Abstract
Parkinson's disease (PD) is a progressive neurodegenerative disorder with complicated pathophysiologic mechanisms. Endoplasmic reticulum (ER) stress appears to play a critical role in the progression of PD. We demonstrated that basic fibroblast growth factor (bFGF), as a neurotropic factor, inhibited ER stress-induced neuronal cell apoptosis and that 6-hydroxydopamine (6-OHDA)-induced ER stress was involved in the progression of PD in rats. bFGF administration improved motor function recovery, increased tyrosine hydroxylase (TH)-positive neuron survival, and upregulated the levels of neurotransmitters in PD rats. The 6-OHDA-induced ER stress response proteins were inhibited by bFGF treatment. Meanwhile, bFGF also increased expression of TH. The administration of bFGF activated the downstream signals PI3K/Akt and Erk1/2 in vivo and in vitro. Inhibition of the PI3K/Akt and Erk1/2 pathways by specific inhibitors partially reduced the protective effect of bFGF. This study provides new insight towards bFGF translational drug development for PD involving the regulation of ER stress.
Keywords: Parkinson's disease, ER stress, bFGF, 6-OHDA
Parkinson’s disease (PD) is the second most common neurodegenerative disorder. It is characterized pathologically by the loss of dopaminergic neurons, primarily in the substantia nigra pars compacta (SNpc), and by the presence of ubiquitinated protein deposits in the neuronal cytoplasm. Another pathological feature of PD is the formation of Lewy bodies, which contain a variety of proteins including ubiquitin and alpha-synuclein (α-syn) [1,2]. According to previous studies, factors such as endoplasmic reticulum (ER) stress, oxidative stress, and genetic factors may culminate in dopaminergic neurodegeneration, thus leading to the neuronal death that occurs in PD [3]. Therefore, these factors have become the focus of treatment strategies for PD.
ER stress triggers an adaptive program called the unfolded protein response (UPR), which may inhibit early protein synthesis to reduce the load of the ER. However, when mis-folded proteins accumulate continuously, they overload the ER, and apoptotic cell death often ensues [4]. The ER stress response is characterized by changes in specific proteins (GRP78, ATF6, XBP-1 and apoptosis protein CHOP and caspase-12), which cause translational attenuation, induction of ER chaperones, and degradation of mis-folded proteins [5,6]. The PD-inducing neurotoxins 6-hydroxydopamine (6-OHDA), 1-methy-l-4-phenylpyridinium (MPP+) and rotenone have been reported to induce the UPR in ER chaperones such as BiP, PDI, calreticulin, IRE1α, and PErk, indicating that ER stress plays a causative role in neuronal cell death [7,8]. Accumulation of polyubiquitinated proteins and UPR activation has been observed in the postmortem brains of PD patients [9]. Altogether, this evidence suggests that suppressing ER stress may be beneficial to PD patients.
Basic fibroblast growth factor (bFGF) is a member of the FGF family, which includes at least 23 members. As a multifunctional protein, bFGF presents therapeutic potential in CNS repair, inducing the proliferation and differentiation of neural progenitor cells, enhancing the survival and axonal sprouting of neural tissue, and promoting the survival of neural precursors. Several studies have focused on the relevance of bFGF in the dopaminergic nigrostriatal system. Chadi et al. reported that bFGF promotes dopaminergic cell survival in human fetal tissue strands transplanted into immunosuppressed 6-OHDA-lesioned rats [10]. Another group showed that bFGF protects against rotenone-induced dopaminergic cell death [11]. In our previous study, we found that bFGF inhibits ER stress-induced neuronal cell apoptosis in spinal cord injury and brain ischemia/reperfusion injury [12,13]. Hence, inhibition of ER stress as an underlying mechanism of bFGF for treatment of PD requires further investigation.
In the current study, we investigated the effect of bFGF on ER stress in 6-OHDA-induced PD both in vivo and in vitro. Our data demonstrates that the neuroprotective effect of bFGF is related to the inhibition of ER stress-induced apoptosis in PD. This study contributes towards elucidating new therapeutic targets and treatment strategies in PD research.
MATERIALS & METHODS
Animals and treatment paradigm
Young adult male SD rats (280-320 g) were purchased from the Animal Center of the Chinese Academy of Sciences and cared for in accordance with the National Institutes for Health Guide for the Care and Use of Laboratory Animals. All procedures were approved by the Institutional Animal Care and Use Committee of Wenzhou Medical University. The rats were maintained in a temperature- and humidity-controlled environment under a 12-h light/dark cycle with ad libitum access to food and water. The animals were anesthetized with 5% chloral hydrate and shaved, and the back of their heads were disinfected. Then, longitudinal cuts were made along the midline of the back of the head, and the fascia was removed by means of 30% hydrogen peroxide etching, thereby exposing the bregma of the skull. The rats were then fixed to a stereotaxic apparatus (KOPF Company, Germany). The PD rat model was reproduced by injecting 6-OHDA (10 μl, 1.5 μg/μl, dissolved in 0.2% ascorbic acid saline solution, Sigma-Aldrich, St. Louis, MO, USA) unilaterally into the right striatum at a constant rate (coordinates: A: +0.7 mm from bregma, L: +2.8 mm from midline and H: +5.5 mm). The sham group rats were injected with 0.2% ascorbic acid in saline at the same location.
One week after right striatum stereotaxic injection of 6-OHDA, animals were subjected to rotational behavior testing. Rats were injection subcutaneously with apomorphine hydrochloride (0.5 mg/kg), placed in a round kettle (40 cm diameter), and the number of contralateral turns in a 30-min period was recorded. The rats with more than 7 contralateral turns per minute were used as valid PD pathology animal models. The PD rats were randomly assigned to two groups, which included the bFGF (80 μg/kg/day) group and vehicle control group. A sham-lesion control was also included. bFGF (Grostre Biotech Co., Wenzhou, China) in distilled water was injected via the tail vein for 2 weeks. The rotational behavior testing of rats was repeated at 1, 2, and 3 weeks after the first bFGF administration.
Primary hippocampal neurons culture and treatment
Primary hippocampal neuron cultures were established from the brains of neonatal Sprague-Dawley rats (>4 h). Hippocampi were dissected from the brains and rinsed in ice-cold dissection buffer. Blood vessels and white matter were removed, and tissues were treated with 0.125% trypsin in Hank’s balanced salt solution for 20 min at 37°C. The whole solution was filtered through stainless steel (200 mesh, hole-width 95 µm). The cell suspension was centrifuged twice at 1000 rpm for 10 min, and the cell pellets were resuspended in the DMEM/F-12 with 20% fetal bovine serum, 100 U/l penicillin, 100 mg/l streptomycin and 0.5 mM glutamine. Cells were seeded at a density of 1-5 × 105/ml in 96-well plates or coverslips precoated with poly-L-lysine and kept at 37°C in a 5% CO2 incubator. The culture medium was changed after 24 h and again every two or three days thereafter. Arabinosylcytosine (10 mg/l) was added at 72 h to prevent the growth of non-neuronal cells. All experiments were performed at 8-11 days after plating. The purity of neurons was measured by staining with anti-microtubule associated protein 2 (MAP-2) antibody (1:50) using immunofluorescence staining.
