Abstract
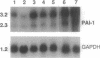
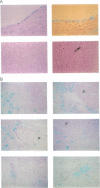
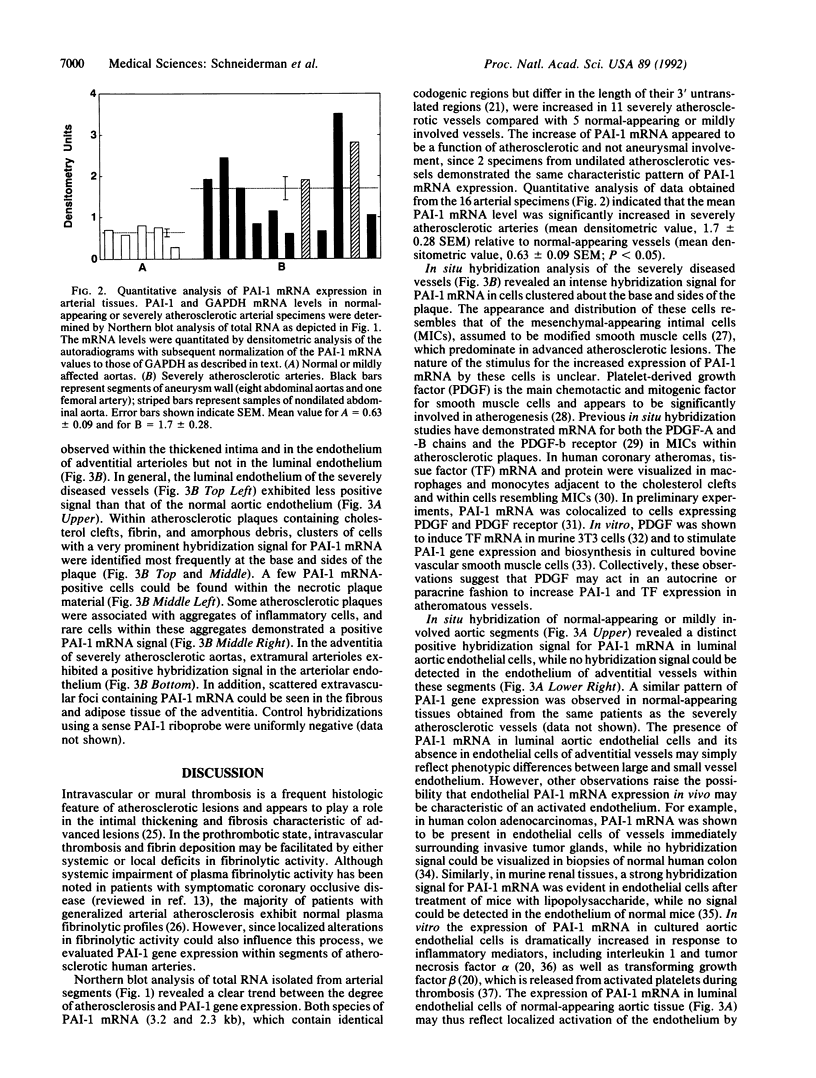
Decreased fibrinolytic capacity has been suggested to accelerate the process of arterial atherogenesis by facilitating thrombosis and fibrin deposition within developing atherosclerotic lesions. Type 1 plasminogen activator inhibitor (PAI-1) is the primary inhibitor of tissue-type plasminogen activator and has been found to be increased in a number of clinical conditions generally defined as prothrombotic. To investigate the potential role of this inhibitor in atherosclerosis, we examined the expression of PAI-1 mRNA in segments of 11 severely diseased and 5 relatively normal human arteries obtained from 16 different patients undergoing reconstructive surgery for aortic occlusive or aneurysmal disease. Densitometric scanning of RNA (Northern) blot autoradiograms revealed significantly increased levels of PAI-1 mRNA in severely atherosclerotic vessels (mean densitometric value, 1.7 +/- 0.28 SEM) compared with normal or mildly affected arteries (mean densitometric value, 0.63 +/- 0.09 SEM; P less than 0.05). In most instances, the level of PAI-1 mRNA was correlated with the degree of atherosclerosis. Analysis of adjacent tissue sections from the same patients by in situ hybridization demonstrated an abundance of PAI-1 mRNA-positive cells within the thickened intima of atherosclerotic arteries, mainly around the base of the plaque. PAI-1 mRNA could also be detected in cells scattered within the necrotic material and in endothelial cells of adventitial vessels. In contrast to these results, PAI-1 mRNA was visualized primarily within luminal endothelial cells of normal-appearing aortic tissue. Our data provide initial evidence for the increased expression of PAI-1 mRNA in severely atherosclerotic human arteries and suggest a role for PAI-1 in the progression of human atherosclerotic disease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asakura H., Jokaji H., Saito M., Uotani C., Kumabashiri I., Morishita E., Yamazaki M., Matsuda T. Changes in plasma levels of tissue-plasminogen activator/inhibitor complex and active plasminogen activator inhibitor in patients with disseminated intravascular coagulation. Am J Hematol. 1991 Mar;36(3):176–183. doi: 10.1002/ajh.2830360304. [DOI] [PubMed] [Google Scholar]
- Assoian R. K., Sporn M. B. Type beta transforming growth factor in human platelets: release during platelet degranulation and action on vascular smooth muscle cells. J Cell Biol. 1986 Apr;102(4):1217–1223. doi: 10.1083/jcb.102.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auwerx J., Bouillon R., Collen D., Geboers J. Tissue-type plasminogen activator antigen and plasminogen activator inhibitor in diabetes mellitus. Arteriosclerosis. 1988 Jan-Feb;8(1):68–72. doi: 10.1161/01.atv.8.1.68. [DOI] [PubMed] [Google Scholar]
