Abstract
Members of the skipper tribe Baorini generally resemble each other and are characterized by dark brown wings with hyaline white spots. These shared characteristics have caused difficulties with revealing the relationships among genera and species in the group, and some conflicting taxonomic views remain unresolved. The present study aims to infer a more comprehensive phylogeny of the tribe using molecular data, to test the monophyly of the tribe as well as the genera it includes in order to clarify their taxonomic status, and finally to revise the current classification of the group. In order to reconstruct a phylogenetic tree, the mitochondrial COI-COII and 16S genes as well as the nuclear EF-1α and 28S genes were analyzed using parsimony, maximum likelihood, and Bayesian inference. The analysis included 67 specimens of 41 species, and we confirmed the monophyly of Baorini, and revealed that 14 genera are well supported. The genus Borbo is separated into three clades: Borbo, Pseudoborbo, and Larsenia gen. nov. We confirmed that Polytremis is polyphyletic and separated into three genera: Polytremis, Zinaida, and Zenonoida gen. nov., and also confirmed that the genus Prusiana is a member of the tribe. Relationships among some genera were strongly supported. For example, Zenonia and Zenonoida were found to be sister taxa, closely related to Zinaida and Iton, while Pelopidas and Baoris were also found to cluster together.
Introduction
The family Hesperiidae, commonly known as skippers or skipper butterflies, comprises approximately 4000 species belonging to 540 genera worldwide [1] and is defined by the following unique morphological character states: an “eye ring”, a wide head, an area of small and specialized scales on the upper side of the hindwing base, and a large thorax, resulting in the mesoscutellum overhanging the metanotum [2]. These unique character states support monophyly of the family [2–4], which has also been supported by molecular data [5]. The higher classification of Hesperiidae had long remained unchanged until Warren et al. challenged it with phylogenetic scheme [2, 5]. The traditional framework of six subfamilies was rearranged into seven: Coeliadinae, Euschemoninae, Eudaminae, Pyrginae, Heteropterinae, Trapezitinae, and Hesperiinae. Moreover, they reinterpreted Evans’ [6] genus groups as tribes, and furthermore the subfamily Hesperiinae was subdivided into six tribes, and the tribe Baorini was proposed [5]. Monophyly of the tribe was strongly supported by both a study using only molecular data [5] as well as another that combined molecular and morphological data [2].
The tribe Baorini was originally introduced as Baorina, one of the subfamilies of Hesperiadae [sic] [7]. The subfamily level designation was also used by Bell [8] in which Baoris Moore, 1881, Caltoris Swinhoe, 1893, Chapra Moore, 1881 (a junior subjective synonym of Pelopidas Walker, 1870), Parnara Moore, 1881, Gegenes Hübner, 1819, and Iton de Nicéville, 1895 were included. Evans [6] placed these genera in his Gegenes group. Subsequently, Eloit named it the Pelopidas group [9, 10], and Chou employed the tribe name Gegenini [11, 12]. According to Code article 23.3.1 and 34.1 of International Commission on Zoological Nomenclature [13], the designation Baorini is more appropriate than Gegenini.
Most members of the group resemble each other and have dark brown wings and hyaline white spots. Mainly due to this simple wing pattern, researchers have struggled to determine which species were related and should be assigned to the same genus. In the renowned Die Gross Schmetterlinge der Erde, all members, except for Gegenes, were placed in Parnara [14–16]. Simultaneously, Zenonia zeno was considered a species of Padraona, whose markings are orange and yellow. It is worth noting that Mabille described the genus Polytremis in 1904 [17], but this description was not reflected in a later publication in 1909 [14]. Evans [18, 19] also initially published contradictory definitions of the taxonomy within the group. Initially, he assigned almost all species to Baoris, except for some that were placed in the genera Iton and Gegenes, even though all of the major genera had been previously described. Evans worked extensively on the group until he finally settled on eight genera [6].
In most current taxonomic studies, the six genera mentioned above as well as two African allies, Zenonia and Brusa, are treated as members of the same group, regardless of the name used. However, Prusiana, Pseudoborbo, and Zinaida are exceptions and further explanation clarifying why they are distinct is necessary.
Although Evans recognized that the genitalia of the genus Prusiana were the same as the Gegenes group, he still treated the genus as a member of his Taractrocera group and placed it after the genus Cephrenes [6]. De Jong considered Prusiana to be a rather enigmatic group due to its unclear relationship to other genera [20]. Maruyama, regarded the difference in genitalia morphology to be an important taxonomic character, and moved the genus into the Pelopidas group [21], which is currently generally followed in classification schemes [2, 22].
Hesperia bevani Moore, 1878 was assigned previously to various genera, such as Baoris [23–26], Parnara [14, 16, 27–38], Caltoris [39, 40], or Pelopidas [19]. Since Evans described the genus Borbo [6], this species is usually placed in this genus [10, 22, 41–44]. Subsequently, Lee described the genus Pseudoborbo based on the adult and immature morphological characters of Borbo bevani and then reclassified this species as his monotypic genus [45]. Some subsequent authors, however, did not support Lee’s arrangement and considered the genus Pseudoborbo to be a synonym of Borbo [2, 5, 46, 47], while others followed Lee’s classification [1, 11, 12, 48–52].
The genus Zinaida was described by Evans with Parnara nascens Leech, 1893 as its type species. In addition to the type species, Z. theca Evans, 1937 was described and Pamphila caerulescens Mabille, 1876 and Pamphila mencia Moore, 1877 were also included in the genus [19]. Without any explanation, however Evans treated Zinaida as a synonym of the genus Polytremis Mabille, 1904 [6]. Subsequent authors also followed this classification scheme [1, 10–12, 41, 42, 52].
Few phylogenetic analyses involving the tribe Baorini have been published. Dodo et al. analyzed mitochondrial ND5 and COI of Japanese skippers, and concluded that the genera Pelopidas and Parnara were monophyletic groups [53], which we have confirmed in this study. Warren et al. investigated the phylogenetic relationships of subfamilies and the circumscription of tribes of the family Hesperiidae based on molecular data [5]. Baorini included only four species belonging to three genera—Pelopidas, Iton, and Polytremis—and it was concluded that the monophyly of the Baorine clade was strongly supported. Warren et al. used 49 morphological characters and molecular data to revise the classification of the family Hesperiidae and confirmed the robust monophyly of the tribe Baorini [2], although, only the above three genera were included. A molecular phylogenetic study of Chinese skippers, which sampled only six species across three genera (Parnara, Pelopidas, and Polytremis), provided evidence that the tribe is monophyletic [54].
Jiang et al. constructed a phylogeny of the genus Polytremis from China using one mitochondrial and two nuclear derived genes and claimed that the monophyly of the genus was supported [55]. Yuan et al. analyzed three mitochondrial genes of three species from China, but could not confirm these findings [54]. Our results also contradict the conclusions made by Jiang et al. [55].
The objectives of the present study were to infer a more comprehensive phylogeny of the tribe Baorini using molecular data, to test the monophyly of the tribe Baorini, to clarify the taxonomic status of multiple genera, and to revise the current classification within this tribe if necessary. A well-resolved phylogeny of the tribe Baorini will enhance the understanding of the evolution and biology among species within this group.
Materials and Methods
Taxon sampling
Samples were obtained from all major genera in the tribe Baorini except for Brusa. When possible, the type species was included and multiple species were chosen in controversial genera to correctly clarify taxonomic status. In total, 67 specimens representing 41 species across 11 genera of the tribe Baorini were selected as ingroup taxa. Specifically, we included the genus Pseudoborbo, which has been considered a synonym of Borbo by some authors; Prusiana, which was considered a member of Taractrocera group [6]; and Polytremis nascens, the type species of the genus Zinaida, believed to be a synonym of Polytremis. An additional six species, including single representatives from two genera of the Taractrocerini tribe, Taractrocera and Telicota, as well as the genera Aeromachus, Ampittia, Daimio, and Tagiades were used as outgroups to assess the status of the genus Prusiana and the stability of basal relationships among ingroup lineages. Voucher specimens representing all sampled species were deposited in the Insect Collection of the South China Agricultural University (SCAU). Specimen information and location data are presented in Table 1.
