ABSTRACT
Background: Economic evaluations should form part of the basis for public health decision making on new vaccine programs. While Canada's national immunization advisory committee does not systematically include economic evaluations in immunization decision making, there is increasing interest in adopting them. We therefore sought to examine the extent and quality of economic evaluations of vaccines in Canada. Objective: We conducted a systematic review of economic evaluations of vaccines in Canada to determine and summarize: comprehensiveness across jurisdictions, studied vaccines, funding sources, study designs, research quality, and changes over time. Methods: Searches in multiple databases were conducted using the terms “vaccine,” “economics” and “Canada.” Descriptive data from eligible manuscripts was abstracted and three authors independently evaluated manuscript quality using a 7-point Likert-type scale scoring tool based on criteria from the International Society for Pharmacoeconomics and Outcomes Research (ISPOR). Results: 42/175 articles met the search criteria. Of these, Canada-wide studies were most common (25/42), while provincial studies largely focused on the three populous provinces of Ontario, Quebec and British Columbia. The most common funding source was industry (17/42), followed by government (7/42). 38 studies used mathematical models estimating expected economic benefit while 4 studies examined post-hoc data on established programs. Studies covered 10 diseases, with 28/42 addressing pediatric vaccines. Many studies considered cost-utility (22/42) and the majority of these studies reported favorable economic results (16/22). The mean quality score was 5.9/7 and was consistent over publication date, funding sources, and disease areas. Conclusions: We observed diverse approaches to evaluate vaccine economics in Canada. Given the increased complexity of economic studies evaluating vaccines and the impact of results on public health practice, Canada needs improved, transparent and consistent processes to review and assess the findings of the economic evaluations of vaccines.
KEYWORDS: Canada, cost-effectiveness, economics, review, vaccine
Introduction
Canadian public health expenditures on vaccines amount to approximately $450 million annually and are expected to grow considerably in the decade to come.1 While the majority of vaccines have traditionally been cost-saving, the cost-effectiveness of new vaccines – such as those protecting against meningococcal and pneumococcal disease – have recently been at the center of considerable debate.2 As such, decision makers looking to adopt new vaccines into health care systems are faced with evaluating the economic value of these new vaccines relative to other alternative uses of health care budgets.
In Canada and abroad, wide scale adoption of some vaccines post-licensure has not occurred because their economic value has been questioned. The World Health Organization (WHO) recommends that countries establish technical advisory committees that serve as a resource to health authorities and as deliberative bodies to formulate guidance enabling evidence based decisions.3 These independent “National Immunization Technical Advisory Groups” (NITAGs) are to advise on vaccine adoption based on obvious factors such as vaccine efficacy and safety, but also on economic considerations, such as the cost-effectiveness of the immunization program and affordability.3 Federal countries with a tradition in evidence-based public health practice such as the US, UK, Australia and Canada have longstanding NITAGs that provide recommendations to their respective jurisdictions.4-7 All of these countries, with the exception of Canada, have coherent and transparent processes for the evaluation of economic evidence in immunization decision making. This clear gap in Canadian vaccine evaluation capacity is not mirrored on the drug evaluation process. Both the Common Drug Review (CDR) and the Pan-Canadian Oncology Drug Review (pCODR) have world class capabilities and processes for the review of economic evidence necessary for drug adoption decisions.8 The gap has been highlighted in a published report by multiple Canadian stakeholders including the Public Health Agency of Canada, the vaccine industry committee and academics.9
In the absence of a technical adjudicating committee on the economics of vaccines, national and provincial immunization advisory bodies in Canada may be relying heavily on available published studies and on unpublished presentations made by industry or academic researchers. Given the potential unfiltered impact of the literature on decision making, we set out to conduct a systematic review of economic evaluations published on vaccines in Canada. Our goal was to critically assess the comprehensiveness across jurisdictions, studied vaccines, funding sources, study quality, changes over time, and to summarize their major findings.
Methods
Search strategy
To identify all published economic evaluation studies of vaccines and vaccination programs in Canada, we conducted a literature search in MEDLINE (National Library of Medicine), EconLit (ProQuest), Web of Science (Thomson Reuters), EMBASE (Elsevier), the Cochrane Collaboration, Scopus (Elsevier), and HealthSTAR (Ovid). MEDLINE searches were performed via PubMed using the combination of “Vaccines” and “Economics & Statistics and Numerical Data” MeSH terms. We limited our search results to publications in Canada. Other databases were searched using a combination of terms: economic evaluations and appraisals; immunization programs and vaccines; and Canada. The detailed search strategies are described in Table S1.
Inclusion and exclusion criteria
Studies were included if they were published in English language peer-reviewed journals, generated original economic evaluation estimates of specific vaccines or immunization programs, and were applied to the Canadian setting. Research articles were screened in two stages. Titles and abstracts of the all retrieved citations were first reviewed by two authors (MS and VHN) for relevance against inclusion and exclusion criteria. In the second stage, the full text articles of included citations were screened for relevance against inclusion and exclusion criteria by 4 authors (AC, PG, JW, and JML). See Fig. 1 for further details of the screening procedure.
Figure 1.