Viability assay
Cells were grown in DMEM medium supplemented with 10% fetal bovine serum and 1% antibiotics and then incubated in a humidified atmosphere containing 5% CO2 at 37 °C. Primary hippocampal neurons were seeded into 96-well plates and treated with 6-OHDA (50, 100, 150, 200 and 400 μM) for 24 h with or without bFGF (10, 20, 40 and 80 ng/mL). After 24 h incubation, cell viability was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. During the final 4 h, MTT (600 mM) was added to the media. Cells were then washed with PBS (pH 7.4), DMSO was added to solubilize the formazan crystals, and the absorbance was measured at 570 nm. Optimal conditions of 150 μM 6-OHDA and 20 ng/ml bFGF were used for subsequent experiments. To further evaluate the effect of PI3K/Akt and Erk1/2 activation on oxidative injury, cells were pretreated for 2 h with specific inhibitors LY294002 (20 μM) and U0126 (20 μM) before the addition of bFGF. Cell signaling and cell survival were then analyzed. Pretreatment compounds were not removed from the media before successive treatment conditions. All experiments were performed in triplicate.
High-Performance Liquid Chromatography Electrolytic Conductivity Detector (HPLC-ED) Analysis
Three weeks after the first bFGF administration, 10 rats were selected from each group for measurement of monoamine neurotransmitters. To collect samples for off-line analysis, the microdialysis probes were implanted into the rat striatum and were perfused with Ringer’s solution. The samples obtained within the first 90 min from each rat were discarded from analysis. Subsequently, the samples were collected and immediately frozen. Before HPLC-ED analysis and injection, the sample was thawed at 4°C. DA, 3, 4-dihydroxyphenyl acetic acid (DOPAC), homovanillic acid (HA), 5-hydroxyindole acetic acid (5-HIAA), 5-hydroxytryptamine (5-HT), epinephrine (E), and norepinephrine (NE), were purchased from Sigma-Aldrich Ltd. (St. Louis, MO, USA). Liquid chromatography experiments were performed using an Agilent 1100 HPLC (Agilent Technologies, Santa Clara, CA, USA), with an Agilent Eclipse XDB-C18 column (2.1 mm × 150 mm, 5 μm particle, Agilent Technologies, Santa Clara, CA, USA). The working electrode was a glassy carbon disc electrode (3 mm in diameter) or an acetylene black nanoparticle (ABN)-modified electrode. The rat’s corpus striatum was weighed and placed in a centrifuge tube with 10 ml/g of 0.1 M perchloric acid solution and homogenized on ice. The soluble fraction was obtained by two cycles of centrifugation at 12,000 rpm for 10 min and 4 °C. Before HPLC-ED analysis, the sample was thawed at 4 °C prior to injection. The standard curve was calculated using Clarity v.2.6.4.402 software for each sample in various concentrations of neurotransmitters; GraphPad Prism 5.0 was used for the analysis.
Immunohistochemistry
Three weeks after the first bFGF administration, six rats were selected from each group, their striata were quickly dissected and placed on ice immediately after euthanasia, and immunohistochemistry was performed as described previously [12, 13]. Briefly, the rats were anesthetized with 4% choral hydrate (10 ml/kg, intraperitoneal, IP) and then perfused with 4% paraformaldehyde in a 0.1 M phosphate buffer. Brains were dissected and post-fixed in the same fixative overnight at 4 °C. The substantia nigra was embedded in paraffin and sectioned. To prepare for staining, the tissue was rehydrated, dewaxed, and antigen retrieval was performed at a high temperature and pressure. The sections were then incubated with 3% hydrogen peroxide for 30 min and then with 1% bovine serum albumin (BSA) for 30 min at room temperature. Sections were then incubated overnight with primary antibodies against CHOP (1:150), GRP78 (1:200), caspase-12 (1:600) and TH (1:300) at 4 °C. The sections were then washed with PBS three times and incubated with horseradish peroxidase-conjugated secondary antibodies for 2 h at 37 °C. Finally, the sections were incubated with 3, 3-diaminobenzidine (DAB, 5% diluted with PBS) for 5 min. The saline injection group was considered the negative control. The results were analyzed by counting the number of positive cells at 40× magnification using a Nikon ECLPSE 80i (Nikon, Tokyo, Japan). The optical density of CHOP, GRP78, caspase-12 and TH in the substantia nigra was counted in 5 randomly selected fields per sample.
Flow cytometry analysis
To quantify the number of apoptotic cells in each group, primary hippocampal neurons were seeded in 60-mm plates and were incubated with 150 μM 6-OHDA and 20 ng/ml bFGF for 24 h. The cells were then harvested after incubation. After triple centrifugation and washing in cold PBS, cells were re-suspended in 1× binding buffer, double-stained with PI/Annexin V-FITC kit (Invitrogen, Carlsbad, CA, USA) for 15 min at room temperature in the dark, and the apoptosis level was analyzed using a FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA).
Western blot analysis
Brain tissues and primary hippocampal neurons were homogenized in lysis buffer as described previously [13]. The cultured cells were lysed in a protein extraction reagent with protease and phosphatase inhibitors. The equivalent of 50 μg of protein was boiled for 10 min and resolved by SDS-PAGE using 10% Tris/Tricine gel (Bio-rad, Hercules, CA) and then transferred onto a PVDF membrane and processed for immunolabeling the proteins of interest. Membranes were incubated with the following antibodies: XBP-1 (1:300), CHOP (1:300), GRP78 (1:300), ATF6 (1:300) and caspase-12 (1:1000 Santa Cruz Biotech, Santa Cruz, CA, USA) overnight at 4 °C. The next day, the membranes were washed three times with TBST, incubated with secondary IgG-HRP antibodies (1:3000) for 1 h, and then washed again with TBST. Lastly, immunoreactive protein bands were visualized with a ChemiDocTM XRS+ Imaging System (Bio-Rad Laboratories, Hercules, CA, USA), and band densities were quantified using Multi-Gauge Software of Science Lab 2006 (FUJIFILM Corporation, Tokyo, Japan).
Statistical analysis
All values are expressed as the mean ± SEM. Data from at least 3 sets of independent experiments were analyzed by one-way analysis-of-variance (ANOVA) tests, followed by a Dunnett’s post hoc test. Values of P < 0.05 were considered significant.
RESULTS
bFGF alleviates 6-OHDA-induced motor dysfunction and increases the levels of neurotransmitters in PD rats
To evaluate the protective effects of bFGF on 6-OHDA-induced PD rats, they were injected with exogenous bFGF through the tail vein every day for 14 days. PD makes rats rotate from the contralateral side to the affected side in response to apomorphine. As shown in Fig. 1A, both the PD control rats and bFGF-treated rats demonstrated this rotational behavior, whereas the sham group did not. However, the number of turns for bFGF administered rats decreased remarkably to just 4.56 ± 0.83 turns/min by day 21, which was significantly less than that for PD control rats (P < 0.05).
Figure 1.