Table 1. Species information and GenBank accession numbers.
| Taxon | Locality | Voucher | The type species | GenBank Accession Nos. | |||
|---|---|---|---|---|---|---|---|
| 16S | COI-COII | 28S | EF-1a | ||||
| Parnara guttata (Bremer & Grey, 1853) 1 | China: Guangdong, Yingde | He001 | ● | JX971164 | JX989082 | JX989114 | KX151612 |
| Parnara guttata (Bremer & Grey, 1853) 2 | China: Guangdong, Yingde | He003 | ● | JX971165 | JX989083 | JX989115 | KX151613 |
| Parnara ganga Evans, 1937 1 | China: Hainan, Jianfengling | He028 | JX971166 | JX989084 | JX989116 | KX151609 | |
| Parnara ganga Evans, 1937 2 | China: Hainan, Jianfengling | He029 | JX971167 | JX989085 | JX989117 | KX151610 | |
| Parnara ganga Evans, 1937 3 | China: Hainan, Jianfengling | He030 | JX971168 | JX989086 | JX989118 | KX151611 | |
| Parnara bada (Moore, 1878) | China: Guangxi, Maoershan | He012 | JX971169 | JX989087 | JX989119 | KX151608 | |
| Polytremis lubricans (Herrich-Schäffer, 1869) 1 | China: Guangdong, Nanling | He095 | ● | JX971170 | JX989088 | JX989120 | KX151619 |
| Polytremis lubricans (Herrich-Schäffer, 1869) 2 | China: Hainan, Jianfengling | He160 | ● | JX971171 | JX989089 | JX989121 | KX151620 |
| Polytremis lubricans (Herrich-Schäffer, 1869) 3 | Malaysia: Perak,Kinta Highland | He549 | ● | KX151512 | KX151572 | - | KX151621 |
| Polytremis lubricans (Herrich-Schäffer, 1869) 4 | Malaysia, Perak,Kinta Highland | He550 | ● | KX151513 | - | KX151545 | KX151622 |
| Polytremis lubricans (Herrich-Schäffer, 1869) 5 | Malaysia, Perak,Kinta Highland | He551 | ● | KX151514 | KX151573 | KX151546 | KX151623 |
| Polytremis caerulescens (Mabille, 1876) | China: Sichuan, Luding, Moxi | He087 | JX971172 | JX989090 | JX989122 | KX151616 | |
| Polytremis zina zina (Evans, 1932) | China: Guangdong, Nanling | He037 | JX971173 | JX989091 | JX989123 | KX151631 | |
| Polytremis zina taiwana Murayama, 1981 | Taiwan | He545 | KX151519 | KX151578 | KX151551 | KX151632 | |
| Polytremis theca theca (Evans, 1937) | China: Shaanxi, Qinling | He503 | KX151518 | KX151577 | KX151550 | KX151630 | |
| Polytremis theca fukia Evans, 1940 | China: Guangdong, Nanling | He009 | JX971174 | JX989092 | JX989124 | KX151629 | |
| Polytremis suprema Sugiyama, 1999 | China:Guangdong, Nanling | He070 | JX971175 | JX989093 | JX989125 | KX151628 | |
| Polytremis nascens (Leech, 1893) | China: Sichuan, Baoxing | He100 | ● | JX971176 | JX989094 | JX989126 | KX151626 |
| Polytremis gotama Sugiyama, 1999 | China: Yunnan, luguhu | He010 | JX971177 | - | JX989127 | - | |
| Polytremis mencia (Moore, 1878) | China: Jiangxi, Lushan | He502 | KX151516 | KX151575 | KX151548 | KX151625 | |
| Polytremis matsuii Sugiyama, 1999 | China: Sichuan, Hailuogou | He484 | KX151515 | KX151574 | KX151547 | KX151624 | |
| Polytremis pellucida (Murray, 1874) | Janpan: Kumamoto | He392 | KX151517 | KX151576 | KX151549 | KX151627 | |
| Polytremis discreta (Elwes & Edwards, 1897) 1 | Vietnam: Dac Lae, Chu Yang Sin | He447 | KX151506 | - | KX151539 | - | |
| Polytremis discreta (Elwes & Edwards, 1897) 2 | China: Sichan, Hanyuan | He448 | KX151507 | - | KX151540 | - | |
| Polytremis discreta (Elwes & Edwards, 1897) 3 | China: Sichuan, Yaan | He481 | KX151508 | KX151570 | KX151541 | KX151617 | |
| Polytremis eltola (Hewitson, 1869) 1 | China: Hunan, Mangshan | He104 | KX151509 | - | KX151542 | - | |
| Polytremis eltola (Hewitson, 1869) 2 | Vietnam:Dac Lae, Chu Yang Sin | He446 | KX151510 | - | KX151543 | - | |
| Polytremis eltola (Hewitson, 1869) 3 | China: Hunan, Mangshan | He509 | KX151511 | KX151571 | KX151544 | KX151618 | |
| Borbo borbonica (Boisduval, 1833) | Kenya: Embu | JS064 | ● | KX151490 | KX151557 | KX151525 | - |
| Borbo cinnara (Wallace, 1866) 1 | China: Guangdong, Nanling | He017 | JX971178 | JX989095 | JX989128 | KX151587 | |
| Borbo cinnara (Wallace, 1866) 2 | China: Hainan, Jianfengling | He017’ | JX971179 | JX989096 | JX989129 | KX151588 | |
| Borbo fatuellus (Hopffer, 1855) 1 | Liberia: Nimba mountains | Tok17 | KX151492 | KX151559 | KX151527 | - | |
| Borbo fatuellus (Hopffer, 1855) 2 | Liberia: Nimba mountains | VA35 | KX151491 | KX151558 | KX151526 | KX151589 | |
| Borbo gemella (Mabille, 1884) | Liberia: Nimba mountains | GA13 | KX151493 | KX151560 | KX151528 | KX151590 | |
| Borbo holtzi (Plötz,1883) 1 | Liberia: Nimba mountains | VA24 | KX151494 | KX151561 | - | KX151591 | |
| Borbo holtzi (Plötz,1883) 2 | Liberia: Nimba mountains | VA40 | KX151495 | KX151562 | - | - | |
| Borbo perobscura (Druce, 1912) | Liberia: Nimba mountains | GA8 | KX151496 | KX151563 | KX151529 | - | |
| Borbo ratek (Boisduval, 1833) 1 | Madagascar | JS071 | KX151497 | KX151564 | KX151530 | KX151592 | |
| Borbo ratek (Boisduval, 1833) 2 | Madagascar | SZS-BOR-004 | KX151498 | KX151565 | KX151531 | KX151593 | |
| Borbo ratek (Boisduval, 1833) 3 | Madagascar | SZS-BOR-006 | KX151499 | - | KX151532 | KX151594 | |
| Borbo sp. | Liberia: Nimba mountains | GA19 | KX151500 | KX151566 | KX151533 | KX151595 | |
| Pseudoborbo bevani (Moore, 1878) | China: Guangdong, Yingde | He018 | ● | JX971180 | JX989097 | JX989130 | KX151633 |
| Pelopidas mathias (Fabricius, 1798) | China: Fujian | He194 | JX971181 | JX989098 | JX989131 | KX151615 | |
| Pelopidas agna (Moore, 1866) | China: Hainan, Jianfengling | He013 | JX971182 | JX989099 | JX989132 | KX151614 | |
| Pelopidas thrax Hübner,1821* | Ghana: Ashanti Region | ● | - | EU364491* | - | EU364286* | |
| Caltoris bromus (Leech, 1893) 1 | China: Guangdong, Yingde | He002 | JX971183 | JX989100 | JX989133 | KX151596 | |
| Caltoris bromus (Leech, 1893) 2 | China: Guangxi, Maoershan | He024 | JX971184 | JX989101 | JX989134 | KX151597 | |
| Caltoris bromus (Leech, 1893) 3 | China: Guangxi, Maoershan | He025 | JX971185 | JX989102 | JX989135 | KX151598 | |
| Caltoris bromus (Leech, 1893) 4 | China: Hainan, Jianfengling | He032 | JX971186 | JX989103 | JX989136 | KX151599 | |
| Caltoris cahira (Moore, 1878) 1 | China: Guangxi, Maoershan | He022 | JX971187 | JX989104 | JX989137 | KX151601 | |
| Caltoris cahira (Moore, 1878) 2 | China: Guangxi, Maoershan | He023 | JX971188 | JX989105 | JX989138 | KX151602 | |
| Caltoris kumara (Moore, 1878) | Java: Mt. Pagoberan | He540 | ● | KX151504 | KX151569 | KX151537 | KX151605 |
| Caltoris malaya (Evans, 1926) | Malaysia: Perak | He541 | KX151505 | - | KX151538 | KX151606 | |
| Caltoris brunnea (Snellen, 1876) | Java: Mt. Pagoberan | He542 | KX151501 | KX151567 | KX151534 | KX151600 | |
| Caltoris cormasa (Hewitson, 1876) 1 | Java: Mt. Pagoberan | He543 | KX151502 | - | KX151535 | KX151603 | |
| Caltoris cormasa (Hewitson, 1876) 2 | Malaysia: Perak | He544 | KX151503 | KX151568 | KX151536 | KX151604 | |
| Baoris farri (Moore, 1878) 1 | China: Hainan, Jianfengling | He091 | JX971189 | JX989106 | JX989139 | KX151584 | |
| Baoris farri (Moore, 1878) 2 | China: Guangdong, Guangzhou | He049 | JX971190 | JX989107 | JX989140 | - | |
