Summary
Context
Autoantibodies to 21-hydroxylase (21OH-AA) precede onset of autoimmune Addison's disease (AD). Progression to AD can take months to years, and early detection of metabolic decompensation may prevent morbidity and mortality.
Objective
To define optimal methods of predicting progression to overt AD (defined by subnormal peak cortisol response to Cosyntropin) in 21OH-AA+ individuals.
Design, Setting and Participants
Individuals were screened for 21OH-AA at the Barbara Davis Center from 1993 to 2011. Subjects positive for 21OH-AA (n = 87) were tested, and the majority prospectively followed for the development of Addison's disease, including seven diagnosed with AD upon 21OH-AA discovery (discovered), seven who progressed to AD (progressors) and 73 nonprogressors.
Main Outcome Measured
Plasma renin activity (PRA), ACTH, baseline cortisol, peak cortisol and 21OH-AA were measured at various time points relative to diagnosis of AD or last AD-free follow-up.
Results
Compared with nonprogressors, in the time period 2 months–2 years prior to the onset of AD, progressors were significantly more likely to have elevated ACTH (11–22 pm, P < 1E-4), with no significant differences in mean PRA (P = 0·07) or baseline cortisol (P = 0·08), and significant but less distinct differences seen with 21OH-AA levels (P < 1E-4) and poststimulation cortisol levels (P = 6E-3).
Conclusion
Moderately elevated ACTH is a more useful early indicator of impending AD than 21OH-AA, PRA or peak cortisol, in the 2 months–2 years preceding the onset of AD.
Introduction
Autoimmune Addison's disease (AD), the primary cause of primary adrenal insufficiency in the developed world, is a relatively rare disorder.1,2 At disease onset, AD can be fatal (Addisonian Crisis) if not properly recognized and treated. The presence of 21-hydroxylase autoantibodies (21OH-AA) usually precedes the development of AD in the absence of symptoms and is a marker for risk of progression to clinical disease.3,4 The natural history of disease progression after the appearance of 21OH-AA has been described2,5,6 with recommendations on clinical management and follow-up based on risk factors associated with progression.7–9
Like other related organ-specific autoimmune diseases (e.g. type 1 diabetes and autoimmune thyroid disease), autoantibody formation precedes disease progression with a variable amount of time from the detection of autoantibodies to the detection of tissue destruction. Progressive deterioration of adrenal gland function is reported to be typified by increased plasma renin activity (PRA) and ACTH and culminates in overt cortisol deficiency10 with a reported 40% of young subjects having positive antiadrenal autoantibodies developing AD within 10 years.9,11
Risk of development of 21OH-AA and progression to overt AD is associated with multiple factors including age (adults more than children), gender (females more than males) and signs of adrenal dysfunction upon discovery of antiadrenal autoantibodies.9 In contrast to autoimmune polyendocrine syndrome type 1 (APS-1, a rare monogenic disorder comprised of hypoparathyroidism and muco-cutaneous candidiasis) in which risk is mainly associated with the AIRE gene, idiopathic autoimmune AD and autoimmune polyendocrine syndrome type 2 (APS-2) are considered polygenic with risk lying mainly in the human leukocyte antigen (HLA) region. In the latter, the presence of other autoimmune disease (including type 1 diabetes and autoimmune thyroid disease) in 21OH-AA+ individuals is a marker for risk,1,12–14 as are specific HLA class I and II alleles4,15–20 [specifically DRB1*0301-DQB1*0201 (DR3) and DRB1*04-DQB1*0302 (DR4)].1,4 The highest risk for the progression to AD in 21OH-AA+ individuals is reported to be conferred by a highly conserved extended haplotype that includes DR3 and HLA-B8 (but not HLA-A1).4,18,21,22 Homozygosity of MICA 5·1 (which is in strong linkage disequilibrium with HLA-B8) is also associated with progression.11,17,22,23 HLA-B15 in one study to date is associated with protection from progression to overt AD in 21OH-AA+ populations.24
A model for stages of AD progression following the development of 21OH-AA has been proposed.2 In this model, elevation of PRA is the first biomarker to become abnormal (increase) following the appearance of 21OH-AA. This is followed by abnormalities of peak cortisol following cortrosyn stimulation (decrease), and finally by serum ACTH (increase) and basal cortisol (decrease) before progression to overt clinical disease in which ACTH greatly increases and peak stimulated and basal cortisol are greatly decreased.10 Serum level of 21OH-AA has also been reported to increase as adrenal function deteriorates.25 Based on this model, it is proposed that risk can be stratified with appropriate follow-up intervals.9 There is little data in the literature from prospective studies documenting quantitative changes in ACTH, PRA, baseline cortisol and peak cortisol in 21OH-AA+, non-APS-1 individuals over an extended period of time.
