abstract
HPV vaccinations were recommended with the backing of a Japanese government subsidy program in 2010, and were included in the National Immunization Program in April 2013. However, the Ministry of Health, Labour, and Welfare withdrew the recommendation for the HPV vaccination in June 2013. We investigated HPV vaccine injury compensation programs for both the national and local governments. Approximately 3.38 million girls were vaccinated, and 2,584 complained of health problems. The majority of these received the vaccine shot as a non-routine vaccination. In total, 98 people developed health problems and applied for assistance from 2011 to 2014, but no cases have been processed since October 2014. Several local governments are providing their own compensation program for cases of vaccine adverse reactions, but the number is extremely low (16 of 1,741 municipalities and 1 of 47 prefectures). The local governments that are providing compensation are largely those where HPV vaccine victim support groups are prominent. The confusion regarding the national program for HPV vaccine injury was caused by the discrepancy between the compensation programs for those vaccinated under the immunization law and for those who received voluntary vaccinations. The establishment of a new compensation program might be key to finding a lasting resolution.
Keywords: cervical cancer, compensation program, HPV, immunisation program, Japan, public health, vaccination, voluntary vaccines
Background
In 2010, a free HPV vaccination program was introduced in Japan, and in April 2013, the HPV vaccination was added by the Japanese Ministry of Health, Labour, and Welfare (MHLW) to the National Immunization Program (NIP) schedule for Japanese girls aged 12–16 y. However, in May and June 2013, there were a number of Japanese media reports concerning the vaccine's potential for adverse effects, including complex regional pain syndrome. Following this, on June 14, 2013, MHLW withdrew their recommendation for the HPV vaccination and instructed local governments to cease all vaccine promotion.1,2 This led to public distrust and a dramatic decrease in HPV vaccination rates.3,4
The National Vaccine Injury Compensation Program in Japan is a no-fault system, and there has been a gap between the medical expense assistance provided to those who experience adverse effects from non-routine vaccinations and that for people who have suffered negative effects as a result of routine vaccinations. Claims of compensation due to negative effects after routine vaccination are made through local governments to the MHLW, whereas claims associated with voluntary vaccination are made to the Pharmaceuticals and Medical Devices Agency (PMDA) directly, and compensation is lower compared with that for cases of routine vaccination.5
Objective
The objective of this study was to investigate the HPV vaccine injury compensation programs of the national and local governments in Japan. We considered factors that affected the establishment of compensation programs by local governments, including in areas where activities of the HPV vaccine victim support groups are prominent.
Methods
We reviewed Japanese newspaper articles covering the compensation programs for HPV vaccination via Nikkei Telecom 21. We also searched the internet using Google.
Results
Approximately 3.38 million girls between 6th grade and the first year of high school have been vaccinated. Of these, 2,584 (0.08%) have complained of health problems, and according to the MHLW, 186 (0.005%) have not recovered.6 The majority (90%) of these received the vaccine as a non-routine vaccination, and no significant differences in severe adverse events were observed between 2vHPV and 4vHPV.
From 2011 to July 2015, 98 people who developed health problems after non-routine HPV vaccinations applied for assistance, and 27 cases were settled (18 cases received compensation, and 9 cases were declined). However, no cases have been processed since October 2014, and the national program for HPV vaccine injury has been suspended (with 71 cases pending).7
Due to the suspension of the national compensation system, several local governments are trying to provide their own compensation programs for cases of adverse reactions to the vaccine, but the number of such cases is extremely low (16 of the 1,741 municipalities and 1 of the 47 prefectures in Japan; Fig. 1, Table 1).
Figure 1.
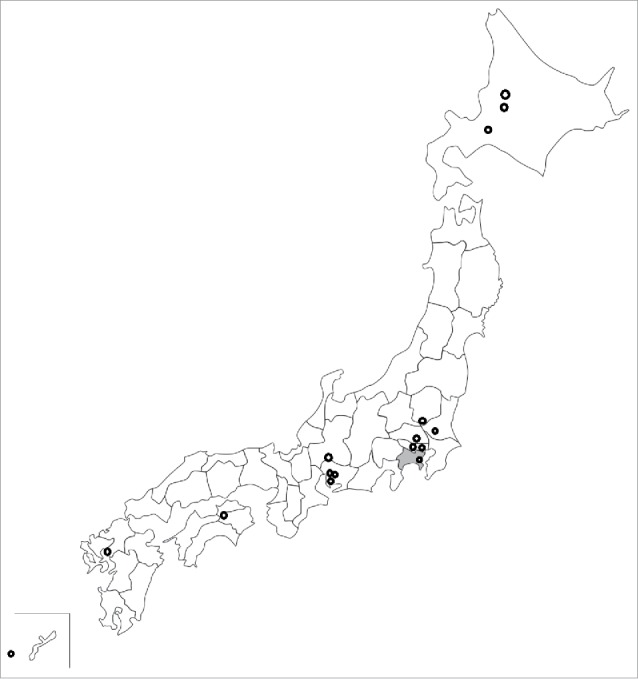
Distribution of local governments that provide their own compensation programs for cases of vaccine adverse reactions. The 15 circles represent municipalities, and the gray areas represent prefectures.