| Baoris penicillata (Moore, 1881) | China: Hainan, Yinggeling | He112 | JX971191 | JX989108 | JX989141 | KX151586 | |
| Baoris leechii (Elwes & Edwards, 1897) | China: Guangdong, Nanling | He093 | KX151489 | - | KX151524 | KX151585 | |
| Iton semamora (Moore, 1866) | Indonesia: Sumatra | He239 | ● | JX971192 | JX989109 | JX989142 | KX151607 |
| Iton watsonii (de Nicéville, 1890) | Thailand: Chiang Mai | - | EU364490* | - | EU364285* | ||
| Gegenes nostrodamus (Fabricius, 1793) | Morocco: Marrakech | He240 | JX971193 | - | JX989143 | - | |
| Prusiana prusias matinus (Fruhstorfer, 1911) 1 | Philippines: Leyte | He241 | ● | JX971194 | - | JX989144 | - |
| Prusiana prusias matinus (Fruhstorfer, 1911) 2 | Philippines: C. Palawan | He393 | ● | KX151520 | KX151579 | KX151552 | KX151634 |
| Zenonia zeno (Trimen, 1864) 1 | Cameroon: N. Cameroon | SZS-ZEN-001 | ● | KX151521 | KX151580 | KX151553 | KX151635 |
| Zenonia zeno (Trimen, 1864) 2 | Kenya: Nairobi | SZS-ZEN-002 | ● | - | KX151581 | KX151554 | - |
| Aeromachus stigmatus (Moore, 1878) | China: Yunnan, Hutiaoxia | He434 | ● | KX151522 | KX151582 | KX151555 | KX151636 |
| Ampittia virgata Leech, 1890 | China: Guangdong, Nanling | He008 | KX151523 | KX151583 | KX151556 | KX151637 | |
| Telicota augias | China: Guangdong, Yingde | He082 | JX971195 | JX989110 | JX989145 | KX151638 | |
| Potanthus trachala | China: Guangxi, Guiling | He346 | JX971196 | JX989111 | JX989146 | KX151639 | |
| Tagiades menaka | China: Guangdong, Yingde | He004 | JX971197 | JX989112 | JX989147 | KX151640 | |
| Daimio tethys | China: Guangxi, Maoershan | He384 | JX971198 | JX989113 | JX989148 | KX151641 | |
* GenBank accession numbers correspond to specimens in Warren et al. (2008).
DNA extraction, PCR amplification, and sequencing
Total genomic DNA was extracted from the thorax of specimens preserved in ethanol, or from one to three legs of dried specimens. The tissues were macerated in 500 μL Proteinase K solution (10 mM Tris HCl, 10 mM EDTA, 150 mM NaCl, and 0.5 mg/mL proteinase K), and incubated at 55°C for 2–3 h. The resulting solution was extracted once with phenol saturated with TE buffer (10 mM Tri-HCl [pH 8.0] and 1 mM EDTA), once with phenol/chloroform (1:1), and once with chloroform/isoamyl alcohol (24:1). The total DNA was precipitated by adding twice the volume of 100% ethanol and one-tenth the volume of 3 M sodium acetate to the supernatant, washed with 70% ethanol, dried, and then dissolved in 80–100 μL TE buffer. DNA from Pseudoborbo bevani, Iton semamora, Prusiana kuehni, and Zenonia zeno specimens was extracted from legs using the Qiagen DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany) following the manufacturer’s protocol for animal tissue.
Four target regions were amplified by PCR using the primers listed in Table 2. PCR reactions were performed in 25 μL volumes containing 2.5 μL 10×PCR buffer (2.0 mM MgCl2), 2 μL dNTPs (containing 2.5 mM of each dNTP), 1 μL of each primer (10 pmol/μL), 1 μL of template DNA, and 1.25 units Taq DNA polymerase (Takara Inc, Shiga, Japan). The amplification cycle was 95°C for 5 min, and for the 16S and 28S genes was followed by 35 cycles of 94°C for 30 sec, then 47°C (16S) or 50°C (28S) for 30 sec and 72°C for 1.5 min. For the COI-COII and EF-1α, the initial 95°C at 5 min was followed by 35 cycles of 94°C for 1 min, 46°C (COI-COII) or 55°C (EF-1α) for 1 min and 72°C for 2 min. All amplification cycles included a final extension period of 72°C for 7 min. Successful amplification was verified using agarose gel electrophoresis.
Table 2. Primers used for amplification and sequencing in this study.
| Gene | Primer | Sequence (5′–3′) | Reference |
|---|---|---|---|
| COI-COII | Gary | TAGGAATAATTTATGCMATAATAGC | Warren et al.[5] |
| Susan | TTGTTGTTCTAATARAAATCG | Warren et al.[5] | |
| 16S rRNA | LR-J-12887 | CCGGTTTGAGCTCAGATCA | Simon et al.[56] |
| LR-N-13398 | CGCCTGTTTATCAAAAACAT | Simon et al.[56] | |
| EF-1α | ef44 | GCYGARCGYGARCGTGGTATYAC | Monteiro and Pierce [57] |
| efrcM4 | ACAGCVACKGTYTGYCTCATRTC | Monteiro and Pierce[57] | |
| 28S rRNA | 28S-01 | GACTACCCCCTGAATTTAAGCAT | Kim et al.[58] |
| 28SR-01 | GACTCCTTGGTCCGTGTTTCAAG | Kim et al.[58] |
PCR products were purified with a Gel DNA purification kit (Takara Inc), and were directly sequenced with the same primers listed in Table 2, or cloned and then sequenced. For cloning, the purified PCR products were cloned into the pMD18-T vector (Takara Inc) using Escherichia coli TG-1 as the host. At least three positive clones were selected for sequencing to correct for PCR errors. Sequencing was performed using the ABI 3730 automated sequencer. DNA sequences were assembled and edited with SeqManII in the DNASTAR package (DNASTAR Inc, Wisconsin, USA) and checked manually. All sequences were deposited in GenBank, and the accession numbers for each sequence are listed in Table 1.
Data analyses
Alignments of the rRNA gene sequences were conducted with MAFFT (version 7) using separate gene partitions (16S and 28S) via the online sever (http://mafft.cbrc.jp/alignment/server/). We used the Q-INS-I strategy, which accounts for the secondary structure of the RNA and small data sets (with less than 200 sequences), and ‘1PAM/κ = 2’, which is recommended for aligning closely related DNA sequences and the offset was set at 0.1 when large gaps were not expected based on preliminary analyses [59–61]. Both the COI-COII (only one 3-bp gap) and EF-1α sequences were aligned using the Clustal X [62] with the default settings. All base frequencies and molecular character statistics were calculated using MEGA 6.0 [63]. Homogeneity of the base frequencies across taxa was tested using the Chi-square test implemented in PAUP* 4.0b10 [64]. The incongruence length difference (ILD) test [65] in PAUP* was conducted to evaluate the congruence of mitochondrial (COI-II and 16S) and nuclear (EF-1α and 28S) markers and determine if they could be analyzed together. Only taxa with sequence information for all four target regions were included in this analysis. Saturation for each gene and for the codon positions of COI, COII, and EF-1α were assessed using the substitution saturation test [66, 67] in the program DAMBE [68].
Phylogenetic trees were constructed using the maximum parsimony (MP), maximum Likelihood (ML), and Bayesian inference (BI) methods. MP analyses were conducted using TNT version 1.1 [69] with the following options: parsimony-informative characters were unordered and equally weighted, gaps were treated as missing data, searches heuristic using a “driven search” until the minimum length was hit ten times by means of a combination of TreeFusion, Ratchet, Tree Drifting, and Sectorial searches under default parameters [70]. Branch support was assessed using the bootstrap test [71] with 1000 replicates.