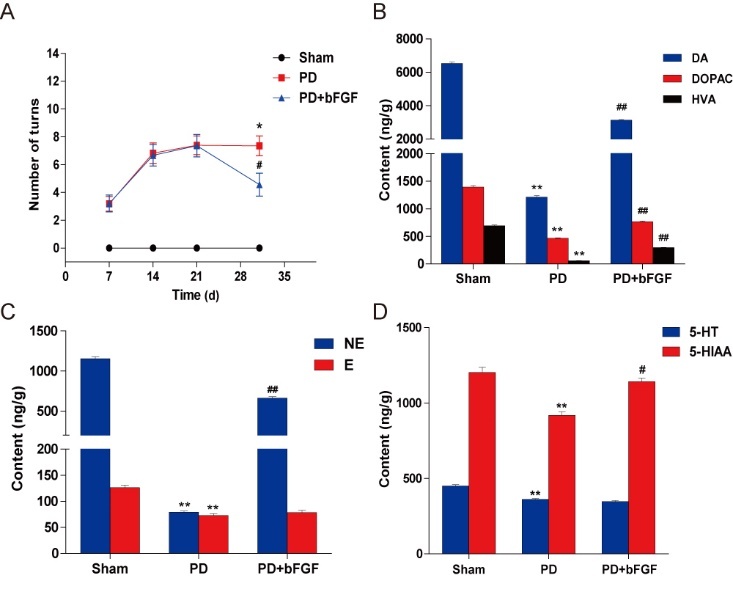
Effects of bFGF infusions on amphetamine-induced rotation and neurotransmitter levels in the striatum of PD model rats. (A) Effects of bFGF administration on the apomorphine (APO)-induced ipsilateral rotations measured at 1, 2 and 3 weeks after lesion. **P < 0.01 versus sham group, # P < 0.05 and ## P < 0.01 versus PD group. (B), (C), (D) The levels of monoamine neurotransmitters in the striatum detected by HPLC-ED at 3 weeks post-lesion. **P < 0.01 versus sham group, #P < 0.05 and ##P < 0.01 versus PD group. Values are presented as the mean ± SD (n = 10).
In addition, to rule out the possibility that bFGF exerted a beneficial effect by increasing the monoamine neurotransmitters in the corpus striatum, HPLC-ECD was performed to detect these levels. Compared with the sham group, the levels of neurotransmitters in the PD group, including dopamine, DOPAC, HVA, NE, E, 5-HT, and 5-HIAA, decreased remarkably (Fig. 1B-1D). However, treatment with 80 μg/kg/day bFGF resulted in significant increases in the levels of DA, DOPAC, HVA, NE and 5-HIAA. It should be noted that levels of E and 5-HT were not significantly increased by bFGF. Taken together, these findings indicate that bFGF alleviates 6-OHDA-induced motor dysfunction and increases secretion of some neurotransmitters.
bFGF protects TH-positive neurons and inhibits ER stress-induced cell death in PD rats
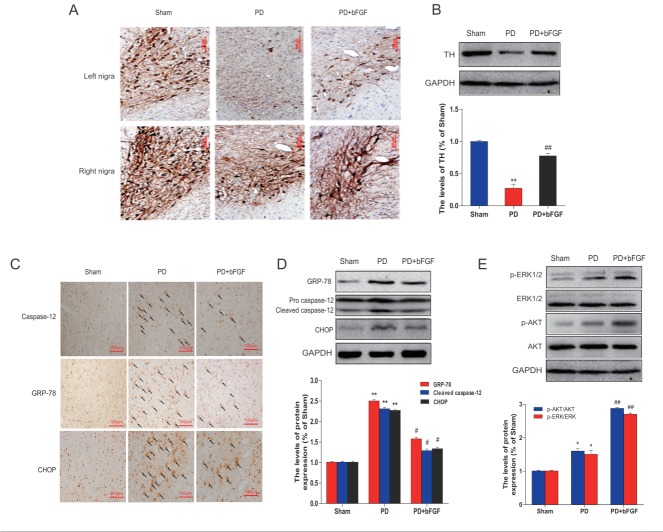
TH staining was performed to evaluate the survival of dopaminergic neurons. As shown in Fig. 2A, the cytoplasm and fibers of dopaminergic neurons in the sham group were intensely stained, and the cellular processes were evident. In contrast, rats in the PD group showed a marked loss of dopamine-containing substantia nigra neurons in the right side, but few TH-positive cells were detected and the cellular processes were absent for most cells. bFGF administration resulted in a 3-fold increase in TH-positive cells showing a cell morphology similar to those in the sham group (Fig. 2B), indicating that bFGF could protect dopaminergic neurons from 6-OHDA neurotoxicity in rats.
Figure 2.
Effects of bFGF on TH levels and ER stress-related proteins at 3 weeks post-lesion in PD rats. (A) Immunohistochemistry of TH-positive cells in the right and left nigra. (B) TH levels analyzed by Western blot. **P < 0.01 versus sham group, ##P < 0.01 versus PD group. (C) Immunohistochemical analysis of GRP78, CHOP and caspase-12 in the left nigra. (D) GRP78, CHOP and caspase-12 levels analyzed by Western blot. (E) Erk1/2 and Akt analyzed by Western blot. **P < 0.01 versus sham group, #P < 0.05 and ##P < 0.01 versus PD group (n = 6).
To determine whether the neuroprotective effect of bFGF is related to ER stress, we measured the expression of ER stress-related proteins. Our immunohistochemical results indicated that very low levels of ER stress-related proteins CHOP, GRP78, and cleaved caspase-12 were present in the cells of the right nigral and corpus striatum region of the sham group. However, in PD rats, the cells positive for CHOP, GRP78 and cleaved caspase-12 were significantly increased in the same regions when compared with the sham group. Moreover, bFGF treatment suppressed by more than 50% the activation of these ER stress-related proteins in PD rats (Fig. 2C). The expression of CHOP, GRP78 and cleaved caspase-12 were also verified by Western blot analysis. Consistent with the immunohistochemical results, the protein levels of CHOP, GRP78 and cleaved caspase-12 were significantly upregulated in the right nigral region in PD rats, whereas treatment with bFGF reduced the activation of ER stress-related proteins when compared with the respective control group (Fig. 2D).
To further understand the underlying mechanism behind the effect of bFGF on PD model rats, the activation of PI3K/Akt and Erk1/2 downstream signals were also analyzed by Western blot. As expected, bFGF treatment increased the phosphorylation of Akt and Erk1/2 when compared with controls (Fig. 2E). Taken together, these results demonstrate that the protective role of bFGF in PD is related to the inhibition of ER stress through activation of the PI3K/Akt and Erk1/2 signaling pathways.
Exogenous bFGF inhibits 6-OHDA-induced apoptosis in primary hippocampal neurons
Primary hippocampal neurons were treated with 6-OHDA to mimic the PD model in vitro. There was a dose-dependent decrease in the viability of primary hippocampal neurons exposed to 6-OHDA (Fig. 3A). Cell viability significantly decreased by approximately 45% and 40% after primary hippocampal neurons cells were treated with 150 μM 6-OHDA for 24 h. Conversely, cells treated with various concentrations of bFGF prior to the addition of 6-OHDA significantly increased cell viability. It should be noted that we did not observe a dose-dependent effect on cell proliferation with bFGF concentrations ranging from 10 ng/ml to 80 ng/ml. (Fig. 3B). To confirm the effect of bFGF on 6-OHDA-induced apoptosis in primary hippocampal neurons, flow cytometry was performed. After 24 h of incubation with 150 μM 6-OHDA, the percentage of cells showing early apoptosis markers increased significantly. Although 6-OHDA-induced apoptosis was reduced by approximately 70% and 50% by treatment with exogenous bFGF (20 ng/ml), bFGF alone did not display an obvious effect (Fig. 3C, 3D). Changes in nuclear morphology were observed with Hoechst 33258 staining. The normal nuclei showed homogeneous staining, bearing regular rounded shapes. However, following exposure to 150 µM 6-OHDA for 24 h, most cells showed asymmetrical, brightly fluorescent nuclei, and the number of condensed nuclei also increased. Collectively, these data indicate that bFGF inhibits 6-OHDA-induced apoptosis in primary hippocampal neurons.