Materials and methods
Subjects
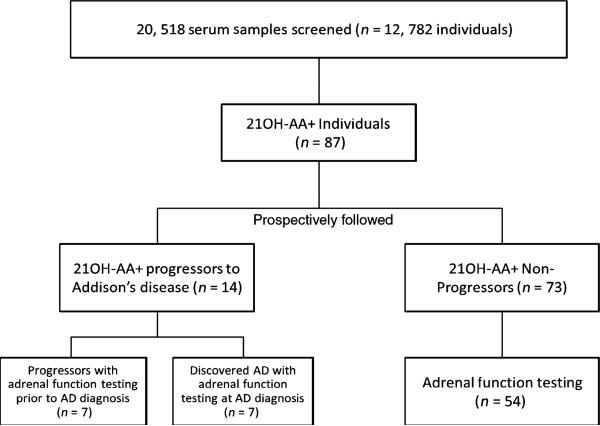
In 1993, we began 21-hydroxylase autoantibody (21OH-AA) testing of relatives of AD individuals and individuals/relatives of individuals with T1DM. Follow-up for progression to AD in 21OHAA+ individuals continues through 2011. To date, we have screened 20 519 serum samples in 12 782 individuals for the expression of 21OH-AA. As illustrated in Fig. 1, we have enrolled a total of 87 individuals (0·6% of the total number of individuals tested) with persistently positive 21OH-AA (defined as more than one consecutive 21OH-AA level > 0·15 measured at least 1 month apart).
Fig. 1.
Flow Diagram depicting the participants of the study.
The mean age at first 21OH-AA detection was 22·6 years in nonprogressors and 17 years in progressors (P = 0·23). Time from first 21OH-AA+ to last event (either last negative clinical follow-up or progression to AD) was 5·4 (±0·49 years, SEM) in nonprogressors and 3·2 (±1·1 years, SEM) in progressors (P = 0·07).
Subjects were prospectively followed for clinical signs and symptoms of AD as well as changes in adrenal function and/or ACTH in 69 of the enrolled 87 individuals. Subjects with 21OH-AA positivity were followed from the date of first positive 21OH-AA detection until (i) onset of AD ‘progressors’ to AD (normal adrenal function testing at first 21OH-AA+, n = 7) and ‘discovered’ AD (adrenal function testing diagnostic of disease onset at first 21OH-AA+, n = 7) or (ii) last follow-up without AD (‘non-progressors’, n = 73; 54 with adrenal function testing). In progressors, mean time to disease progression was 3·2 years (CI 95%, 2·2–4·2 years) from first detection of 21OH-AA. Nonprogressors were followed for a mean of 5·4 years (CI 95%, range 4·9–6·9 years) from the time of 21OH-AA detection, with the absence of AD typically confirmed via clinical history and cortisol levels post cortrosyn stimulation. Since our last publication,24 one additional individual has progressed to clinical AD.
The HLA genotypes and demographic information in this group (including age, gender and the presence of other autoimmune disease) have been previously characterized.24 For those who volunteered race information (n = 77), 75 were white non-Hispanic, one was Hispanic and one was ‘biracial’ (white and Hispanic). There were no significant differences between progressors and nonprogressors for the presence of T1DM (>80% in both groups). Patients or their parents provided informed consent with Institutional Review Board (IRB) oversight at the University of Colorado Denver.
All patients in this study were 21OH-AA-positive (index > 99th percentile of normal) using previously defined methods.3,4 Patients with the clinical diagnosis of APS-1 (with distinct signs, symptoms and abnormalities in the AIRE gene) were excluded, and all included patients were Interferon alpha autoantibody negative (confirming absence of APS-1).26 Amongst 21OH-AA+ nonprogressor cohort, 78% (57/73) had type 1 diabetes vs 86% (12/14) of progressors (P-value not significant). Only 12 individuals from either cohort had no other autoimmune disorders reported.
ACTH stimulation test and AD diagnosis
Individuals were admitted into the Clinical Translational Research Center the morning of testing, placed in a supine position, and an IV catheter was inserted 30 min before baseline laboratory work. At baseline, blood was drawn for 21OH-AA, PRA, ACTH and cortisol. At time 0, 1-μg Cosyntropin (Amphastar Pharmaceuticals, Rancho Cucamonga, CA, USA) (low-dose Cosyntropin stimulation) was given and immediately followed by a normal saline flush; 30 min later, serum was obtained for cortisol measurement. Then, a dose of 250-μg Cosyntropin (high-dose stimulation) was given by IV catheter, and cortisol was measured 30 and 60 min later. The normal range values for ACTH are 0–10 pm (Siemens/DPC Immulite 1000 Assay, Deerfield, IL, USA),27 and the PRA normal range is 0– 3·7 μg/l/h (standard radioimmunoassay from DiaSorin).28 Diagnosis of AD was made with a peak cortisol level after 250-μg Cosyntropin of ≥497 nm or a fasting cortisol of ≥82·8 nm (Beckman Coulter Access II one-step competitive assay, Brea, CA, USA)29,30 The highest cortisol level after stimulation was considered the peak level. If symptoms of adrenal disease developed between stimulation testing or were present at the determination of 21OH-AA positivity, limited testing at the discretion of the attending endocrinologist may have been performed (nonstimulated ACTH and cortisol).
Data analysis
The statistical analyses including mean, median and quartile values were performed using GraphPad Prism 4·0 (La Jolla, CA, USA). Level of significance in all testing was P < 0·05 unless noted. P-values were derived using the Mann–Whitney U-test accounting for non-normal distribution of the data. Time points for evaluation were set at 2 months–2 years prior to ‘event’ and >2 years prior to ‘event’, with testing on the ‘day of diagnosis’ specified if it is being used in the analysis.