Prior to ML and BI analyses, the best-fit model of nucleotide substitution was selected using jModeltest 2.1.7 [72] for each gene region (COI (GTR+I+G), tRNAleu (HKY+I), COII (GTR+I+G), 16S (GTR+I+G), EF-1α (SYM+I+G), and 28S (GTR+I+G)), and by codon position for COI, COII and EF-1α (seven partitions: 1st+2nd (GTR+I+G) and 3rd codon positions (GTR+I+G) of the mitochondrial protein coding genes COI and COII together, same for the nuclear gene EF-1α (positions (1+2): SYM+I+G, position 3: GTR+I+G), the mitochondrial RNA genes tRNAleu and 16S, and also the nuclear 28S gene) under the Akaike Information Criterion [73].
ML analyses were carried out using RAxML version 8 [74] on a concatenated data set of all genes, with 1000 rapid bootstraps using both GTR+G and GTR+I+G. The topologies of the trees were consistent, and support values for the clades only differed slightly. Here, we have only presented the results from the analysis using the GTR+G model. Bayesian analyses were conducted using MrBayes 3.2.2 [75] using the best-fit model determined using the two above-mentioned schemes. Four simultaneous chains were run for 5×106 generations, and trees were sampled every 100 generations with the first 25% of sampled trees discarded as burn-in. The convergence of the analyses was determined with the program Tracer v1.6 [76] and Bayesian posterior probabilities were used to evaluate branch support. Both MrBayes and RAXML runs were carried out on the online CIPRES Science Gateway resource [77].
Bootstrap support values (BP, for MP; BS, for ML) and posterior probabilities (PP for BI) were used to assess the robustness of the results. In order to discuss the results, we have delimited the support values as strongly, moderately, and weakly supported. In the MP and ML analyses, we regard clades with bootstrap values of 69 and below to be weakly supported, 70–89 to be moderately supported, and 90 and above to be strongly supported. In the BI analyses, we considered clades with posterior probabilities of 0.79 and below to be weakly supported, those with probabilities of 0.80–0.94 to be moderately supported, and those with probabilities of 0.95 and above to be strongly supported.
Nomenclature Acts
The electronic edition of this article conforms to the requirements of the amended International Code of Zoological Nomenclature, and hence the new names contained herein are available under that Code from the electronic edition of this article. This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix“http://zoobank.org/”. The LSID for this publication is: urn:lsid:zoobank.org:pub: 89BFF498-46F3-4007-87A9-F826290724C7. The electronic edition of this work was published in a journal with an ISSN, and has been archived and is available from the following digital repositories: PubMed Central, LOCKSS.
Results
Sequence Characteristics
From a total of 71 samples, we obtained 60, 70, 58, and 69 sequences for COI-COII, 16S, EF-1α and 28S sequences, respectively. In addition, we included an additional four sequences from two species from GenBank (Table 1).
The COI-COII (929 bp) region was composed of 703 bp of the COI gene, the entire 70 bp of the intervening tRNAleu (including one 3-bp gap since Pelopidas agna has a three-base-pair insertion), and 156 bp of the COII gene. Due to several small indels in some species, the 16S and 28S sequence lengths varied between 512–520 bp and 825–840 bp, respectively. In total, the alignment of the four regions consisted of a total of 3380 bp (929, 531, 1066, and 854 bp of the COI-COII, 16S, EF-1α and 28S genes, respectively), of which 975 positions were variable, and 747 were parsimony-informative. We failed to obtain sequences for some specimens, and the missing data were designated as a ‘?’ in the alignment. Within the ingroup, average base composition was T = 30.4%, C = 21.1%, A = 28.8%, and G = 19.7%. The Chi-square test revealed no significant base composition heterogeneity across samples employed (df = 150, P = 1.00). For all three codon positions of COII and EF-1α as well as for the three regions tRNAleu, 16S, and 28S, the value of the substitution saturation index (Iss) was much smaller than the critical value (Iss. c), assuming either a symmetrical topology or an asymmetrical topology. These results show that these data subsets are unlikely to have reached saturation. For COI, only the third codon position reveals that Iss is larger than Iss.c, assuming an asymmetrical topology. Therefore, there is little substitution saturation in our sequence data.
The ILD test revealed no significant incongruence between the two data sets (mtDNA COI-II and 16S vs. rDNA EF-1α and 28S, P = 0.19), indicating that the sequences could be combined in the phylogenetic reconstruction.
Phylogenetic analyses
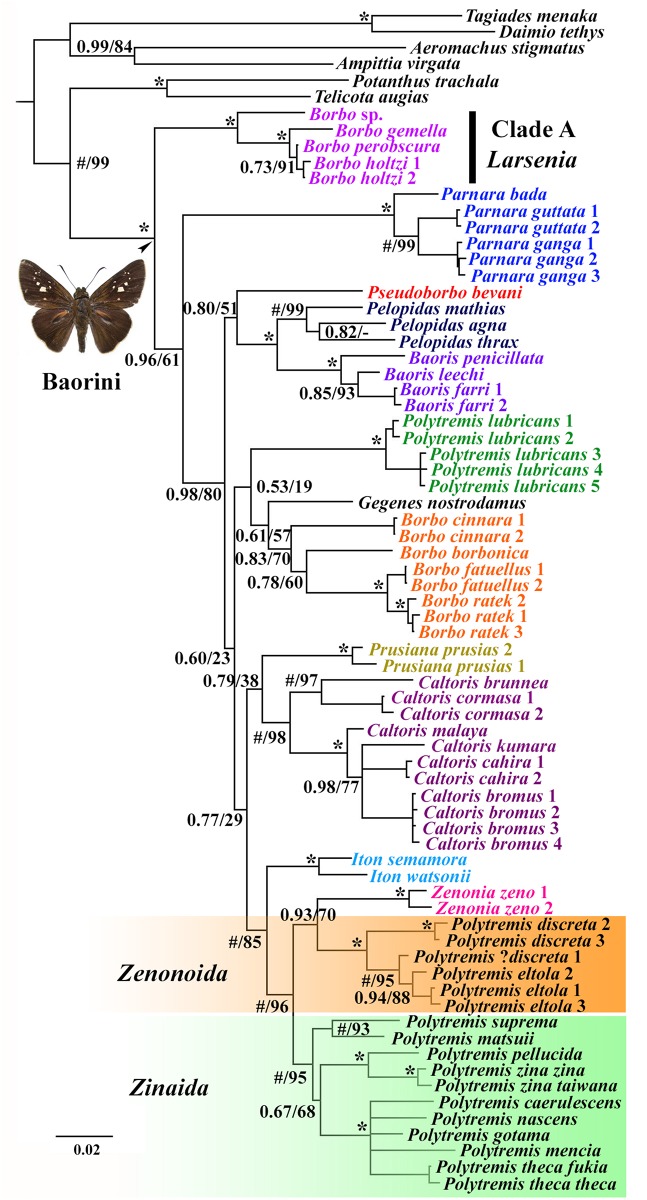
The three concatenated analyses (BI, ML, and MP) revealed similar topologies, differing mainly in branch support (Fig 1, S1 Fig); however, the monophyly of the tribe Baorini is strongly supported in all methods (PP = 1.00, BS = 100, BP = 100). Within the tribe, although support for some basal clades is low, the monophyly of the seven traditionally established genera (Parnara, Pelopidas, Baoris, Caltoris, Prusiana, Iton, and Zenonia) is strongly supported in all phylogenetic analyses. On the other hand, contrary to conventional taxonomy, the genera Borbo and Polytremis are not monophyletic. Members of Borbo did not form a cluster, but instead formed three clades—Clade A, the Borbo clade, and the Pseudoborbo clade (which only included the species P. bevani, which was previously placed within Borbo). Clade A is a strongly supported monophyletic group (PP = 1.00, BS = 100, BP = 100) that consists of the following species: B. sp., B. gemella, B. holtzi, and B. perobscura and is, by this analysis, sister to the other remaining Baorini. We designated the clade to have a new genus status, Larsenia gen. nov. The genus Pseudoborbo has a controversial taxonomic status and according to all of the methods is sister to Pelopidas and Baoris, which is moderately supported in BI analysis (PP = 0.80). We determined that Pelopidas is sister to Baoris (PP = 1.00, BS = 100, BP = 97). The genus Borbo, excluding Larsenia and Pseudoborbo, was moderately supported in the BI and ML analyses (PP = 0.83, BS = 70). For the genus Polytremis, all members analyzed here except for P. lubricans, together with the genus Zenonia, formed a strongly supported monophyletic clade (PP = 1.00, BS = 96, BP = 91), which is sister to the genus Iton (PP = 1.00, BS = 85, BP = 65). Within the clade, P. eltola and P. discreta formed a strongly supported monophyletic group (PP = 1.00, BS = 100, BP = 100), which is sister to the genus Zenonia, with moderate support (PP = 0.93, BS = 70, BP = 66). We recognized the P. eltola and P. discrete clade to have a new genus status. Other species of Polytremis sensu Evans [6] including P. nascens (the type species of Zinaida) appeared to form a monophyletic group with strong support (PP = 1.00, BS = 95, BP = 81). P. lubricans, the type species of Polytremis, formed a separate clade from other Polytremis sensu Evans [6] species. Consequently, we propose that the genus Zinaida Evans, 1937 be reinstated. Based on highly supported monophyly of these genera, together with morphological characters, herein we have designated the following fourteen clades as genera: Larsenia gen. nov., Parnara, Gegenes, Borbo, Pelopidas, Baoris, Caltoris, Pseudoborbo, Polytremis, Prusiana, Iton, Zenonia, Zenonoida gen. nov., and Zinaida.