Table 1.
Local governments providing their own compensation program.
| Name of local gov | Type of local gov | Budget* | Adverse Events Cases | Vaccinated Cases | Start date |
|---|---|---|---|---|---|
| Suginami Ward | Municipality | N/A | N/A | N/A | 2013/4/13 |
| Yokohama City | Municipality | 14000000 | 27 | 75000 | 2014/6/1 |
| Bibai City | Municipality | N/A | N/A | 618 | 2015/1/1 |
| Eniwa City | Municipality | N/A | N/A | N/A | 2015/1/1 |
| Ushiku City | Municipality | 3950000 | 1 | N/A | 2015/1/8 |
| Musashino City | Municipality | N/A | 7 | 1951 | 2015/3/30 |
| Fujimino City | Municipality | 6250000 | 3 | 2500 | 2015/5/25 |
| Miyakojima City | Municipality | N/A | N/A | 1632 | 2015/6/1 |
| Tochigi City | Municipality | 1126000 | 1 | 3300 | 2015/6/18 |
| Kariya City | Municipality | 11020000 | 2 | 4082 | 2015/7/1 |
| Hekinan City | Municipality | 3500000 | 2 | 1953 | 2015/7/1 |
| Chiryu City | Municipality | 3970000 | 2 | 1650 | 2015/7/1 |
| Kanagawa Prefecture | Prefecture | 31690000 | 112 | N/A | 2015/7/1 |
| Iwamizawa City | Municipality | 1636000 | 1 | 2130 | 2015/7/1 |
| Okawa City | Municipality | 4720000 | 2 | 909 | 2015/8/27 |
| Miyoshi City | Municipality | 5730000 | N/A | 697 | 2015/10/1 |
| Kakamigahara City | Municipality | N/A | N/A | 3800 | 2015/12/1 |
Name of local gov: Name of local government; Type of local gov: Type of local government (municipality or prefecture).
Budget: Yen.
The nationwide HPV vaccine victim support group liaison office in Tokyo was established on March 25, 2013. From May 2013 to September 2015, 13 local HPV vaccine victim support groups have been established (Fig. 2, Table 2).
Figure 2.
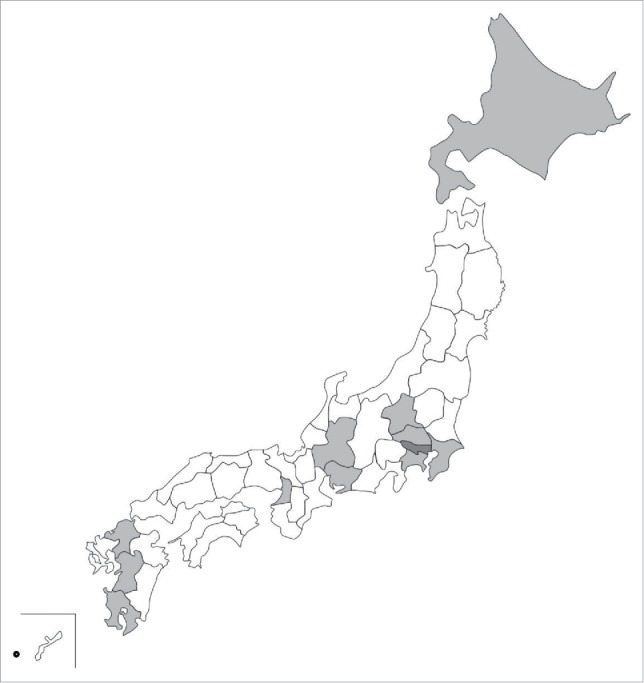
Distribution of local HPV vaccine victim support groups. The gray areas represent 12 prefectures where local HPV vaccine victim support groups are established. The names of the prefectures are Hokkaido, Gunma, Saitama, Kanagawa, Ibaraki, Chiba, Aichi, Gifu, Osaka, Fukuoka, Kumamoto, and Kagoshima (light gray). The nationwide HPV vaccine victim support group liaison office is located in Tokyo (dark gray). The circle represents one municipality, Miyakojima City.
