ABSTRACT
This study compared the economic value of pediatric immunisation programmes for influenza to those for rotavirus (RV), meningococcal disease (MD), pneumococcal disease (PD), human papillomavirus (HPV), hepatitis B (Hep B), and varicella reported in recent (2000 onwards) cost-effectiveness (CE) studies identified in a systematic review of PubMed, health technology, and vaccination databases. The systematic review yielded 51 economic evaluation studies of pediatric immunisation — 10 (20%) for influenza and 41 (80%) for the other selected diseases. The quality of the eligible articles was assessed using Drummond's checklist. Although inherent challenges and limitations exist when comparing economic evaluations of immunisation programmes, an overall comparison of the included studies demonstrated cost-effectiveness/cost saving for influenza from a European-Union-Five (EU5) and United States (US) perspective; point estimates for cost/quality-adjusted life-years (QALY) from dominance (cost-saving with more effect) to ≤45,444 were reported. The economic value of influenza programmes was comparable to the other vaccines of interest, with cost/QALY in general considerably lower than RV, Hep B, MD and PD. Independent of the perspective and type of analysis, the economic impact of a pediatric influenza immunisation program was influenced by vaccine efficacy, immunisation coverage, costs, and most significantly by herd immunity. This review suggests that pediatric influenza immunisation may offer a cost effective strategy when compared with HPV and varicella and possibly more value compared with other childhood vaccines (RV, Hep B, MD and PD).
KEYWORDS: cost-effectiveness, economic evaluation, influenza, pediatric immunisation program, vaccines
Introduction
Influenza infections pose a significant health concern and have been responsible for substantial mortality and morbidity worldwide.1 In many countries, influenza immunisation strategies exist, targeting those at risk of significant complications or death from influenza infections; such groups are the primary target of immunisation programmes across all European countries.2 However, the burden in the pediatric population is under-represented (even with increased awareness) in many childhood immunisation programmes, despite the fact that children are believed to be the major transmitters.3 For example, in young children aged less than 2 years, hospitalisation rates for influenza-related events are similar to those observed for other vulnerable groups considered to be at a higher risk of influenza-related complications, including the elderly population.4
Numerous studies have modeled the health, clinical, and economic implications of influenza prevention strategies including pediatric immunisation coverage.5–8 The broad consensus of these studies is that childhood immunisation is cost-effective or cost-saving and should be prioritised.7 Infants and young children are at a higher risk of influenza-related hospitalisations and complications. Decreasing influenza virus transmission among children attending day care centers and schools has been shown to reduce the burden of influenza, providing both direct and indirect protection in the wider community.6,9
The aim of this study was to compare the economic value of pediatric influenza immunisation programmes with other commonly implemented immunisation programmes, based on articles retrieved from the systematic review conducted here. To allow comparison with recent pediatric immunisation programmes in similar contexts, vaccines for rotavirus (RV), meningococcal disease (MD), pneumococcal disease (PD), human papillomavirus (HPV), hepatitis B (Hep B) and varicella have been considered in this study.
Results
Overview of the included studies
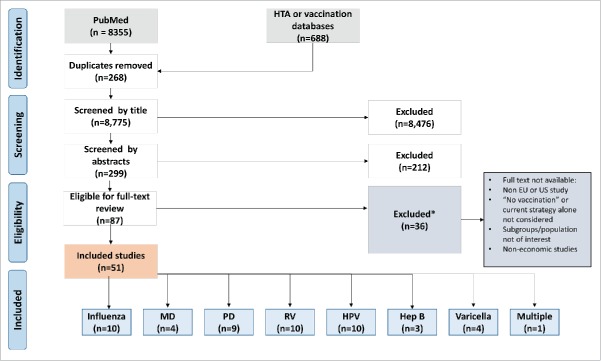
The literature search identified in total 9,043 articles, 8,335 in PubMed and 688 in other databases (Fig. 1). Following the removal of duplicates (n = 268), and evaluation of the titles (n = 8436) and abstracts (n = 212), 87 studies were considered eligible for full-text screening. Of these, 9 full texts were unavailable and 27 were excluded because they included non-pediatric or alternative populations not of interest, reviews for models, framework and systematic reviews, or intervention comparisons excluding immunisation strategies of ‘no immunisation’ (alone) and studies conducted outside the US- or EU5-setting. In total, 51 full economic evaluations were included in the review. Of the 51 studies included, 16 (31%) were conducted in the US, 10 (20%) in the UK, and 7 (14%) in Italy; the remaining 18 studies (35%) were conducted in Germany, Spain or France or in more than a single country (including the UK). With the exception of one study on multiple diseases,10 all included studies reported on single diseases. Influenza,6,9,11–17 HPV18–27 and RV28–37 were covered in 10 studies each, constituting 59% of all included studies. Hep B38-40 was covered in the lowest number of included studies (6%).
Figure 1.
PRISMA flowchart of the literature search, selection process and study inclusion. EU European Union, Hep B Hepatitis B, HPV Human papillomavirus, HTA Health technology assessment, MD Meningococcal disease, PD Pneumococcal disease, PRISMA Preferred Reporting Items for Systematic Reviews and Meta-Analyses, RV Rotavirus, US United States.
Overview of the economic evaluations
The studies included in the review largely described economic models of hypothetical patient cohorts, where literature values are used to populate model parameters – a few studies11,13,14 applied clinical trial data to the model framework. The majority (43/51, 84%) of the studies provided outcomes for QALY (Fig. 2a–2c), LYG (life years gained) or LYS (life years saved) and consisted of cost-effectiveness analysis (CEA) (42/43, 98%) and cost-utility analysis (CUA) (1/43, 2%) studies. Many studies (23/32, 72%) reported QALY outcomes below the willingness to pay threshold (WTP) within each country setting. Lower cost per QALY outcomes were influenced by herd immunity (5/32, 16%) and high risk group stratification (4/32, 13%). The remaining studies (8/51, 16%) reported overall savings (CEA: 2/8, 25% and cost-benefit analysis [CBA]: 3/8, 38%) and the number of cases averted (CEA: 3/8, 38%); studies on influenza vaccines reported the number of events averted for hospitalisations, influenza-related-events or mortality. While many perspectives were considered, most studies took a societal (26/51, 51%) or healthcare perspective (24/51, 48%); some considered third party payer or insurer perspectives (common in Germany) and others considered more than one perspective. The economic analyses largely included both direct and indirect costs — some also mentioned the direct medical and non-medical costs and have been reported, where relevant, in the data-extraction tables (Table 1 and Table 2).
Figure 2.
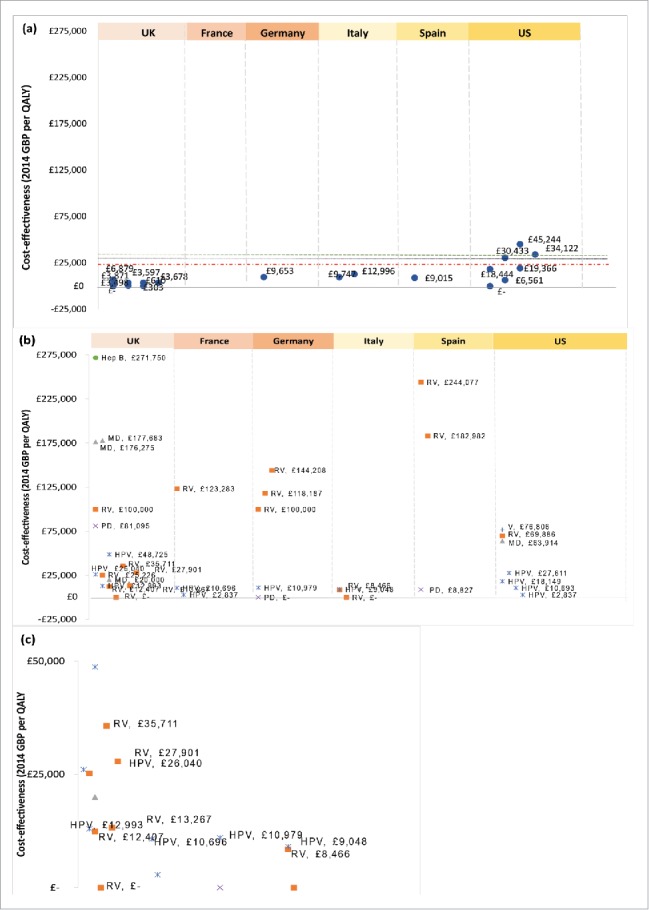
Cost-effectiveness by country per quality adjusted life year (QALY) of vaccinating the pediatric population against (a) influenza or (b) all other selected indications (rotavirus, pneumococcal disease, meningococcal disease, hepatitis B, human papillomavirus and varicella). A detailed view of cost/QALY between ≤0–50,000 in (b) can be found in (c). Willingness to pay (WTP) thresholds are represented by black (UK, ≤30,000), red (remaining EU5 countries) and green (US) dashed horizontal lines. GBP Great British Pound, ICER Incremental cost-effectiveness ratio, EU5 European Union 5, Hep B Hepatitis B, HPV Human papillomavirus, MD Meningococcal disease, PD Pneumococcal disease, QALY Quality adjusted life year, RV Rotavirus, UK United Kingdom, US United States, V Varicella aCost savings are denoted by ≤- bICER thresholds are represented by dashed horizontal lines for the UK (black —-), US (green - - -) and EU5 (red _ _ _).
Table 1.