Fig 1. Majority-rule consensus tree from the Bayesian analysis (BI) of the concatenated COI-COII, 16S, EF-1α, and 28S sequences.
Values at nodes represent the posterior probabilities (PP) of BI and the bootstrap support (BS) values of the maximum likelihood (ML) analysis, respectively. Asterisks indicate branches supported 100% by both PP and BS. “#” indicates that PP = 100. Colors highlight recognized genera.
Discussion
Although the basal relationships within Baorini were poorly resolved, proximal clades were strongly supported across all analyses. Of the 14 major lineages we defined here as genera, eight (Parnara, Gegenes, Pelopidas, Baoris, Caltoris, Prusiana, Iton, and Zenonia) are concordant with traditionally established genera, while the others are inconsistent with the previously described genera.
Larsenia Chiba, Fan & Sáfián gen. nov.
urn:lsid:zoobank.org:act:E3CA9226-4199-48BC-9D92-7A1A3F293E49
Type species. Hesperia holtzi Plötz, 1883 Male [78]
Diagnosis. Length of antennae less than half that of costa, with apiculus small and bent. Third segment of palpi short and bent slightly forward. New genus differing from other genera of tribe Baorini by harboring bifid uncus and developed socius.
Etymology. The genus is named after the late Dr. Torben Larsen, the leading expert on African butterfly taxonomy, who was a member of this project. He passed away suddenly in May 2015 and therefore did not see the final results of this research; with respect, we would like to name the new genus after him.
In our analyses, four species currently treated as members of the genus Borbo, namely B. gemella, B. perobscura, B. holtzi, and an unidentified species formed a distinct group that is basal and sister to the rest of Baorini. Based on these results, we established Larsenia as a new genus. Before describing Borbo, Evans [19] divided brown skippers into Baoris and Pelopidas. The three species above were all assigned to Pelopidas. After describing Borbo, he divided members into two groups: one with smooth mid-tibia and the other with spined mid-tibia [79]. Both B. perobscura and B. holtzii have spined mid-tibia but not B. gemella. These three species are autapomorphous with respect to their male genitalia, with developed socius. Although it is beyond the scope of this study, a detailed description of the new genus is in preparation pending further research determining which members of the African Borbo that were not included in this study should be assigned to the new genus.
Pseudoborbo Lee, 1966 confirmed status
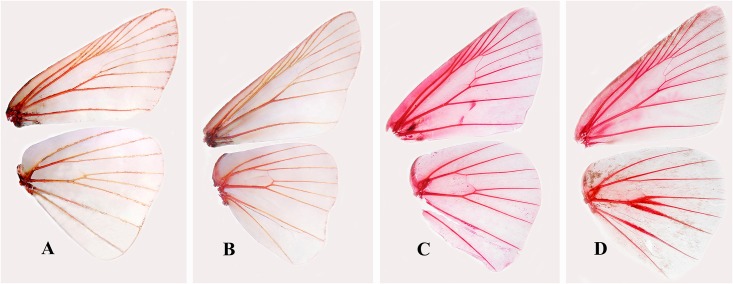
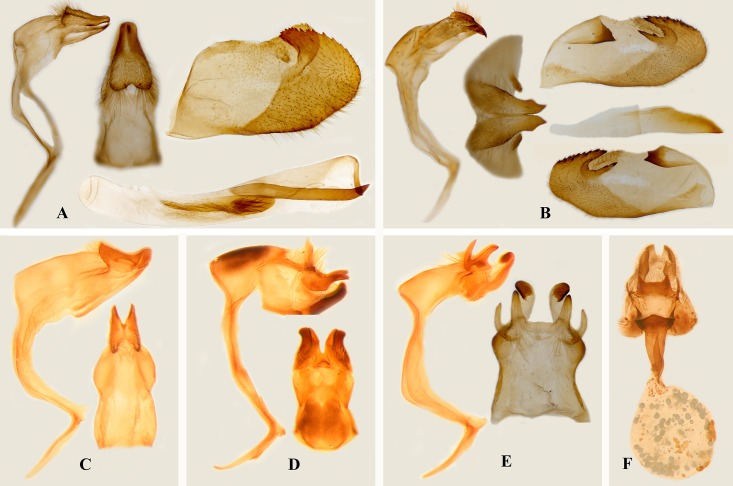
Our morphological study shows that the type species of both genera are greatly different in wing venation and male genitalia. Specifically regarding wing venation (Fig 2A and 2B) on the forewing, the origin of M3 is branched midway between M2 and CuA1 while on the hindwing, the origin of vein CuA1 is distinctly closer to M3 than to CuA2 in Pseudoborbo. Simultaneously, on the forewing, the origin of the vein M3 is distinctly closer to M2 than to CuA1, and on the hindwing, the origin of vein CuA1 is branched midway between M2 and CuA2 in Borbo. In the male genitalia (Fig 3A and 3B) of Pseudoborbo, the uncus not separated at tip, while the gnathos is developed and nearly reaches the tip of uncus; the valva lacks transtilla, and the aedeagus is characterized by a thick, long spine and an uneven cornuti. However, in Borbo, the uncus is bifid and bent ventrally at the tip, the gnathos is far from reaching to tip of uncus, the valva harbors transtilla, and the aedeagus is simple without cornuti. Eight species of traditional Borbo, including the type species Hesperia borbonica Boisduval, 1833, as well as the type and sole species of Pseudoborbo, were analyzed in our molecular study. The results revealed that Pseudoborbo bevani is located separately from the two clades of the other members of Borbo. The relationship of P. bevani to the sister clades Pelopidas and Baoris is closer than its relationship to Borbo. Morphologically, Pseudoborbo is also much more similar to Pelopidas and Baoris, especially with regard to the male genitalia.
Fig 2. Wing venation of Baorini.
(A) Pseudoborbo bevani (Moore, 1878); (B) Borbo borbonica (Boisduval, 1833); (C) Zinaida nascens Leech, 1893; (D) Polytremis lubricans (Herrich-Schäffer, 1869).
Fig 3. Genitalia of Baorini (A-E, male; F, female).
(A) Pseudoborbo bevani (Moore, 1878); (B) Borbo borbonica (Boisduval, 1833); (C) Zinaida nascens Leech, 1893; (D) Zenonoida elota (Hewitson, 1869); (E & F) Polytremis lubricans (Herrich-Schäffer, 1869).
Based on molecular evidence as well as morphological characters, we propose that the genus Pseudoborbo Lee, 1966 is valid.
Borbo Evans, 1949
Currently, the genus Borbo consists of five Indo-Australian and 18 African species [22]. These species vary extensively in the morphology of the male genitalia, and, therefore, it is necessary to divide them into several groups according to their characteristic genitalia structures [78]. Our analyses clearly indicate that the eight species analyzed here are polyphyletic. Although Borbo, excluding Clade A and Pseudoborbo, forms a moderately supported clade, the relationship among the three sublineages (B. cinnara, B. borbonica, and B. fatuellus+B. ratek) is unclear. We did find that B. fatuellus is sister to B. ratek and each sublineage differs according to male genitalia morphology. Evans [19] determined that Baoris included B. ratek and B. fatuellus and Pelopidas included P. borbonica. Again, mid-tibial characteristics do not appear to be informative, since B. ratek and B. fatuellus have smooth mid-tibia while P. borbonica has a spined mid-tibia. However, since the sample size is not sufficient and the support for the Borbo clade is relatively low (PP = 0.83, B = 70), additional species sampling and gene sequencing are necessary to resolve the phylogeny of Borbo in the future.