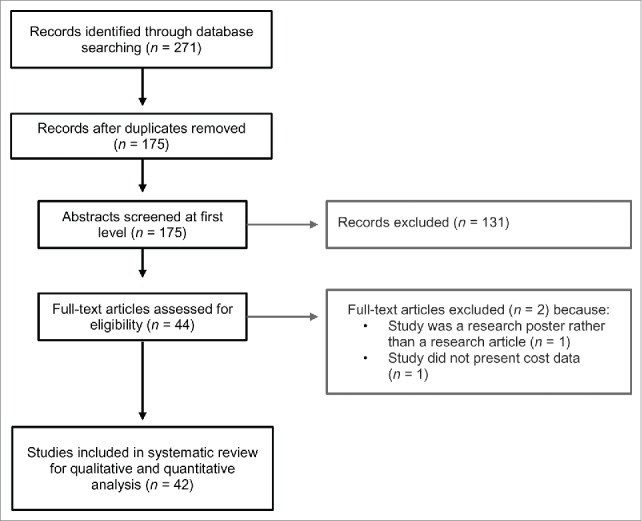
Flowchart of study selection for systematic review of vaccine economics in Canada.
Data collection and quality scoring
A standardized template was used in the abstraction of the research articles that satisfied our screening criteria. Three authors (MS, JL and AC) reviewed each article and performed the data abstraction that collected descriptive characteristics on the following: study objective, study population, intervention(s), time horizon, outcome measures, cost measurements, discount rates for outcomes and costs, geographical location, vaccine type, type of mathematical model used, intervention effectiveness measure, herd effect consideration, study perspective, sensitivity analyses used, prices and costs, and threshold values or conclusions on economic efficiency. We summarized the descriptive characteristics and the findings of the cost-utility studies.
A scoring tool was developed to assess the quality of the economic evaluation methods used in each article. Using the criteria from the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) 10-19 a Likert scale (1-7) 20 was created to assign scores to three components: clinical and epidemiological evidence, mathematical model, and economic analysis. Three reviewers; JML, JW and PG, independently evaluated and scored the articles along these three dimensions. JML scored the articles for the quality of the epidemiological evidence, JW scored the articles for the quality of the mathematical model, and PG scored the articles for the quality of the economic analysis. Each article therefore had three Likert scores. These scores were then averaged to produce an overall quality score for each paper. Table SII contains the scoring tool used by the reviewers.
To study factors that may impact study quality, we examined the correlation of quality with publication date, analysis type (cost benefit, cost effectiveness or cost utility), funding source, industry author participation, and journal impact factor. In addition we used the Mann-Whitney non-parametric rank test to explore if industry funded cost-utility studies would report more favorable results compared to cost-utility studies not funded by industry. In assessing whether the vaccines evaluated in the cost-utility studies were cost effective, we elected to use the implicit Canadian cost-effectiveness threshold (between $20,000/QALY to $100,000/QALY),21 rather than the WHO recommended threshold.22-23 Since there is no explicit economic threshold in Canada, there is no mandate for the government to adopt or reject a technology because it crosses a pre-specified cost-effectiveness value. Further, we reviewed statements of Canada's National Advisory Committee on Immunization (NACI) to determine if any of the retrieved studies were cited in recommendation statements. NACI was chosen as they are Canada's national technical advisory group on vaccines and their recommendations have long been followed closely at the provincial level in Canada.24
Results
The overall characteristics of the 42 studies that met our screening criteria, published between 1993 and 2013, are summarized in Table 1 (Table SIII provides detailed descriptions of each individual study we have identified). We observed an increase in the number of studies per year over time (Fig. 2 panel A). Vaccine manufacturers funded 40% of the studies and government funded 17%. Only 5% of studies were funded through academic research grants (Table 1), although there appears to be an increase in co-funded studies in recent years (Fig. 2 panel A). The majority of studies (60%) adopted a national perspective, 35% the perspective of the 3 most populous Canadian provinces (Ontario, Quebec and British Columbia) and only 4% of studies examined programs from the perspective of the remaining 7 provinces and 3 territories in Canada (Table 1). We observed studies against 10 diseases (Table 1), although there were no reported studies of older vaccines such as Smallpox, Tetanus, Diphtheria, Polio, Rubella, Mumps, and Haemophilus influenzae. Further, there were no studies of commonly recommended travel and specialty vaccines such as Typhoid fever, Yellow fever, Cholera, Japanese Encephalitis, Rabies and Tuberculosis.
Table 1.
Characteristics of canadian health economic studies on vaccines.
| Number | % | |
|---|---|---|
| Study Design | ||
| Modeling research | 38 | 90% |
| Experimental research | 4 | 10% |
| Disease | ||
| Pneumococcal Disease | 8 | 19% |
| Influenza | 6 | 14% |
| Hepatitis B | 5 | 12% |
| HPV/Cervical Cancer | 5 | 12% |
| Pertussis | 5 | 12% |
| Meningococcal Disease | 4 | 10% |
| Zoster | 2 | 5% |
| Rotavirus | 2 | 5% |
| Varicella | 2 | 5% |
| Measles | 1 | 2% |
| Hepatitis A | 1 | 2% |
| Hepatitis C | 1 | 2% |
| Focus of Study | ||
| Canada | 25 | 60% |
| Ontario | 6 | 14% |
| Quebec | 6 | 14% |
| British Columbia | 3 | 7% |
| Alberta | 1 | 2% |
| Manitoba | 1 | 2% |
| Population | ||
| Pediatric | 28 | 67% |
| All ages | 8 | 19% |
| Adolescents | 3 | 7% |
| Adults ≥ 65 y | 3 | 7% |
| Funding Source | ||
| Industry only | 17 | 40% |
| Government only | 7 | 17% |
| Multiple Sources | 5 | 12% |
| Research Grants only | 2 | 5% |
| Non-Profit only | 1 | 2% |
| Not Determined | 10 | 24% |
| Analysis Type | ||
| Cost-utility | 22 | 52% |
| Cost-effectiveness | 39 | 93% |
| Cost-benefit | 42 | 100% |
Figure 2.