Overview of economic evaluations of annual pediatric influenza immunisation when compared with no immunisation/existing strategies in the UK, France, Germany, Italy, Spain and the US.
| Study | Type of analysis | Alternatives* | Country | Perspective | Cohort | Coverage | Time horizon | Effectiveness measure | Cost measures | Sensitivity analysis | Outcomes65-67 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Luce et al. 200111 | CEA | (1) Immunisation No immunisation | US | Societal | 15–71 m | 80% (1 dose) 20% (2 doses) | 2 y | Cases averted | Direct and indirect | One-way & PSA Model sensitive to the cost of vaccine, proportion of children needing 2 doses per year and group setting | Cost/averted case: cost saving (if vaccine cost was <£21) |
| Muennig et al. 200115 | CEA | Immunisation Treatment No immunisation | US | Societal | 15–64 y | 95% | 1 y | QALY | Direct and indirect (1997, $US) | One-way & PSA Model sensitive to ILI incidence, transportation costs, caregiver costs and cost of vaccine | Cost/QALY: cost saving with immunisation vs. treatment/ support care |
| Marchetti et al. 200712 | CEA | (1) Immunisation No immunisation | IT | Healthcare and societal | (1) 6–60 m 6–24 m | 30% | 5 y | Cases averted and QALY | Direct and indirect (2005, €) | One-way & PSA Model sensitive to protection rate of vaccines for households | Cost/QALY: Healthcare £9,747 (6–60 months) £12,996 (6–24 months) Societal Net savings – £62 million |
| Hibbert et al. 200713 | CEA | (1) Immunisation No immunisation | US | Societal | 12–23 m | 47% | 2 y | Saving | Direct and indirect (2007, $US) | One-way Model sensitive to attack rate, duration of work absenteeism and duration of child or adult sickness | Cost saving: £4–116/child vaccinated |
| Navas et al. 200714 | CEA | (1) Immunisation No immunisation | ES | Provider and societal | 3–14 y | — | 6 m | LYS, QALY loss & BCR | Direct and indirect (2006, €) | One-way Model sensitive to vaccine price and cost of work absenteeism | Cost/QALY loss (provider): £9,015 Cost/LYS (provider):£11 NPV (societal): £7,179 BCR (societal): 1.80 |
| Baguelin et al. 20106 | CEA | (1) Immunisation No immunisation | EW | NHS | (1) <1 y 1–4 y 5–14 y 25–44 y 45–64 y 65 y and over | 70% (high risk) 40% (low risk) | Lifetime | QALY | Direct (2008, £) | One-way & PSA Model sensitive to overall size of epidemic without vaccination, QALY loss, hospitalisation rates, costs and case-fatality ratios | Cost/QALY £3,871 (0–4 years) £3,498 (5–14 years) £3,597 (0–14 years) £3,678 (0–14 and 65+) Extending to school children is the most cost effective. |
| Prosser et al. 201116 | CEA | (1) Immunisation No immunisation | US | Societal | 0.5 – 64 y | — | 1 y | QALY | Direct and indirect (2009, $US) | One-way Model was sensitive to number of doses, vaccine price and time of vaccine delivery | Cost/QALY: Cost saving (high risk subgroups) £6,561–45,244 (low risk subgroups risk) |
| Prosser et al. 201117 | CEA | (1) Immunisation(s) No immunisation | US | Societal | <5 y | — | 5 y | QALY | Direct and indirect (2006, $US) | One-way & PSA Model sensitive to probability of hospitalisation | Cost/QALY: £18,444–30,433 (LAIV) £19,366–34,122 (IIV) |
| Lugner et al. 201218 | CEA | (1) Immunisation(s) No immunisation | DE, NL and UK | Payer and societal | (1) 5–19 y (high risk) 65 y and over | 90% | Duration of pan-demic flu 2009 | QALY | Direct and indirect (2008, €) | One-way Model sensitive to vaccine price, coverage and pre-existing immunity. | Cost/QALY 5–19 years £6,879 (UK) £9,653 (Germany) |
| Pitman et al. 20139 | CEA | (1) Immunisation No immunisation | EW | NHS | (1) 0–1 y 2–4 y 5–10 y 11–18 y 19–49 y 50–64 y 65 y and older | 50% | Lifetime | QALY | Direct (2008, £) | One-way & PSA Model sensitive to coverage | Cost/QALY LAIV Cost saving (2–4 years) £610 (2–10 years) £303 (2–18 years) TIV Dominated* (TIV) |
BCR Benefit–cost ratio, CEA Cost–effectiveness analysis, DE Germany, ES Spain, EW England and Wales, FR France, IIV Inactivated influenza vaccination, ILI Influenza-Like Illness, IT Italy, LAIV Live attenuated influenza vaccination. LYG Life year gained, LYS Life years saved, m months, NHS National Health Service, NL The Netherlands, NPV Net present value, PSA Probabilistic sensitivity analysis, QALY Quality adjusted life years, TIV Trivalent influenza vaccination, UK United Kingdom, US United States, y years.
Exchange rate 1 EUR = £0.79 and 1 USD = £0.63; all costs have been converted to 2014 GBP (where possible) 66.
No immunisation refers to either baseline standard of care or the absence of routine immunisation policies within the pediatric-population.
Dominated: More costly and less effective than comparator.
Table 2.
Overview of economic evaluations of pediatric immunisation for rotavirus (RV), meningococcal disease (MD), pneumococcal disease (PD), human papillomavirus (HPV), hepatitis B (Hep B), varicella (V) and multiple indications (Mult.) in the UK, France, Germany, Italy, Spain and the US.
| Disease | Study | Type of analysis | Alternatives* | Country | Perspective | Cohort | Coverage | Time horizon | Effectiveness measure | Cost measures | Sensitivity analysis | Outcomes 65-67 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RV | Melliez et al. 200829 | CEA | (1) Immunisation (2) No immunisation | FR | Societal | Birth cohort | 75% | 3 y | Cases averted, LYS & QALY | Direct cost (2005, €) | One-wayModel sensitive to coverage, disease incidence, probability of death, diarrhea complications and discount rates | Cost/QALY: £123,283Cost/LYS: £266,219 |
| RV | Jit et al. 200930 | CEA | (1) Immunisation (2) No immunisation | Europe (BE, EW, FI, FR and NL) | Healthcare | 0- 5 y | BE: 97.5–98%EW: 95–95.18%FI: 97%FR:75%NL: 97% | 5 y | QALY | Direct and indirect (2005–2006, €) | One-wayModel is sensitive to number of carers and herd immunity | Cost/QALY: >£100,000 (E&W and France) |
| RV | Giammanco et al. 200931 | CEA | (1) Immunisation(2) No immunisation | IT | NHS Societal | 0–5 y | 90% | 5 y | Cases averted and net savings | Direct and indirect(2004–2005, €) | One-wayModel sensitive to coverage | Net savings: >–£9m (NHS) >£24 (societal) |
| RV | Panatto et al. 200932 | CEA | (1) Immunisation(2) No immunisation | IT | RHSSocietal | 0–5 y | 90% | 1 y | QALY | Direct and indirect(2009, €) | – | Cost/QALY: £ 8,466 (RHS)Cost-saving (societal) |
| RV | Martin et al. 200933 | CEA | (1) Immunisation(2) No immunisation | UK | NHS | Birth cohort (< 5 y) | 88% | Lifetime | Cases averted & QALY | Direct cost (2006–07, £) | One-way & PSAModel sensitive to hospitalisation costs, number of GP visit and duration of absenteeism | Cost/QALY: £25,226 (NHS)£12,407 (societal) |
| RV | Shim et al. 200934 | CEA | (1) Immunisation(2) No immunisation | US | NHSSocietal | < 5 y | 75% | 20 y | Cases averted & QALY | Direct and indirect (2007, US$) | One-wayModel sensitive to coverage and vaccine efficacy | Cost/QALY: £69,886Cost/averted case: £51.91 |
| RV | Atkins et al. 201235 | CEA | (1) Immunisation(2) No immunisation | EW | NHS | < 5 y | 95% | 50 y | QALY | Direct costs (2010–11, £) | One-way & PSAModel sensitive to the vaccine price, immunity waning and vaccine administration cost | Cost/QALY: Static model (without herd immunity)Cost saving - £21,077 (£45/ course)£9,313 – 35,711 (£60/course)Dynamic model (with herd immunity)Cost-saving - £13,267 (£45/course)£6,841 – 27,901 (£60/course) |
| RV | Knoll et al. 201336 | CEA | (1) Immunisation(2) No immunisation | DE | SHI | Birth cohort | 100% | 5 y | Cases averted and savings | Direct and indirect (2011, €) | One-way & PSAModel sensitive to the frequency of seeking medical advice, RV disease and hospital visits | Net saving: >£9 million |
| RV | Imaz et al. 201337 | CUA | (1) Immunisation(2) No immunisation | ES | Societal Healthcare | Birth cohort | 100% | 5 y | QALY | Direct and indirect (2011, €) | One-wayModel sensitive to the vaccine price, vaccine efficacy and utility values | Cost/QALY:£244, 077 (healthcare)£182,982 (societal) |
| RV | Aidelsburger et al. 201438 | CEA | (1) Immunisation(s) (2) No immunisation | DE | SHI | < 5 y | 80% | 5 y | QALY | Direct and indirect costs (2010, £) | One- and 2-wayModel sensitive to herd immunity, utility values, the vaccine price and administration costs | Cost/QALY: £118,187–144,208 |
| MD | Trotter et al. 200242 | CEA | (1) Immunisation (2) NoImmunisation | EW | NHS | 0–17 y | <1 y: 89%1–4 y:82%5–13 y: 97%14–15 y: 83%16–17 y: 65% | Lifetime | Cases averted & LYS | Direct costs (2000, £) | One-way & PSAModel sensitive to disease incidence | Cost/LYS: £8,467 |
| MD | Ortega–Sanchez et al. 200843 | CEA | (1) Immunisation + catch upNo immunisation | US | Payer and societal | 11–17 y | 70% | 10 y | Cases averted, LYS & QALY | Direct and indirect (2005, US$) | One-way & PSAModel sensitive to herd immunity, vaccine price and vaccination campaign | Cost/QALY: £63,914 (societal)Cost/LYS:£92,240 (payer), £106,766 (societal) |
| MD | Christensen et al. 201344 | CEA | (1) Immunisation (2) No immunisation | UK | NHSPSS | Birth cohort | 91% | Lifetime | Cases averted & QALY | Direct (2008, £) | One-way & PSAModel sensitive to disease incidence, vaccine efficacy, immunity and case-fatality | Cost/QALY: £176,275 (early infant vaccination); £177,683 (late infant vaccination) |