Figure 3.
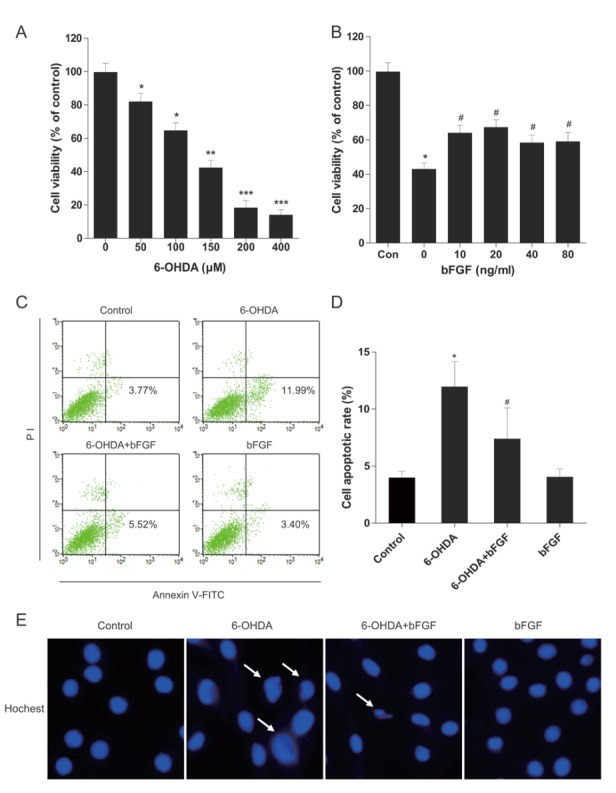
Effects of bFGF on 6-OHDA-induced apoptosis in primary hippocampal neurons. (A) Primary hippocampal neurons were treated with different concentrations of 6-OHDA for 24 h, and then cell viability was assessed by MTT assay. (B) Primary hippocampal neurons were treated with 6-OHDA (150 µM) and different concentrations of bFGF for 24 h, and then cell viability was assessed by MTT assay. (C) Primary hippocampal neurons were treated with 6-OHDA (150 µM) and bFGF (20 ng/ml) for 24 h, and then cells were stained with annexin V-FITC/propidium iodide and detected by flow cytometry; the lower right panel indicates the apoptotic cells. (D) Levels of cell apoptosis. *P < 0.05 versus control group, **P < 0.01, ***P< 0.001, #P < 0.05 versus 6-OHDA group (n = 3).
Exogenous bFGF inhibits 6-OHDA-induced ER stress in primary hippocampal neurons
To determine whether the molecular mechanisms of bFGF are related to the regulation of ER stress in these two cell types, the protein expression of ER stress-induced apoptosis was analyzed using Western blot. As shown in Fig. 4A, the expression of GRP78, XBP-1, ATF-6, cleaved caspase-12 and CHOP was significantly increased in the 6-OHDA incubated cells when compared with the control group. bFGF treatment alone had no effect on the level of ER stress response proteins. However, GRP78, CHOP, cleaved caspase-12, XBP-1 and ATF-6 were down-regulated in the group exposed to 6-OHDA plus bFGF. Furthermore, treatment with bFGF significantly increased the expression of TH, which was decreased by 6-OHDA. Taken together, the results suggest that the protective role of bFGF may involve the inhibition of ER stress-induced proteins.
Figure 4.
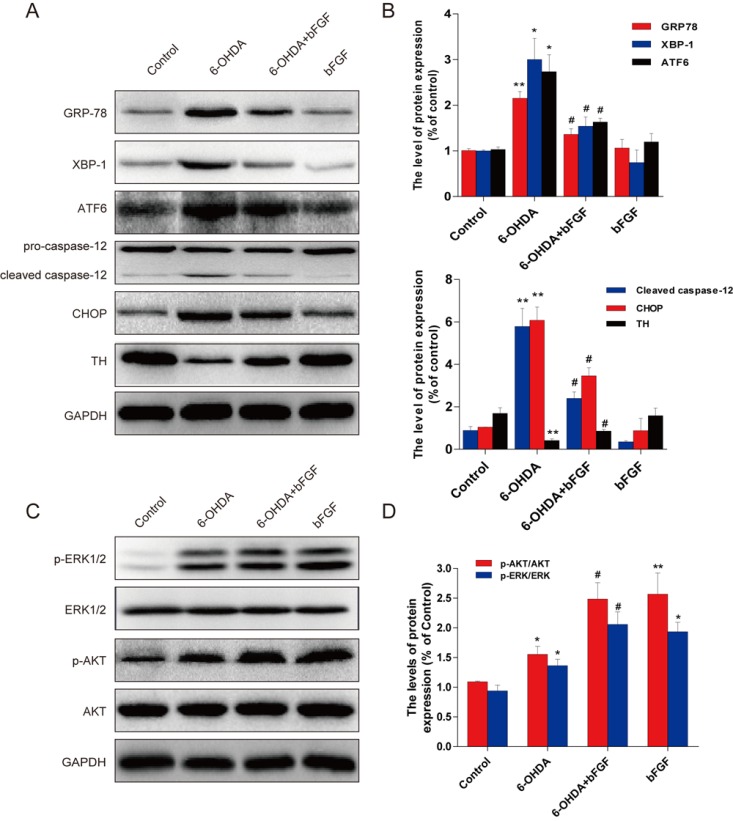
Effect of bFGF on 6-OHDA-induced ER stress and Erk/Akt phosphorylation in primary hippocampal neurons. (A) Primary hippocampal neurons were treated with 6-OHDA (150 µM) and bFGF (20 ng/ml) for 24 h. Cells were then collected, and ATF6,GRP78, XBP-1, caspase12, CHOP were analyzed by Western blot. (B) Optical density analysis of ER stress-related proteins. (C) p-Erk1/2, Erk1/2, p-Akt and Akt were analyzed by Western blot. (D) Optical density analysis of p-Erk/Erk and p-Akt/Akt. *P < 0.05 and **P < 0.01 versus control group, #P < 0.05 versus 6-OHDA group (n = 3).
The activation of PI3K/Akt and Erk1/2 signals is crucial for the protective role of bFGF
PI3K/Akt and Erk1/2 pathways are the main downstream signals of bFGF; therefore, we hypothesized that the PI3K/Akt and Erk1/2 pathways may be involved in the downstream effects of bFGF-induced inhibition of ER stress in the 6-OHDA-induced PD model. As shown in Figure 4C, an increase in p-Akt and p-Erk1/2 was observed in both primary hippocampal neurons exposed to 6-OHDA when compared with control cells. Pre-treatment with bFGF significantly increased the activation of the PI3K/Akt and Erk1/2 pathways in the primary hippocampal neurons exposed to 6-OHDA (Figure 4C, 4D). These data suggest that both the PI3K/Akt and Erk1/2 pathways are involved in the protective effect of bFGF.