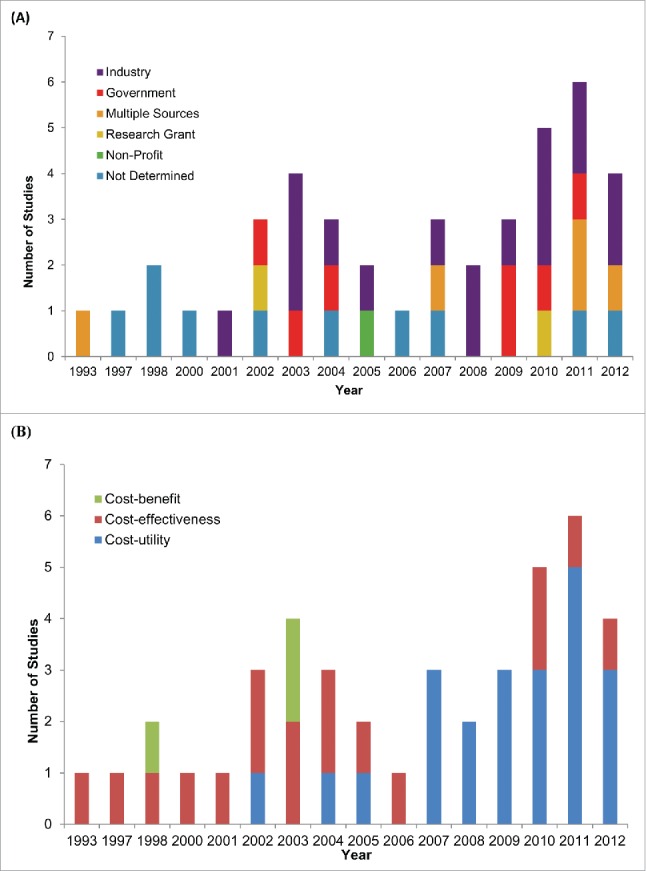
Evolution of Canadian economic evaluations of vaccines over time. Temporal trends in (A) funding source and (B) study design of 42 studies published from 1993-2012.
All economic analyses took the form of decision analysis, with the majority of studies reporting benefit in the form of quality adjusted life years (QALYs). The proportion of these cost-utility studies increased over time and as of 2007 they became the most widely used economic evaluation (Fig. 2 panel B). Table 2 summarizes the results of the 22 cost-utility studies.25-46 Only 2 of these studies reported results that were not cost-effective based on the implicit Canadian cost-effectiveness thresholds,30,39 with 7 studies reporting that the vaccine was dominant (the intervention costs less and is at least as effective as the comparator).27-28,38,42-44,46 13 out of 22 studies did not report results from a societal perspective which underestimate the full benefit of the vaccines.28-29,31-34,36-41,43-45 Out of these studies, only one reported that the vaccine was not cost-effective (as per the WHO threshold),39 while 4 others reported dominance.28,38,43,44 In the Mann-Whitney rank test to explore if industry funding was associated with higher ICERs, the mean rank for industry funded studies was 11.38, while the mean rank of non-industry funded studies was 11.6. This would indicate that industry funding was not associated with more favorable results when compared to studies that relied on non-industry sources of funding.
Table 2.
Summary of Canadian cost-utility studies.
| Base Case Median Incremental Cost-Effectiveness Ratio ($/QALY*) | |||||
|---|---|---|---|---|---|
| Publication Year |
Reference |
Vaccine Type |
Societal Perspective |
Payer Perspective |
Study Conclusion |
| 2002 | 25 | Meningococcal | 68,000 | NR | Cost-effective‡ |
| 2004 | 26 | Meningococcal | 42,000 | NR | Cost-effective |
| 2005 | 27 | Hepatitis C | D§ | NR | Dominant |
| 2007 | 28 | Hepatitis A | NR† | D | Dominant |
| 2007 | 29 | HPV | NR | 25,786 | Probably Cost-effective‡ |
| 2007 | 30 | Meningococcal | 113,000 | NR | Not Cost-effective |
| 2008 | 31 | Herpes Zoster | NR | 33,000 | Probably Cost-effective |
| 2008 | 32 | HPV | NR | 1,249-3,291 | Cost-effective |
| 2009 | 33 | Herpes Zoster | NR | 41,709 | Probably Cost-effective |
| 2009 | 34 | HPV | NR | 27,398 | Probably Cost-effective |
| 2009 | 35 | Pneumococcal | 466 | 18,000 | Cost-effective |
| 2010 | 36 | Influenza | NR | 9,388 | Cost-effective |
| 2010 | 37 | Influenza | NR | 12,154 | Cost-effective |
| 2010 | 38 | Pneumococcal | NR | D | Dominant |
| 2011 | 39 | Hepatitis B | NR | 3,648,123 | Not Cost-effective‡ |
| 2011 | 40 | HPV | NR | 1,839 | Cost-effective |
| 2011 | 41 | Influenza | NR | 1,612 | Cost-effective |
| 2011 | 42 | Influenza | D | NR | Dominant |
| 2011 | 43 | Pertussis | NR | D | Dominant |
| 2012 | 44 | HPV | NR | D | Dominant |
| 2012 | 45 | Rotavirus | NR | 2,400 | Cost-effective |
| 2012 | 46 | Rotavirus | D | 115,000 | Dominant |
QALY: Quality-adjusted Life Years
NR: Not reported
D: Dominant: The intervention costs less and is at least as effective as the comparator.
Cost-effectiveness: defined by Canada's implicit threshold of $20,000/QALY – 100,000/QALY
Categories of cost-effectiveness:
Cost-effective – less than $20,000 CAD/QALY;
Probably cost-effective – between $20,000 CAD/QALY –$100,000 CAD/QALY;
Not cost-effective – greater than $100,000 CAD/QALY.
