Abstract
To study whether focal angiogenesis is induced in aged rodents after permanent distal middle cerebral artery occlusion (MCAO), young adult (3-month-old) and aged (24-month-old) Fisher 344 rats underwent MCAO and sacrificed up to two months after MCAO. Immunohistochemistry and synchrotron radiation microangiography were performed to examine the number of newly formed blood vessels in both young adult and aged rats post-ischemia. We found that the number of capillaries and small arteries in aged brain was the same as young adult brain. In addition, we found that after MCAO, the number of blood vessels in the peri-infarct region of ipsilateral hemisphere in aged ischemic rats was significantly increased compared to the aged sham rats (p<0.05). We also confirmed that ischemia-induced focal angiogenesis occurred in young adult rat brain while the blood vessel density in young adult ischemic brain was significantly higher than that in the aged ischemic brain (p<0.05). Our data suggests that focal angiogenesis in aged rat brain can be induced in response to ischemic brain injury, and that aging impedes brain repairing and remodeling after ischemic stroke, possible due to the limited response of angiogenesis.
Keywords: aging, angiogenesis, focal cerebral ischemia, rat, brain
Although many neuroprotective, anti-oxidative, or anti-inflammatory drugs show dramatic efficacy in the experimental animal models of ischemic stroke, none of these drugs could be applied in clinical. Therefore, ischemic stroke remains the major cause of death worldwide. One of the key reasons for the failure of clinical trial is that animal models of stroke are often performed in healthy young adult rather than aged animals, while stroke predominantly occurs in the aging population [1]. Using aged animal for the ischemic animal model is therefore the critical issue to translate experimental result to the clinical application.
Aging is a critical factor in the occurrence and development of cerebrovascular diseases especially in ischemic stroke [2]. Studies have shown that the aging process is associated with cellular function alteration, metabolic rearrangements, organ structural and tissue morphological changes, which were detected in kidney, brain, heart and the vasculature system [3, 4]. Aging could significantly alter the cerebrovascular response to various stress and injuries [5]. For example, collateral circulation to the infarct-related artery in elderly patients with acute myocardial infarction was reduced [6], which could reduce recovery compared to the young adult. Therefore, using aged rodents in experimental cerebral ischemia research appear appropriate because the physiological conditions in these animals mimic clinical patients closely.
Several mechanisms are involved in the self-repair and remodeling after ischemic stroke such as promoting focal angiogenesis and neurogenesis [7], in which angiogenesis is one of important processes for the reduction of infarct volume and improvement of long-term neurobehavioral recovery after ischemic stroke [8, 9]. It is well documented that focal angiogenesis is induced in young adult animals after cerebral ischemia [10, 11]. However, whether ischemia induced angiogenesis also occurred in aged animal brain is unclear.
In this study, we investigated whether angiogenesis was also induced in aged brain as did in young adult brain after focal cerebral ischemia and whether there was difference of ischemia-induced angiogenesis between young adult brain and aged brain using immunohistochemistry and synchrotron radiation microangiography.
MATERIALS AND METHODS
Distal middle cerebral artery occlusion (MCAO)
Animal work in this study was approved by the Institutional Animal Care and Use Committee (IACUC) of University of North Texas Health Science Center at Fort Worth, Texas and Shanghai Jiao Tong University, Shanghai, China. 3-month-old and 24-month-old male Fisher 344 rats (n=6 in each group) were obtained from National Institute of Health, National Institute of Aging (NIH/NIA). Animals were anesthetized with 4% isoflurane in 70% nitrogen/30% oxygen gas mixture via an anesthesia machine (MIDMARK, Versailles Ohio). Body temperature was maintained at 37±0.5°C using a heating pad. Focal cerebral ischemia was induced by occlusion of distal middle cerebral artery (MCAO) through a small burr hole on the skull as previously described [12]. Briefly, under the surgical microscope, a 2 cm incision was made between the left orbit and the tragus, a 3-mm diameter craniotomy was made just rostral to the foramen ovale. The dura was incised and the distal middle cerebral artery (MCA) was exposed. The left MCA was coagulated via an electro-coagulator at approximate the rhinal fissure level and was occluded immediately distal to the lenticulostriate branches such that infarction would be confined to the neocortex. In sham operated rats, the MCA was exposed only without coagulation. After surgery the animals received warmed Ketoprofen fluids (100 mg/ml diluted in 0.9% Sodium Chloride) SQ, at a dose of 5mg/kg body weight, prior to the awakening from anesthesia. Then they were placed on a 37? warming tray, food and water were accessible inside the cage. The weight and appearance were monitored every day during the first week, and ketoprofen were given for three consecutive days.