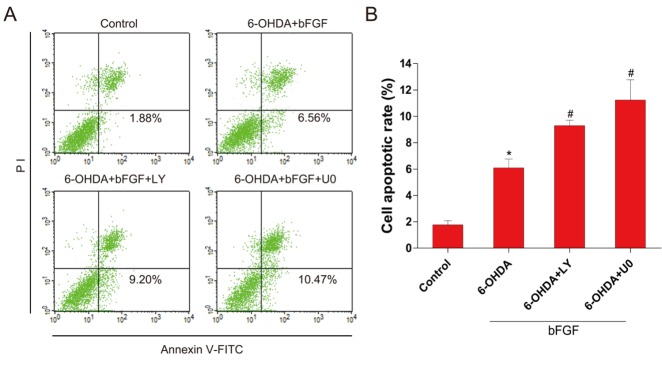
To demonstrate the molecular mechanisms of bFGF in the ER stress-induced cell apoptosis model, two classic signal inhibitors, LY294002 for PI3K/Akt and U0126 for Erk1/2, were added to the cell model. Neither of these inhibitors affected cell death when used. [14,15] The activation of XBP-1, GRP78, ATF-6 and cleaved caspase-12 by 6-OHDA treatment was inhibited by the addition of bFGF; however, this protective effect was abolished by LY294002 and U0126. The levels of TH, p-Akt and p-Erk1/2 also increased with bFGF treatment and decreased with the addition of the inhibitors (Fig. 5A-5E). Moreover, as shown in Fig. 6, the addition of LY294002 or U0126 significantly increased cell apoptosis when compared with the bFGF group. These results suggest that the protective effect of bFGF is mediated by both the PI3K/Akt and Erk1/2 signaling pathways.
Figure 5.
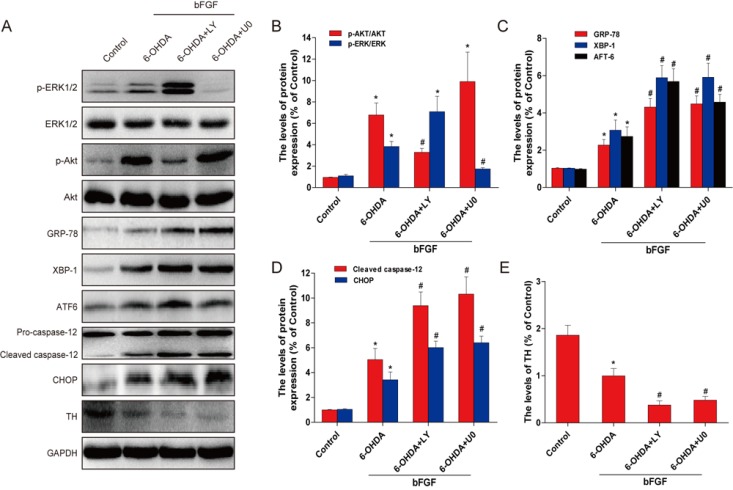
Inhibition of PI3K/Akt and Erk1/2 pathways partially attenuates the reduction of the ER stress by bFGF in primary hippocampal neurons. (A) Primary hippocampal neurons were treated with or without the specific inhibitors LY294002 (20 μM) and U0126 (20 μM). Cell lysates were then analyzed by Western blotting for the expression of p-Akt, Akt, p-Erk, Erk, GRP78, XBP-1, ATF6, cleaved-caspase-12, and TH. Bar diagrams of (B) p-Akt/Akt and p-Erk/Erk; (C) GRP78, XBP-1, and ATF6; (D) cleaved caspase-12 and CHOP; and (E) TH from three Western blot analyses. *P < 0.05 versus control group, #P < 0.05 versus 6-OHDA group (n = 3).
Figure 6.
Inhibition of PI3K/Akt and Erk1/2 pathways partially impairs the protective effects of bFGF in 6-OHDA-induced primary hippocampal neurons. (A) Primary hippocampal neurons were collected and stained with annexin V-FITC/PI and detected by flow cytometry; the lower right panel indicates apoptotic cells. (B) Cell apoptosis levels from three separate experiments. ***P < 0.001 versus control group, #P < 0.05 versus 6-OHDA group (n = 3).
DISCUSSION
The main pathological features of PD include the degeneration of the dopaminergic neurons in the SNpc and the formation of Lewy bodies, which consist of α-synuclein aggregates. Growing evidence from clinical studies of PD patients and genetic and toxicology animal models indicate that ER stress is a common feature of the disease and contributes to neurodegeneration [16,17]. A previous study that examined the brains of PD patients showed higher levels of phosphorylated PErk and downstream to eIF2α [18]. In another study, 6-OHDA and MPP+ were found to increase the phosphorylation of UPR proteins, XBP-1, P-Erk and eIF2α in an MN9D cell line [19]. Grp78 and CHOP are well-known indicators of ER stress, and their increased levels indicate ER stress induction; moreover, the CHOP pathway is a major regulator of ER stress-induced apoptosis, as CHOP-/- cells exhibit a lower frequency of programmed cell death [20]. CHOP is the initial signal that triggers the apoptosis pathway, and it represses the promoter of the bcl-2 gene, which renders cells sensitive to the pro-apoptotic effects [21,22]. In this study, we investigated ER stress induced by 6-OHDA in vivo and in vitro. As reported in previous studies [23,24], we found that exposure to 6-OHDA decreased TH expression. We also found that cell viability decreased with increased expression of GRP78, CHOP and caspase-12, suggesting that ER stress plays a potential role in 6-OHDA-induced neuron death in PD models.
It should be noted that 6-OHDA can also cause mitochondrial damage that can trigger many different cellular pathological pathways, including ER stress. The possible underlying mechanism of neurotoxicity induced by 6-OHDA may be related to oxidative stress and mitochondrial respiratory dysfunction, which is caused by the production of hydroxyl radicals during autoxidation [25] and the inhibition of complex I [26], resulting in excessive oxidative stress and eventually neuronal death. In fact, ER stress is closely related to oxidative stress [27] and mitochondrial damage in PD models. ER stress can lead to oxidative damage by activating oxidative protein folding enzymes, such as ERO1, which participates in protein disulfide bond formation during protein refolding in the ER to relieve ER stress [28]. ER stress can also induce mitochondrial damage, resulting in a bioenergetic deficit and mitochondrial fragmentation. Conversely, mitochondrial stress can induce ER stress, which is reflected by the induction of UPR [29]. Therefore, we focus on 6-OHDA-induced ER stress signaling pathways in this study. However, further studies are required to identify the precise function of communication between the ER and mitochondria in 6-OHDA-induced PD model.