The majority of published studies were mathematical modeling studies that simulate expected costs and benefits through decision models. There were only 4 evaluations that did not use a mathematical model but instead empirically measured actual costs and benefits of established immunization programs (Table SIII). We noted an increase in the complexity of the mathematical models employed over time through the introduction of several dynamic transmission models in the latter years (Table SIII).
On the 7 point scale, the mean quality score was 5.9 (95% CI: 5.6, 6.2). The mean 5-year impact factor of the journals in which the studies were published was 3.8 (95% CI: 3.1, 4.6). Both mean quality score and mean impact factor did not vary by publication year, disease type or funding source, or industry author participation (Fig. 3; Table SIV, Figs. SI and SII). Notably, 43% of the studies were published in the journal Vaccine, and the mean quality of these studies was 6.2, with a range from 5.3 to 7.0. We observed a slight positive correlation (Slope: y = 0.0977x + 5.5479, R2: 0.0841) between journal impact factor and study quality (Fig. SIII). While economic evaluations are not within the purview of NACI, 5 economic evaluations were cited in 8 NACI recommendations, on the topics of Meningococcal disease, influenza, varicella and Herpes Zoster. Compared to the broader group of articles, NACI cited articles have a similar mean quality score (6.3 vs. 6.0) and a slightly higher mean 5-year impact factor of (5.95 vs. 3.83). The details of the NACI statements and the economic studies that are cited by these statements are summarized in Table SV.
Figure 3.
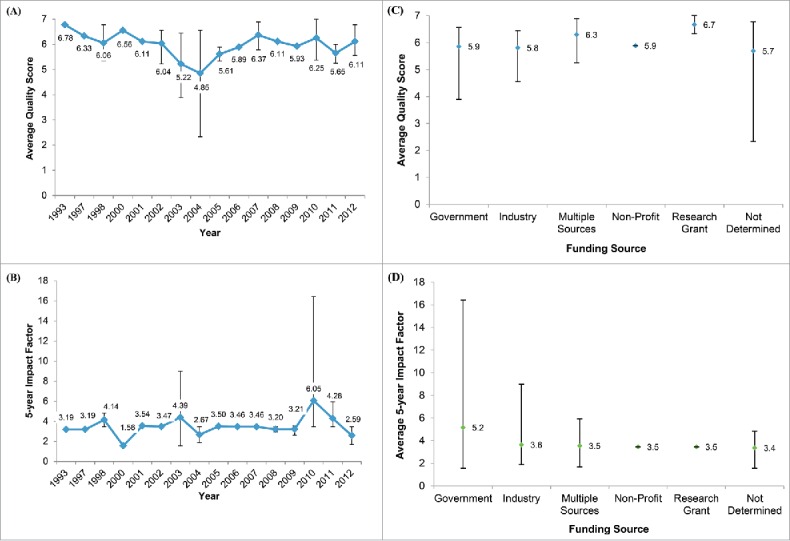
Quality and impact factor of Canadian economic evaluations of vaccines. The temporal trends in (A) mean quality score and (B) 5-year impact factor of the 42 publications from 1993-2012 identified in this study. Also presented are the average (C) quality score and (D) 5-year impact factor of the studies grouped by funding source. Quality scores were determined by an expert panel evaluating the clinical and epidemiological evidence, mathematical model, and economic analyses performed in each publication. Note: error bars denote the range of scores for a given year or funding source.
Discussion
This is the first systematic review of the literature on economic evaluations of vaccines in Canada. We found that Industry funded the majority of the studies in the literature (17/42 studies), followed by government (7/42 studies), while academic research grants only funded a few (2/42) studies. 22/42 studies considered cost-utility analysis and 16/22 of these reported favorable economic results, i.e. below the Canadian implicit cost-effectiveness threshold of $20,000 - $100,000/QALY. However, had the WHO-recommended cost-effectiveness threshold (up to 3x the per-capita GDP/QALY) been used in our analysis, all but one cost-utility study would have been reported favorable results. 38/42 studies employed mathematical models and we noted an increase in model complexity over time. Canada-wide economic evaluations made up most of the literature (25/42 studies), while the few provincial level studies focused on populous provinces. Overall, the quality of the literature was good, with a mean quality score of 5.9 on a 7 point Likert scale. This was consistent over time, across funding sources, and disease areas.
In line with the mean quality score, the mean score for the modeling component was 5.9/7. We found that most Canadian vaccine studies were aligned to the guidance offered in the report of the ISPOR-SMDM modeling task force with regard to communicable diseases.14 Compared to the broader group of articles, NACI cited articles have a similar mean quality score (6.3 vs. 6.0) and come from journals with a slightly higher mean 5-year impact factor of (5.95 vs. 3.83). However, the higher 5-year impact factor is driven by the Sander et al. article 36 which was published in a journal with 5-year impact factor of 16.43. Indeed, this article is an outlier when considering all articles in our study as the next highest 5-year impact factor score is 4.83 and the lowest value in our overall study range is 1.56.
There are no widely adopted standards to assign a quality score to health economic evaluations and authors attempting to asses literature quality have taken a variety of approaches.47-48 Our approach was to task three Canadian experts, one in each of the three major disciplines in health economic evaluations: 1) clinical epidemiology, 2) modeling and 3) economics; to assess the papers on these three critical dimensions. We believe that this approach is relatively robust as the experts each have over 20 years' experience in their relevant discipline and are all professors at Canadian academic institutions. Our approach could have been improved through blinding reviewers to the study authors and employing a larger number of reviewers to generate multiple scores per category. However, these improvements would be challenging to implement. Notably, the small nature of the scholarly community studying the economics of vaccines in Canada makes it challenging to find suitable reviewers and to blind the ones enlisted in the reviews.