Prusiana Evans, 1937 confirmed status
Prusiana, a small genus with only three species, is obviously a monophyletic group with a synapomorphy in which the males have a brand at the base of the space M1 on the hindwing [6, 22]. Nevertheless, the taxonomic position of Prusiana has been controversial, as mentioned above. Based on morphology rather than molecular evidence, Warren et al. included Prusiana in Baorini [2]. The molecular phylogeny presented here clearly indicates that Prusiana is a member of Baorini and that its sister-group relation to Catoris is weakly supported in the BI phylogeny (PP = 0.79, BS = 38).
Polytremis Mabille, 1904
In our present analyses (Fig 1, S1 Fig), twelve species of Polytremis, sensu Evans [6], were not determined to be a monophyletic group but were split into three strongly supported and very distant clades, of which the clade with the type species P. lubricans harbors five representative individuals from China and Malaysia. Therefore, we now recognize Polytremis Mabille, 1904 to be a monotypic genus (type species Goniloba lubricans Herrich-Schäffer). Morphologically, the genus is distinguishable based on the male genitalia (where the lateral process of the uncus, which is divided and horn-like, is clearly separated at its base (Fig 3E)) and the female genitalia (with sclerotized fingerlike projections between the anterior and posterior lamella (Fig 3F)).
Zinaida Evans, 1937 reinstated status
Our morphological study shows that Zinaida is quite different from Polytremis in wing venation and genitalia. Unique characteristics in wing venation in Zinaida (Fig 2C) include the forewing, in which the origin of R1 follows that of CuA2 and is located nearly midway between CuA1 and CuA2, and the hindwing, in which the origin of Rs is before that of CuA2. However, in Polytremis (Fig 2D), the origin of vein R1 is opposite CuA2 and the origin of Rs is opposite CuA2. In addition, males of most species have a stigma in space CuA2 on the upper side of the forewing, and in Polytremis males, the hindwing expanded at middle A, basal M3, CuA1, and CuA2. The male genitalia (Fig 3C and 3E) in Zinaida are unique since the uncus is V-shaped, projects at the left and right and is attached at its base, while the gnathos is straight and has an attached uncus. In Polytremis, the uncus is completely separated, and the gnathos is elbow-shaped and located far from the uncus.
Of the 18 species included in Polytremis sensu Evans [6], 12 species, including the type species of both Polytremis and Zinaida, were analyzed in our study. Three clades were defined using all methods. One clade consisted of five individuals of P. lubricans; P. discreta and P. eltola and formed a strongly supported clade (PP = 1.00, BS = 100, BP = 100), which is sister to Zenonia with moderate support (PP = 0.93, BS = 70, BP = 66). The other samples, including P. nascens, formed a strongly supported monophyletic group. Our study thus suggests that the monophyly of Polytremis presented by Evans should be rejected and the genus Zinaida reinstated. Our result contradicts that of Jiang et al. [55]. In their analysis, the monophyly of the genus Polytremis is weekly supported in ML analysis (BS = 52 on the concatenated data; and BS = 73 on COI sequence), even though they claim that the monophyly is strongly supported. On the other hand, the clade including P. lubricans, P. eltola, and P. discreta is strongly supported (BS = 99 for both the COI sequence and combined data set). The DNA markers and samples (ingroup and outgroup) selected are essentially why the results are different. First, they used one mitochondrial gene COI (490 bp) and two nuclear genes (the D3 region of 28S rRNA gene and the V4 and V7 regions of the 18S rRNA gene, in total 1048 bp). The trees derived from the separate analyses of COI as well as the concatenated sequences (COI+rDNA) have roughly similar topologies; however, we determined that the COI gene contributed more to the phylogenetic signal, and combined analyses yielded lower resolution. This is because the two slowly evolving rDNA genes are usually used in higher taxonomic levels studies [80, 81]. Additionally, different genes are phylogenetically informative at various taxonomic levels [82]. Therefore, choosing suitable genetic markers is a key element in reconstructing improved molecular phylogenies. We chose COI-COII and 16S rRNA from mitochondrial DNA, rDNA EF-1α, and 28S rRNA as molecular markers. All of these markers have been previously used successfully to elucidate the relationships among many groups within the Lepidoptera, including at the levels of genera, tribe, and subfamily [5, 57, 82–89]. Second, 15 Chinese species were used as the ingroup and four Baorine genera as the outgroup. Despite the relatively large number of samples included in the ingroup, the result of molecular phylogeny analysis is not ideal due to the unsuitable outgroup. Since relationships among genera in Baorini are unclear and Polytremis is a morphologically diverse group, all available genera should be included as the outgroup in analyses instead of only four. Our study included nearly all the major genera within Baorini all over the world. In order to test previous analyses, our study included 12 species, allowing for a broad representation of lineages within Polytremis, and containing more than three individuals for P. lubricans, P. eltola, and P. discreta. Although our species sampling is less extensive than in previous studies, the present trees (Fig 1, S1 Fig) are better resolved than those from Jiang et al. [55] and reveal that that Polytremis sensu Evans [6] is not a monophyletic group, P. eltola as sister group to P. discreta rather than to P. lubricans.
Zenonoida Fan and Chiba gen. nov.
urn:lsid:zoobank.org:act:8CA5AEF0-E81D-4F74-8CA1-F62C407A5FBA
Type species. Hesperia eltola Hewitson, 1869 (Male)
Diagnosis. New genus superficially similar to Polytremis Mabille, 1904 and Zinaida Evans, 1937, though distinguishable from other two genera as follows: palpi characterized by short third segment, stout and barely protruding; forewing cell spots conjoined or upper cell spot absent. Uncus with central-basal area membranous; gnathos elbow-shaped, sclerotized except for a narrow distal membranous band.
Etymology. The scientific name, Zenonoida is derived from the genus Zenonia since the new genus is significantly similar to Zenonia with respect to the male genitalia.
In our analyses, P. eltola and P. discreta were assigned to Polytremis sensu Evans [6], which is distantly located from both Polytremis and Zinaida. Thus, we describe Zenonoida as a new genus, and move P. elota and P. discreta from Polytremis sensu Evans [6] to the new genus: Z. elota com. nov., Z. discreta comb. nov.
Supporting Information
The numbers indicate bootstrap values. Colors highlight recognized genera.