With the relationship between ER stress and PD confirmed, finding new targets for the treatment of PD based on ER stress-related mechanisms has become a hot topic for research. In organotypic hippocampal slice cultures, mithramycin was found to provide resistance to ER stress-induced neurotoxicity [30]. Takano et al. demonstrated that methoxyflavones could protect cells against ER stress caused by neurotoxins in PD models [31]. We previously showed that bFGF could protect against oxidative injury or ischemia/reperfusion injury in stroke and spinal cord injury that were related to the inhibition of ER stress-induced neurocyte apoptosis [12, 13]. In the present study, the levels of these ER stress-induced apoptosis proteins were assessed following dopaminergic neuronal injury to investigate the mechanistic benefit of bFGF in PD. A suitable concentration of bFGF also suppressed the upregulation of ER stress response proteins and significantly decreased 6-OHDA-induced cell apoptosis. We also detected an increase in the ER stress response proteins GRP78, CHOP, caspase-12 in the cytoplasm and fibers of dopaminergic neurons in PD rats. Moreover, treatment with bFGF demonstrated a protective effect by increasing TH-positive neuron cells and increasing locomotor activity in the PD rats. These results indicate that ER stress was involved in PD and could be inhibited by exogenous bFGF administration.
In this study, an ABN-modified electrode was used for HPLC-ED, which has excellent catalytic activity for the oxidation of monoamine neurotransmitters and their metabolites, as well as a relatively high sensitivity, stability, and long life [32]. Our results indicated that 6-OHDA reduced the levels of neurotransmitters in PD rats. However, bFGF reduced the down-regulation of dopamine, DOPAC, HVA, NE and 5-HIAA, suggesting that bFGF exerts neuroprotective activity in this PD animal model. This study provides valuable information towards performing neurochemical measurements of monoamine neurotransmitters and their metabolites in the striatum of conscious and freely moving PD rats. We found that these levels could be easily assessed when combined with in vivo microdialysis.
The PI3K/Akt and Erk1/2 pathways are two main downstream signaling pathways activated by bFGF. The PI3K/Akt pathway is necessary for mediating the trophic factor-induced survival of several neuronal cell types [33]. Activated Erk kinase has also been shown to induce the neuronal transdifferentiation of dopaminergic neuron cells [34,35]. For instance, Akt and Erk phosphorylates the pro-apoptotic Bcl-2 family member Bax and thereby inhibits its pro-apoptotic functions [36,37]. Although PI3K/Akt and Erk1/2 signal activation is generally considered to be prosurvival, the role of the PI3K/Akt and MAPK/Erk cascades towards the protective effects of bFGF and ER stress in 6-OHDA-induced PD models requires further characterization. In the present study, bFGF showed a neuronal protective effect in a PD rat model and in 6-OHDA-induced primary hippocampal neurons via the activation of both PI3K/Akt and Erk1/2 signals, which was consistent with the findings of Hsuan et al. and Clough et al. [11,38]. To further confirm that these two pathways are essential for the protective effect of bFGF, we used the PI3K/Akt inhibitor LY294002 or the Erk1/2 inhibitor U0126 to treat primary hippocampal neurons and showed that they abolished the anti-apoptotic effects of bFGF. We found it interesting that Akt inhibition greatly upregulated Erk1/2 phosphorylation in the presence of bFGF (Fig. 5A), and we speculate that Akt inhibition may provide partial compensation of Erk1/2 signals. Moreover, these inhibitors reversed the levels of the ER stress response proteins CHOP, cleaved caspase-12, XBP-1 and ATF-6 attenuated by bFGF in 6-OHDA-induced primary hippocampal neurons, suggesting that the activation of Akt and Erk is essential for bFGF inhibition of ER stress in PD. Some studies suggest that this may be related to the translocation of Bim to the ER in response to ER stress, which is an important mechanism for the activation of caspase-12 and the initiation of ER stress-induced apoptosis [39]. However, further studies are necessary to elucidate whether the protective effect of bFGF and activation of the PI3K/Akt pathway are also mediated by reduction of Bim translocation to the ER. ER stress knock-out mice could help to address this question.
bFGF has generated considerable excitement as a promising therapy for neurodegenerative diseases, including PD [11], Huntington’s disease [40] and Alzheimer’s disease [41]. bFGF appeared to be efficacious in two Phase II clinical trials for stroke, but failed in the large scale Phase III trial [42, 43]. The issue also arises that bFGF is highly unstable and undergoes rapid enzymatic degradation, resulting in the loss of biological activity. Our lab used gelatin nanostructured lipid carriers that mediated intranasal delivery of bFGF to the brain, and they displayed a good safety profile and an obvious therapeutic effect in PD [44]. Intravenous bFGF can penetrate the blood-brain barrier (BBB) in an ischemia-reperfusion animal model [45], though it may not cross the BBB in PD model [46]. Thus, our lab used modified bFGF by covalently attaching polyethylene glycol (PEG) polymers to improve their superior pharmacological activity. This addition resulted in greater permeability through the BBB and better in vivo stability compared to native bFGF [47]. Additional studies are needed to explore the appropriate therapeutic window, dosage, and combinations with other therapeutic agents or with biomaterials, which may improve the efficacy of bFGF.
It should be noted that our group also reported that acidic fibroblast growth factor (FGF-1, aFGF) can effectively improve monoamine neurotransmitter levels and improve the symptoms of PD [48]. Administration of aFGF also significantly reduced damage to 6-OHDA-induced PD and inhibition of ER stress-induced cell apoptosis. However, compared with aFGF, bFGF may play more important roles in PD. First, the volumes of the substantia nigra were enlarged in both bFGF-/- and in bFGF transgenic mice, suggesting that bFGF is required for establishing the proper number of DA neurons and a normal sized substantia nigra during development [49]. Second, astrocyte-derived bFGF is required for regulation of DA differentiation of stem cells and may provide a strategy for targeting astrocytes for the treatment of PD [50]. Third, the neuroprotective effects of bFGF might be caused by directly promoting endothelium-derived upregulation and secretion of neuronal trophic factors, such as brain-derived neurotrophic factor (BDNF) [51] and nerve growth factor (NGF). Thus, bFGF exhibits greater potential for clinical applications in PD than aFGF.
Conclusion
In summary, the present study demonstrated that bFGF significantly ameliorated 6-OHDA-induced brain injury, as evidenced by improvements in the rotational test (apomorphine-induced circling) and pathological lesions in TH-positive substantia nigra neurons. Furthermore, bFGF reduced ER stress-induced apoptosis through activation of the PI3K/Akt and Erk1/2 pathways. Our study provides new evidence to support therapeutic strategies for PD in which bFGF is used to target ER stress. More effort is required to expound the detailed mechanisms and develop an effective drug delivery system to fully realize the clinical potential of bFGF.
Acknowledgments
This study was partly supported by research grants from the Zhejiang Provincial Natural Science Funding (LY14H150010, LY14H090013, Y2090834), National Natural Science Funding of China (81560375, 81472165, 81302775), Zhejiang Provincial Program for the Cultivation of High-level Innovative Health talents (to J.X.), Ninbo City Natural Science Funding (2015A610208), Zhejiang Pharmaceutical Association Project (2014ZYY36). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflicts of interest
The authors confirm that the content of this paper has no conflicts of interest.