Immunohistochemistry
Histological sections (4 µm in thickness) prepared from paraffin-embedded tissue samples of the brain were used for immunohistochemical analysis. Endothelial cells were identified by immunohistochemical staining with lectin (1:200 dilutions, Vector Labs, Burlingame, CA) or a polyclonal antibody against rat Glut-1 (1:200 dilutions, Thermo scientific, Waltham, MA). Smooth muscle cells were determined by staining with a polyclonal antibody against rat alpha smooth muscle actin (aSMA, 1:300 dilutions, Abcam, Cambridge, UK).
Immunohistochemistry was performed as previously described [13]. Briefly, brain sections were incubated with primary antibodies over night at 4°C, after washing, they were incubated with biotinylated antibody at room temperature for one hour and with avidin-biotin complex for another one hour. Uniformly timed application of the peroxidase substrate 3, 3-diaminobenzidine (DAB) was used for all studies. Some of immunostained sections were counterstained with hematoxylin. After staining, the sections were dehydrated with a graded series of ethanol, cleaned with xylene, mounted and visualized by light microscopy. For immunofluorescent staining, after brain samples were incubated with primary antibodies, the sections were washed and incubated with Alexa flour 594-labeled secondary antibodies. In all experiments, secondary antibody alone served as a negative control.
Blood vessel Counting
Blood vessel counting through lectin staining blood vessel density was a simple and reproducible way to morphologically identify the number of blood vessels [14-16]. Numerous studies of manual and computer-assisted methods confirm minimal interobserver variability in the blood vessel counting [17]. Two brain coronal sections from the lectin fluorescein staining brain, 1 mm frontier and 1 mm posterior from the ischemic core, were chosen. Three areas of blood vessels, just in the peri-focal region, including cortex and striatum, were chosen at low power objective lens (20X) and blood vessel counting was carried out on these pictures. Two investigators blinded to the experimental group assessed blood vessel counts separately. Only vessels with a clearly defined lumen or a well-defined linear vessels shape was taken into account. Single endothelial cells were ignored. The number of blood vessels was calculated as the mean of the blood vessel counts obtained from the three pictures [10].
Synchrotron radiation angiography (SRA)
SRA was performed at Beam line 13W of Shanghai Synchrotron Radiation Facility (SSRF). A charge coupled device (CCD) camera with a resolution of 9μm was used to obtain real-time angiographic imaging in this study. X-ray energy was 33.3keV, just above the iodine K-edge energy. The sample-detector distance was 650 mm. Contrast agent (350 mgl/ml, Ipamiro, Shanghai, China) was injected into the external carotid artery and common carotid artery bifurcation at a speed of 133.3 μl/sec. Images were snapped at 4 frames/second. Acquired images were processed by Matlab software (Mathworks Ltd., Natick, Mass).
Statistical analysis
The values were given as Mean ± Standard Deviation. Number of blood vessels were compared with non-parametric test by the Mann-Whitney U test. A probability value of less than 5% was considered statistically significant.
RESULTS
To determine the effect of aging on angiogenesis, we first examined the number of blood vessels on normal young adult and aged brain sections using immunohistochemistry with lectin and Glut-1 antibodies. As shown in Fig. 1A and 1B, lectin- and Glut-1-positive cells were found in both normal young adult and aged rat brain, and lectin-positive cells expressed Glut-1 (Fig. 1C), indicating that these immune-positive cells were endothelial cells. Interestingly, we found that the number of lectin- and Glut-1-positive blood vessels in aged brain was the same as young adult brain (Fig. 1D and E, p>0.05), suggesting that angiogenesis in the brain was not increased during aging process.
Figure 1.