| MD | Christensen et al. 201445 | CEA | (1) Immunisation (2) No immunisation | UK | NHSPSS | Birth cohort | 88% | Lifetime | Cases averted & QALY | Direct and indirect (2011, £) | One-wayModel sensitive to herd immunity and disease incidence | Cost/QALY: <£20,000 |
| PD | Claes et al. 200346 | CEA | (1) Immunisation(2) No immunisation | DE | Healthcare payer, public authority and societal | < 2 y | 100% | 10 y | Cases averted & LYS | Direct and indirect costs (1999–2000,€) | One-wayModel sensitive to coverage, productivity loss, vaccine price and discount rates | Cost/LYS:£62,147 (healthcare) |
| PD | Melegaro et al. 200447 | CEA | (1) Immunisation(2) No immunisation | EW | NHS | Birth cohort | – | Lifetime | Burden reduction, LYG & QALY | Direct costs (2002, £) | One-way & PSAModel sensitive to disease incidence, vaccine price and herd immunity | Cost/QALY: £81,095Cost/LYG: £153,182 |
| PD | Ray et al. 200648 | CEA | (1) Immunisation(2) No immunisation | US | Societal-Healthcare | 0–23 m | 70% | 5 y | Cases averted & LYS | Direct and indirect (2004, US$) | One-wayModel sensitive to herd immunity, perspective and pneumonia events | Cost/LYS:£83,955 (without herd effects)£5,622 (with herd immunity) |
| PD | Lieu et al. 200049 | CEA | (1) Immunisation(2) No immunisation | US | Societal | Infants and young children | 100% | 5 y | Cases averted & LYS | Direct and indirect costs (1997, US$) | One-wayModel sensitive to disease incidence, vaccine efficacy and administration costs | Cost/LYS:£64,440 (societal) |
| PD | Lloyd et al. 200850 | CEA | (1) Immunisation(2) No immunisation | DE | Healthcare payer | Birth cohort | 83% | Lifetime | Cases averted & LYG | Direct costs (2004, €) | One-wayModel sensitive to herd immunity, vaccine efficacy and pneumonia events | Cost/LYG:£67,696 (entire cohort without herd immunity)£25,711 (high risk) |
| PD | Ray et al. 200951 | CEA | (1) Immunisation(2) No immunisation | US | Healthcare | (1) < 5 y(2) 5 y and over | 76% (children born 2000–2002)85% (children born 2003–2006) | 5 y | Cases averted & LYS | Direct costs (2006, US$) | One-wayModel sensitive to disease incidence, herd immunity and hospital visits | Cost/LYS:£141,633 (without herd immunity)£7,328 (with herd immunity) |
| PD | Giorgi- Rossi et al. 200952 | CEA | (1) Immunisation(2) No immunisation | IT | Public healthcare | Birth cohort | 12 m: 80%24 m: 82% | 10 y | Cases averted, LYG & DALY | Direct costs (2005, €) | One-way & PSAModel sensitive to disease incidence, mortality, vaccine price and vaccine efficacy | Cost/LYG: £108,668Cost/DALY: £50,193Cost/averted case: £830 £172,324/ IPD£669,022/ meningitis£3,791,124/ death |
| PD | Claes et al. 200953 | CEA | (1) Immunisation(2) No immunisation | DE | SHI | Birth cohort | 70% | Lifetime | Cases averted, LYS & QALY | Direct costs (2005–2007, €) | One-wayModel sensitive to vaccine price, coverage and schemes | Cost/QALY: cost-savingCost/LYG: cost-saving |
| PD | Díez- Domingo et al. 201154 | CEA | (1) Immunisation(2) No immunisation | ES | Payer | < 1 y | 95% | Lifetime | LYG & QALY | Direct (2009, €) | One-wayModel sensitive to herd immunity, disease incidence, hospital events, vaccine price and coverage | Cost/QALY: £8,827Cost/LYG: £10,851 |
| HPV | Sanders et al. 200319 | CEA | (1) Immunisation(2) No immunisation | US | Payer | 12 y | 70% | Lifetime | Case averted & QALY | Direct costs (2001, US$) | One-way & PSAModel sensitive to vaccine efficacy, vaccine price and disease incidence | Cost/QALY: £18,149 |
| HPV | Jit et al. 200820 | CEA | (1) Immunisation + catch up(2) No immunisation | UK | NHS | 12 y | 80% | Lifetime | QALY | Direct (2006–07, £) | One-way & PSAModel sensitive to vaccine efficacy, immunity, vaccine price & QALY loss | Cost/QALY: £26,040 |
| HPV | Insinga et al. 200821 | CBA | (1) Immunisation(2) No immunisation | US | Health economic | 16–23 y | – | 2.5 y | Healthcare costs | Direct (2006, US$) | One-wayModel sensitive to disease incidence, resource use and costs | Reduction of £28 per patient |
| HPV | Goldhaber-Fiebert et al. 200822 | CEA | (1) Immunisation(2) No immunisation | US | Societal | 9–12 y | 100% | Lifetime | QALY | Direct and indirect cost (2004, US$) | One-way, 2-way & PSAModel sensitive to screening tests | Cost/QALY: £27,611 |
| HPV** | Chesson et al. 200823 | CEA | (1) Immunisation + screening(2) No immunisation | US | Societal | 12 y | 70% | Lifetime | QALY | Direct medical costs (2005, US$) | One-wayModel sensitive to herd immunity, discount rates and time horizon | Cost/QALY: £10,693 (without herd immunity) £ 2,837 (with herd immunity) |
| HPV | Bergeron et al. 200824 | CEA | (1) Immunisation(2) No immunisation | FR | Direct and third party | 14 y | 80% | Lifetime | QALY & LYG | Direct (2004, €) | One-wayModel sensitive to discount rates | Cost/QALY: £10,696 (direct)£2,837 (third party)Cost/LYG£14,447 (direct)£18,839 (third party) |
| HPV | Mennini et al. 200925 | CEA | (1) Immunisation(2) No immunisation | IT | NHS | 12 y | 80% | Lifetime | QALY | Direct costs (2004–2005,€) | One-way & PSAModel sensitive to vaccine efficacy, coverage % discount rates | Cost/QALY: £9,048 |
| HPV | Hillemanns, et al. 200926 | CEA | (1) Immunisation +Screening(2) No immunisation | DE | Healthcare | 12 y | 80% | Lifetime | QALY & LYG | Direct costs (2006, €) | One-wayModel sensitive to vaccine protection, booster vaccination and discount rates | Cost/QALY: £10,979Cost/LYG: £16,353 |
| HPV | Diaz et al. 201027 | CEA | (1) Immunisation(2) No immunisation | ES | Societal | 11–14 y | 90% | Lifetime | LYS | Direct and indirect (2006, €) | One-wayModel sensitive to vaccine price, coverage and immunity | Cost/LYS: £19,686 |
| HPV | Jit et al. 201128 | CEA | (1) Immunisation(s)(2) No immunisation | UK | NHS | 12–75 y | 80% | Lifetime | QALY | Direct costs (2008–09, £) | One-way & PSAModel sensitive to discount rates | Cost/QALY: £12.993–48,725 (depending on vaccination and specific cancer prevention) |
| Hep B | Szucs et al. 200039 | CEA | (1) Immunisation(2) No immunisation | DE | Third party | (1) 1–15 y (2) 11–15 y | 100% | 30 y | Cases averted and savings | Direct costs (1997, DM) | – | Cost/averted: £100,365Net saving: £55,758 |
| Hep B | Siddiqui et al. 201140 | CEA | (1) Immunisation(2) No immunisation | UK | NHS | Infants and adolescents | 90% | Lifetime | QALY | Direct (2006, £) | One-way & PSAModel sensitive to vaccine protection and discount rates | Cost/QALY: £271,750 |
| Hep B | Boccalini et al. 201341 | CBA | (1) Immunisation(2) No immunisation | IT | NHS and societal | New-borns and 12 y | 95% | 20 y | BCR & ROI | Direct and indirect costs (2010, €) | One-wayModel sensitive to coverage and number of symptomatic patients | BCR: 2.46 |
| V | Thiry et al. 200455 | CEA | (1) Immunisation(s) + no screening/ blood tests(2) No immunisation | IT | Societal and payer | 11 y | 70% | Lifetime | LYG | Direct or indirect costs (2002 , €) | One-wayModel sensitive to the vaccine price | Cost/LYG: £8,928 |
| V | Lenne et al. 200656 | CEA | (1) Immunisation(2) Immunisation + catch up No immunisation | ES | Healthcare & Societal | 1–2 y | 12m: 90% 24 m: 97% | Lifetime | Cases averted & LYG | Direct and indirect(2004, €) | One-way & PSAModel sensitive to coverage, vaccine efficacy and discount rates | Cost/LYG: £3,393 (healthcare), £5,909(societal + catch-up) |
| V | Coudeville et al. 200857 | CEA | (1) Immunisation(2) Immunisation + catch up No immunisation | FRDE | Societal Third party | 1–2 y | 90% 70%45% | Lifetime | Cases averted & LYG | Direct and indirect costs (2002, €) | One-way & PSAModel sensitive to the vaccine price and cost of varicella episodes | Cost/LYG: France & Germany– cost saving (both) |
| V | Zhou et al. 200858 | CEA | (1) Immunisation(2) No immunisation | US | Societal | Infants | 95% | Lifetime | BCR, QALY | Direct and indirect(2006, US$) | One-wayModel sensitive to discount rates | Cost/QALY: £76,806BCR:2.73 |
| Mult | Zhou et al. 201410 | CBA | (1) Immunisation (2) No immunisation | US | Payer and societal | Birth cohort | 53% | Lifetime | Cases averted & BCR | Direct and indirect (2009, US$) | One-wayModel sensitive to administration cost | BCR: >1 PCV<1 for RV |
BCR Benefit–cost ratio, BE Belgium, CBA Cost–benefit analysis, CEA Cost-effectiveness analysis, CER Cost-effectiveness ratio, CUA Cost-utility analysis, DALY Daily adjusted life years, DE Germany, DM Deutsche Mark, EW England & Wales, ES Spain, FR France, Hep B Hepatitis B, HPV Human papillomavirus, IT Italy, IPD Invasive pneumococcal disease, LYG Life year gained, LYS Life years saved, m months, MD Meningococcal disease, Mult. Multiple, NHS National Health Service, NHSPSS National Health Service Personal Social Services, NL The Netherlands, PCV Pneumococcal conjugate vaccination, PD Pneumococcal disease, RHS Regional Health Service, ROI Return on investment, RV Rotavirus, SHI Statutory Health Insurance, PSA Probabilistic sensitivity analysis, QALY Quality adjusted life years, UK United Kingdom, US United States, V Varicella, y years.