Table 2.
Place and date of the establishment of the HPV vaccine victim support groups.
| Place Established | Date of Establishment |
|---|---|
| *Tokyo Prefecture | 2013/3/25 |
| Chiba Prefecture | 2013/10/3 |
| Kanagawa Prefecture | 2013/10/15 |
| Gunma Prefecture | 2013/10/22 |
| Saitama Prefecture | 2014/6/1 |
| Hokkaido Prefecture | 2014/6/29 |
| **Kumamoto Prefecture | N/A |
| Osaka Prefecture | 2014/10/18 |
| Aichi Prefecture | 2014/10/19 |
| Ibaraki Prefecture | 2014/11/15 |
| Fukuoka Prefecture | 2015/1/31 |
| Miyakojima City | 2015/6/16 |
| Gifu Prefecture | 2015/8/18 |
| Kagoshima Prefecture | 2015/9/14 |
The nationwide HPV vaccine victim support group liaison office was established March 25, 2013, in Tokyo.
The HPV vaccine victim support group in Kumamoto Prefecture was established prior to October 2014.
Discussion
In Japan, the national program for HPV vaccine injury has been suspended but the situation is confusing due to the discrepancy in HPV vaccine injury compensation between those who received vaccinations under the immunization law and those who received them voluntarily. HPV vaccine injury compensation programs differ between patients who were vaccinated before and those who were vaccinated after April 2013, when the Preventive Vaccination Law was amended.6 Patients who receive routine vaccination can be reimbursed for medical expenditures for both outpatient and inpatient treatment from MHLW. However, in the case of non-routine vaccinations, patients can receive financial assistance from PMDA only if the health repercussions warrant the equivalent of hospitalisation.
MHLW is now planning to correct this discrepancy by increasing the level of assistance provided to those suffering from adverse effects of non-routine vaccinations. The ministry will consult an expert panel soon to draw up its relief program.6-8 No-fault compensation following adverse events attributed to vaccination is key for vaccine implementation,9 and the establishment of a new compensation program might be key to calming an anxious public in Japan.
For an individual to be eligible to receive money for medical expenses, the government must agree that a “causal relationship between the individual's health problem and the vaccination cannot be denied.” In August 2015, MHLW also issued a list of medical institutions where people can go if they have symptoms after HPV vaccination. In addition, guidelines for the evaluation and management of symptoms that begin after HPV vaccination were issued to healthcare professionals by the Japan Medical Association (JMA) and the Japanese Association of Medical Sciences (JAMS).10
The areas where local governments have stepped in are correlated with those where the HPV vaccine victim support groups are prominent (Figs. 1 and 2), and the timing of the initiation of the compensation program seems related to the date of groups' establishment (Tables 1 and 2). The source of funding for vaccine-injury compensation schemes largely reflects where the decision-making power lies.9 A typical example is Suginami Ward, Tokyo, which started their own compensation program in April 2013. Suginami Ward is one of the earliest municipalities to have provided funding for the HPV vaccine, having begun doing so in 2009. Parents of a 14-year-old girl claimed compensation for suspected adverse events after the vaccination, but the national government refused to provide compensation for the family. After the case was reported at a local assembly meeting in April 2013, Suginami Ward announced a compensation budget for the next fiscal year.11-12 Associations for parents of cervical cancer vaccination “victims” had a strong impact on the decision-making processes regarding the compensation schemes. The number of vaccinated cases, the rate of adverse events, and the local governments' finances do not seem to be related to the establishment of compensation programs.
The safety and efficacy of the HPV vaccination is well established,13 and the suspension of the recommendation for HPV vaccination has a negative impact on global health.14 The Japanese government must manage the symptoms that sometimes follow the vaccination, restore public confidence, and provide a strong, evidence-based communication plan for the HPV vaccine.