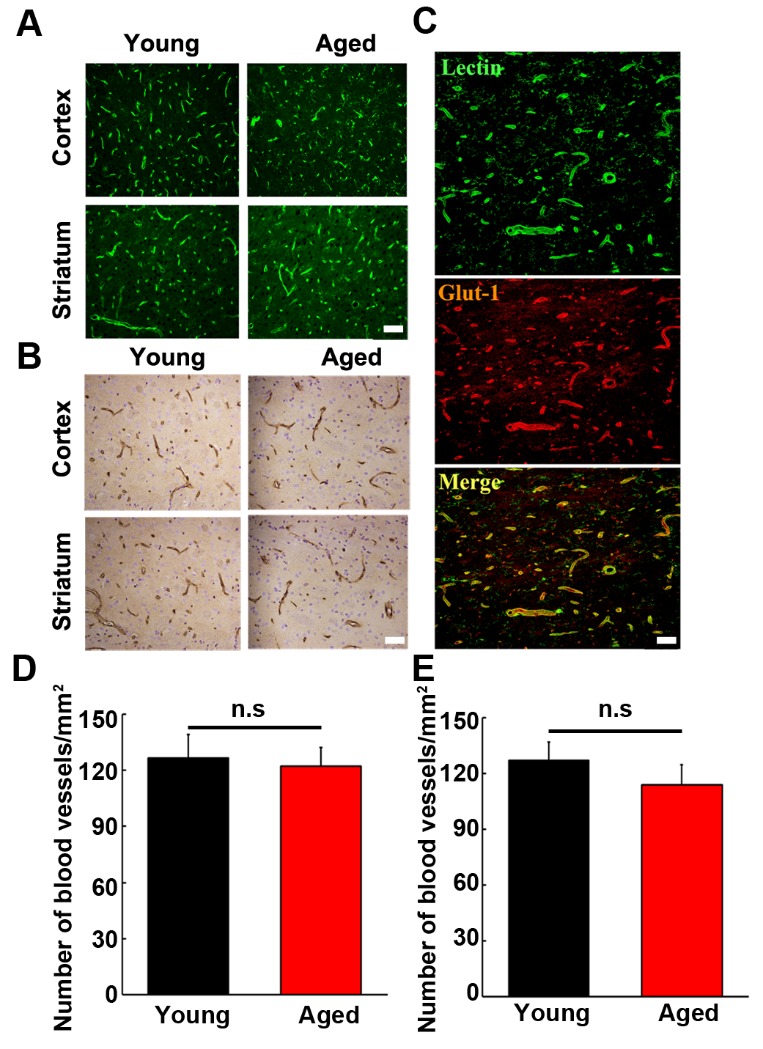
The number of blood vessels in normal young adult and aged rat brain. Photomicrographs showed lectin (A) and Glut-1 (B) staining in the cortex and striatum of the normal young adult (left panel) and aged (right panel) rat brain. Lectin-positive cells was well merged with Glut-1 staining (C), indicating these are endothelial cells and blood vessels. Scale bar=20 µm. The quantitation of the blood vessels in the normal young and aged rats was assessed with either lectin (D) or Glut-1 (E) staining. Data were presented as mean±SD. n.s. p>0.05, the number of blood vessels in normal young vs. that in normal aged rats. N=6 per group.
Next, we investigated whether angiogenesis was also increased in aged brain after focal ischemia as did in young adult brain. Aged rats underwent permanent MCAO and sacrificed 2 months later. Immunohistochemistry showed that the number of blood vessels in the ipsilateral hemisphere after two months of MCAO in aged rats was increased compared to the aged sham rats (Fig. 2; p<0.05). We then asked whether there was the difference of angiogenesis in aged and young adult brain in response to focal ischemia. MCAO was performed in both young adult and aged rats and angiogenesis was evaluated 2 months after MCAO. As shown in Fig. 3, although angiogenesis was induced in both young adult and aged brain, greater number of blood vessels was found in the young adult ischemic brain comparing to the aged ischemic brain (p<0.05).
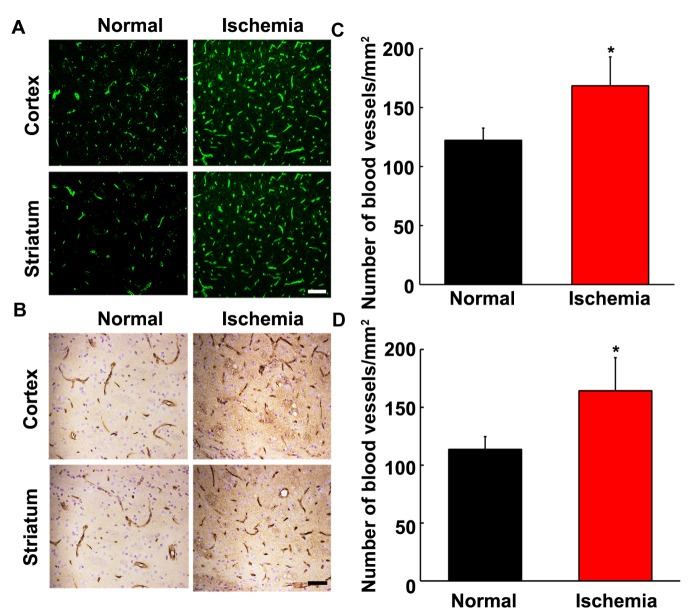
Figure 2.