(TIF)
Acknowledgments
We are grateful to the late Dr. Torben B. Larsen for discussion on African species. Had it not been for him, this project would not be as substantial as it currently is. We also thank Dr. Houshuai Wang (SCAU) for valuable assistance regarding the implementation of molecular methods and data analysis. The following people and institution provided materials for the study: Michael Yeh (Ipoh, Malaysia), Chia-Ying Yen (Taipei, Taiwan), Alexander Monastyrskii (Hanoi, Vietnam), and Kyushu University Museum (Fukuoka, Japan).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Natural Science Foundation of China (grant numbers:31172136 and 31471984), XLF received the funding. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bridges CA. Catalogue of the Family-Group, Genus-Group and Species-Group Names of the Hesperiidae (Lepidoptera) of the World. Urbana: Published by Author; 1994. [Google Scholar]
- 2.Warren AD, Ogawa JR, Brower AVZ. Revised classification of the family Hesperiidae (Lepidoptera: Hesperioidea) based on combined molecular and morphological data. Syst Entomol. 2009; 34: 467–525. [Google Scholar]
- 3.de Jong R, Vane-Wright RI, Ackery PR. The higher classification of butterflies (Lepidoptera): problems and prospects. Entomol Scan. 1996; 27: 65–101. [Google Scholar]
- 4.Ackery PR, de Jong R, Vane-Wright RI. The butterflies: Hedyloidea, Hesperioidea and Papilionoidea In: Kristensen NP, editor. Lepidoptera, Moths and Butterflies. 1. Evolution, Systematics, and Biogeography. Handbook of Zoology. 4 (35), Lepidoptera. Berlin: de Gruyter; 1999. pp. 263–300. [Google Scholar]
- 5.Warren AD, Ogawa JR, Brower AVZ. Phylogeneticre lationships of subfamilies and circumscription of tribes in thefamily Hesperiidae (Lepidoptera: Hesperioidea). Cladistics. 2008; 24: 642–676. [Google Scholar]
- 6.Evans WH, A Catalogue of the Hesperiidae from Europe, Asia, and Australia in the British Museum (Natural History). London: The British Museum; 1949. [Google Scholar]
- 7.Doherty W. A list of butterflies taken in Kumaon. J Asiatic Soc Beng. 1886; 55: 103–140. [Google Scholar]
- 8.Bell TR. The common butterflies of the plains of India (including those met with in the hill stations of the Bombay presidency). J Bomb Nat Hist Soc. 1920–1927; 27: 26–32 (part 26, 1920), 211–227 (part 27, 1920), 431–447 (part 27, 1921), 778–793 (part 28, 1921); 28: 429–455 (part 29, 1923), 703–717 (part 30, 1923), 921–946 (part 31, 1923); 30: 132–150 (part 32, 1925), 285–305 (part 33, 1925), 561–586 (part 34, 1925), 822–837 (part 35, 1925); 31: 323–351 (part 36, 1926), 655–686 (part 37, 1926), 951–974 (part 38, 1927). [Google Scholar]
- 9.Eliot JN. [descriptions and revisions] In: Corbet AS, Pendlebury HM. The Butterflies of the Malay Peninsula, 3rdedn Kuala Lumpur: Malayan Nature Society; 1978. [Google Scholar]
- 10.Eloit JN. [descriptions and revisions] In: Corbet A.S., Pendlebury H.M. The Butterflies of the Malay Peninsula, 4rdedn Kuala Lumpur: Malayan Nature Society; 1992. [Google Scholar]
- 11.Chou I. (Ed.) Monographia Rhopalocerorum Sinensium. Henan, China: Henan Scientific and Technological Publishing House; 1994. [Google Scholar]
- 12.Chou I. Classification and Identification of Chinese Butterflies. Henan, China: Henan Scientific and Technological Publishing House; 1998. [Google Scholar]
- 13.International Commission on Zoological Nomenclature (ICZN). International Code of Zoological Nomenclature, 4th edn London: The International Trust for Zoological Nomenclature; 1999. [Google Scholar]
- 14.Mabille P. Die palaearctica Tagfalter. Grypocera In: Seitz A, editor. Grossschmett Erde 1. 1909. pp. 329–354. [Google Scholar]
- 15.Aurivillius C. Die afrikanischen Tagfalter In: Seitz A, editor. Grossschmett. Erde 13. 1925. pp. 11–613. [Google Scholar]
- 16.Seitz A. Die Indo-Australische Tagfalter. Grypocera Grossschmetterlinge der Erde 9. Stuttgart: Alfred Kernen; 1927. [Google Scholar]
- 17.Mabille P. Lepidoptera Rhopalocera. Family Hesperiidae. Gen Insecorum. 1903–1904; 17a: 1–78 (1903); 17b: 79–142 (1904); 17c: 143–182(1904); 17d: 183–210 (pls. 1904).
- 18.Evans WH. The identification of Indian butterflies. Second edition revised Madras: Bombay Nat Hist Soc. 1932. [Google Scholar]
- 19.Evans WH. A catalogue of the Arican Hesperiidae. London: The British Museum; 1937. [Google Scholar]
- 20.de Jong R. Some aspects of the biogeography of the Hesperiidae (Lepidoptera, Rhopalocera) of Sulawesi In: Knight WJ, Holloway JD, editors. Insects and the Rain Forests of South East Asia (Wallacea). R Entomol Soc Lond; 1990; 35–42. [Google Scholar]
- 21.Maruyama K. Butterflies of Borneo 2(2), Hesperiidae. Tokyo: Tobishima Corporation; 1991. [Google Scholar]
- 22.de Jong R, Treadaway CG. Hesperiidae of Philippines Islands In: Bauer E, Frankenbach T, editors. Butterflies of the World, Supplement 15. Keltern: Goecke & Elvers; 2007. pp. 1–72. [Google Scholar]
- 23.Watson EY. A proposed classification of the Hesperiidae, with a revision of the genera. Proc zool Soc Lond. 1893; 1: 3–132. [Google Scholar]
- 24.Watson EY. A key to the Asiatic genera of the Hesperiidae. J Bombay Nat Hist Soc. 1895; 9(4): 411–437. [Google Scholar]
- 25.Aitkin EH, Comber E. A list of Butterflies of Konkan. J Bombay Nat Hist Soc. 1903; 15: 42–55. [Google Scholar]
- 26.Evans WH. Lepidoptera-Rhopalocera obtained by Mme J. Wisser-Hooft of the Hague (Holland) during an exploration of the unknown country in the western Karakorum, NW India 1925. Tijdschr Entomol. 1927; 70: 158–162. [Google Scholar]
- 27.de Nicéville L. Descriptions of some new Indian Rhopalocera. J Asia Soc Bengal, Part 2–Nat Sci. 1885; 54: 117–124. [Google Scholar]
- 28.de Nicéville L. On new and little-known butterflies from the Indian region, with description of three new genera of Hesperiidae. J. Bombay nat Hist Soc. 1890; 5(3): 199–225. [Google Scholar]
- 29.Swinhoe C. On the Lepidoptera of Bombay and the Deccan. Proc zool Soc Lond. 1885; 53(2): 287–307. [Google Scholar]
- 30.Swinhoe C. On the Lepidoptera of Mhow, in Central India. Zoological Society of London. 1886. [Google Scholar]
- 31.Moore F. On the lepidopterous insects of Bengal. Proc zool Soc Lond. 1866; 1865(3): 755–822. [Google Scholar]
- 32.Elwes HJ. On butterflies collected by Mr. W. Doherty in the Naga and Karen Hills and in Perak, Part II. Proc zool Soc Lond. 1892; 1892(4): 617–664. [Google Scholar]
- 33.Davidson J, Aitken E H. Notes on the larvae and pupae of some butterflies of the Bombay Presidency. J. Bomb. Nat. Hist. Soc. 1890; 5: 260–278, 349–375. [Google Scholar]
- 34.Watson EY. Hesperiidae indicae. Vest and Co; Madras: 1891. [Google Scholar]
- 35.Betham JA. The butterflies of the Central Provinces. Part VI. (Hesperiidae). J Bombay nat Hist. Soc. 1893; (4): 425–429. [Google Scholar]
- 36.Elwes HJ, Edwards J. A revision of the Oriental Hesperiidae. Trans zool Soc Lond. 1897; 14(4): 101–324. [Google Scholar]
- 37.Hannyngton F. The butterflies of Kumaon. J Bombay Nat Hist Soc. 1910; 20: 130–142. [Google Scholar]
- 38.Evans WH. A List of the Butterflies of the Palni Hills with descriptions of two new species. J. Bombay Nat Hist Soc. 1910; 20(2): 380–390. [Google Scholar]
- 39.Swinhoe C. A list of the Lepidoptera of the Khasia Hills. Part I. Trans. ent. Soc. Lond. 1893; 41(3): 267–330. [Google Scholar]
- 40.Swinhoe C. In: Moore F. Lepidoptera Indica. London: L. Reeve & Co; 1912–1913. [Google Scholar]
- 41.Shirôzu T. Butterflies of Formosa in Colour. Osaka: Hoikusha; 1960. [Google Scholar]
- 42.Pinratana A, Eliot JN. Butterflies in Thailand, Vol. 5 Hesperiidae. Bangkok: Viratham Press; 1985. [Google Scholar]
- 43.Bascombe MJ, Johnston G, Bascombe FS. The butterflies of Hong Kong. Great Britain: Harcourt Brace & Company; 1999. [Google Scholar]
- 44.Chiba H. Sipper of Hainan (Lepidoptera: Hesperiidae). Report on Insect Inventory Project in Tropical Asia (TAIIV). 2008; 337–344.