Result
Serum 21OH-AA
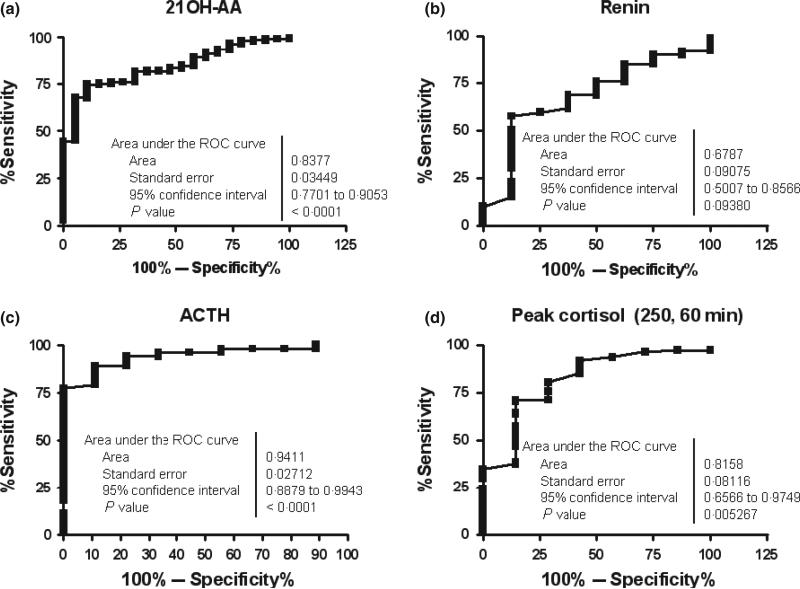
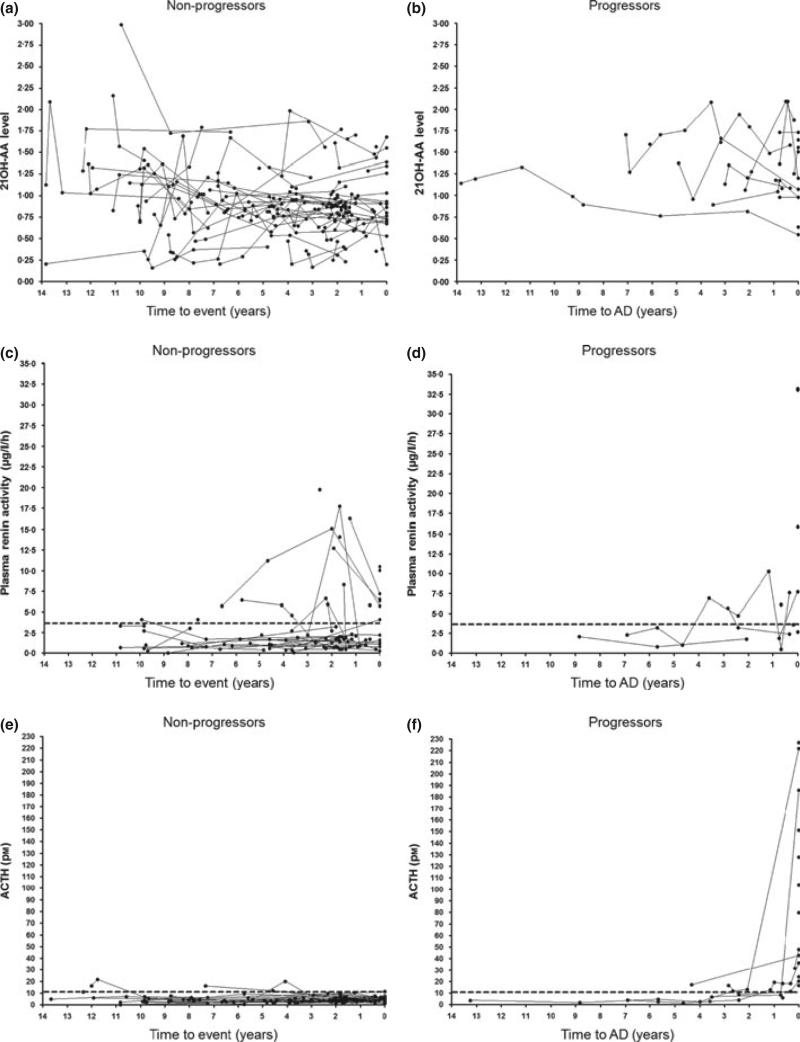
As shown in Table 1, mean index value of serum 21OH-AA in nonprogressors was 0·90 (±0·03 SEM). In progressors/discovered, mean 21OH-AA was 1·14 ± 0·14 on the day of diagnosis (P = 0·04 vs nonprogressors). Mean 21OH-AA in progressors at 2 months– 2 years prior to diagnosis was 1·40 ± 0·09 (P < 1E-4 vs nonprogressors) and at >2 years prior to diagnosis of overt AD was 1·33 ± 0·08 (P < 1E-4). When looking at individuals over time, there is significant overlap between the nonprogressor and the progressor groups (Fig. 1a,b). As illustrated in Fig. 3a, ROC analysis of nonprogressors and progressors at 2 months–2 years prior to AD diagnosis indicates an area under the curve of 0·84 with optimal specificity (89%) and sensitivity (75%) at a cut-off of >1·044 (P < 1E-4).
Table 1.