Exchange rate 1 EUR = £0.79 and 1 USD = £0.63; all costs have been converted to 2014 GBP (where possible)66.
No immunisation refers to either baseline standard of care or the absence of routine immunisation policies within the pediatric-population.
The study adopts a societal perspective and includes all direct medical costs and benefits regardless of who incurred the costs or received the benefits.
From the included studies, 10 (20%) investigated pediatric influenza immunisation programmes6,9,11–17 (Table 2) and the remaining 41 (80%) studies investigated programmes for RV,28–37 MD,41–44 PD,45–53 HPV,18–27 Hep B,38–40 varicella54–57 and multiple indications10 (Table 2). The primary alternatives considered were immunisation and no-immunisation, which refers to either baseline standard of care or the absence of routine immunisation policies within the pediatric-population; 2 studies considered an additional alternative treatment for influenza6 or a catch-up campaign for varicella.57
The included studies followed cohorts of various ages for various time horizons using a range of cost-effectiveness measures. Studies on influenza immunisation considered pediatric populations primarily aged less than 5 years in 4 studies11–13,16 and a wider pediatric age range in 5 studies,6,9,13,15,17 following these between 1 year and lifetime horizons. The primary effectiveness measures were cost per QALY, LYS and cases averted.
Studies on RV immunisation strategies28–37 primarily followed birth cohorts for up to 5 years across29,30,35–37 various perspectives including statutory health insurance (SHI)35,37 and regional health service (RHS).31 Cost per QALY and cases averted were the most common effectiveness measures provided. Studies on MD41–44 and PD45–53 vaccination programmes followed birth cohorts (with the exceptions of Ortega-Sanchez et al. [2008]42 [cohort of 11–17 year olds] and Trotter et al. [2002]41 [0–17 years] for MD and Ray et al. [2006]47 [cohort of < 5 year olds and > 5 year olds], Lieu et al. [2000]48 [cohort of infants and young children], Ray et al. [2009]50 and Diez-Domingo et al. [2011]53 [cohort of <1 year olds], for PD) between 5 years and lifetime horizons. The primary effectiveness measures included cost per QALY, cases averted, and LYS.
Studies on HPV vaccines followed mainly cohorts of children aged 12 years and above over a lifetime horizon.18–20,22–25,27 Common effectiveness measures were cost per QALY and LYG.
Studies on Hep B and varicella vaccines followed cohorts of infants and adolescent age groups for up to 30 years and lifetime durations.38-40,54-56 From the small number of studies, cost per QALY, cases averted, LYG, and benefit–cost ratio (BCR) were used to determine the cost-effectiveness outcomes.
The study on multiple indications considered vaccination for PD and RV in birth cohorts followed over a lifetime horizon with effectiveness measures of BCR and cases averted was used to determine the health benefit.10
Assessment of the included studies with Drummond's checklist (Table 3)58 showed that in all studies, the research question was stated (item 1), relevant alternatives were compared (item 30), the study question was given (item 33) and conclusions followed from the data reported (item 34). All but 6 studies reported the primary outcomes (item 11); the majority of studies stated the alternatives described (item 5), the form of economic evaluation used (item 6), methods for estimation of quantities and unit costs (item 17) and currency and price data (item 18). The productivity changes (item 14) were not relevant to 40 studies, as the focus was the pediatric population. Some studies did consider the impact of vaccine programmes on parents and work loss days from caring for children — this was common in the RV studies which considered children under the age of 5 years.32,33 Several studies including Hibbert et al.(2009),13 Jit et al.(2010)29 and Giammanco et al. (2009),30 did not mention some of the main features of interest including the inflation rates, effectiveness measure and type of sensitivity analysis used.
Table 3.
Quality appraisal of included studies (based on Drummond's checklist68).
| Yes | No | Unclear | Inappropriate | ||
|---|---|---|---|---|---|
| 1 | The research question is stated | 51 (100%) | 0 (0%) | 0 (0%) | 0 (0%) |
| 2 | The economic importance of the research is stated | 29 (56%) | 17 (33%) | 5 (10%) | 0 (0%) |
| 3 | The viewpoint(s) of the analysis are clearly stated and justified | 49 (96%) | 2 (4%) | 0 (0%) | 0 (0%) |
| 4 | The rationale for choosing the alternative programmes or interventions compared is stated | 15 (29%) | 35 (69%) | 1 (2%) | 0 (0%) |
| 5 | The alternatives being compared are clearly described | 45 (88%) | 5 (10%) | 1 (2%) | 0 (0%) |
| 6 | The form of economic evaluation used is stated | 46 (90%) | 5 (10%) | 0 (0%) | 0 (0%) |
| 7 | The choice of form of economic evaluation is justified in relation to the questions addressed | 10 (20%) | 35 (69%) | 5 (10%) | 1 (2%) |
| 8 | The source(s) of effectiveness estimates used are stated | 40 (78%) | 5 (10%) | 5 (10%) | 1 (2%) |
| 9 | Details of the design and results of effectiveness study are given (if based on a single study) | 25 (49%) | 9 (18%) | 5 (10%) | 12 (24%) |
| 10 | Details of the method of synthesis or meta-analysis of estimates are given (if based on an overview of a number of effectiveness studies) | 0 (0%) | 0 (0%) | 0 (0%) | 51 (100%) |
| 11 | The primary outcome measure(s) for the economic evaluation are clearly stated | 45 (88%) | 4 (8%) | 2 (4%) | 0 (0%) |
| 12 | Methods to value health states and other benefits are stated | 39 (76%) | 7 (14%) | 5 (10%) | 0 (0%) |
| 13 | Details of the subjects from whom valuations were obtained are given | 25 (49%) | 20 (39%) | 6 (12%) | 0 (0%) |
| 14 | Productivity changes (if included) are reported separately | 9 (18%) | 0 (0%) | 2 (4%) | 40 (78%) |
| 15 | The relevance of productivity changes to the study question is discussed | 9 (18%) | 5 (10%) | 2 (4%) | 35 (67%) |
| 16 | Quantities of resources are reported separately from their unit costs | 26 (51%) | 22 (43%) | 3 (6%) | 0 (0%) |
| 17 | Methods for estimation of quantities and unit costs are described | 44 (86%) | 0 (0%) | 7 (14%) | 0 (0%) |
| 18 | Currency and price data are recorded | 45 (88%) | 4 (8%) | 2 (4%) | 0 (0%) |
| 19 | Details of currency of price adjustment for inflation or currency conversion are given | 38 (75%) | 8 (16%) | 5 (10%) | 0 (0%) |
| 20 | Details of any model used are given | 43 (84%) | 6 (12%) | 2 (4%) | 0 (0%) |
| 21 | The choice of model used and the key parameters on which it is based are justified | 10 (20%) | 34 (67%) | 7 (14%) | 0 (0%) |
| 22 | Time horizon of costs and benefits is stated | 38 (75%) | 6 (12%) | 7 (14%) | 0 (0%) |
| 23 | The discount rate(s) is stated | 43 (84%) | 5 (10%) | 3 (6%) | 0 (0%) |
| 24 | The choice of rate(s) is justified | 26 (51%) | 22 (43%) | 3 (6%) | 0 (0%) |
| 25 | An explanation is given if costs or benefits are not discounted | 2 (4%) | 2 (4%) | 0 (0%) | 47 (92%) |
| 26 | Details of statistical tests and confidence intervals are given for stochastic data | 15 (29%) | 30 (59%) | 6 (12%) | 0 (0%) |
| 27 | The approach to sensitivity analysis is given | 41 (80%) | 5 (10%) | 5 (10%) | 0 (0%) |
| 28 | The choice of variables for sensitivity analysis is justified | 30 (59%) | 15 (29%) | 6 (12%) | 0 (0%) |
| 29 | The ranges over which the variables are varied are stated | 30 (59%) | 10 (20%) | 11 (22%) | 0 (0%) |
| 30 | Relevant alternatives are compared | 51 (100%) | 0 (0%) | 0 (0%) | 0 (0%) |
| 31 | Incremental analysis is reported | 38 (75%) | 10 (20%) | 3 (6%) | 0 (0%) |
| 32 | Major outcomes are presented in a disaggregated as well as aggregated form | 36 (71%) | 15 (29%) | 0 (0%) | 0 (0%) |
| 33 | The answer to the study question is given | 51 (100%) | 0 (0%) | 0 (0%) | 0 (0%) |
| 34 | Conclusions follow from the data reported | 51 (100%) | 0 (0%) | 0 (0%) | 0 (0%) |
| 35 | Conclusions are accompanied by the appropriate caveats | 38 (75%) | 8 (16%) | 5 (10%) | 0 (0%) |
The relative percentages may not equate to 100% due to rounding errors
Comparison of economic evaluations in the included studies
Pediatric influenza
Compared with no immunisation, the implementation of influenza immunisation in the pediatric-population offers an overall cost-effective strategy in each of the EU-5 or US countries of up to ≤19,366, with the exception of Prosser et al. (2011)15 who reported cost per QALY up to ≤45,244 in the US, and in some cases provides a cost-saving potential when compared to treatment with supportive care or vaccination in those aged 6 months – 64 years (Muennig et al. [2001]14 and Prosser et al. [2011]16). Pitman et al. (2013)9 and Lugner et al. (2012)17 measured the indirect protection of the rest of the population via herd immunity by considering both pediatric and older population groups. Extending the immunisation either to a select or full pediatric population will benefit individuals within the targeted age group and also provide wider protection in other non-targeted age groups who may come into contact with the pediatric population.12,14,16
Other diseases relative to influenza
The included studies on pediatric immunisation programmes for the other selected diseases reported a wide range of cost-effectiveness, with cost per QALY mostly higher compared to that of influenza (Fig. 2a–2c). For RV, the cost per QALY was in general substantially higher (Table 2) than those presented for influenza. The values ranged from cost-saving from an Italian31 to ≤244, 077/QALY from a Spanish healthcare perspective.36 Similar to RV, studies of PD and MD immunisation yielded a wide range of cost-effectiveness, from cost saving to ≤177,683/QALY,43 with differences in outcomes notably influenced by the inclusion or exclusion of measurements of herd immunity. Studies of HPV immunisation yielded a similar range of cost-effectiveness to influenza of between ≤2,83723 and ≤48,725/QALY,27 with differences in outcomes influenced by vaccine type, perspective, and cancer-specific prevention. Varicella immunisation57 yielded a cost per QALY of ≤76,806 and a BCR of 2.73 for varicella prevention, demonstrating a high number of cases averted in the pediatric population. Hep B prevention is associated with a ≤271,750/QALY and a similar BCR of 2.43 (new-borns and 12-year-olds);39 however, the limited number of studies in the pediatric population included in this review may not be truly reflective of the extent to which vaccines may prevent disease burden, particularly for varicella and Hep B vaccines, as the small number of studies provide inaccurate estimates of impact.
