CI −0.32 to 0.55; 6MWT: r = 0.05, P = .82, CI −0.44 to 0.59; LEFM: r = 0.15, P = .54, CI −0.35 to 0.57). Estimated prestroke peak Vo2 in women showed a strong, significant relationship with LEFM (r = 0.73, P = .005, CI 0.41 to 0.96) (Fig. 2), but moderate, nonsignificant relationships with the PPT (r = 0.53, P = .06, CI −0.13 to 0.91) (Fig. 3), and 6MWT (r = 0.47, P = .10, CI −0.14 to 0.91) (Fig. 4) when controlling for initial lesion volume. Mean lesion volume was 2.6 (7.0) cm3.
Fig. 2.
Estimated prestroke peak Vo2 versus discharge FMA score.
Fig. 3.
Estimated prestroke peak Vo2 versus discharge PPT score.
Fig. 4.
Estimated prestroke peak Vo2 versus discharge 6MWT score.
DISCUSSION
This study used a nonexercise estimation equation to predict prestroke peak Vo2 as an assessment of CR fitness in people with acute stroke during their hospital stay. The mean length of stay for our participants was 2.5 days, which may make conducting an exercise test during the acute hospital stay difficult. Previous data support that approximately 50% of patients with acute stroke return home and do not receive any information regarding CR fitness.38 These findings support previous work that CR fitness is low in acute stroke, specifically among women.11 Using a nonexercise estimation of peak Vo2 would allow rehabilitation professionals, such as physical therapists, the opportunity to evaluate and discuss with their patients the importance of a prescribed home exercise program wherever appropriate.
In humans, there is some suggestion that prestroke physical activity can have a positive effect on stroke recovery after 8 days,19 3 months,21 and 2 years20 after stroke. One study enrolled participants within 30 days of stroke and asked about their physical activity levels during the year before their stroke. The outcome measures were collected by telephone at 2 months after stroke and were “dichotomized into ‘good’ and ‘bad’ categories according to instrument-specific cut-off points.”21 They report that individuals who engaged in moderate or high levels of activity were more likely to have a “good” outcome at 3 months after stroke. Based on the evidence in the literature and in an attempt to better understand the relationship between CR fitness and stroke recovery, we conducted this study. We hypothesized that estimated prestroke peak Vo2 would be moderately and significantly related to discharge PPT, 6MWT, and LEFM, when controlling for initial lesion volume. Our reported results did not support our hypothesis. Our results showed only a weak, nonsignificant correlation between estimated prestroke peak Vo2 and 2 of our outcome measures at discharge. This could be due to an overestimation or underestimation of physical activity before stroke. This would influence the predicted peak Vo2.
The acute stroke period is a sensitive time where an individual may not remember their typical physical activity. It is possible that men and women may account for daily physical activity differently. Therefore, in a post hoc analysis, we examined whether sex differences exist between estimated prestroke peak Vo2 and functional performance at the time of discharge. A novel finding was the sex difference related to the estimated prestroke peak Vo2 and the functional outcome measures. The results indicated that, in women, estimated prestroke peak Vo2 was related to functional outcomes but only the LEFM was significantly related. There was no relationship between estimated prestroke peak Vo2 and functional outcome measures in men. These results could be due to an overestimation of self-selected physical activity levels in men or underestimation in women. There could be an influence of stroke size and location on men and women.