Cost-utility studies, which account for more than half of the literature, point to a potential lost opportunity in Canada. These studies are unique in that their results can be compared to other interventions across the healthcare system.17 This is facilitated through the use of the QALY as the unit of benefit.49 A rational healthcare system is one that seeks to maximize QALY gains and minimize incremental cost.17 Health technologies, policies and interventions will all have a different incremental costs/QALY gained and health care resources should be diverted to areas demonstrating the lowest incremental cost/QALY.49 As a pragmatic course of action the WHO recommend the adoption of health technologies that fall below a specific cost/utility threshold.22 In this study we used the implicit Canadian threshold to make this assessment. We observed that all but 2 of the cost-utility studies reported results that would be considered higher than the upper limit of the Canadian threshold $100,000/QALY. Several of these programs, however, are not universally adopted across Canada. For instance, quadrivalent Meningococcal (42,000/QALY – $113,206/QALY), Herpes Zoster ($33,000/QALY), and Rotavirus (cost-saving) vaccine programs have seen limited to no adoption across Canadian jurisdictions. It should be noted that these vaccines are approved by Health Canada, and NACI statements are supportive of the use of these vaccines in Canada.50-52
In our limited data set we did not observe that industry funded studies were of lower quality than non-industry funded ones. Nor did we observe a bias in industry funded studies toward reporting more favorable results. Further, an analysis of studies which included industry authors showed similar results. Industry authorship was not associated with lower quality or a lower journal impact factor. These findings are not surprising. A report by Barbieri and Drummond 53 outlined that industry bias in economic evaluation is generally observed in the selection of technologies to study rather than in the economic evaluations themselves. Industry is less likely to invest resources in studying the economics of established vaccines and is less likely to report a vaccine as not being cost-effective given industry's ability to modify prices.
It was not evident from our literature review or from the NACI statements why vaccines reported to be cost-effective in the literature were not widely adopted in Canada. While there might be good reasons for this, the rationale for such decisions are not publically accessible. The absence of a formal and transparent national process for the review of economic evaluations of vaccines in Canada is therefore noteworthy, especially given the contrasts with the robust infrastructure for review of some other health technologies. For example, the pan Canadian Oncology Drug Review (pCODR) process, which is used by all provinces with the exception of Quebec, has established early engagement with industry to plan for new technology introduction; capacity to conduct technical reviews in a pre-specified time frame; the ability to complete full review and recommendations in parallel with the Health Canada market authorization process; and transparency on technical review details and recommendation deliberations. Many countries have established processes for including economic evaluations into immunization decision making. The United States Advisory Committee on Immunization Practices (ACIP), for example, has explicit standards and processes for economic evidence that can be considered in the recommendation process.5 As such, ACIP recommendations are based on thoroughly vetted economic data. Upon a positive ACIP recommendation, the vaccine is then provided by law. In the UK, the Joint Committee on Vaccines and Immunization (JCVI) sets an explicit monetary threshold for cost-effectiveness of vaccines for public health programs.6 In Australia the Pharmaceutical Benefits Scheme (PBS) reviews the economic evidence for both drugs and vaccines in Australia.7 These established methodologies can serve as templates for Canada, or other countries, that seek to incorporate economic evidence into their vaccine program planning.24
Our review has two limitations. First, there may be a tendency to published studies with favorable or desired results. For instance, industry might not be motivated to publish results that indicate that a vaccine is not cost-effective. Similarly, government might not be motivated to publish on a vaccine that is cost-effective if they are unable to fund it. A second limitation is related to the overall small number of studies. This made quantitative analysis difficult and we could not make any robust conclusion on the statistical significance of any of our trend analysis.
In conclusion, we observed diverse approaches to evaluate vaccine economics in Canada. Since economic analyses may influence public health decision making about immunization programs, it is important to be aware of how varying methodologies can affect results, and interpretation of results. Canada does not have a systematic process to review and assess the findings of the economic evaluations of vaccines; as such, there is some risk of variation in understanding published economic analyses and their implications.
Supplementary Material
Disclosure of potential conflicts of interest
A Chit, JK Lee, and R Van Exan are employees of Sanofi Pasteur. P Grootendorst has received financial and in-kind research support from pharmaceutical companies. JM Langley's institution has received research funding from Merck, Sanofi Pasteur, GlaxoSmithKline, Novartis, and Pfizer. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Notes on contributors
Dr. Langley holds the Canadian Institutes of Health Research-GlaxoSmithKline Chair in Pediatric Vaccinology at Dalhousie University.
Funding
This study was funded by Sanofi Pasteur.