Due to the paucity of data and variability across the indications, comparisons of other outcome measures, such as LYG and cases averted (hospitalisations or mortality events) are not conclusive enough to be presented here but are summarised in Table 1 and Table 2.
Discussion
Overview
In 2005 the World Health Organization (WHO) published guidelines on policy issues to help decision makers consider the broader implications of adding a vaccine to a national immunisation program. In addition to economic and financial questions, other aspects such as the public health priority of particular vaccines, the disease burden, public health surveillance and comparisons with other interventions should be taken into consideration.59 Subsequently, in 2008, the WHO published specific advice for standardising economic evaluations of vaccination programmes for current and emerging diseases (including pandemic influenza) to meet decision-makers' needs for relevant, reliable and consistent economic information in this area.60 This is because compared to most drugs assessed by health economic analyses, vaccines have characteristics that require special considerations when evaluating their cost effectiveness. These characteristics are related to herd immunity, quality-of-life losses in young children, parental care and associated work loss, time preference, uncertainty, eradication, macroeconomics and tiered pricing.61 Specific to infant influenza, complicating factors that contribute to uncertainty are seasonal variations in incidence, severity of disease and vaccine efficacy.62 Against this background, this review compared the economic value of pediatric influenza immunisation and a selection of existing pediatric immunisation programmes within similar contexts. For influenza, all studies demonstrated that pediatric immunisation offers a valuable health intervention demonstrating, in most cases, a cost-effective or cost-saving potential across societal, payer, National Health Service (NHS), and provider perspectives with incremental cost- effective ratios (ICER) below the respective thresholds in the US and EU5 (cost per QALY up to ≤19,366 reported) — consistent with the literature within this area.2,5–8
The derived ICER for pediatric influenza immunisation suggests that it fits well within the overall cost range of recent pediatric immunisation programmes already in place, particularly to HPV and varicella and across the other indications (MD, PD, RV, Hep B) considered here. The cost-effectiveness ratios derived from the included studies on the other diseases of interest (MD, PD, HPV, Hep B, varicella, and RV) demonstrated an overall cost per QALY range between cost-saving up to ≤271,75039 across all indications. For HPV, the modeled vaccines targeted those aged 12 years or older over a lifetime horizon, in most cases; similarly for varicella the average age ranges from those aged 1–2 and 11 years old, also modeled over a lifetime-horizon (in most cases) although cost-effectiveness outcomes varied considerably. The difference in age groups considered in these studies may provide further challenges when comparing cost-effectiveness across studies. Chesson et al. (2008)22 modeled the impact of an HPV immunisation program, given in addition to current cervical screening, in the US and concluded that it was a cost-effective strategy, with an ICER of ≤10,693 when herd immunity was ignored and an ICER of ≤2,837 when herd immunity was included. Jit et al.(2011)27 used a model to compare bivalent and quadrivalent HPV immunisations versus no immunisation in the UK and concluded that both vaccines were cost effective when protection against anal, penile, and oropharyngeal cancers was assumed. For the licensed endpoints including the incidence of cervical cancers bivalent HPV immunisation exceeds the UK's ≤30,000 WTP threshold. It is ≤48,725 per QALY. Coudeville et al.(2005)56 found that routine childhood varicella immunisation represents a cost saving health intervention in both France and Germany. Similarly, Lenne et al.(2006)55 concluded that routine varicella immunisation in Spain is cost saving from the societal perspective and highly cost-effective from the healthcare perspective.
Besides demonstrating cost effectiveness compared to other vaccinations, the included studies on pediatric influenza vaccinations also demonstrate the importance and impact on the cost-effectiveness outcomes with herd immunity. Analyses modeling the direct effects of pediatric vaccines (immunising children aged less than 5 years old)11,13,16 or both pediatric and adult vaccines6,9,17 on the wider population demonstrated a lower cost-effectiveness ratio associated with herd immunity.
While the quality of the analyses across the included studies was fairly robust the differences between age groups and characteristics of each disease may limit the comparability of cost-effectiveness within and across indications. Indeed the reported cost-effectiveness values fall within a wide range, which may be explained by factors such as vaccine efficiency but also by choice of study design, including (1) difference in time horizon, (2) inclusion of herd immunity, (3) coverage, (4) pediatric population age groups, for example variations in the patient cohort (e.g. <5 years and 5–19 years), and (5) perspectives, which are presented as part of the scenario/sensitivity analyses.
Furthermore, the lack of a single outcome measure to quantify the impact of pediatric immunisation may lead to inconsistencies when comparing overall results and benefits (Table 1). The outcome measures chosen by the included studies consist of (1) cases averted, (2) overall net saving or BCR (value >1 implies incremental benefits exceed incremental costs), (3) cost per QALY, (4) cost per LYG, and (5) cost per LYS. The non-inclusion of certain costs may also result in different estimates of cost-effectiveness; particularly with pediatric diseases the impact on parents/guardians is not considered in many studies included in this review. This may understate the overall cost-effectiveness outcome, potentially underestimating the true impact of these diseases and the associated benefit of routine childhood immunisation.
Limitations
The approach used to conduct this review has several limitations. The search was conducted on selected databases. Conference proceedings and other sources, such as bibliographies from included studies, were excluded in the preparation of this review. Studies selected were based on availability of full texts and those exclusive to, or inclusive of the pediatric population. The selection process excluded subgroups, such as children with asthma, where the impact of immunisation programmes could highlight an even greater economic benefit, particularly so in the case of influenza. Studies were only selected for inclusion if they were conducted after 2000, were limited to EU5 and US settings and involved comparisons between immunisation and no immunisation(s). Existing systematic reviews for each indication or combined indications were also excluded from the review. Such reviews could have potentially provided a wider scope of studies conducted in this area and highlighted those studies not identified from the literature search per se. Many existing reviews in these indications have not been limited to geographical locations although the results presented here can be compared/found in existing reviews.2,42,55,63,64 One of the key issues in this review was the economic measure used to determine the cost-effectiveness of immunisation across the studies and indications. Although most studies provided an economic value (before final negotiated price by the national governments) predominantly expressed as an ICER (cost/QALY), where QALY is a well-accepted generic measure of mortality and morbidity across different indications, the lack of other standardised measures compromises comparability across studies. If studies had been limited to those providing ICER values only, the number of studies included in the review would have been limited further. The approach carried out here was consistent with existing systematic reviews where all effectiveness measures were considered.63,64 Comparability across the studies was further compromised by differences in age groups, time horizons, and perspectives.
Implications for new research
Future considerations, particularly for influenza immunisation studies, should try to capture the full population to allow comparisons within and across different age groups and incorporate the impact of herd immunity to illustrate the wider protection to society.
When comparing economic measures in the same or across different indications, the context of each cost-per-QALYs should be recognized to allow a reasonable comparison of one indication vs. another, for example influenza and HPV. Results should be interpreted with caution and some authorities will require supporting/further evidence of the benefits of routine immunisations beyond the comparison of cost-per-QALYs.64 Keeping in mind that economic evaluations offer useful tools to inform decisions and price discussions of implementing pediatric immunisation programmes, future studies should also report, or at least acknowledge, the available funding and policy implications for routine immunisation programmes, to help assess the discrepancies in each country setting and the cost-effectiveness outcomes considered.