- Aznar J., Estellés A., Tormo G., Sapena P., Tormo V., Blanch S., España F. Plasminogen activator inhibitor activity and other fibrinolytic variables in patients with coronary artery disease. Br Heart J. 1988 May;59(5):535–541. doi: 10.1136/hrt.59.5.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barath P., Fishbein M. C., Cao J., Berenson J., Helfant R. H., Forrester J. S. Detection and localization of tumor necrosis factor in human atheroma. Am J Cardiol. 1990 Feb 1;65(5):297–302. doi: 10.1016/0002-9149(90)90291-8. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Collen D., Juhan-Vague I. Fibrinolysis and atherosclerosis. Semin Thromb Hemost. 1988 Apr;14(2):180–183. doi: 10.1055/s-2007-1002773. [DOI] [PubMed] [Google Scholar]
- Colucci M., Paramo J. A., Collen D. Generation in plasma of a fast-acting inhibitor of plasminogen activator in response to endotoxin stimulation. J Clin Invest. 1985 Mar;75(3):818–824. doi: 10.1172/JCI111777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cybulsky M. I., Gimbrone M. A., Jr Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991 Feb 15;251(4995):788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Neufeld G., Schweigerer L. Fibroblast growth factor. Mol Cell Endocrinol. 1986 Aug;46(3):187–204. doi: 10.1016/0303-7207(86)90001-8. [DOI] [PubMed] [Google Scholar]
- Gram J., Jespersen J., Kluft C., Rijken D. C. On the usefulness of fibrinolysis variables in the characterization of a risk group for myocardial reinfarction. Acta Med Scand. 1987;221(2):149–153. doi: 10.1111/j.0954-6820.1987.tb01259.x. [DOI] [PubMed] [Google Scholar]
- Hamsten A., Wiman B., de Faire U., Blombäck M. Increased plasma levels of a rapid inhibitor of tissue plasminogen activator in young survivors of myocardial infarction. N Engl J Med. 1985 Dec 19;313(25):1557–1563. doi: 10.1056/NEJM198512193132501. [DOI] [PubMed] [Google Scholar]
- Hartzell S., Ryder K., Lanahan A., Lau L. F., Nathan D. A growth factor-responsive gene of murine BALB/c 3T3 cells encodes a protein homologous to human tissue factor. Mol Cell Biol. 1989 Jun;9(6):2567–2573. doi: 10.1128/mcb.9.6.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhan-Vague I., Alessi M. C., Vague P. Increased plasma plasminogen activator inhibitor 1 levels. A possible link between insulin resistance and atherothrombosis. Diabetologia. 1991 Jul;34(7):457–462. doi: 10.1007/BF00403280. [DOI] [PubMed] [Google Scholar]
- Juhan-Vague I., Vague P., Alessi M. C., Badier C., Valadier J., Aillaud M. F., Atlan C. Relationships between plasma insulin triglyceride, body mass index, and plasminogen activator inhibitor 1. Diabete Metab. 1987 Jul;13(3 Pt 2):331–336. [PubMed] [Google Scholar]
- Juhan-Vague I., Valadier J., Alessi M. C., Aillaud M. F., Ansaldi J., Philip-Joet C., Holvoet P., Serradimigni A., Collen D. Deficient t-PA release and elevated PA inhibitor levels in patients with spontaneous or recurrent deep venous thrombosis. Thromb Haemost. 1987 Feb 3;57(1):67–72. [PubMed] [Google Scholar]