Statistical analysis of biomarkers (21 OH-AA, PRA, ACTH, baseline Cortisol and peak Cortisol following cortrosyn stimulation). Time in progressors is divided into >2 years and 2 months–2 years prior to the onset of AD. These progressor means are compared to each other and to nonprogressor values
| n (samples) | Minimum | 25% | Median | 75% | Maximum | Mean | SEM | CI 95% | |
|---|---|---|---|---|---|---|---|---|---|
| (A) | |||||||||
| 21OH-AA | |||||||||
| NP | 236 | 0·16 | 0·7 | 0·9 | 1·1 | 3·0 | 0·9 | 0·027 | 0·86–0·97 |
| Progressor > 2 years | 23 | 0·76 | 1·0 | 1·3 | 1·7 | 2·1 | 1·3 | 0·077 | 1·16–1·50 |
| Progressor 2 months–2 years | 18 | 0·82 | 1·1 | 1·3 | 1·8 | 2·1 | 1·4 | 0·090 | 1·20–1·58 |
| Renin | |||||||||
| NP | 99 | 0 | 0·8 | 1·6 | 3·3 | 19·7 | 3·1 | 0·408 | 2·3–4·0 |
| Progressor >2 years | 9 | 0·8 | 1·6 | 3·2 | 5·2 | 6·9 | 3·3 | 0·694 | 1·7–5·0 |
| Progressor 2 months–2 years | 8 | 0·5 | 1·9 | 3·0 | 6·9 | 10·3 | 4·3 | 1·200 | 1·4–7·1 |
| ACTH | |||||||||
| NP | 135 | 0 | 2·7 | 4·0 | 5·7 | 21·6 | 4·8 | 0·288 | 4·2–5·3 |
| Progressor >2 years | 14 | 1·5 | 2·4 | 3·9 | 10·2 | 17·2 | 6·3 | 1·412 | 3·2–9·3 |
| Progressor 2 months–2 years | 10 | 5·9 | 9·9 | 13·0 | 18·8 | 31·7 | 15·1 | 2·301 | 9·9–20·3 |
| Baseline cortisol | |||||||||
| NP | 125 | 80 | 193·2 | 276 | 386·4 | 993·6 | 298·7 | 13·1 | 272·8–324·7 |
| Progressor > 2 years | 14 | 166 | 248·4 | 409·9 | 560·3 | 1380 | 474·7 | 83·6 | 294·1–655·3 |
| Progressor 2 months–2 years | 10 | 237 | 266·3 | 309·1 | 510·6 | 679 | 372·3 | 47·3 | 265·4–479·3 |
| Peak cortisol | |||||||||
| NP | 107 | 442 | 690·0 | 800·4 | 910·8 | 1297 | 808·2 | 18·3 | 771·9–844·4 |
| Progressor > 2 years | 12 | 469 | 666·5 | 772·8 | 869·4 | 2180 | 887·3 | 131·5 | 598–1177 |
| Progressor 2 months–2 years | 7 | 466 | 469·2 | 552 | 706·6 | 855·6 | 597·3 | 54·7 | 463·5–731·2 |
| (B) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 21 OH-AA |
Renin |
ACTH |
Baseline cortisol |
Peak cortisol |
||||||
| 21 OH-AA+ group | Mean difference | P | Mean difference | P | Mean difference | P | Mean difference | P | Mean difference | P |
| NP vs progressor 2 months–2 years | –0·48 | <lE-4 | –1·15 | 0·1 | –10·29 | <1E-4 | –73·60 | 0·12 | 210·90 | 5·E-03 |
| NP vs progressor > 2 years | 0·41 | <lE-4 | 0·19 | 0·13 | 1·54 | 0·7 | 176·00 | 0·01 | 79·10 | 0·83 |
| Progressor 2 months–2 years vs progressor > 2 years | 0·07 | 0·49 | 0·95 | 0·74 | 8·75 | 3·E-03 | –102·40 | 0·52 | –290·00 | 0·05 |
P-values derived from Mann-Whitney U-Test. 21 OH-AA, 21-hydroxylase autoantibodies; AD, Addison's disease; PRA, plasma renin activity; NP, nonprogressor values.
Fig. 3.
(a–d) ROC curves for four biomarkers comparing values in progressors (at 2 months–2 years prior to the onset of Addison's disease) and comparing with nonprogressor values.
Plasma renin activity
Individual renin values for progressors and nonprogressors are shown in Fig. 2c,d. Mean values in progressors for 2 months– 2 years and >2 years (prior to AD diagnosis) were 4·3 μg/l/h (±1·2 SEM) and 3·3 ± 0·7 μg/l/h respectively, compared with 3·1 ± 0·4 μg/l/h in nonprogressors (overall P = 0·7). There is an overall increase in renin activity within 2 months–2 years prior to AD onset (median 3·0 μg/l/h, IQR 1·9–6·9 μg/l/h); however, extensive overlap exists when they are compared with nonprogressors (median 1·6, IQR 0·8–3·2 μg/l/h). Mann–Whitney comparison between all groups was not significant (Table 1). Fig. 3b illustrates the ROC curve area for renin (calculated using all non progressor values compared with progressor values 2 months– 2 years prior to diagnosis) was 0·67 (P = 0·09), with the upper limit of normal (3·7 μg/l/h) giving a sensitivity of 75% and a specificity of 50%. Optimal cut-off value from the ROC curve was determined to be >1·75 μg/l/h with a sensitivity of 58% and specificity of 87%.
Fig. 2.