The number of blood vessels in aged rat brain after focal cerebral ischemia. Photomicrographs showed lectin and Glut-1 staining in aged normal (A and B, left panel) and aged ischemic brain (right panel). Scale bar=20 µm. Bar graph showed that the quantitative number of blood vessels in ipsilateral hemisphere of ischemic and normal rat brain from lectin (C) and Glut-1 (D) staining. Data were presented as mean±SD. *, p<0.05, the number of blood vessels in aged normal rats vs. aged ischemic rats. N=6 per group.
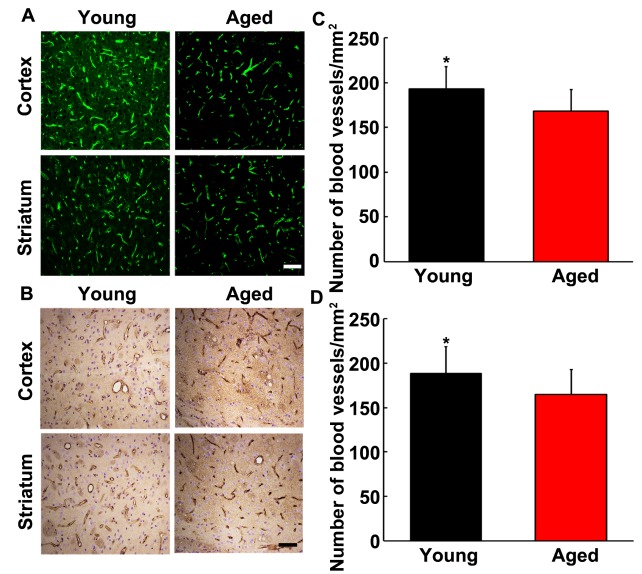
Figure 3.
The number of blood vessels in young adult and aged rat brain after focal ischemia. Photomicrographs showed lectin and Glut-1 staining in the ipsilateral hemisphere in young adult ischemic (A and B, left panel) and aged ischemic brain (right panel). Scale bar =20 µm. Bar graph showed that the quantitative number of blood vessels in ipsilateral hemisphere of young adult and aged rat brain from lectin (C) and Glut-1 (D) staining. Data were presented as mean±SD. *, p<0.05, the number of microvessels of ipsilateral hemisphere in young adult ischemic vs. that in aged ischemic rats. N=6 per group.
To assess the effect of aging in small arteries after MCAO, immunohistochemistry was performed on young adult and aged brain using aSMA antibody. As shown in Fig. 4, the density of aSMA-positive arteries in normal young adult brain and aged brain was similar (p>0.05), while increased number of small arteries were found in the ipsilateral hemisphere in both young adult and aged brain compared to their sham-operated control (p<0.01), suggesting ischemia could promote small artery formation in both young and aged rats.
Figure 4.
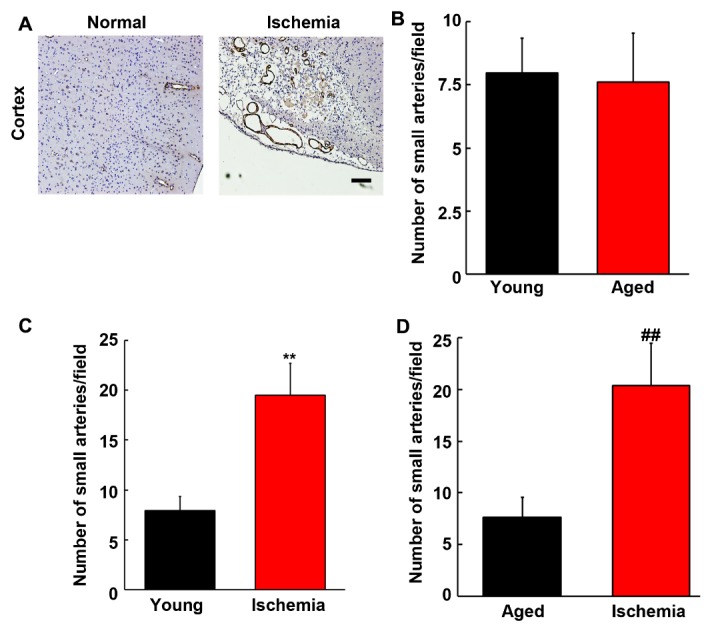
Quantification of small arteries in young adult and aged rat brain. Photomicrographs showed that SMA-positive small arteries in normal aged and ischemic aged rat brains (A). Scale bar=20 µm. Bar graph showed that semi-quantitative number of small arteries in normal young adult and normal aged brain (B), young sham-operated and young ischemic rat brain (C), and aged sham-operated and aged ischemic rats (D). Data were presented as mean±SD. **, p<0.01, the number of small arteries in normal young vs. that in ischemic young brain; ##, p<0.01, the number of small arteries in normal aged vs. that in ischemic aged brain. N=6 per group.