Conclusion
The findings of this review suggest that pediatric influenza immunisation could provide a valuable health intervention with cost-effective potential from both healthcare and societal perspectives when compared with no immunisation or existing policies. Influenza immunisation programmes were located within the lower range of overall ICER values across immunisation programmes of the selected indications; although various age groups were considered within the same and across indications (e.g., <5 and 5–19 years). For influenza, extending immunisation to the full pediatric population (major transmitters) was generally the most cost-effective strategy.9,65 Although, it could be argued that the most efficient way to implement pediatric influenza immunisation programmes would be from programmes that offer cost-saving potential; possibly achieved with increased vaccination coverage to allow optimal herd immunity. Many factors remain unclear or confounding, such as the level of vaccination coverage needed and differences in existing country policies and in this respect further research is required.
Methods
This review is reported in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) recommendations,66 and compares economic evaluations of the vaccines of interest according to their licensed indications and contraindications as defined in each study.
Searches
A comprehensive literature review of published evidence on economic evaluations (cost-effectiveness analysis [CEA], cost–benefit analysis [CBA] or cost-utility analysis [CUA]) across the 7 disease areas was conducted using an electronic medical-journal database (PubMed), health technology databases (NIHR CRD [National Institute for Health Research Centers for reviews and dissemination], HAS [French National Authority for Health], IqWiG [Institute for Quality and Efficiency in Healthcare], Medicare, and vaccination databases (JCVI [Joint Committee on Vaccination and Immunisation], STIKO [Standing Committee on Vaccination], CTV [Technical Vaccination Committee]).
The search strategy grouped keywords into categories for disease, economic outcomes, model type, and programmes. Search terms for the disease category were “influenza a virus; influenza b virus,” “pneumococcus; pneumococcal,” “meningitis,” “varicella zoster virus; chicken pox,” “hepatitis B,” “human papillomavirus vaccines” and “rotavirus;” for the economic outcomes category “cost-effectiveness,” “cost utility,” “cost benefit” and “economic evaluation;” for the model type category ”economic models,” “decision tree,” “economic decision models,” “Markov,” “transmission model” and “SEIR;” and for the programmes category “immunisation,” “vaccine” and “vaccination.” Combined searches in PubMed were performed for “disease AND (economic outcomes OR model types) AND programmes.” Searches conducted in the other databases combined “disease AND economic outcomes.”
Inclusions
Studies were included in the review if they considered human subjects, were published in English language between 1st January 2000 to 16th December 2014 and had abstracts available. Studies were included according to the target group, comparisons, type of economic evaluation, and country perspectives. The target group is defined as the pediatric population (either the overall pediatric population aged less than 18 years, smaller age ranges such as 5–9 year olds, or pediatric groups consisting of both pediatric as well as adult population —an example being 5–9 and 18–65 year olds) without specific conditions, such as asthma, or other co-existing illnesses. Comparisons with no-intervention or current vaccination policy were included. Complete economic evaluation of CEA, CBA and CUA assessing both the benefits and costs of influenza, RV, MD, HPV, Hep B, PD and varicella, either independently or across multiple diseases, were included. Studies reporting on the UK, France, Germany, Italy and Spain (collectively known as the European Union Five, or EU5), and the US were included.
Full-text articles were retrieved and assessed to determine the final inclusion of studies. Articles were excluded: (1) if they reported systematic reviews on the economic evaluation across the 7 diseases either independently or combined; (2) if full-text articles were unavailable; (3) if studies were conducted outside the EU5 and US; and (4) if studies were exclusively based on the non-pediatric population. Additionally, studies that reported the impact of the disease in terms of epidemiology or clinical trial data were also excluded.
All included papers assess the impact of vaccination on standard vaccination schedules, which for pediatric influenza immunization is an annual event. While mismatches between vaccine strains and circulating strains may occur and have an impact on vaccine effectiveness, mismatch averages out over the duration of the modeling time horizon for the health economic outcomes of adding pediatric immunisation programmes into current practice. In addition, the effectiveness results are usually not statistically significant due to the low numbers for individual vaccine and age groups in influenza.
Data extraction
Eligible articles were independently screened by 2 researchers (NB and BS) on the basis of titles and abstracts retrieved by the search from the electronic databases (Fig. 1), alternative extraction methods of screening reference list from relevant studies or hand searching key journals and conference proceedings were not adopted in this review. In the case of disagreement a third reviewer was consulted (SR) for discussions on population(s), setting, immunisation programmes and type of studies included. Data on economic evaluations was extracted at the full text stage by 2 researchers (NB and BS) and entered into an extraction table comparing cost-effectiveness outcomes according to the type of analysis, immunisation strategies, country, perspective, vaccination coverage, time horizon, effectiveness measure, cost measure, sensitivity analysis and outcome measures. All monetary outcomes were converted based on currency conversions taken from the XE website and inflated accordingly to 2014 GBP using the European Central Bank (ECB) and Medical Expenditure Panel Survey (MEPS) databases.67–69
Willingness to pay thresholds
WTP were used to assess and compare cost-effectiveness of pediatric immunisation programmes within each country setting and across the vaccines considered. The WTP for the UK was ≤30,000.70 The assumed WTP for all other European countries,26 including Italy, is €30,000 = ≤23,780 (€1 = $0.79), and for the US is $50,00021= ≤31,780 ($1 = ≤0.63) based on currency conversions taken from the XE website (20 December 2014) and inflated to 2014 GBP.67–69
Quality assessment
The quality of each included study was assessed independently by 2 researchers (NB and BS) using Drummond's checklist.58 Discrepancies were resolved by discussion and consensus with a third investigator if needed (EG) for clarity on choice of economic evaluation, parameter description and subjects considered in the studies. Table 3 presents the overall results for each item on Drummond's list categorised by study design (items 1–7), data collection and analysis (items 8–21), and interpretation of results (items 22–35). Responses to the items in the list were completely satisfied (yes), not satisfied (no), unclear or not-applicable (inappropriate).
Disclosure of potential conflicts of interest
This work was funded by AstraZeneca (AZ) who currently has a licensed Live Attenuated influenza vaccine (Fluenz® Tetra or Flumist Quadrivalent). AS, JH and SR are current employees of AZ. EG and NB are employed by Wickenstones Ltd (at the time of the study BS was employed by Wickenstones Ltd) and were financed by AZ to complete this study. AZ have also funded EG to develop an economic model for pediatric influenza vaccination strategies.
Acknowledgments
We are grateful to Prof. Ken Redekop for his comments and recommendations on an earlier draft.
Funding
All funding for this study was provided by AstraZeneca (employees of whom are also authors and contributors to this paper) as per conflict of interest statement.
References
- [1].Lewis DB. Avian flu to human influenza. Annu Rev Med 2006; 57:139-54; PMID:16409141; http://dx.doi.org/ 10.1146/annurev.med.57.121304.131333 [DOI] [PubMed] [Google Scholar]
- [2].De Waure C, Veneziano MA, Cadeddu C, Capizzi S, Specchia ML, Capri S, Ricciardi W. Economic value of influenza vaccination. Hum Vaccin Immunother 2012; 8:78-88; http://dx.doi.org/ 10.4161/hv.8.1.18420 [DOI] [PubMed] [Google Scholar]
- [3].Glezen WP, Couch RB. Interpandemic influenza in the Houston area, 1974–76. N Engl J Med 1978; 298:587-92; PMID:628375; http://dx.doi.org/ 10.1056/NEJM197803162981103 [DOI] [PubMed] [Google Scholar]