We imposed a control for the initial lesion volume but we also acknowledge that the location of the lesion could influence functional performance such as walking during the 6MWT. We also acknowledge that participant fatigue could have influenced individual performance and the functional measures, especially the 6MWT which measures walking endurance. We worked with the hospital therapy team to coordinate our testing with therapy sessions to avoid overlap, but previous therapy sessions could have affected performance. In addition, on the day of discharge from the hospital, the participant was busy before leaving and this could have affected patient performance. It is important to acknowledge that our study was likely underpowered when considering previous work where the sample sizes were much larger.19,21
These pilot data will provide important information so that future work can be adequately powered to determine whether estimated prestroke peak Vo2 is related to outcome measures, such as the PPT or walking endurance (6MWT), and has laid the foundation for future work. The Jurca equation to estimate peak Vo2 has provided an objective measure for CR fitness in patients after stroke. Although we did not compare the predicted peak Vo2 to measured Vo2, one previous study by McAuley et al39 found that these measures were correlated in older adults with chronic disease. The authors used the Jurca equation to investigate the association of the nonexercise estimation with peak Vo2 measures of CR fitness (maximal exercise test and 1-mile timed walk).39 They found that the estimation equation was significantly correlated with the maximal exercise test and the 1-mile timed walk. McAuley et al39 suggests that their results serve as evidence that the Jurca equation has use in other populations, but that future work needs to be performed to determine whether any relationships exist in other domains. The current work adds to the literature by describing the relationship of the Jurca nonexercise prediction equation and measures of function in a small sample of individuals with acute stroke. McAuley et al39 highlight that assessing CR fitness is important in both healthy and diseased populations and may give deeper insight into other domains of health and acknowledges that exercise testing in older adults and individuals with high CV risk is difficult because of the risks involved, time constraints, and expenses such as obtaining proper equipment, personnel, and medical oversight. Therefore, we believe that there is a need for a short nonexercise estimation of Vo2 that is easily obtained in the acute clinical setting.
This study is novel in several ways. First, we enrolled individuals early after stroke, within 48 hours of admission to the stroke unit, whereas previous work has enrolled participants using a wider window of 30 days21 or between 5 and 24 days after stroke.20 Second, rather than using the Likert scale for physical activity, we chose to use a continuous measure of CR fitness, nonexercise estimated peak Vo2, which incorporates many variables, including sex, age, self-reported physical activity, and rHR. We acknowledge that rHR may be different before and after stroke, although we demonstrated in a subset of our participants that rHR was not significantly different following stroke when compared to prestroke values. Because of a small sample size, we acknowledge that these data may not be generalizable for all patients who have had a stroke.
We examined the relationship between estimated prestroke peak Vo2 and selected outcome measures at discharge from the hospital. Although most studies have used categorical levels of self-reported physical activity, the intention was to be more specific by using peak Vo2, which is a continuous variable. To our knowledge, this is the first investigation to examine the relationship between estimated prestroke peak Vo2 and objective measures of functional outcome at discharge from an acute stroke unit.
This study is the first study conducted on acute stroke using an estimated peak Vo2 prediction equation. These data provide important information for assessing CR fitness as recommended by the American Heart Association.10 The data have provided a foundation for future work to rigorously examine the relationship between peak Vo2 (estimated or measured) and its outcome at discharge following stroke.
Limitations
We must address the large number of participants not enrolled and the potential for systematic bias. We had many participants on bed rest, and 238 were ineligible because of the anticipation of discharge before the study team conducted baseline testing. We recognize that this may seem to be a systematic bias towards nonenrollment for those with mild stroke, which would bias the study towards accepting the null hypothesis. The reason for nonenrollment lies with the parent study,40 for which we aimed to consent, perform baseline testing, and participants with equip accelerometers all within 48 hours of admission to the hospital. Participants also needed a confirmed diagnosis of stroke by neuroimaging. The challenge we faced was the short lengths of stay (mean of 2.5 days) for all patients regardless of stroke severity. If the study team was unable to consent and conduct the baseline testing within 48 hours, many potential participants were not approached for consent. This could have been due to the individual being unavailable because he was out for other medical tests, so our study team did not get the neuroimaging report within 48 hours or we had current participant testing and could not screen and consent with the potential participant in a timely manner. We believe we enrolled participants with a wide range of function from mild to severe stroke (Table 1 and Figs. 2–4). It is important to note that although the functional performance of participants at discharge was varied, the propensity for low peak Vo2 values, especially in women is observed across the x-axis for estimated prestroke Vo2 (Figs. 2–4). This may be a result of an underestimation of physical activity before stroke or that people who have had a stroke tend to have low levels of aerobic fitness.41 Finally, we must acknowledge that this study was powered for the parent study and not powered to detect the relationship between the functional outcome measures and estimated prestroke peak Vo2. This study does provide data for future studies to adequately power and address scientific questions in this area of research.