As shown in Fig. 5, using synchrotron radiation angiography in living animals, we found that penetrating arteries of left MCA in ischemic rats were greater than that in the sham groups; in addition, increased blood vessels were found in young ischemic rats than in aged ischemic rats, which was consistent with immunohistochemical results.
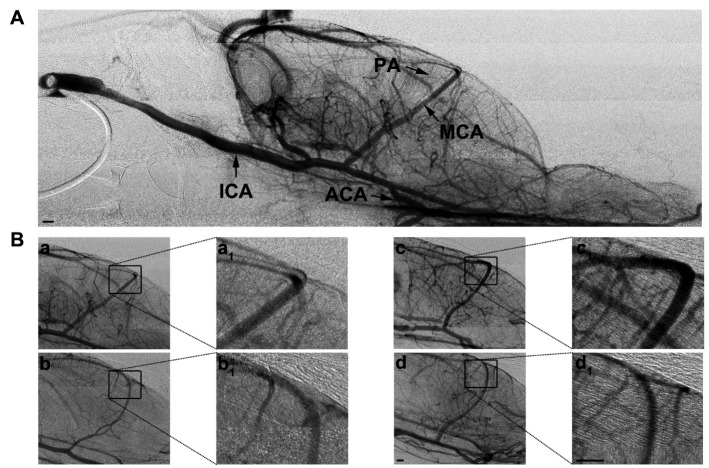
Figure 5.
Live SRA of blood vessels in young and aged rat brain after focal ischemia. A representative SRA image of young sham rat (A). MCA, ICA, ACA and PA indicate middle cerebral artery, internal cerebral artery, anterior cerebral artery and penetrating artery, respectively. B. Photomicrographs showed the perfusion of the MCA territory in normal young rat (a), normal aged rat (b), young ischemic rat (c) and aged ischemic rat (d). a1, b1, c1 and d1 show magnified view of the boxed region. More functional branches of MCA were detected in the young ischemic rats. Bar=500 µm.
DISCUSSION
In the present study, we demonstrated that: 1) aging did not affect capillary and small artery density in normal rat brain; 2) focal ischemia promoted angiogenesis in the peri-focal regions of both young and aged brain, but the number of blood vessels in aged brain was less than that in the young adult brain after ischemia; 3) aging did not affect ischemia-stimulated small artery formation. Our data suggests that promoting angiogenesis after cerebral ischemia in aged brain may be crucial for the repairing and remodeling after ischemic brain injury.
Angiogenesis is one of the fundamental processes during development and some disease processes. Many brain diseases are closely linked to the angiogenic changes such as brain tumors, stroke, arteriovenvous malformations [18]. Induction or inhibition of angiogenesis could provide a therapeutic approach for prevention and treatment of these diseases. Our previous study demonstrated that angiogenesis occurred during focal cerebral ischemia in adult brain [19]. Angiogenesis is a vital process for the functional recovery after ischemic brain injury [20], and the factor of aging may be an important implication for the therapeutic strategies in older patients who indeed represent the population subset suffered from ischemic stroke. Our results showed that under the normal condition, the number of microvessels was similar in aged brain as in young adult brain. After focal ischemia, the number of blood vessels and small arteries in the peri-focal region in both young and aged ischemic rats was significantly increased. Previous study stated that ischemic injury stimulated angiogenesis in the ischemic penumbra regions of young adult animal during the first two weeks after transient MCAO [21]. Treatment with VEGF and Ang/Tie2 could promote neovascularization in rat brain two hours after transient focal cerebral ischemia [22]. Angiogenesis is also induced in patients with cerebral ischemic stroke by analyzing microvessel density [23]. In addition, arteriogenesis in the young adult brain is observed in four-vessel occlusion-induced hypoperfused rat model via increasing granulocyte-macrophage colony-stimulating factor [24, 25]. However, these previous studies were focused in young adult animals, aged animal models were not involved in those work. Whether angiogenesis is induced in aged animal after cerebral ischemia is still unknown.
In our present work, we found that although angiogenesis increased in both young adult and aged brain following MCAO, the number of microvessels adjunct to the lesion area was much more in young adult brain than that in aged brain, indicating aging impeded angiogenesis during repairing process, which may be caused by two potential reasons. First, aged animals after stroke sustain more severe brain injuries compared to young animals [26-28], and it’s highly probable that different brain damage will induce different angiogenesis. Second, intravascular flow rate is an important mediator for angiogenesis, and previous studies showed that aging is greatly related to blood flow velocity and flow volume [29].