References
- [1].BIOTECanada Vaccine Industry Committee Building on the Legacy of Vaccines in Canada: Value, Opportunities, and Challenges Series: Paper 2 – The Current Canadian Vaccine Environment. 2010 [Google Scholar]
- [2].Kim J. The role of cost-effectiveness in US vaccination policy. N Engl J Med. 2011. November 10; 365(19):1760-1; http://dx.doi.org/ 10.1056/NEJMp1110539 [DOI] [PubMed] [Google Scholar]
- [3].Duclos P. National Immunization Technical Advisory Groups (NITAGs): guidance for their establishment and strengthening. Vaccine 2010; 28(Suppl 1):A18-25; PMID:20412991; http://dx.doi.org/ 10.1016/j.vaccine.2010.02.027 [DOI] [PubMed] [Google Scholar]
- [4].Giesecke J. Modern infectious disease epidemiology. Second edition. London: Arnold, a member of the Hodder Headline group, 2002 [Google Scholar]
- [5].Smith JC. The structure, role, and procedures of the US. Advisory Committee on Immunization Practices (ACIP). Vaccine 2010; 28(Suppl 1):A68-75; PMID:20413002; http://dx.doi.org/ 10.1016/j.vaccine.2010.02.037 [DOI] [PubMed] [Google Scholar]
- [6].Hall AJ. The United Kingdom Joint Committee on Vaccination and Immunisation. Vaccine 2010; 28(Suppl 1):A54-7; PMID:20412998; http://dx.doi.org/ 10.1016/j.vaccine.2010.02.034 [DOI] [PubMed] [Google Scholar]
- [7].Nolan TM. The Australian model of immunization advice and vaccine funding. Vaccine 2010; 28(Suppl 1):A76-83; PMID:20413003; http://dx.doi.org/ 10.1016/j.vaccine.2010.02.038 [DOI] [PubMed] [Google Scholar]
- [8].Canadian Agency for Drugs and Technologies in Health (CADTH) Guidelines for the Economic Evaluation of Health Technologies. CADTH; Ottawa:Canada: 2006 [Google Scholar]
- [9].Langley JM, Krahn M, Husereau D, Spika J, Fisman DN, Chit A, Van Exan R. Incorporating economic evaluation into immunization decision making in Canada: a workshop. Expert Rev Vaccines 2014. November; 13(11):1291-6; http://dx.doi.org/ 10.1586/14760584.2014.939637 [DOI] [PubMed] [Google Scholar]
- [10].Weinstein MC, O'Brien B, Hornberger J, Jackson J, Johannesson M, McCabe C, Luce BR. Principles of Good Practice for Decision Analytic Modeling in Health-Care Evaluation: Report of the ISPOR Task Force on Good Research Practices – Modeling Studies. Value Health 2003; 6(1):9-17; PMID:12535234; http://dx.doi.org/ 10.1046/j.1524-4733.2003.00234.x [DOI] [PubMed] [Google Scholar]
- [11].Berger ML, Mamdani M, Atikns D, Johnson ML. Good research practices for comparative effectiveness research: defining, reporting and interpreting nonradomized studies of treatment effects using secondary data sources: the ISPOR Good Research Practices for Retrospective Database Analysis Task Force Report – Part I. Value Health. 2009; 12(8):1044-52; PMID:19793072; http://dx.doi.org/ 10.1111/j.1524-4733.2009.00600.x [DOI] [PubMed] [Google Scholar]
- [12].Cox E, Martin BC, Van Staa T, Garbe E, Siebert U, Johnson ML. Good research practices for comparative effectiveness research: approaches to mitigate bias and confounding in the design of nonrandomized studies of treatment effect using secondary data sources: the International Society of Pharmacoeconomics and Outcomes Research Good Research Practices for Retrospective Database Analysis Task Force Report - Part II. Value Health 2009; 12(8):1053-61; PMID:19744292; http://dx.doi.org/ 10.1111/j.1524-4733.2009.00601.x [DOI] [PubMed] [Google Scholar]
- [13].Johnson ML, Crown W, Martin BC, Dormuth CR, Siebert U. Good research practices for comparative effectiveness research: analytic methods to improve causal inference from nonrandomized studies of treatment effects using secondary data sources: the ISPOR Good Research Practices for Retrospective Database Analysis Task Force Report—Part III. Value Health 2009; 12(8):1062-73; PMID:19793071; http://dx.doi.org/ 10.1111/j.1524-4733.2009.00602.x [DOI] [PubMed] [Google Scholar]
- [14].Pitman R, Fisman D, Zaric GS, Postma M, Kretzschmar M, Edmunds J, Brisson M. Dynamic Transmission Modeling: A Report of the ISPOR-SMDM Modeling Good Research Practices Task Force-5. Value Health 2012; 15:828-34; PMID:22999132; http://dx.doi.org/ 10.1016/j.jval.2012.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Briggs A, Weinstein MC, Fenwick EAL, Karnon J, Sculpher MJ, Paltiel D. Model parameter estimation and uncertainty analysis: A report of the ISPOR-SMDM Modeling Good Research Practices Task Force Working Group. J Med Decis Making 2012; 32:722-32 [DOI] [PubMed] [Google Scholar]
- [16].Drumond M, Brown R, Fendrick MA, Fullerton P, Neumann P, Taylor R, Barbieri M. Use of pharmacoeconomics information—Report of the ISPOR task force on use of pharmacoeconomic/health economic information in health-care decision making. Value Health 2003; 6(4):407-16; PMID:12859580; http://dx.doi.org/ 10.1046/j.1524-4733.2003.64245.x [DOI] [PubMed] [Google Scholar]
- [17].Drumond M, Barbieri M, Cook J, Glick H, Lis J, Malik F, Reed SD, Rutten F, Sculpher M, Severens J. Transferability of economic evaluations across jurisdictions: ISPOR good research practices task force report. Value Health 2009; 12(4):409-18; PMID:19900249; http://dx.doi.org/ 10.1111/j.1524-4733.2008.00489.x [DOI] [PubMed] [Google Scholar]