CONCLUSIONS
There is limited information regarding CR fitness early after stroke, especially during a short hospital stay. The authors believe that given the challenges to conduct a maximal exercise test within the first few days after stroke, a prediction equation for assessing CR fitness (peak Vo2) was a reasonable tool to use during the acute stroke hospital stay. The results show weak nonsignificant relationships between estimated prestroke peak Vo2 early after stroke and our selected functional outcome measures. Females demonstrated a stronger relationship with the functional measures at the time of discharge but only the LEFM was significantly related. This study lays the groundwork for future exploration in this area of research.
Acknowledgments
Sandra A. Billinger was supported in part by award number K01HD067318 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Anna E. Mattlage, Sara A. Redlin, and Jason-Flor V. Sisante were supported in part by award number T32HD057850 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Anna E. Mattlage was also supported in part by award number 14PRE20040026 from the American Heart Association. REACH laboratory space is supported by the Georgia Holland Endowment Fund. We would also like to thank the participants and their families for their time and dedication to this study and Lindsay Loyd, DPT, and Virginia Rader, DPT. KUMC Human Subjects Committee #13386.
Footnotes
Michael G. Abraham is a consultant for Stryker Neurovascular and is listed in the speaker bureau for Boehringer Ingelhelm. The remaining authors declare no conflicts of interest.
REFERENCES
- 1.Roos MA, Rudolph KS, Reisman DS. The structure of walking activity in people after stroke compared with older adults without disability: a cross-sectional study. Phys Ther. 2012;92:1141–1147. doi: 10.2522/ptj.20120034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gordon NF, Gulanick M, Costa F, et al. Physical activity and exercise recommendations for stroke survivors: an American Heart Association scientific statement from the council on clinical cardiology, subcommittee on exercise, cardiac rehabilitation, and prevention; the council on cardiovascular nursing; the council on nutrition, physical activity, and metabolism; and the stroke council. Circulation. 2004;109:2031–2041. doi: 10.1161/01.CIR.0000126280.65777.A4. [DOI] [PubMed] [Google Scholar]
- 3.Mackay-Lyons MJ, Makrides L. Exercise capacity early after stroke. Arch Phys Med Rehabil. 2002;83:1697–1702. doi: 10.1053/apmr.2002.36395. [DOI] [PubMed] [Google Scholar]
- 4.Macko RF, Katzel LI, Yataco A, et al. Low-velocity graded treadmill stress testing in hemiparetic stroke patients. Stroke. 1997;28:988–992. doi: 10.1161/01.str.28.5.988. [DOI] [PubMed] [Google Scholar]
- 5.Billinger SA, Taylor JM, Quaney BM. Cardiopulmonary response to exercise testing in people with chronic stroke: a retrospective study. Stroke Res Treat. 2012;2012:987637. doi: 10.1155/2012/987637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ACSM . Guidelines for Exercise Testing and Prescription. 8th Lippincott Williams & Wilkins; Philadelphia, PA: 2010. [Google Scholar]
- 7.Fleg JL, Forman DE, Berra K, et al. Secondary prevention of atherosclerotic cardiovascular disease in older adults: a scientific statement from the American Heart Association. Circulation. 2013;128:2422–2446. doi: 10.1161/01.cir.0000436752.99896.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Billinger SA, Vidoni ED, Honea RA, et al. Cardiorespiratory response to exercise testing in individuals with Alzheimer’s disease. Arch Phys Med Rehabil. 2011;92:2000–2005. doi: 10.1016/j.apmr.2011.07.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mattlage AE, Redlin SA, Rippee MA, et al. Use of accelerometers to examine sedentary time on an acute stroke unit. J Neurol Phys Ther. 2015;39:166–171. doi: 10.1097/NPT.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaminsky LA, Arena R, Beckie TM, et al. The importance of cardiorespiratory fitness in the united states: the need for a national registry: a policy statement from the American Heart Association. Circulation. 2013;127:652–662. doi: 10.1161/CIR.0b013e31827ee100. [DOI] [PubMed] [Google Scholar]