Conclusion
In Japan, the national program for HPV vaccine injury has been suspended, and the result has been confusing to the public. This confusion is caused by the discrepancy in HPV vaccine injury compensation between those immunized under the immunization law and those who received voluntary vaccinations. Several local governments are trying to provide their own compensation programs for adverse reactions caused by the vaccine, but the number of such cases is extremely low, and efforts at compensation seem to be influenced primarily by the activities of HPV vaccine victim support groups. The establishment of a new national compensation program based on a scientific debate regarding benefits and risks, might be a key to finding a lasting resolution.
Disclosure of potential conflicts of interest
There are no potential conflicts of interest to disclose.
Funding
This study is supported by Grant-in-Aid for Scientific Research (No: 23590779).
References
- [1].Gilmour S, Kanda M, Kusumi E, Tanimoto T, Kami M, Shibuya K. HPV vaccination programme in Japan. Lancet. 2013; 382(9894):768; PMID:23993189; http://dx.doi.org/ 10.1016/S0140-6736(13)61831-0 [DOI] [PubMed] [Google Scholar]
- [2].Larson HJ, Wilson R, Hanley S, Parys A, Paterson P. Tracking the global spread of vaccine sentiments: the global response to Japan's suspension of its HPV vaccine recommendation. Hum Vaccin Immunother. 2014; 10(9):2543-50; PMID:25483472; http://dx.doi.org/ 10.4161/21645515.2014.969618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hanley SJ, Yoshioka E, Ito Y, Kishi R. HPV vaccination crisis in Japan. Lancet. 2015; 385(9987):2571; PMID:26122153; http://dx.doi.org/ 10.1016/S0140-6736(15)61152-7 [DOI] [PubMed] [Google Scholar]
- [4].Ueda Y, Enomoto T, Sekine M, Egawa-Takata T, Morimoto A, Kimura T. Japan's failure to vaccinate girls against human papillomavirus. Am J Obstet Gynecol. 2015; 212(3):405-6; PMID:25434842; http://dx.doi.org/ 10.1016/j.ajog.2014.11.037 [DOI] [PubMed] [Google Scholar]
- [5].Ihara T. The National Vaccine Injury Compensation Program in Japan. Nihon Rinsho. 2011; 69(9):1645-50; PMID:21922768 [PubMed] [Google Scholar]
- [6].Ministry of Health, Labour and Welfare The results of follow-up investigation of adverse reaction cases. The 15th meeting of Adverse Reaction Review Committee of Immunization Vaccination. September 17, 2015. http://www.mhlw.go.jp/file/05-Shingikai-10601000-Daijinkanboukouseikagakuka-Kouseikagakuka/0000097681.pdf (Accessed September. 18, 2015) [Google Scholar]
- [7].Ishii K. MHLW to expand relief measures for those with health problems after HPV vaccination. Asahi Shimbun. September 4, 2015 [Google Scholar]
- [8].Ministry of Health, Labour and Welfare The 15th meeting of Adverse Reaction Review Committee of Immunization Vaccination to be held (Information). September 14, 2015. http://www.mhlw.go.jp/stf/shingi2/0000097233.html (Accessed September. 18, 2015) [Google Scholar]
- [9].Looker C, Kelly H. No-fault compensation following adverse events attributed to vaccination: a review of international programmes. Bull World Health Organ. 2011;89(5):371-8; PMID:21556305; http://dx.doi.org/ 10.2471/BLT.10.081901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chustecka Z. Japan scheme for managing symptoms after HPV vaccine. Medscape Oncology. September 4, 2015. http://www.medscape.com/viewarticle/850436 (Accessed September. 18, 2015) [Google Scholar]
- [11].Anon. Severe adverse events after HPV vaccine. Asahi Shimbun March 8, 2013. [Google Scholar]
- [12].Wilson R, Paterson P. Larson H. The HPV Vaccination in Japan: Issues and Options. A Report of the CSIS Global Health Policy Center. May 15, 2014. http://csis.org/publication/hpv-vaccination-japan (Accessed September. 18, 2015) [Google Scholar]
- [13].Kash N, Lee MA, Kollipara R, Downing C, Guidry J, Tyring SK. Safety and efficacy data on vaccines and immunization to human papillomavirus. J Clin Med. 2015;4(4):614-33; PMID:26239350; http://dx.doi.org/ 10.3390/jcm4040614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wilson R, Paterson P. Chiu J, Schulz W, Larson H. HPV Vaccination in Japan: The Continuing Debate and Global Impacts. A Report of the CSIS Global Health Policy Center. April 23, 2015. http://csis.org/publication/hpv-vaccination-japan-0 (Accessed September. 18, 2015) [Google Scholar]