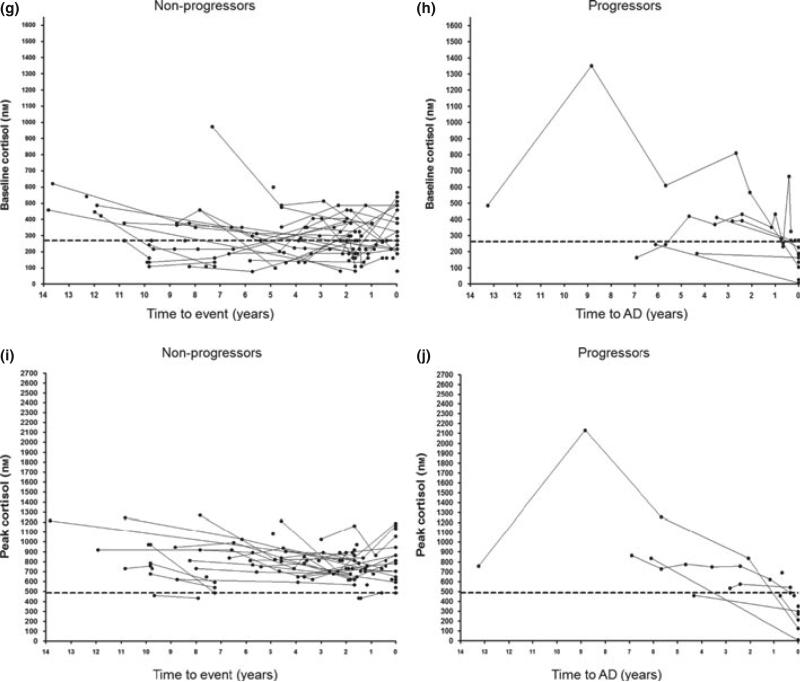
(a–j): Changes in adrenal function in discovered (n = 7), progressor (n = 7) and nonprogressor (n = 54) 21OH-AA-positive individuals over time. Heavy dashed lines represent ‘high’ ACTH and plasma renin activity values and ‘low’ peak and baseline cortisol values.
ACTH
High ACTH was defined as >11 pm (Fig. 2e,f). Mean values for nonprogressors, progressors at >2 years prior to AD diagnosis and progressors at 2 months–2 years were 4·8 (±0·28 SEM), 6·3 ± 1·4 pm (P = 0·57 vs nonprogressors) and 15·1 ± 2·3 (P < 1E-4 vs nonprogressors), respectively. Mean progressor ACTH between 2 months and 2 years was also greater vs progressors >2 years prior to diagnosis had a difference of 8·75 pm (P = 3E-3). There was no significant difference between nonprogressors and progressor measurements >2 years prior to AD onset.
ROC area under the curve (Fig. 3c) using nonprogressor values vs progressors at 2 months–2 years is 0·94 (P < 1E-4), with sensitivity and specificity at an ACTH value of >11·2 pm of 94% and 78%, respectively. Sensitivity of 89% and specificity of 89% are found at an ACTH value of >8·2 pm.
When the progressor time course is evaluated (including values on the day of diagnosis, illustrated in Fig. 2e,f), the ACTH value in progressors >2 years prior to AD diagnosis is typically below 11 pm, but 2 months–2 years prior to diagnosis values are between 11 and 22 pm. On the day of diagnosis (including seven progressors and seven discovered, n = 14), values are typically over 22 pm with a mean value on the day of diagnosis 99 pm (range 16·5–226·8 pm).
Baseline cortisol
‘Low’ baseline cortisol level is indicated as <276 nm with levels ‘diagnostic’ for adrenal failure <80 nm (Fig. 2g,h). Mean baseline cortisol (Table 1) in nonprogressors was 299 (±13·1 SEM) nm, in progressors 2 months–2 years prior to diagnosis was 372 ± 47 nm and in progressors >2 years prior to diagnosis was 475 ± 84 nm.
Median baseline cortisol for nonprogressors was 276 nm (IQR 193–386 nm) compared with progressors at 2 months–2 years prior to AD diagnosis (median 309 nm, IQR 256–511 nm) and progressors >2 years prior to diagnosis (median 410 nm, IQR 248– 560 nm). ROC analysis (not pictured) in nonprogressors vs progressors at 2 months–2 years prior to AD diagnosis showed an area under the curve of 0·65 (P = 0·1).
Peak cortisol
High-dose Cosyntropin (250 μg) cortisol measurements at 30 or 60 min (whichever was highest) were considered ‘peak cortisol’ with ‘low’ being defined as less than or equal to 497 nm (Fig. 2i,j). All values on the day of diagnosis in progressors were by definition <497 nm (maximum 414 nm, mean 238 ± 36 nm SEM), and considered diagnostic of AD. Nonprogressor mean peak cortisol values was 808 nm (±18 nm SEM), and progressor peak cortisol of 597 nm (±55 nm SEM) at 2 months–2 years prior to diagnosis and 887 nm (±131 nm SEM) at >2 years prior to diagnosis (Table 1). As illustrated in Table 1, data were significantly different in progressors 2 months–2 years prior to AD diagnosis vs nonprogressors (mean difference 211 nm, P = 5E-3) as well as progressors 2 months–2 years vs >2 years prior to AD diagnosis (mean difference 290 nm, P = 0·05).
Median peak cortisol was highest in nonprogressors (800 nm, IQR 690–911 nm) compared with progressors at >2 years (773 nm, IQR 667–869 nm), 2 months–2 years (552 nm, IQR 469–707 nm) and on the day of AD diagnosis (276 nm, IQR 174–304 nm). Area under the curve in ROC analysis (Fig. 3d) was 0·81 (P = 5E-3). Sensitivity of 71% with specificity of 86% was found at a peak cortisol of <709 nm, with the ‘low’ value of <497 nm having a sensitivity of 96% and specificity of 29%.