- 11.MacKay-Lyons MJ, Makrides L. Cardiovascular stress during a contemporary stroke rehabilitation program: is the intensity adequate to induce a training effect? Arch Phy Med Rehabil. 2002;83:1378–1383. doi: 10.1053/apmr.2002.35089. [DOI] [PubMed] [Google Scholar]
- 12.Jurca R, Jackson AS, LaMonte MJ, et al. Assessing cardiorespiratory fitness without performing exercise testing. Am J Prev Med. 2005;29:185–193. doi: 10.1016/j.amepre.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Mailey EL, White SM, Wojcicki TR, et al. Construct validation of a non-exercise measure of cardiorespiratory fitness in older adults. BMC Public Health. 2010;10:59. doi: 10.1186/1471-2458-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calvert JW, Condit ME, Aragon JP, et al. Exercise protects against myocardial ischemia-reperfusion injury via stimulation of beta(3)-adrenergic receptors and increased nitric oxide signaling: role of nitrite and nitrosothiols. Circ Res. 2011;108:1448–1458. doi: 10.1161/CIRCRESAHA.111.241117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding YH, Ding Y, Li J, et al. Exercise pre-conditioning strengthens brain microvascular integrity in a rat stroke model. Neurol Res. 2006;28:184–189. doi: 10.1179/016164106X98053. [DOI] [PubMed] [Google Scholar]
- 16.Liebelt B, Papapetrou P, Ali A, et al. Exercise preconditioning reduces neuronal apoptosis in stroke by up-regulating heat shock protein-70 (heat shock protein-72) and extracellular-signal-regulated-kinase 1/2. Neuroscience. 2010;166:1091–1100. doi: 10.1016/j.neuroscience.2009.12.067. [DOI] [PubMed] [Google Scholar]
- 17.Davis W, Mahale S, Carranza A, et al. Exercise pre-conditioning ameliorates blood-brain barrier dysfunction in stroke by enhancing basal lamina. Neurol Res. 2007;29:382–387. doi: 10.1179/016164107X204701. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Luan X, Clark JC, et al. Neuroprotection against transient cerebral ischemia by exercise pre-conditioning in rats. Neurol Res. 2004;26:404–408. doi: 10.1179/016164104225016038. [DOI] [PubMed] [Google Scholar]
- 19.Deplanque D, Masse I, Libersa C, et al. Previous leisure-time physical activity dose dependently decreases ischemic stroke severity. Stroke Res Treat. 2012;2012:614925. doi: 10.1155/2012/614925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krarup LH, Truelsen T, Gluud C, et al. Prestroke physical activity is associated with severity and long-term outcome from first-ever stroke. Neurology. 2008;71:1313–1318. doi: 10.1212/01.wnl.0000327667.48013.9f. [DOI] [PubMed] [Google Scholar]
- 21.Stroud N, Mazwi TM, Case LD, et al. Prestroke physical activity and early functional status after stroke. J Neurol Neurosurg Psychiatry. 2009;80:1019–1022. doi: 10.1136/jnnp.2008.170027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Majersik JJ, Cole JW, Golledge J, et al. Recommendations from the international stroke genetics consortium, part 1: standardized phenotypic data collection. Stroke. 2015;46:279–284. doi: 10.1161/STROKEAHA.114.006839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newman GC. Clarification of abc/2 rule for ICH volume. Stroke. 2007;38:862. doi: 10.1161/01.STR.0000257309.50643.0a. [DOI] [PubMed] [Google Scholar]
- 24.Sherman SE, Reuben D. Measures of functional status in community-dwelling elders. J Gen Intern Med. 1998;13:817–823. doi: 10.1046/j.1525-1497.1998.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reuben DB, Valle LA, Hays RD, et al. Measuring physical function in community-dwelling older persons: a comparison of self-administered, interviewer-administered, and performance-based measures. J Am Geriatr Soc. 1995;43:17–23. doi: 10.1111/j.1532-5415.1995.tb06236.x. [DOI] [PubMed] [Google Scholar]