References
- [1].Redgrave P, Rodriguez M, Smith Y, Rodriguez-Oroz MC, Lehericy S, Bergman H, et al. (2010). Goal-directed and habitual control in the basal ganglia: implications for Parkinson's disease. Nat Rev Neurosci, 11: 760-772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Schwarz J, Storch A (2010). Transplantation in Parkinson's disease: will mesenchymal stem cells help to reenter the clinical arena. Transl Res, 155: 55-56 [DOI] [PubMed] [Google Scholar]
- [3].Aminzadeh MA, Sato T, Vaziri ND (2012). Participation of endoplasmic reticulum stress in the pathogenesis of spontaneous glomerulosclerosis-Role of intra-renal angiotensin system. Transl Res, 160: 309-318 [DOI] [PubMed] [Google Scholar]
- [4].Li S, Yang L, Selzer ME, Hu Y (2013). Neuronal endoplasmic reticulum stress in axon injury and neurodegeneration. Ann Neurol, 74: 768-777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cheng B, Gong H, Xiao H, Petersen RB, Zheng L, Huang K (2013). Inhibiting toxic aggregation of amyloidogenic proteins: a therapeutic strategy for protein misfolding diseases. Biochim Biophys Acta, 1830: 4860-4871 [DOI] [PubMed] [Google Scholar]
- [6].Mercado G, Valdés P, Hetz C (2013). An ERcentric view of Parkinson's disease. Trends Mol Med, 19: 165-175 [DOI] [PubMed] [Google Scholar]
- [7].Galehdar Z, Swan P, Fuerth B, Callaghan SM, Park DS, Cregan SP (2010). Neuronal apoptosis induced by endoplasmic reticulum stress is regulated by ATF4-CHOP-mediated induction of the Bcl-2 homology 3-only member PUMA. J Neurosci, 30: 16938-16948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ryu EJ, Harding HP, Angelastro JM, Vitolo OV, Ron D, Greene LA (2002). Endoplasmic reticulum stress and the unfolded protein response in cellular models of Parkinson's disease. J Neurosci, 22: 10690-10698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hoozemans J, Van Haastert E, Eikelenboom P, De Vos R, Rozemuller J, Scheper W (2007). Activation of the unfolded protein response in Parkinson’s disease. Biochem Biophys Res Commun, 354: 707-711 [DOI] [PubMed] [Google Scholar]
- [10].Chadi G, Silva C, Maximino JR, Fuxe K, da Silva GO (2008). Adrenalectomy counteracts the local modulation of astroglial fibroblast growth factor system without interfering with the pattern of 6-OHDA-induced dopamine degeneration in regions of the ventral midbrain. Brain Res, 1190: 23-38 [DOI] [PubMed] [Google Scholar]
- [11].Hsuan SL, Klintworth HM, Xia Z (2006). Basic fibroblast growth factor protects against rotenone-induced dopaminergic cell death through activation of extracellular signal-regulated kinases 1/2 and phosphatidylinositol-3 kinase pathways. J Neurosci, 26: 4481-4491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhang HY, Zhang X, Wang ZG, Shi HX, Wu FZ, Lin BB, et al. (2013). Exogenous basic fibroblast growth factor inhibits ER stress-induced apoptosis and improves recovery from spinal cord injury. CNS Neurosci Ther, 19: 20-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang ZG, Zhang HY, Xu X, Shi HX, Yu X, Wang X, et al. (2012). bFGF inhibits ER stress induced by ischemic oxidative injury via activation of the PI3K/Akt and ERK1/2 pathways. Toxicol Lett, 212: 137-146 [DOI] [PubMed] [Google Scholar]
- [14].Zhang HY, Wu FZ, Kong XX, Yang J, Chen H, Deng L, et al. (2014). Nerve growth factor improves functional recovery by inhibiting endoplasmic reticulum stress-induced neuronal apoptosis in rats with spinal cord injury. J Transl Med, 12: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Xu R, Chen J, Cong X, Hu S, Chen X (2008). Lovastatin protects mesenchymal stem cells against hypoxia and serum deprivation induced apoptosis by activation of PI3K/Akt and ERK1/2. J Cell Biochem, 103: 256-269 [DOI] [PubMed] [Google Scholar]
- [16].Zhou JX, Zhang HB, Huang Y, He Y, Zheng Y, Anderson JP, et al. (2013). Tenuigenin Attenuates α-Synuclein Induced Cytotoxicity by Down-Regulating Polo-Like Kinase 3. CNS Neurosci Ther, 19: 688-694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hoozemans JJ, Scheper W (2012). Endoplasmic reticulum: the unfolded protein response is tangled in neurodegeneration. Int J Biochem Cell Biol, 44: 1295-1298 [DOI] [PubMed] [Google Scholar]
- [18].Holtz WA, O'Malley KL (2003). Parkinsonian mimetics induce aspects of unfolded protein response in death of dopaminergic neurons. J Biol Chem, 278: 19367-19377 [DOI] [PubMed] [Google Scholar]
- [19].Silva RM, Ries V, Oo TF, Yarygina O, Jackson Lewis V, Ryu EJ, et al. (2005). CHOP/GADD153 is a mediator of apoptotic death in substantia nigra dopamine neurons in an in vivo neurotoxin model of parkinsonism. J Neurochem, 95: 974-986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhang HY, Wang ZG, Lu XH, Kong XX, Wu FZ, Lin L, et al. (2014). Endoplasmic reticulum stress: relevance and therapeutics in central nervous system diseases. Mol Neurobiol, 51: 1343-1352 [DOI] [PubMed] [Google Scholar]
- [21].Wan XS, Lu XH, Xiao YC, Lin Y, Zhu H, Ding T, et al. (2014). ATF4-and CHOP-dependent induction of FGF21 through endoplasmic reticulum stress. Biomed Res Int, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tanaka KI, Fukuoka S, Kawahara S, Kimoto N, Ogawa N (2013). Effect of cabergoline on increase of several ER stress-related molecules in 6-OHDA-lesioned mice. Neurol Sci, 34: 259-261 [DOI] [PubMed] [Google Scholar]
- [23].Luo F, Wei L, Sun C, Chen X, Wang T, Li Y, et al. (2012). HtrA2/Omi is involved in 6-OHDA-induced endoplasmic reticulum stress in SH-SY5Y cells. J Mol Neurosci, 47: 120-127 [DOI] [PubMed] [Google Scholar]
- [24].Soto-Otero R, Méndez-Álvarez E, Hermida-Ameijeiras Á, Muñoz-Patiño AM, Labandeira-Garcia JL (2000). Autoxidation and Neurotoxicity of 6-Hydroxydopamine in the Presence of Some Antioxidants. J Neurochem, 74: 1605-1612 [DOI] [PubMed] [Google Scholar]
- [25].Glinka YY, Youdim MB (1995). Inhibition of mitochondrial complexes I and IV by 6-hydroxydopamine. Eur J Pharmacol, 292: 329-332 [DOI] [PubMed] [Google Scholar]