The levels of 21OH-AA, ACTH, PRA and serum cortisol are statistically significantly different in progressors vs. nonprogressors. However, significant overlap limits the utility of several of the biomarkers for differentiating the two groups. Therefore, we find that the most appropriate early predictor of overt AD in the 2 years preceding clinical onset is increased ACTH. Increased PRA and 21OH-AA, both of which have been reported to indicate subclinical AD progression, were not significantly associated with AD onset in progressor vs nonprogressors over time.
Discussion
Autoimmune AD (isolated or in the form of APS) is a relatively rare disorder in which the time from initial serologic signs of disease (namely 21OH-AA formation) and overt disease onset can take years. Early detection is an important tool in mitigating significant morbidity and mortality related to undiagnosed disease. According to previous literature, biomarkers associated with adrenal dysfunction in individuals with adrenal autoimmunity appear (and remain) consistently abnormal in the following sequence: PRA (increase), peak cortisol (decrease) and finally ACTH (increase) and baseline cortisol (decrease). Quantitative changes for each of the aforementioned biomarkers, specifically as they relate to AD progression over time, could serve as useful tools to better predict progress to overt AD.
In the period of more than 2 years prior to AD onset, there was little difference between mean progressor and nonprogressor values, specifically for PRA, ACTH and peak cortisol. As expected, on the day of diagnosis, ACTH values were over twice (and in most cases between five and 20 times) the upper limit of normal. Similarly, most of the PRA values were above the upper limit of normal; however, there was at least one progressor with a PRA value well within the normal range. While this does not exclude the use of PRA in supporting the diagnosis of AD, it does raise concern about the consistency of elevation in individuals who are clinically progressing to AD. As the study was conducted at a large type 1 diabetes centre, it is possible that study participants may have been taking ACE inhibitors or angiotensin receptor blockers, which can lower PRA levels and are common in longstanding T1DM individuals.
In the period 2 months–2 years prior to the onset of AD, the biomarkers 21OH-AA, baseline cortisol and PRA all displayed a large degree of variability in nonprogressor and progressor individuals with no clear pattern of significant change over time. Serum 21OH-AA values are significantly higher in progressors at all time points vs nonprogressors, with progressor values almost all remaining >1 throughout follow-up. Although the P-values comparing progressors and nonprogressors are significant (P < 1E-4), we do not find a clear pattern of continuing elevation for 21OH-AA as individuals progress to adrenal failure (i.e. as the markers ACTH and peak and basal cortisol change). We also find no significant difference in the mean PRA or baseline cortisol values relative to nonprogressor data in either ROC analysis or analysis of mean and median values. Therefore, while persistently elevated 21OH-AA may be of use in indicating eventual progression to AD, none of these biomarkers (21OH-AA, PRA or baseline cortisol) appear to be useful temporal prognostic indicators.
Conversely, ACTH at 2 months–2 years prior to AD onset displayed a significant difference between those individuals who eventually progressed and those who did not. When individual progressor patterns are examined in this time period, ACTH values rise above 11 pm (2–3 times the average nonprogressor values) and remain between 11 and 22 pm until approximately 1–2 months prior to AD diagnosis at which time they rise up to 20-fold. This is in contrast to nonprogressor individuals, in whom isolated ACTH values >11 pm rarely occur. Transient elevations in ACTH with no subsequent evidence of adrenal deterioration make it essential that a diagnosis of AD not be based upon ACTH alone in 21OH-AA+ individuals. Low basal cortisol levels and ACTH stimulated cortisol levels remain the gold standard in AD diagnosis.
As part of this study of 21OH-AA+, non-APS-1 individuals, we performed cortrosyn stimulation testing every 1–2 years. We would now use ACTH as an early (‘sentinel’) indicator of impending AD onset (vs 21OH-AA levels or PRA) in 21OH-AA+ individuals. All 21OH-AA+ patients would be educated on clinical signs and symptoms of AD and would have adrenal function tested if they became symptomatic. A rise in ACTH above 11 pm (but often below 22 pm) would prompt more frequent ACTH level measurements (e.g. every 3–6 months). Elevation in serial measurements would trigger formal cortrosyn testing, and higher vigilance against acute metabolic decompensation (Addisonian Crisis).
Limitations to this study include a lack of patients with the rare APS-1. The use of 21OH-AA levels to predict onset of autoimmune AD has been reported, but complicated by the lack of standardization of 21OH-AA measurements. The basic study design relied on regular clinical and biochemical follow-up of asymptomatic individuals for, in some cases, over a nearly 15-year period. Although a large number of nonprogressors have been sequentially tested, only seven individuals who we prospectively followed progressed to AD. This is more reflective of the patient population and overall rate of progression in non-APS-1, 21OH-AA-positive individuals than the study design. There is the potential that in the future some nonprogressors will progress. Many nonprogressors (n = 26) have the protective HLA-B15 allele, where to date we have observed only one 21OH-AA+ progressor. Thus, a major subset may never progress, although vigilance in this group is essential with approximately 10% of patients with AD having HLA-B15.24
This study, in modified form, is ongoing with plans to continue follow-up of 21OH-AA+ patients. Nonprogressors will continue to be followed annually with 21OH-AA and ACTH levels. Adrenal function testing will be performed if there is a persistent rise in ACTH or there are clinical signs or symptoms of Addison's disease. A larger population, more frequent and longer follow-up of the current series, as well as studies from other centres would be helpful in better defining temporal and quantitative guidelines for predicting progression to overt AD in 21OH-AA+ individuals.