- [4].Ruf BR, Knuf M. The burden of seasonal and pandemic influenza in infants and children. Eur J Pediatr 2014; 173:265-76; PMID:23661234; http://dx.doi.org/ 10.1007/s00431-013-2023-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ashkenazi S, Vertruyen A, Arístegui J, Esposito S, McKeith DD, Klemola T, Biolek J, Kühr J, Bujnowski T, Desgrandchamps D, et al.. Superior relative efficacy of live attenuated influenza vaccine compared with inactivated influenza vaccine in young children with recurrent respiratory tract infections. Pediatr Infect Dis J 2006; 25:870-9; PMID:17006279; http://dx.doi.org/ 10.1097/01.inf.0000237829.66310.85 [DOI] [PubMed] [Google Scholar]
- [6].Baguelin M, Van Hoek AJ, Jit M, Flasche S, White PJ, Edmunds WJ. Vaccination against pandemic influenza A/H1N1v in England: A real-time economic evaluation. Vaccine 2010; 28:2370-84; PMID:20096762; http://dx.doi.org/ 10.1016/j.vaccine.2010.01.002 [DOI] [PubMed] [Google Scholar]
- [7].Ambrose CS, Wu X, Knuf M, Wutzler P. The efficacy of intranasal live attenuated influenza vaccine in children 2 through 17 years of age: A meta-analysis of 8 randomized controlled studies. Vaccine 2012; 30:886-92; PMID:22155144; http://dx.doi.org/ 10.1016/j.vaccine.2011.11.104 [DOI] [PubMed] [Google Scholar]
- [8].Nichol KL. Cost-effectiveness and socio-economic aspects of childhood influenza vaccination. Vaccine 2011; 29:7554-8; PMID:21820477; http://dx.doi.org/ 10.1016/j.vaccine.2011.08.015 [DOI] [PubMed] [Google Scholar]
- [9].Pitman RJ, Nagy LD, Sculpher MJ. Cost-effectiveness of childhood influenza vaccination in England and Wales: Results from a dynamic transmission model. Vaccine 2013; 31:927-42; PMID:23246550; http://dx.doi.org/ 10.1016/j.vaccine.2012.12.010 [DOI] [PubMed] [Google Scholar]
- [10].Zhou F, Shefer A, Wenger J, Messonnier M, Wang LY, Lopez A, Moore M, Murphy T V, Cortese M, Rodewald L. Economic evaluation of the routine childhood immunization program in the United States, 2009. Pediatrics 2014; 133:577-85; PMID:24590750; http://dx.doi.org/ 10.1542/peds.2013-0698 [DOI] [PubMed] [Google Scholar]
- [11].Luce BR, Zangwill KM, Palmer CS, Mendelman PM, Yan L, Wolff MC, Cho I, Marcy SM, Iacuzio D, Belshe RB. Cost-effectiveness analysis of an intranasal influenza vaccine for the prevention of influenza in healthy children. Pediatrics 2001; 108:E24; PMID:11483834; http://dx.doi.org/ 10.1542/peds.108.2.e24 [DOI] [PubMed] [Google Scholar]
- [12].Marchetti M, Kühnel UM, Colombo GL, Esposito S, Principi N. Cost-effectiveness of adjuvanted influenza vaccination of healthy children 6 to 60 months of age. Hum Vaccin 2007; 3:14-22; PMID:17245134; http://dx.doi.org/ 10.4161/hv.3.1.3657 [DOI] [PubMed] [Google Scholar]
- [13].Hibbert CL, Piedra PA, McLaurin KK, Vesikari T, Mauskopf J, Mahadevia PJ. Cost-effectiveness of live-attenuated influenza vaccine, trivalent in preventing influenza in young children attending day-care centres. Vaccine 2007; 25:8010-20; PMID:17936446; http://dx.doi.org/ 10.1016/j.vaccine.2007.09.018 [DOI] [PubMed] [Google Scholar]
- [14].Muennig PA, Khan K. Cost-effectiveness of vaccination versus treatment of influenza in healthy adolescents and adults. Clin Infect Dis 2001; 33:1879-85; PMID:11692300; http://dx.doi.org/ 10.1086/324491 [DOI] [PubMed] [Google Scholar]
- [15].Prosser LA, Lavelle TA, Fiore AE, Bridges CB, Reed C, Jain S, Dunham KM, Meltzer MI. Cost-effectiveness of 2009 Pandemic influenza A(H1N1) vaccination in the United States. PLoS One 2011; 6:e22308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Prosser LA, Meltzer MI, Fiore A, Epperson S, Bridges CB, Hinrichsen V, Lieu TA. Effects of adverse events on the projected population benefits and cost-effectiveness of using live attenuated influenza vaccine in children aged 6 months to 4 years. Arch Pediatr Adolesc Med 2011; 165:112-8; PMID:20921341; http://dx.doi.org/ 10.1001/archpediat-rics.2010.182 [DOI] [PubMed] [Google Scholar]
- [17].Lugner AK, van Boven M, de Vries R, Postma MJ, Wallinga J. Cost effectiveness of vaccination against pandemic influenza in European countries: mathematical modelling analysis. Bmj 2012; 345:e4445-e4445; PMID:22791791; http://dx.doi.org/ 10.1136/bmj.e4445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sanders GD, Taira A V. Cost effectiveness of a potential vaccine for Human papillomavirus. Emerg Infect Dis 2003; 9:37-48; PMID:12533280; http://dx.doi.org/ 10.3201/eid0901.020168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jit M, Choi YH, Edmunds WJ. Economic evaluation of human papillomavirus vaccination in the United Kingdom. BMJ 2008; 337:a769; PMID:18640957; http://dx.doi.org/ 10.1136/bmj.a769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Insinga R, Allen S, Carides G, Myers E. Reductions in human papillomavirus-disease resource use and costs with quadrivalent vaccination: The FUTURE study economic evaluation. Value Heal 2008; in press 11(7):1022-32; http://dx.doi.org/ 10.1111/j.1524-4733.2008.00342.x [DOI] [PubMed] [Google Scholar]
- [21].Goldhaber-Fiebert JD, Stout NK, Salomon JA, Kuntz KM, Goldie SJ. Cost-effectiveness of cervical cancer screening with human papillomavirus DNA testing and HPV-16,18 vaccination. J Natl Cancer Inst 2008; 100:308-20; PMID:18314477; http://dx.doi.org/ 10.1093/jnci/djn019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chesson HW. Cost-Effectiveness of Human Papillomavirus Vaccination in the United States. Emerg Infect Dis 2008; 14:244-51; PMID:18258117; http://dx.doi.org/ 10.3201/eid1402.070499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bergeron C, Largeron N, McAllister R, Mathevet P, Remy V. Cost-effectiveness analysis of the introduction of a quadrivalent human papillomavirus vaccine in France. Int J Technol Assess Health Care 2008; 24:10-9; PMID:18218164; http://dx.doi.org/ 10.1017/S0266462307080026 [DOI] [PubMed] [Google Scholar]
- [24].Mennini FS, Giorgi Rossi P, Palazzo F, Largeron N. Health and economic impact associated with a quadrivalent HPV vaccine in Italy. Gynecol Oncol 2009; 112:370-6; PMID:19041125; http://dx.doi.org/ 10.1016/j.ygyno.2008.09.031 [DOI] [PubMed] [Google Scholar]
- [25].Hillemanns P, Petry KU, Largeron N, McAllister R, Tolley K, Büsch K. Cost-effectiveness of a tetravalent human papillomavirus vaccine in Germany. J Public Health (Bangkok) 2009; 17:77-86; http://dx.doi.org/ 10.1007/s10389-008-0228-3 [DOI] [Google Scholar]
- [26].Diaz M, De Sanjose S, Ortendahl J, O'Shea M, Goldie SJ, Bosch FX, Kim JJ. Cost-effectiveness of human papillomavirus vaccination and screening in Spain. Eur J Cancer 2010; 46:2973-85; PMID:20638840; http://dx.doi.org/ 10.1016/j.ejca.2010.06.016 [DOI] [PubMed] [Google Scholar]
- [27].Jit M, Chapman R, Hughes O, Choi YH. Comparing bivalent and quadrivalent human papillomavirus vaccines: economic evaluation based on transmission model. Bmj 2011; 343:d5775-d5775; PMID:21951758; http://dx.doi.org/ 10.1136/bmj.d5775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Melliez H, Levybruhl D, Boelle PY, Dervaux B, Baron S, Yazdanpanah Y. Cost and cost-effectiveness of childhood vaccination against rotavirus in France. Vaccine 2008; 26:706-15; PMID:18166250; http://dx.doi.org/ 10.1016/j.vaccine.2007.11.064 [DOI] [PubMed] [Google Scholar]
- [29].Jit M, Bilcke J, Mangen M-JJ, Salo H, Melliez H, Edmunds WJ, Yazdan Y, Beutels P. The cost-effectiveness of rotavirus vaccination: Comparative analyses for five European countries and transferability in Europe. Vaccine 2009; 27:6121-8; PMID:19715781; http://dx.doi.org/ 10.1016/j.vaccine.2009.08.030 [DOI] [PubMed] [Google Scholar]
- [30].Giammanco MD, Coniglio MA, Pignato S, Giammanco G. An economic analysis of rotavirus vaccination in Italy. Vaccine 2009; 27:3904-11; PMID:19446934; http://dx.doi.org/ 10.1016/j.vaccine.2009.04.002 [DOI] [PubMed] [Google Scholar]
- [31].Panatto D, Amicizia D, Ansaldi F, Marocco A, Marchetti F, Bamfi F, Giacchino R, Tacchella A, Del Buono S, Gasparini R. Burden of rotavirus disease and cost-effectiveness of universal vaccination in the Province of Genoa (Northern Italy). Vaccine 2009; 27:3450-3; PMID:19200850; http://dx.doi.org/ 10.1016/j.vaccine.2009.01.054 [DOI] [PubMed] [Google Scholar]
- [32].Martin A, Batty A, Roberts JA, Standaert B. Cost-effectiveness of infant vaccination with RIX4414 (RotarixTM) in the UK. Vaccine 2009; 27:4520-8; PMID:19446594; http://dx.doi.org/ 10.1016/j.vaccine.2009.05.006 [DOI] [PubMed] [Google Scholar]
- [33].Shim E, Galvani AP. Impact of transmission dynamics on the cost-effectiveness of rotavirus vaccination. Vaccine 2009; 27:4025-30; PMID:19389452; http://dx.doi.org/ 10.1016/j.vaccine.2009.04.030 [DOI] [PubMed] [Google Scholar]
- [34].Atkins KE, Shim E, Carroll S, Quilici S, Galvani AP. The cost-effectiveness of pentavalent rotavirus vaccination in England and Wales. Vaccine 2012; 30:6766-76; PMID:23000223; http://dx.doi.org/ 10.1016/j.vaccine.2012.09.025 [DOI] [PubMed] [Google Scholar]
- [35].Knoll S, Mair C, Benter U, Vouk K, Standaert B. Will vaccination against rotavirus infection with RIX4414 be cost-saving in Germany? Health Econ Rev 2013; 3:27; PMID:24246029; http://dx.doi.org/ 10.1186/2191-1991-3-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Imaz I, Rubio B, Cornejo AM, González-Enríquez J. Budget impact and cost-utility analysis of universal infant rotavirus vaccination in Spain. Prev Med (Baltim) 2014; 61:116-21; http://dx.doi.org/ 10.1016/j.ypmed.2013.12.013 [DOI] [PubMed] [Google Scholar]