- 45.Lee CL. On the Chinese species of Borbo Evans (Lep. Hesperiidae). Acta Zool Sinica. 1966; 18: 221–231. [Google Scholar]
- 46.Parsons MJ. The Butterflies of Papua New Guinea Their Systematics and Biology. London: Academic Press; 1999. [Google Scholar]
- 47.Vane-Wright RI, de Jong R. The butterflies of Sulawesi: annotated checklist for a critical island fauna. Zool Verh. 2003; 343: 1–267. [Google Scholar]
- 48.Lee CL, Zhu BY. Atlas of Chinese butterflies. Shanghai: Shanghai Far East Publishers; 1992. [Google Scholar]
- 49.Osada S, Umura Y, Uehara J. An Illustrated Checklist of the Butterflies of Laos P D R. Tokyo: Mokuyo-sha; 1999. [Google Scholar]
- 50.Braby MF. Butterflies of Australia: Their Identification, Biology and Distribution. Melbourne: CSIRO Publishing; 2000. [Google Scholar]
- 51.Huang BK. Fauna of Insects in Fujian Province of China. Vol. 4 Fuzhou: Fujian Scientific and Technological Publishing House; 2001. [Google Scholar]
- 52.Yuan F, Yuan XQ, Xue GX. Fauna Sinica (Insecta: Lepidoptera: Hesperiidae). China: Science Press; 2015. [Google Scholar]
- 53.Dodo YT, Saigusa T, Chiba H, Nishiyama T, Ishii M, Yagi T, et al. Molecular phylogeny of Japanese skippers (Lepidoptera, Hesperiidae) based on mitochondrial ND5 and COI gene sequences. Trans Lep Soc Jap. 2008; 59: 29–41. [Google Scholar]
- 54.Yuan XQ, Gao K, Yuan F, Wang P, Zhang YL. Phylogenetic relationships of subfamilies in the family Hesperiidae (Lepidoptera: Hesperioidea) from China. Scientific reports. 2015; 5: 11140 10.1038/srep11140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiang W, Zhu JQ, Xiao CS, Li Y, Yang Y, Yu WD. Molecular Phylogeny of the Butterfly Genus Polytremis (Hesperiidae, Hesperiinae, Baorini) in China. PLoS One. 2013; 8(12): e84098 10.1371/journal.pone.0084098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simon C, Frati F, Beckenbach A, Crespi B, Liu H, Flook P. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann Entomol Soc Am. 1994; 87: 651–701. [Google Scholar]
- 57.Monteiro A, Pierce NE. Phylogeny of Bicyclus (Lepidoptera: Nymphalidae) Inferred from COI, COII, and EF-1a Gene Sequences. Mol Phy Evol. 2001; 1–18. [DOI] [PubMed] [Google Scholar]
- 58.Kim CG, Zhou HZ, Imura Y, Tominaga O, Su ZH, Osawa S. Pattern of morphological diversification of the Leptocarabus ground beetles as deduced from mitochondrial ND5 gene and nuclear 28S rDNA sequences. Mol Biol Evol. 2000; 17: 137–145. [DOI] [PubMed] [Google Scholar]
- 59.Katoh K, Kuma K, Toh H, Miyata T. MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Res. 2005; 33(2): 511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Katoh K, Misawa K, Kuma K, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002; 30(14): 3059–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Katoh K, Toh H. Recent developments in the MAFFT multiple sequence alignment program. Brief Bioinform. 2008; 9: 286–298. 10.1093/bib/bbn013 [DOI] [PubMed] [Google Scholar]
- 62.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The Clustal X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997; 24: 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013; 30: 2725–2729. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Swofford DL. PAUP * 4.0: Phylogenetic Analysis Using Parsimony and Other Methods (Software). Sunderland, Massachusetts: Sinauer Associates; 2003. [Google Scholar]
- 65.Farris JS, Källersjö M, Kluge AG, Bult C. Testing significance of incongruence. Cladistics. 1995; 10: 315–319. [Google Scholar]
- 66.Xia X, Xie Z, Salemi M, Chen L, Wang Y. An index of substitution saturation and its application. Mol Phylogenet Evol. 2003; 26: 1–7. [DOI] [PubMed] [Google Scholar]
- 67.Xia X, Lemey P. Assessing substitution saturation with DAMBE In: Lemey P, Salemi M, Vandamme AM, editors. The Phylogenetic Handbook: A PracticalApproach to DNA and Protein Phylogeny, second ed Cambridge: Cambridge University Press; 2009. pp. 615–630. [Google Scholar]
- 68.Xia X, Xie Z. DAMBE: data analysis in molecular biology and evolution. J Hered. 2001; 92: 371–373. [DOI] [PubMed] [Google Scholar]
- 69.Goloboff PA, Farris JS, Nixon KC. TNT, a free program for phylogenetic analysis. Cladistics. 2008; 24: 774–786. [Google Scholar]
- 70.Goloboff PA. Analyzing large data sets in reasonable times: solutions for composite optima. Cladistics. 1999; 15: 415–428. [DOI] [PubMed] [Google Scholar]
- 71.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 1985; 39: 783–791. [DOI] [PubMed] [Google Scholar]
- 72.Darriba D, Taboada GL, Doalla R, Posada D. jModelTest 2: more models, new heuristics and paralles computing. Nature Methods. 2012; 9(8): 772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Akaike H. A new look at the statistical model identification. IEEE Trans. Automat Control. 1974; 19: 716–723. [Google Scholar]
- 74.Stamatakis A. RAxML Version 8: A tool for Phylogenetic Analysis and Post-Analysis of Large Phylogenies. Bioinformatics. 2014; 10: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ronquist F, Teslenko M, van der Mark P, Ayres D L, Darling A, Hohna S, et al. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst Biol. 2012; 61: 539–542. 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rambaut A, Suchard M A, Xie D, Drummond A J. Tracer v1.6. Available from http://beast.bio.ed.ac.uk/Tracer. 2014.
- 77.Miller MA, Pfeiffer W, Schwartz T. Creating the CIPRES Science Gateway for Inference of Large Phylogenetic Trees. In: SC10 Workshop on Gateway Computing Environments (GCE10). 2010.
- 78.Larsen TB. Two new species in the African skipper genera Borbo (Hesperiinae, Baorini) and Platylesches (Hesperiinae, incertae sedis).Trop Lepid Res. 2013; 23(2): 92–98. [Google Scholar]
- 79.Evans WH. Revisional notes on African Hesperiidae. Ann Mag Nat hist. 1946; (11)13: 641–648. [Google Scholar]
- 80.Caterino MS, Cho S, Sperling FAH. The current state of insect molecular systematics: a thriving tower of Babel. Annu Rev of Entomol. 2000; 45: 1–54. [DOI] [PubMed] [Google Scholar]
- 81.Gillespie JJ, Munro JB, Herat JM, Yoder MJ, Owen AK, Carmichael AE. A Secondary Structural Model of the 28S rRNA Expansion Segments D2 andD3 for Chalcidoid Wasps (Hymenoptera: Chalcidoidea). Mol Biol Evol. 2005; 22(7): 1593–608. [DOI] [PubMed] [Google Scholar]
- 82.Wahlberg N, Zimmermann M. Pattern of phylogenetic relationships among members of the tribe Melitaeini (Lepidoptera: Nymphalidae) inferred from mitochondrial DNA sequences. Cladistics. 2000; 16: 347–363. [DOI] [PubMed] [Google Scholar]
- 83.Niehuis O, Yen SH, Naumann CM, Misof B. Higher phylogeny of zygaenid moths (Insecta: Lepidoptera) inferred from nuclear and mitochondrial sequence data and the evolution of larval cuticular cavities for chemical defence. Mol Phylogenet Evol. 2006; 39: 812–829. [DOI] [PubMed] [Google Scholar]
- 84.Nazari V, Zakharov EV, Sperling FAH. Phylogeny, historical biogeography, and taxonomic ranking of Parnassiinae (Lepidoptera, Papilionidae) based on morphology and seven genes. Mol Phylogenet Evol. 2007; 42: 131–156. [DOI] [PubMed] [Google Scholar]
- 85.Kim MI, Wan XL, Kim MJ, Jeong HC, Ahn NH, Kim KG, et al. Phylogenetic Relationships of True Butterflies (Lepidoptera: Papilionoidea) Inferred from COI,16S rRNA and EF-1α Sequences. Mol Cell. 2010; 30: 409–425. [DOI] [PubMed] [Google Scholar]
- 86.Zaspel JM, Weller SJ, Wardwell CT, Zahiri R, Wahlberg N. Phylogeny and Evolution of Pharmacophagy in Tiger Moths (Lepidoptera: Erebidae: Arctiinae). PLoS One. 2014; 9: e101975 10.1371/journal.pone.0101975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brower AVZ, Freitas AVL, Lee MM, Silva-Brandão KL, Whinnett A, Willmott KR. Phylogenetic relationships among the Ithomiini (Lepidoptera: Nymphalidae) inferred from one mitochondrial and two nuclear gene regions. Syst Entomol. 2006; 31: 288–301. [Google Scholar]
- 88.Lee S, Brown RL. Phylogenetic relationships of Holarctic Teleiodini (Lepidoptera: Gelechiidae) based on analysis of morphological and molecular data. Syst Entomol. 2008; 33: 595–612. [Google Scholar]
- 89.Wang HS, Wahlberg N, Holloway J D, Bergsten J, Fan XL, Daniel H, et al. Molecular phylogeny of Lymantriinae (Lepidoptera, Noctuoidea, Erebidae) inferred from eight gene regions. Cladistics. 2015; 1–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The numbers indicate bootstrap values. Colors highlight recognized genera.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.