- [26].Egawa N, Yamamoto K, Inoue H, Hikawa R, Nishi K, Mori K, et al. (2011). The endoplasmic reticulum stress sensor, ATF6α, protects against neurotoxin-induced dopaminergic neuronal death. J Biol. Chem, 286: 7947-7957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bhandary B, Marahatta A, Kim HR, Chae HJ (2012). An involvement of oxidative stress in endoplasmic reticulum stress and its associated diseases. Int J Mol Sci, 14: 434-456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bouman L, Schlierf A, Lutz A, Shan J, Deinlein A, Kast J, et al. (2011). Parkin is transcriptionally regulated by ATF4: evidence for an interconnection between mitochondrial stress and ER stress. Cell Death Differ, 18: 769-782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kosuge Y, Taniguchi Y, Imai T, Ishige K, Ito Y (2011). Neuroprotective effect of mithramycin against endoplasmic reticulum stress-induced neurotoxicity in organotypic hippocampal slice cultures. Neuropharmacology, 61: 252-261 [DOI] [PubMed] [Google Scholar]
- [30].Takano K, Tabata Y, Kitao Y, Murakami R, Suzuki H, Yamada M, et al. (2007). Methoxyflavones protect cells against endoplasmic reticulum stress and neurotoxin. Am J Physiol Cell Physiol, 292: C353-C361 [DOI] [PubMed] [Google Scholar]
- [31].Lin L, Yang J, Lin R, Yu L, Gao H, Yang S, et al. (2013). In vivo study on the monoamine neurotransmitters and their metabolites change in the striatum of Parkinsonian rats by liquid chromatography with an acetylene black nanoparticles modified electrode. J Pharm Biomed, 72: 74-79 [DOI] [PubMed] [Google Scholar]
- [32].Wang L, Yang HJ, Xia YY, Feng ZW (2010). Insulin-like growth factor 1 protects human neuroblastoma cells SH-EP1 against MPP+-induced apoptosis by AKT/GSK-3β/JNK signaling. Apoptosis, 15: 1470-1479 [DOI] [PubMed] [Google Scholar]
- [33].Zhang Q, Zhang J, Jiang C, Qin J, Ke K, Ding F (2014). Involvement of ERK1/2 pathway in neuroprotective effects of pyrroloquinoline quinine against rotenone-induced SH-SY5Y cell injury. Neuroscience, 270: 183-191 [DOI] [PubMed] [Google Scholar]
- [34].Fieblinger T, Sebastianutto I, Alcacer C, Bimpisidis Z, Maslava N, Sandberg S, et al. (2014). Mechanisms of dopamine D1 receptor-mediated ERK1/2 activation in the parkinsonian striatum and their modulation by metabotropic glutamate receptor type 5. J Neurosci, 34: 4728-4740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sanchez A, Tripathy D, Yin X, Luo J, Martinez J, Grammas P (2012). Pigment epithelium-derived factor (PEDF) protects cortical neurons in vitro from oxidant injury by activation of extracellular signal-regulated kinase (ERK) 1/2 and induction of Bcl-2. Neurosci Res, 72: 1-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Xu Y, Zhang Q, Yu S, Yang Y, Ding F (2011). The protective effects of chitooligosaccharides against glucose deprivation-induced cell apoptosis in cultured cortical neurons through activation of PI3K/Akt and MEK/ERK1/2 pathways. Brain Res, 1375: 49-58 [DOI] [PubMed] [Google Scholar]
- [37].Clough RL, Stefanis L (2007). A novel pathway for transcriptional regulation of α-synuclein. FASEB J, 21: 596-607 [DOI] [PubMed] [Google Scholar]
- [38].Morishima N, Nakanishi K, Tsuchiya K, Shibata T, Seiwa E (2004). Translocation of Bim to the endoplasmic reticulum (ER) mediates ER stress signaling for activation of caspase-12 during ER stress-induced apoptosis. J Biol Chem, 279: 50375-50381 [DOI] [PubMed] [Google Scholar]
- [39].Jin K, LaFevre-Bernt M, Sun Y, Chen S, Gafni J, Crippen D, et al. (2005). FGF-2 promotes neurogenesis and neuroprotection and prolongs survival in a transgenic mouse model of Huntington's disease. Proc Natl Acad Sci U S A, 102: 18189-18194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kiyota T, Ingraham KL, Jacobsen MT, Xiong H, Ikezu T (2011). FGF2 gene transfer restores hippocampal functions in mouse models of Alzheimer's disease and has therapeutic implications for neurocognitive disorders. Proc Natl Acad Sci U S A, 108: E1339-E1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bogousslavsky J, Victor SJ, Salinas EO, Pallay A, Donnan GA, Fieschi C, et al. (2002). Fiblast (trafermin) in acute stroke: results of the European-Australian phase II/III safety and efficacy trial. Cerebrovasc Dis, 14: 239-251 [DOI] [PubMed] [Google Scholar]
- [42].Paciaroni M, Bogousslavsky J (2011). Trafermin for stroke recovery: is it time for another randomized clinical trial. Expert Opin Biol Ther, 11: 1533-1541 [DOI] [PubMed] [Google Scholar]
- [43].Zhao YZ, Li X, Lu CT, Lin M, Chen LJ, Xiang Q, et al. (2014). Gelatin nanostructured lipid carriers-mediated intranasal delivery of basic fibroblast growth factor enhances functional recovery in hemiparkinsonian rats. Nanomedicine, 10: 755-764 [DOI] [PubMed] [Google Scholar]
- [44].Cuevas P, Carceller F, Muñoz-Willery I, Giménez-Gallego G (1998). Intravenous fibroblast growth factor penetrates the blood-brain barrier and protects hippocampal neurons against ischemia-reperfusion injury. Surg Neurol, 49: 77-84 [DOI] [PubMed] [Google Scholar]
- [45].Lee H, Pienaar IS (2013). Disruption of the blood-brain barrier in Parkinson's disease: curse or route to a cure. Front Biosci, 19: 272-280 [DOI] [PubMed] [Google Scholar]
- [46].Zhu G, Chen G, Shi L, Feng J, Wang Y, Ye C, et al. (2015). PEGylated rhFGF-2 Conveys Long-term Neuroprotection and Improves Neuronal Function in a Rat Model of Parkinson’s Disease. Mol Neurobiol, 51: 32-42 [DOI] [PubMed] [Google Scholar]
- [47].Wei X, He S, Wang Z, Wu J, Zhang J, Cheng Y, et al. (2014). Fibroblast growth factor 1attenuates 6-hydroxydopamine-induced neurotoxicity: an in vitro and in vivo investigation in experimental models of parkinson’s disease. Am J Transl Res, 6: 664. [PMC free article] [PubMed] [Google Scholar]
- [48].Timmer M, Cesnulevicius K, Winkler C, Kolb J, Lipokatic-Takacs E, Jungnickel J, et al. (2007). Fibroblast growth factor (FGF)-2 and FGF receptor 3 are required for the development of the substantia nigra, and FGF-2 plays a crucial role for the rescue of dopaminergic neurons after 6-hydroxydopamine lesion. J Neurosci, 27: 459-471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Yang F, Liu Y, Tu J, Wan J, Zhang J, Wu B, et al. (2014). Activated astrocytes enhance the dopaminergic differentiation of stem cells and promote brain repair through bFGF. Nat Commun, 17:5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Soto I, Rosenthal JJ, Blagburn JM, Blanco RE (2006). Fibroblast growth factor 2 applied to the optic nerve after axotomy up-regulates BDNF and TrkB in ganglion cells by activating the ERK and PKA signaling pathways. J Neurochem, 96: 82-96 [DOI] [PubMed] [Google Scholar]
- [51].Yoshida K, Gage FH (1991). Fibroblast growth factors stimulate nerve growth factor synthesis and secretion by astrocytes. Brain Res, 538: 118-126 [DOI] [PubMed] [Google Scholar]