Acknowledgments
This study was supported by Diabetes Endocrine Research Center Clinical Investigations and Bioinformatics Core Grant P30 DK 57516 and the Children's Diabetes Foundation. In addition, research was supported by National Institutes of Health Grants DK 32083 and A146374. Peter R. Baker is a Fellow of the Pediatric Scientist Development Program. The project described was supported by Award K12-HD000850 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Clinical and Translational Research Center was supported by Grants RR-00051 and RR-00069. J.M.B. was supported by the Juvenile Diabetes Research Foundation Grant 11-2005-15. We acknowledge the Clinical and Translational Research Centers for their contribution in performing the adrenal testing and the core laboratories at the University of Colorado Hospital, the Children's Hospital of Denver and the translational unit of the Barbara Davis Center for performing the endocrine assays. Also, we would like to acknowledge the patients and families for their participation and the National Adrenal Diseases Foundation.
Footnotes
Financial disclosure
The authors have nothing to disclose.
References
- 1.Erichsen MM, Lovas K, Skinningsrud B, et al. Clinical, immunological, and genetic features of autoimmune primary adrenal insufficiency: observations from a Norwegian registry. Journal of Clinical Endocrinology and Metabolism. 2009;94:4882–4890. doi: 10.1210/jc.2009-1368. [DOI] [PubMed] [Google Scholar]
- 2.Betterle C, Dal Pra C, Mantero F, et al. Autoimmune adrenal insufficiency and autoimmune polyendocrine syndromes: autoantibodies, autoantigens, and their applicability in diagnosis and disease prediction. Endocrine Reviews. 2002;23:327–364. doi: 10.1210/edrv.23.3.0466. [DOI] [PubMed] [Google Scholar]
- 3.Falorni A, Laureti S, Nikoshkov A, et al. 21-hydroxylase autoantibodies in adult patients with endocrine autoimmune diseases are highly specific for Addison's disease. Clinical and Experimental Immunology. 1997;107:341–346. doi: 10.1111/j.1365-2249.1997.262-ce1153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu L, Brewer KW, Gates S, et al. DRB1*04 and DQ alleles: expression of 21-hydroxylase autoantibodies and risk of progression to Addison's disease. Journal of Clinical Endocrinology and Metabolism. 1999;84:328–335. doi: 10.1210/jcem.84.1.5414. [DOI] [PubMed] [Google Scholar]
- 5.Giordano R, Balbo M, Picu A, et al. Corticotrope hypersecretion coupled with cortisol hypo-responsiveness to stimuli is present in patients with autoimmune endocrine diseases: evidence for subclinical primary hypoadrenalism? European Journal of Endocrinology. 2006;155:421–428. doi: 10.1530/eje.1.02222. [DOI] [PubMed] [Google Scholar]
- 6.Laureti S, Aubourg P, Calcinaro F, et al. Etiological diagnosis of primary adrenal insufficiency using an original flowchart of immune and biochemical markers. Journal of Clinical Endocrinology and Metabolism. 1998;89:3163–3168. doi: 10.1210/jcem.83.9.5103. [DOI] [PubMed] [Google Scholar]
- 7.Betterle C, Volpato M, Smith BR, et al. I. Adrenal cortex and steroid 21-hydroxylase autoantibodies in adult patients with organ-specific autoimmune diseases: markers of low progression to clinical Addison's disease. Journal of Clinical Endocrinology and Metabolism. 1997;82:932–938. doi: 10.1210/jcem.82.3.3819. [DOI] [PubMed] [Google Scholar]
- 8.Betterle C, Volpato M, Rees Smith B, et al. II. Adrenal cortex and steroid 21-hydroxylase autoantibodies in children with organ-specific autoimmune diseases: markers of high progression to clinical Addison's disease. Journal of Clinical Endocrinology and Metabolism. 1997;82:939–942. doi: 10.1210/jcem.82.3.3849. [DOI] [PubMed] [Google Scholar]
- 9.Coco G, Dal Pra C, Presotto F, et al. Estimated risk for developing autoimmune Addison's disease in patients with adrenal cortex autoantibodies. Journal of Clinical Endocrinology and Metabolism. 2006;91:1637–1645. doi: 10.1210/jc.2005-0860. [DOI] [PubMed] [Google Scholar]
- 10.Betterle C, Scalici C, Presotto F, et al. The natural history of adrenal function in autoimmune patients with adrenal autoanti-bodies. Journal of Endocrinology. 1988;117:467–475. doi: 10.1677/joe.0.1170467. [DOI] [PubMed] [Google Scholar]
- 11.Barker JM, Ide A, Hostetler C, et al. Endocrine and immunogenetic testing in individuals with type 1 diabetes and 21-hydroxylase autoantibodies: Addison's disease in a high-risk population. Journal of Clinical Endocrinology and Metabolism. 2005;90:128–134. doi: 10.1210/jc.2004-0874. [DOI] [PubMed] [Google Scholar]
- 12.Eisenbarth GS, Gottlieb PA. Autoimmune polyendocrine syndromes. New England Journal of Medicine. 2004;350:2068–2079. doi: 10.1056/NEJMra030158. [DOI] [PubMed] [Google Scholar]