- [18].Hay JW, Smeeding J, Carroll NV, Drummond M, Garrision L, Mansely EC, Mullins CD, Mycka JM, Seal B, Shi L. Good research practices for measuring drug costs in cost effectiveness analyses: issues and recommendations: The ISPOR drug cost task force report—Part I. Value Health 2010; 13(1):3-7; PMID:19874571; http://dx.doi.org/ 10.1111/j.1524-4733.2009.00663.x [DOI] [PubMed] [Google Scholar]
- [19].Garrison L, Mansley EC, Abbot TA, Bresnahan B, Hay JW, Smeeding J. Good Research Practices for Measuring Drug Costs in Cost-Effectiveness Analyses:A Societal Perspective: The ISPOR Drug Cost Task Force Report—Part II. Value in Health 2010; 13(1):8-13vvhe_663 3..7; PMID:19883405; http://dx.doi.org/ 10.1111/j.1524-4733.2009.00660.x [DOI] [PubMed] [Google Scholar]
- [20].University of Connecticut guide to using the Likert scale. http://www.gifted.uconn.edu/siegle/research/instrument%20reliability%20and%20validity/likert.html. Accessed: 2December2013 [Google Scholar]
- [21].Laupacis A, Feeny D, Detsky AS, Tugwell PX. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. CMAJ 1992; 146(4):473-81; PMID:1306034 [PMC free article] [PubMed] [Google Scholar]
- [22].World Health Organization Cost effectiveness and strategic planning (WHO-CHOICE). World Health Organization; 2014. Available at: http://www.who.int/choice/costs/CER_thresholds/en/. Accessed 5June2014 [Google Scholar]
- [23].World Bank GDP per capita (current US$). World Development Indicators; Washington, DC, USA: Available at: http://data.worldbank.org/indicator/NY.GDP.PCAP.CD. Accessed 5June2014 [Google Scholar]
- [24].Ismail SJ, Langley JM, Harris TM, Warshawsky BF, Desai S, FarhangMehr M. Canada's National Advisory Committee on Immunization (NACI): evidence-based decision-making on vaccines and immunization. Vaccine 2010; 28:A58-63; PMID:20412999; http://dx.doi.org/ 10.1016/j.vaccine.2010.02.035 [DOI] [PubMed] [Google Scholar]
- [25].De Wals P, Erickson L. Economic analysis of the 1992–1993 mass immunization campaign against serogroup C meningococcal disease in Quebec. Vaccine 2002; 20(21):2840-4; PMID:12102036; http://dx.doi.org/ 10.1016/S0264-410X(02)00161-5 [DOI] [PubMed] [Google Scholar]
- [26].De Wals P, Erickson LJ, Guay M, Drapeau J, St-Laurent J. Cost-effectiveness of immunization strategies for the control of serogroup C meningococcal disease. Vaccine 2004; 22(9):1233-40; PMID:15003652; http://dx.doi.org/ 10.1016/j.vaccine.2003.09.022 [DOI] [PubMed] [Google Scholar]
- [27].Krahn MD, John-Baptiste A, Yi Q, Doria A, Remis RS, Ritvo P, Friedman S. Potential cost-effectiveness of a preventive hepatitis C vaccine in high risk and average risk populations in Canada. Vaccine 2005; 23(13):1549-58; PMID:15694507; http://dx.doi.org/ 10.1016/j.vaccine.2004.09.023 [DOI] [PubMed] [Google Scholar]
- [28].Bauch CT, Anonychuk AM, Pham BZ, Gilca V, Duval B, Krahn MD. Cost-utility of universal hepatitis A vaccination in Canada. Vaccine 2007; 25(51):8536-48; PMID:17996339; http://dx.doi.org/ 10.1016/j.vaccine.2007.10.001 [DOI] [PubMed] [Google Scholar]
- [29].Brisson M, Van de Velde N, De Wals P, Boily MC. The potential cost-effectiveness of prophylactic human papillomavirus vaccines in Canada. Vaccine 2007; 25(29):5399-408; PMID:17561316; http://dx.doi.org/ 10.1016/j.vaccine.2007.04.086 [DOI] [PubMed] [Google Scholar]
- [30].De Wals P, Coudeville L, Trottier P, Chevat C, Erickson LJ. Vaccinating adolescents against meningococcal disease in Canada: a cost-effectiveness analysis. Vaccine 2007; 25(29):5433-40; PMID:17560695; http://dx.doi.org/ 10.1016/j.vaccine.2007.04.071 [DOI] [PubMed] [Google Scholar]
- [31].Brisson M, Pellissier JM, Camden S, Quach C, De Wals P. The potential cost-effectiveness of vaccination against herpes zoster and post-herpetic neuralgia. Human Vaccines 2008; 4(3):238-45; PMID:18382137; http://dx.doi.org/ 10.4161/hv.4.3.5686 [DOI] [PubMed] [Google Scholar]
- [32].Debicki D, Ferko N, Demarteau N, Gallivan S, Bauch C, Anonychuk A, Annemans L. Comparison of detailed and succinct cohort modelling approaches in a multi-regional evaluation of cervical cancer vaccination. Vaccine 2008; 26:F16-28; PMID:18992379; http://dx.doi.org/ 10.1016/j.vaccine.2008.02.040 [DOI] [PubMed] [Google Scholar]
- [33].Najafzadeh M, Marra CA, Galanis E, Patrick DM. Cost effectiveness of herpes zoster vaccine in Canada. Pharmacoeconomics 2009; 27(12):991-1004; PMID:19908924; http://dx.doi.org/ 10.2165/11314010-000000000-00000 [DOI] [PubMed] [Google Scholar]
- [34].Anonychuk AM, Bauch CT, Merid MF, Van Kriekinge G, Demarteau N. A cost-utility analysis of cervical cancer vaccination in preadolescent Canadian females. BMC Public Health 2009; 9(1):401; PMID:19878578; http://dx.doi.org/ 10.1186/1471-2458-9-401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Poirier B, De Wals P, Petit G, Erickson LJ, & Pépin J. Cost-effectiveness of a 3-dose pneumococcal conjugate vaccine program in the province of Quebec, Canada. Vaccine 2009; 27(50):7105-09; PMID:19786137; http://dx.doi.org/ 10.1016/j.vaccine.2009.09.057 [DOI] [PubMed] [Google Scholar]