Our finding was concurrent with other animal ischemic models. For example, there was lower capillary density after ischemic in limb in aged New Zealand White rabbits [30]. In the wound-healing process in rats, angiogenesis rate is decreased in the aged group [31]. Interestingly, aging also attenuated angiogenesis in tumor animal models which might be related to the aging environment [32-34].
Regional cerebral blood flow can be measured using autoradiography and laser Doppler flowmetry, but neither method can dynamically monitor changes of cerebral blood flow in deeper tissue during ischemia-reperfusion [35]. Application of immunohistochemistry is limited by the need to kill the animals and this method cannot distinguish functional blood vessels. Synchrotron radiation angiography (SRA) may represent a novel solution to directly and dynamically measure the number of functional blood vessels [36]. In the present study, we used SRA to assess the changes of blood vessels in young and aged rats after focal cerebral ischemia. We found ischemia stimulated angiogenesis in both young and aged rats, in addition, more functional blood vessels were found in young ischemic rats compared to aged ischemic rats, which was consistent with the results acquired from immunohistochemical analysis.
The mechanisms of aging affects angiogenesis are potentially diverse and still unclear. Studies demonstrated that growth factors, particularly endothelial cell mitogens, played important roles in angiogenesis [8, 37]. Among of them, vascular endothelial growth factor and Angiopoietin/Tie2 systems are critical growth factors in therapeutic and pathological angiogenesis [38, 39]. Some studies have indicated that the integrity of endothelial function may be compromised as a function of advanced age [40, 41]. Moreover, previous in vitro studies have indicated that endothelial cell showed impaired proliferation and migration in response to growth factors [42].
Although angiogenesis is induced in the young adult brain after experimental ischemic stroke, its contribution for long-term recovery remains controversial. Newly formed capillaries can supply oxygen and nutrition to the ischemic region, which is extremely important for the tissue repairing and remodeling [43, 44]. On the other hand, newly formed capillaries have high permeability, which increases the risk of micro-hemorrhage [45].
Acknowledgements
This study was supported by research grants from the National Natural Science Foundation of China (81070939, 81471178 and 81371305), National Basic Research Program of China (973 Program 2011CB504405), the Science and Technology Commission of Shanghai Municipality (10JC1408100) and KC Wong Foundation (GYY). We thank Minjie Shen for editorial assistance, and the staff of Neuroscience and Neuroengineering Center (http://med-x.sjtu.edu.cn/english), Shanghai Jiao Tong University for their collaborative support.
Footnotes
Disclosures
None
References
- [1].Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. (2009). Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation, 119: e21-181 [DOI] [PubMed] [Google Scholar]
- [2].Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, et al. (2008). Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation, 117: e25-146 [DOI] [PubMed] [Google Scholar]
- [3].Rauscher F, Goldschmidt-Clermont P, Davis B, Wang T, Gregg D, Ramaswami P, et al. (2003). Aging, progenitor cell exhaustion, and atherosclerosis. Circulation, 108: 457. [DOI] [PubMed] [Google Scholar]
- [4].Chowdhury MH, Nagai A, Bokura H, Nakamura E, Kobayashi S, Yamaguchi S (2011). Age-related changes in white matter lesions, hippocampal atrophy, and cerebral microbleeds in healthy subjects without major cerebrovascular risk factors. J Stroke Cerebrovasc Dis, 20: 302-309 [DOI] [PubMed] [Google Scholar]
- [5].Lakatta EG (2002). Age-associated cardiovascular changes in health: impact on cardiovascular disease in older persons. Heart Fail Rev, 7: 29-49 [DOI] [PubMed] [Google Scholar]
- [6].Kurotobi T, Sato H, Kinjo K, Nakatani D, Mizuno H, Shimizu M, et al. (2004). Reduced collateral circulation to the infarct-related artery in elderly patients with acute myocardial infarction. J Am Coll Cardiol, 44: 28-34 [DOI] [PubMed] [Google Scholar]
- [7].Nudo RJ (2006). Mechanisms for recovery of motor function following cortical damage. Curr Opin Neurobiol, 16: 638-644 [DOI] [PubMed] [Google Scholar]