- [37].Aidelsburger P, Grabein K, Böhm K, Dietl M, Wasem J, Koch J, Ultsch B, Weidemann F, Wichmann O. Cost-effectiveness of childhood rotavirus vaccination in Germany. Vaccine 2014; 32:1964-74; PMID:24561052; http://dx.doi.org/ 10.1016/j.vaccine.2014.01.061 [DOI] [PubMed] [Google Scholar]
- [38].Szucs T. Cost-effectiveness of hepatitis A and B vaccination programme in Germany. Vaccine 2000; 18:S86-9; PMID:10683559; http://dx.doi.org/ 10.1016/S0264-410X(99)00474-0 [DOI] [PubMed] [Google Scholar]
- [39].Siddiqui MR, Gay N, Edmunds WJ, Ramsay M. Economic evaluation of infant and adolescent hepatitis B vaccination in the UK. Vaccine 2011; 29:466-75; PMID:21073988; http://dx.doi.org/ 10.1016/j.vaccine.2010.10.075 [DOI] [PubMed] [Google Scholar]
- [40].Boccalini S, Taddei C, Ceccherini V, Bechini A, Levi M, Bartolozzi D, Bonanni P. Economic analysis of the first 20 y of universal hepatitis B vaccination program in Italy: An a posteriori evaluation and forecast of future benefits. Hum Vaccines Immunother 2013; 9:1119-28; http://dx.doi.org/ 10.4161/hv.23827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Trotter CL, Edmunds WJ. Modelling cost effectiveness of meningococcal serogroup C conjugate vaccination campaign in England and Wales. BMJ 2002; 324:809; PMID:11934772; http://dx.doi.org/ 10.1136/bmj.324.7341.809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ortega-Sanchez IR, Lee GM, Jacobs RJ, Prosser LA, Molinari N-A, Zhang X, Baine WB, McCauley MM, Miller T. Projected cost-effectiveness of new vaccines for adolescents in the United States. Pediatrics 2008; 121 Suppl:S63-78; PMID:18174323; http://dx.doi.org/ 10.1542/peds.2007-1115H [DOI] [PubMed] [Google Scholar]
- [43].Christensen H, Trotter CL, Hickman M, Edmunds WJ. Re-evaluating cost effectiveness of universal meningitis vaccination (Bexsero) in England: modelling study. Bmj 2014; 349:g5725-g5725; PMID:25301037; http://dx.doi.org/ 10.1136/bmj.g5725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Christensen H, Hickman M, Edmunds WJ, Trotter CL. Introducing vaccination against serogroup B meningococcal disease: An economic and mathematical modelling study of potential impact. Vaccine 2013; 31:2638-46; PMID:23566946; http://dx.doi.org/ 10.1016/j.vaccine.2013.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Claes C, Graf Von Der Schulenburg JM. Cost effectiveness of pneumococcal vaccination for infants and children with the conjugate vaccine PnC-7 in Germany. Pharmacoeconomics 2003; 21:587-600; PMID:12751916; http://dx.doi.org/ 10.2165/00019053-200321080-00005 [DOI] [PubMed] [Google Scholar]
- [46].Melegaro A, Edmunds WJ. Cost-effectiveness analysis of pneumococcal conjugate vaccination in England and Wales. Vaccine 2004; 22:4203-14; PMID:15474710; http://dx.doi.org/ 10.1016/j.vaccine.2004.05.003 [DOI] [PubMed] [Google Scholar]
- [47].Ray GT, Whitney CG, Fireman BH, Ciuryla V, Black SB. Cost-effectiveness of pneumococcal conjugate vaccine: evidence from the first 5 years of use in the United States incorporating herd effects. Pediatr Infect Dis J 2006; 25:494-501; PMID:16732146; http://dx.doi.org/ 10.1097/01.inf.0000222403.42974.8b [DOI] [PubMed] [Google Scholar]
- [48].Lieu T a, Ray GT, Black SB, Butler JC, Klein JO, Breiman RF, Miller MA, Shinefield HR. Projected cost-effectiveness of pneumococcal conjugate vaccination of healthy infants and young children. JAMA 2000; 283:1460-8; PMID:10732936; http://dx.doi.org/ 10.1001/jama.283.11.1460 [DOI] [PubMed] [Google Scholar]
- [49].Lloyd A, Patel N, Scott DA, Runge C, Claes C, Rose M. Cost-effectiveness of heptavalent conjugate pneumococcal vaccine (Prevenar) in Germany: Considering a high-risk population and herd immunity effects. Eur J Heal Econ 2008; 9:7-15; http://dx.doi.org/ 10.1007/s10198-006-0013-6 [DOI] [PubMed] [Google Scholar]
- [50].Ray GT, Pelton SI, Klugman KP, Strutton DR, Moore MR. Cost-effectiveness of pneumococcal conjugate vaccine: An update after 7 years of use in the United States. Vaccine 2009; 27:6483-94; PMID:19720366; http://dx.doi.org/ 10.1016/j.vaccine.2009.08.045 [DOI] [PubMed] [Google Scholar]
- [51].Giorgi-Rossi P, Merito M, Borgia P. Cost-effectiveness of introducing the conjugated pneumococcal vaccine to routine free immunizations for infants in Lazio, Italy. Health Policy (New York) 2009; 89:225-38; http://dx.doi.org/ 10.1016/j.healthpol.2008.05.016 [DOI] [PubMed] [Google Scholar]
- [52].Claes C, Reinert RR, Von Der Schulenburg JMG. Cost effectiveness analysis of heptavalent pneumococcal conjugate vaccine in Germany considering herd immunity effects. Eur J Heal Econ 2009; 10:25-38; http://dx.doi.org/ 10.1007/s10198-008-0098-1 [DOI] [PubMed] [Google Scholar]
- [53].Díez-Domingo J, Ridao-López M, Gutiérrez-Gimeno MV, Puig-Barberá J, Lluch-Rodrigo JA, Pastor-Villalba E. Pharmacoeconomic assessment of implementing a universal PCV-13 vaccination programme in the Valencian public health system (Spain). Vaccine 2011; 29:9640-8; http://dx.doi.org/ 10.1016/j.vaccine.2011.10.038 [DOI] [PubMed] [Google Scholar]
- [54].Thiry N, Beutels P, Tancredi F, Roman∫ L, Zanetti A, Bonanni P, Gabutti G, Van Damme P. An economic evaluation of varicella vaccination in Italian adolescents. Vaccine 2004; 22:3546-62; PMID:15315834; http://dx.doi.org/ 10.1016/j.vaccine.2004.03.043 [DOI] [PubMed] [Google Scholar]
- [55].Lenne X, Diez Domingo J, Gil A, Ridao M, Lluch JA, Dervaux B. Economic evaluation of varicella vaccination in Spain-Results from a dynamic model. Vaccine 2006; 24:6980-9; PMID:16860909; http://dx.doi.org/ 10.1016/j.vaccine.2006.04.051 [DOI] [PubMed] [Google Scholar]
- [56].Coudeville L, Brunot A, Szucs TD, Dervaux B. The economic value of childhood varicella vaccination in France and Germany. Value Heal 2005; 8:209-22; http://dx.doi.org/ 10.1111/j.1524-4733.2005.04005.x [DOI] [PubMed] [Google Scholar]
- [57].Zhou F, Ortega-Sanchez IR, Guris D, Shefer A, Lieu T, Seward JF. An economic analysis of the universal varicella vaccination program in the United States. J Infect Dis 2008; 197 Suppl:S156-64; PMID:18419391; http://dx.doi.org/ 10.1086/522135 [DOI] [PubMed] [Google Scholar]
- [58].Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 1996. [cited 2015 May 29]; 313:275-83; PMID:8704542; http://dx.doi.org/ 10.1136/bmj.313.7052.275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].WHO Vaccine Introduction Guidelines: Adding a vaccine to a national immunization programme: decision and implementation. 2005. [Google Scholar]
- [60].Walker D, Beutels P. WHO guide for standardization of economic evaluations of immunization programmes: Immunization , Vaccines and Biologicals. Vaccine 2008; 28:1-116 [DOI] [PubMed] [Google Scholar]
- [61].Beutels P, Scuffham PA, MacIntyre CR. Funding of drugs: do vaccines warrant a different approach? Lancet Infect Dis 2008. [cited 2015 Sep 15]; 8:727-33; PMID:18992409; http://dx.doi.org/ 10.1016/S1473-3099(08)70258-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Hope-Simpson RE. The role of season in the epidemiology of influenza. J Hyg (Lond) 1981. [cited 2015 Sep 15]; 86:35-47; PMID:7462597; http://dx.doi.org/ 10.1017/S0022172400068728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Bin-Chia Wu D, Chaiyakunapruk N, Chong H-Y, Beutels P. Choosing between 7-, 10- and 13-valent pneumococcal conjugate vaccines in childhood: A review of economic evaluations (2006-2014). Vaccine 2015. [cited 2015 Feb 19]; 33:1633-58; PMID:25681663; http://dx.doi.org/ 10.1016/j.vaccine.2015.01.081 [DOI] [PubMed] [Google Scholar]
- [64].Boccalini S, Azzari C, Resti M, Valleriani C, Cortimiglia M, Tiscione E, Bechini A, Bonanni P. Economic and clinical evaluation of a catch-up dose of 13-valent pneumococcal conjugate vaccine in children already immunized with three doses of the 7-valent vaccine in Italy. Vaccine 2011; 29:9521-8; PMID:22008820; http://dx.doi.org/ 10.1016/j.vaccine.2011.10.013 [DOI] [PubMed] [Google Scholar]
- [65].Pitman RJ, Melegaro A, Gelb D, Siddiqui MR, Gay NJ, Edmunds WJ. Assessing the burden of influenza and other respiratory infections in England and Wales. J Infect 2007; 54:530-8; PMID:17097147; http://dx.doi.org/ 10.1016/j.jinf.2006.09.017 [DOI] [PubMed] [Google Scholar]
- [66].Liberati A, Altman DG, Tetzlaff J, Mulrow C, Ioannidis JP a, Clarke M, Devereaux PJ, Kleijnen J, Moher D. Annals of Internal Medicine Academia and Clinic The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Ann Intern Med 2009; 151:W65-94; PMID:19622512; http://dx.doi.org/ 10.7326/0003-4819-151-4-200908180-00136 [DOI] [PubMed] [Google Scholar]
- [67].MEPS . Medical Expenditure Panel Survey; [Google Scholar]
- [68].XE. XE Currency Converter [Google Scholar]
- [69].European Central Bank. Health [Google Scholar]
- [70].Joint Committee on Vaccination and Immunisation. Code of Practice June 2013. 2013; [Google Scholar]