- 26.Rozzini R, Frisoni G, Ferrucci L, et al. The effect of chronis diseases on physical function. Comparison between activities of daily living scales and the physical performance test. Age Ageing. 1997;26:281–287. doi: 10.1093/ageing/26.4.281. [DOI] [PubMed] [Google Scholar]
- 27.Vidoni ED, Billinger SA, Lee C, et al. The physical performance test predicts aerobic capacity sufficient for independence in early-stage Alzheimer disease. J Geriatr Phys Ther. 2012;35:72–78. doi: 10.1519/JPT.0b013e318232bf61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah KR, Carr D, Roe CM, et al. Impaired physical performance and the assessment of dementia of the alzheimer type. Alzheimer Dis Assoc Disord. 2004;18:112–118. doi: 10.1097/01.wad.0000127441.77570.f3. [DOI] [PubMed] [Google Scholar]
- 29.Kosak M, Smith T. Comparison of the 2-, 6-, and 12-minute walk tests in patients with stroke. J Rehabil Res Devel. 2004;41:103. doi: 10.1682/jrrd.2003.11.0171. [DOI] [PubMed] [Google Scholar]
- 30.Flansbjer UB, Holmback AM, Downham D, et al. Reliability of gait performance tests in men and women with hemiparesis after stroke. J Rehabil Med. 2005;37:75–82. doi: 10.1080/16501970410017215. [DOI] [PubMed] [Google Scholar]
- 31.Patterson SL, Forrester LW, Rodgers MM, et al. Determinants of walking function after stroke: differences by deficit severity. Arch Phys Med Rehabil. 2007;88:115–119. doi: 10.1016/j.apmr.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 32.Wevers LE, Kwakkel G, van de Port IG. Is outdoor use of the six-minute walk test with a global positioning system in stroke patients’ own neighbourhoods reproducible and valid? J Rehabil Med. 2011;43:1027–1031. doi: 10.2340/16501977-0881. [DOI] [PubMed] [Google Scholar]
- 33.Perera S, Mody SH, Woodman RC, et al. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 34.Eng JJ, Dawson AS, Chu KS. Submaximal exercise in persons with stroke: test-retest reliability and concurrent validity with maximal oxygen consumption1. Arch Phy Med Rehabil. 2004;85:113–118. doi: 10.1016/s0003-9993(03)00436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fulk GD, Echternach JL. Test-retest reliability and minimal detectable change of gait speed in individuals undergoing rehabilitation after stroke. J Neurol Phys Ther. 2008;32:8–13. doi: 10.1097/NPT0b013e31816593c0. [DOI] [PubMed] [Google Scholar]
- 36.Crapo R, Casaburi R, Coates A, et al. ATS Statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 37.Sanford J, Moreland J, Swanson LR, et al. Reliability of the fugl-meyer assessment for testing motor performance in patients following stroke. Phys Ther. 1993;73:447–454. doi: 10.1093/ptj/73.7.447. [DOI] [PubMed] [Google Scholar]
- 38.Pohl PS, Billinger SA, Lentz A, et al. The role of patient demographics and clinical presentation in predicting discharge placement after inpatient stroke rehabilitation: analysis of a large, US data base. Disabil Rehabil. 2013;35:990–994. doi: 10.3109/09638288.2012.717587. [DOI] [PubMed] [Google Scholar]
- 39.McAuley E, Szabo AN, Mailey EL, et al. Non-exercise estimated cardiorespiratory fitness: associations with brain structure, cognition, and memory complaints in older adults. Ment Health Phys Act. 2011;4:5–11. doi: 10.1016/j.mhpa.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mattlage A, Ashenden A, Lentz A, et al. Submaximal and peak cardiorespiratory response after moderate-high intensity exercise training in subacute stroke. Cardiopulm Phys Ther J. 2013;24:14–20. [PMC free article] [PubMed] [Google Scholar]
- 41.Billinger SA, Arena R, Bernhardt J, et al. Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2532–2553. doi: 10.1161/STR.0000000000000022. [DOI] [PubMed] [Google Scholar]