- [8].Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, et al. (2003). VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest, 111: 1843-1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Li Y, Lu Z, Keogh CL, Yu SP, Wei L (2007). Erythropoietin-induced neurovascular protection, angiogenesis, and cerebral blood flow restoration after focal ischemia in mice. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism, 27: 1043-1054 [DOI] [PubMed] [Google Scholar]
- [10].Yang GY, Xu B, Hashimoto T, Huey M, Chaly T Jr., Wen R, et al. (2003). Induction of focal angiogenesis through adenoviral vector mediated vascular endothelial cell growth factor gene transfer in the mature mouse brain. Angiogenesis, 6: 151-158 [DOI] [PubMed] [Google Scholar]
- [11].Wei L, Erinjeri JP, Rovainen CM, Woolsey TA (2001). Collateral growth and angiogenesis around cortical stroke. Stroke; a journal of cerebral circulation, 32: 2179-2184 [DOI] [PubMed] [Google Scholar]
- [12].Jin K, Mao X, Xie L, Greenberg RB, Peng B, Moore A, et al. (2010). Delayed transplantation of human neural precursor cells improves outcome from focal cerebral ischemia in aged rats. Aging Cell, 9: 1076-1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hao Q, Chen Y, Zhu Y, Fan Y, Palmer D, Su H, et al. (2007). Neutrophil depletion decreases VEGF-induced focal angiogenesis in the mature mouse brain. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism, 27: 1853-1860 [DOI] [PubMed] [Google Scholar]
- [14].Wesseling P, Ruiter DJ, Burger PC (1997). Angiogenesis in brain tumors; pathobiological and clinical aspects. J Neurooncol, 32: 253-265 [DOI] [PubMed] [Google Scholar]
- [15].Bossi P, Viale G, Lee AK, Alfano R, Coggi G, Bosari S (1995). Angiogenesis in colorectal tumors: microvessel quantitation in adenomas and carcinomas with clinicopathological correlations. Cancer Res, 55: 5049-5053 [PubMed] [Google Scholar]
- [16].Harris AL, Zhang H, Moghaddam A, Fox S, Scott P, Pattison A, et al. (1996). Breast cancer angiogenesis--new approaches to therapy via antiangiogenesis, hypoxic activated drugs, and vascular targeting. Breast Cancer Res Treat, 38: 97-108 [DOI] [PubMed] [Google Scholar]
- [17].Folkerth RD (2000). Descriptive analysis and quantification of angiogenesis in human brain tumors. J Neurooncol, 50: 165-172 [DOI] [PubMed] [Google Scholar]
- [18].Machein MR, Plate KH (2000). VEGF in brain tumors. J Neurooncol, 50: 109-120 [DOI] [PubMed] [Google Scholar]
- [19].Zhu Y, Lee C, Shen F, Du R, Young WL, Yang GY (2005). Angiopoietin-2 facilitates vascular endothelial growth factor-induced angiogenesis in the mature mouse brain. Stroke; a journal of cerebral circulation, 36: 1533-1537 [DOI] [PubMed] [Google Scholar]
- [20].Slevin M, Kumar P, Gaffney J, Kumar S, Krupinski J (2006). Can angiogenesis be exploited to improve stroke outcome? Mechanisms and therapeutic potential. Clinical Science, 111: 171-183 [DOI] [PubMed] [Google Scholar]
- [21].Thored P, Wood J, Arvidsson A, Cammenga J, Kokaia Z, Lindvall O (2007). Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke; a journal of cerebral circulation, 38: 3032-3039 [DOI] [PubMed] [Google Scholar]
- [22].Zhang ZG, Zhang L, Tsang W, Soltanian-Zadeh H, Morris D, Zhang R, et al. (2002). Correlation of VEGF and angiopoietin expression with disruption of blood-brain barrier and angiogenesis after focal cerebral ischemia. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism, 22: 379-392 [DOI] [PubMed] [Google Scholar]
- [23].Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM (1994). Role of angiogenesis in patients with cerebral ischemic stroke. Stroke; a journal of cerebral circulation, 25: 1794-1798 [DOI] [PubMed] [Google Scholar]
- [24].Busch HJ, Buschmann IR, Mies G, Bode C, Hossmann KA (2003). Arteriogenesis in hypoperfused rat brain. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism, 23: 621-628 [DOI] [PubMed] [Google Scholar]
- [25].Buschmann IR, Busch HJ, Mies G, Hossmann KA (2003). Therapeutic induction of arteriogenesis in hypoperfused rat brain via granulocyte-macrophage colony-stimulating factor. Circulation, 108: 610-615 [DOI] [PubMed] [Google Scholar]
- [26].Boyko M, Kutz R, Gruenbaum BF, Cohen H, Kozlovsky N, Gruenbaum SE, et al. (2013). The influence of aging on poststroke depression using a rat model via middle cerebral artery occlusion. Cogn Affect Behav Neurosci, 13: 847-859 [DOI] [PubMed] [Google Scholar]