- [36].Sander B, Bauch CT, Fisman D, Fowler RA, Kwong JC, Maetzel A, Krahn M. Is a mass immunization program for pandemic (H1N1) 2009 good value for money? Evidence from the Canadian Experience. Vaccine 2010; 28(38):6210-20; PMID:20643091; http://dx.doi.org/ 10.1016/j.vaccine.2010.07.010 [DOI] [PubMed] [Google Scholar]
- [37].Sander B, Kwong JC, Bauch CT, Maetzel A, McGeer A, Raboud JM, Krahn M. Economic appraisal of Ontario's Universal Influenza Immunization Program: a cost-utility analysis. PLoS Med 2010; 7(4):e1000256; PMID:20386727; http://dx.doi.org/ 10.1371/journal.pmed.1000256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chuck AW, Jacobs P, Tyrrell G, Kellner JD. Pharmacoeconomic evaluation of 10-and 13-valent pneumococcal conjugate vaccines. Vaccine 2010; 28(33):5485-90; PMID:20554066; http://dx.doi.org/ 10.1016/j.vaccine.2010.05.058 [DOI] [PubMed] [Google Scholar]
- [39].Wong WW, Woo G, Jenny Heathcote E, Krahn M. Cost effectiveness of screening immigrants for hepatitis B. Liver Int 2011; 31(8):1179-90; PMID:21745300; http://dx.doi.org/ 10.1111/j.1478-3231.2011.02559.x [DOI] [PubMed] [Google Scholar]
- [40].Tully SP, Anonychuk AM, Sanchez DM, Galvani AP, Bauch CT. Time for change? An economic evaluation of integrated cervical screening and HPV immunization programs in Canada. Vaccine 2012; 30(2):425-35; PMID:22075091; http://dx.doi.org/ 10.1016/j.vaccine.2011.10.067 [DOI] [PubMed] [Google Scholar]
- [41].Fisman DN, Tuite AR. Estimation of the health impact and cost-effectiveness of influenza vaccination with enhanced effectiveness in Canada. PloS One 2011; 6(11):e27420; PMID:22110645; http://dx.doi.org/ 10.1371/journal.pone.0027420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Nosyk B, Sharif B, Sun H, Cooper C, Anis AH, CIHR Canadian HIV Trials Network Influenza Vaccine Research Group . The cost-effectiveness and value of information of three influenza vaccination dosing strategies for individuals with human immunodeficiency virus 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Greer AL, Fisman DN. Use of models to identify cost-effective interventions: pertussis vaccination for pediatric health care workers. Pediatrics 2011; 128(3):e591-9; PMID:21844056 [DOI] [PubMed] [Google Scholar]
- [44].Kohli M, Lawrence D, Haig J, Anonychuk A, Demarteau N. Modeling the impact of the difference in cross-protection data between a human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and a human papillomavirus (HPV)-6/11/16/18 vaccine in Canada. BMC Public Health 2012; 12(1):872; PMID:23061913; http://dx.doi.org/ 10.1186/1471-2458-12-872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Fisman DN, Chan CH, Lowcock E, Naus M, Lee V. Effectiveness and cost-effectiveness of pediatric rotavirus vaccination in British Columbia: A model-based evaluation. Vaccine 2012; 30(52):7601-7; PMID:23107595; http://dx.doi.org/ 10.1016/j.vaccine.2012.10.034 [DOI] [PubMed] [Google Scholar]
- [46].Coyle D, Coyle K, Bettinger JA, Halperin SA, Vaudry W, Scheifele DW, Le Saux N. Cost effectiveness of infant vaccination for rotavirus in Canada. Canadian J Infect Dis Medical Microbiol 2012; 23(2):71; PMID:23730312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Neumann PJ, Stone PW, Chapman RH, Sandberg EA, Bell CM. The quality of reporting in published cost-utility analyses, 1976–1997. Annals Internal Med 2000; 132(12):964-72; PMID:10858180; http://dx.doi.org/ 10.7326/0003-4819-132-12-200006200-00007 [DOI] [PubMed] [Google Scholar]
- [48].Stone PW, Larson E, Kawar LN. A systematic audit of economic evidence linking nosocomial infections and infection control interventions: 1990-2000. Am J Infect Control 2002; 30(3):145-52; PMID:11988708; http://dx.doi.org/ 10.1067/mic.2002.121099 [DOI] [PubMed] [Google Scholar]
- [49].Weinstein MC, Torrance G, McGuire A. QALYs: The Basics. Value Health 2009; 12(1):S5-9; PMID:19250132; http://dx.doi.org/ 10.1111/j.1524-4733.2009.00515.x [DOI] [PubMed] [Google Scholar]
- [50].National Advisory Committee on Immunization Update on the Invasive Meningococcal Disease and Meningococcal Vaccine Conjugate Recommendations. Can Commun Dis Rep 2009; 36(ACS-3):1-40 [PubMed] [Google Scholar]
- [51].National Advisory Committee on Immunization An Advisory Committee Statement (ACS). National Advisory Committee on Immunization (NACI): Update on the use of herpes zoster vaccine. Public Health Agency Canada January 2014. (Catalogue no. HP40-92/2014E-PDF) [Google Scholar]
- [52].National Advisory Committee on Immunization Updated statement of the use of rotavirus vaccines. Can Commun Dis Rep 2010:36(ACS-4):1-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Barbieri M, Drummond MF. Conflict of interest in industry-sponsored economic evaluations: Real or imagined? Curr Oncol Reports 2001; 3(5):410-13; PMID:11489241; http://dx.doi.org/ 10.1007/s11912-001-0027-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