- 13.Falorni A, Laureti S, De Bellis A, et al. Italian addison network study: update of diagnostic criteria for the etiological classification of primary adrenal insufficiency. Journal of Clinical Endocrinology and Metabolism. 2004;89:1598–1604. doi: 10.1210/jc.2003-030954. [DOI] [PubMed] [Google Scholar]
- 14.Badenhoop K, Walfish PG, Rau H, et al. Susceptibility and resistance alleles of human leukocyte antigen (HLA) DQA1 and HLA DQB1 are shared in endocrine autoimmune disease. Journal of Clinical Endocrinology and Metabolism. 1995;80:2112–2117. doi: 10.1210/jcem.80.7.7608264. [DOI] [PubMed] [Google Scholar]
- 15.Alper CA, Larsen CE, Dubey DP, et al. The haplotype structure of the human major histocompatibility complex. Human Immunology. 2006;67:73–84. doi: 10.1016/j.humimm.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Gambelunghe G, Falorni A, Ghaderi M, et al. Microsatellite polymorphism of the MHC class I chain-related (MIC-A and MIC-B) genes marks the risk for autoimmune Addison's disease. Journal of Clinical Endocrinology and Metabolism. 2006;84:3701–3707. doi: 10.1210/jcem.84.10.6069. [DOI] [PubMed] [Google Scholar]
- 17.Gombos Z, Hermann R, Kiviniemi M, et al. Analysis of extended human leukocyte antigen haplotype association with Addison's disease in three populations. European Journal of Endocrinology. 2007;157:757–761. doi: 10.1530/EJE-07-0290. [DOI] [PubMed] [Google Scholar]
- 18.Weetman AP, Zhang L, Tandon N, et al. HLA associations with autoimmune Addison's disease. Tissue Antigens. 1999;38:31–33. doi: 10.1111/j.1399-0039.1991.tb02032.x. [DOI] [PubMed] [Google Scholar]
- 19.Neufeld M, Maclaren NK, Blizzard RM. Two types of autoimmune Addison's disease associated with different polyglandular autoimmune (PGA) syndromes. Medicine (Baltimore) 1981;60:355–362. doi: 10.1097/00005792-198109000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Bilbao JR, Martin-Pagola A, Perez de Nanclares G, et al. HLA-DRB1 and MICA in autoimmunity: common associated alleles in autoimmune disorders. Annals of the New York Academy of Sciences. 2003;1005:314–318. doi: 10.1196/annals.1288.049. [DOI] [PubMed] [Google Scholar]
- 21.Eisenbarth GS, Wilson P, Ward F, et al. HLA type and occurrence of disease in familial polyglandular failure. New England Journal of Medicine. 1978;298:92–94. doi: 10.1056/NEJM197801122980209. [DOI] [PubMed] [Google Scholar]
- 22.Baker PR, Baschal EE, Fain PR, et al. Haplotype analysis discriminates genetic risk for DR3-associated endocrine autoimmunity and helps define extreme risk for Addison's disease. Journal of Clinical Endocrinology and Metabolism. 2010;95:E263–E270. doi: 10.1210/jc.2010-0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Triolo TM, Baschal EE, Armstrong TK, et al. Homozygosity of the polymorphism MICA5Æ1 identifies extreme risk of progression to overt adrenal insufficiency among 21-hydroxylase antibody-positive patients with type 1 diabetes. Journal of Clinical Endocrinology and Metabolism. 2010;94:4517–4523. doi: 10.1210/jc.2009-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker PR, Baschal EE, Fain PR, et al. Dominant suppression of Addison's disease associated with HLA-B15. Journal of Clinical Endocrinology and Metabolism. 2011;96:2154–2162. doi: 10.1210/jc.2010-2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laureti S, De Bellis A, Muccitelli VI, et al. Levels of adrenocortical autoantibodies correlate with the degree of adrenal dysfunction in subjects with preclinical Addison's disease. Journal of Clinical Endocrinology and Metabolism. 1998;83:3507–3511. doi: 10.1210/jcem.83.10.5149. [DOI] [PubMed] [Google Scholar]
- 26.Meager A, Visvalingam K, Peterson P, et al. Anti-inter-feron autoantibodies in autoimmune polyendocrinopathy syndrome type 1. PLoS Medicine. 2006;3:e289. doi: 10.1371/journal.pmed.0030289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horrocks PM, London DR. Diagnostic value of 9 am plasma adrenocorticotrophic hormone concentrations in Cushing's disease. British Medical Journal (Clinical Research Edition) 1982;285:1302–1303. doi: 10.1136/bmj.285.6351.1302. [DOI] [PubMed] [Google Scholar]
- 28.Martinez-Aguayo A, Aglony M, Campino C, et al. Aldosterone, plasma renin activity, and aldosterone/renin ratio in a normotensive healthy pediatric population. Hypertension. 2010;56:391–396. doi: 10.1161/HYPERTENSIONAHA.110.155135. [DOI] [PubMed] [Google Scholar]
- 29.May ME, Carey RM. Rapid adrenocorticotropic hormone test in practice. Retrospective review. American Journal of Medicine. 1985;79:679–684. doi: 10.1016/0002-9343(85)90517-0. [DOI] [PubMed] [Google Scholar]
- 30.Speckart PF, Nicoloff JT, Bethune JE. Screening for adrenocortical insufficiency with cosyntropin (synthetic ACTH). Archives of Internal Medicine. 1971;128:761–763. [PubMed] [Google Scholar]