- [27].Sohrabji F, Bake S, Lewis DK (2013). Age-related changes in brain support cells: Implications for stroke severity. Neurochemistry international, 63: 291-301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tang Y, Wang J, Lin X, Wang L, Shao B, Jin K, et al. (2014). Neural stem cell protects aged rat brain from ischemia-reperfusion injury through neurogenesis and angiogenesis. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism, 34: 1138-1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Scheel P, Ruge C, Schoning M (2000). Flow velocity and flow volume measurements in the extracranial carotid and vertebral arteries in healthy adults: reference data and the effects of age. Ultrasound in medicine & biology, 26: 1261-1266 [DOI] [PubMed] [Google Scholar]
- [30].Rivard A, Fabre JE, Silver M, Chen D, Murohara T, Kearney M, et al. (1999). Age-dependent impairment of angiogenesis. Circulation, 99: 111-120 [DOI] [PubMed] [Google Scholar]
- [31].Yamaura H, Matsuzawa T (1980). Decrease in capillary growth during aging. Exp Gerontol, 15: 145-150 [DOI] [PubMed] [Google Scholar]
- [32].Kreisle RA, Stebler BA, Ershler WB (1990). Effect of host age on tumor-associated angiogenesis in mice. JNCI Journal of the National Cancer Institute, 82: 44. [DOI] [PubMed] [Google Scholar]
- [33].Ershler WB (1993). The influence of an aging immune system on cancer incidence and progression. The Journal of Gerontology, 48: B3. [DOI] [PubMed] [Google Scholar]
- [34].Reed MJ, Karres N, Eyman D, Cruz A, Brekken RA, Plymate S (2007). The effects of aging on tumor growth and angiogenesis are tumor-cell dependent. Int J Cancer, 120: 753-760 [DOI] [PubMed] [Google Scholar]
- [35].Dirnagl U, Kaplan B, Jacewicz M, Pulsinelli W (1989). Continuous measurement of cerebral cortical blood flow by laser-Doppler flowmetry in a rat stroke model. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism, 9: 589-596 [DOI] [PubMed] [Google Scholar]
- [36].Shirai M, Schwenke DO, Eppel GA, Evans RG, Edgley AJ, Tsuchimochi H, et al. (2009). Synchrotron-based angiography for investigation of the regulation of vasomotor function in the microcirculation in vivo. Clin Exp Pharmacol Physiol, 36: 107-116 [DOI] [PubMed] [Google Scholar]
- [37].Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z (1999). Vascular endothelial growth factor (VEGF) and its receptors. FASEB J, 13: 9-22 [PubMed] [Google Scholar]
- [38].Zhang Z, Chopp M (2002). Vascular endothelial growth factor and angiopoietins in focal cerebral ischemia. Trends Cardiovasc Med, 12: 62-66 [DOI] [PubMed] [Google Scholar]
- [39].Greenberg DA (1998). Angiogenesis and stroke. Drug News Perspect, 11: 265-270 [DOI] [PubMed] [Google Scholar]
- [40].Gerhard M, Roddy MA, Creager SJ, Creager MA (1996). Aging progressively impairs endothelium-dependent vasodilation in forearm resistance vessels of humans. Hypertension, 27: 849-853 [DOI] [PubMed] [Google Scholar]
- [41].Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, et al. (1995). Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation, 91: 1981-1987 [DOI] [PubMed] [Google Scholar]
- [42].Garfinkel S, Hu X, Prudovsky IA, McMahon GA, Kapnik EM, McDowell SD, et al. (1996). FGF-1-dependent proliferative and migratory responses are impaired in senescent human umbilical vein endothelial cells and correlate with the inability to signal tyrosine phosphorylation of fibroblast growth factor receptor-1 substrates. J Cell Biol, 134: 783-791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Shen F, Fan Y, Su H, Zhu Y, Chen Y, Liu W, et al. (2008). Adeno-associated viral vector-mediated hypoxia-regulated VEGF gene transfer promotes angiogenesis following focal cerebral ischemia in mice. Gene Ther, 15: 30-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zhu W, Fan Y, Frenzel T, Gasmi M, Bartus RT, Young WL, et al. (2008). Insulin growth factor-1 gene transfer enhances neurovascular remodeling and improves long-term stroke outcome in mice. Stroke; a journal of cerebral circulation, 39: 1254-1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ozawa CR, Banfi A, Glazer NL, Thurston G, Springer ML, Kraft PE, et al. (2004). Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J Clin Invest, 113: 516-527 [DOI] [PMC free article] [PubMed] [Google Scholar]