- Knudsen B. S., Harpel P. C., Nachman R. L. Plasminogen activator inhibitor is associated with the extracellular matrix of cultured bovine smooth muscle cells. J Clin Invest. 1987 Oct;80(4):1082–1089. doi: 10.1172/JCI113164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimuro J., Schleef R. R., Loskutoff D. J. Extracellular matrix of cultured bovine aortic endothelial cells contains functionally active type 1 plasminogen activator inhibitor. Blood. 1987 Sep;70(3):721–728. [PubMed] [Google Scholar]
- Mosse P. R., Campbell G. R., Wang Z. L., Campbell J. H. Smooth muscle phenotypic expression in human carotid arteries. I. Comparison of cells from diffuse intimal thickenings adjacent to atheromatous plaques with those of the media. Lab Invest. 1985 Nov;53(5):556–562. [PubMed] [Google Scholar]
- Ny T., Sawdey M., Lawrence D., Millan J. L., Loskutoff D. J. Cloning and sequence of a cDNA coding for the human beta-migrating endothelial-cell-type plasminogen activator inhibitor. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6776–6780. doi: 10.1073/pnas.83.18.6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson B. O., Dahlén G., Nilsson T. K. Evidence for increased levels of plasminogen activator inhibitor and tissue plasminogen activator in plasma of patients with angiographically verified coronary artery disease. Eur Heart J. 1989 Jan;10(1):77–82. doi: 10.1093/oxfordjournals.eurheartj.a059384. [DOI] [PubMed] [Google Scholar]
- Oseroff A., Krishnamurti C., Hassett A., Tang D., Alving B. Plasminogen activator and plasminogen activator inhibitor activities in men with coronary artery disease. J Lab Clin Med. 1989 Jan;113(1):88–93. [PubMed] [Google Scholar]
- Paramo J. A., Colucci M., Collen D., van de Werf F. Plasminogen activator inhibitor in the blood of patients with coronary artery disease. Br Med J (Clin Res Ed) 1985 Aug 31;291(6495):573–574. doi: 10.1136/bmj.291.6495.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyke C., Kristensen P., Ralfkiaer E., Eriksen J., Danø K. The plasminogen activation system in human colon cancer: messenger RNA for the inhibitor PAI-1 is located in endothelial cells in the tumor stroma. Cancer Res. 1991 Aug 1;51(15):4067–4071. [PubMed] [Google Scholar]
- Reilly C. F., McFall R. C. Platelet-derived growth factor and transforming growth factor-beta regulate plasminogen activator inhibitor-1 synthesis in vascular smooth muscle cells. J Biol Chem. 1991 May 25;266(15):9419–9427. [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Saksela O., Moscatelli D., Rifkin D. B. The opposing effects of basic fibroblast growth factor and transforming growth factor beta on the regulation of plasminogen activator activity in capillary endothelial cells. J Cell Biol. 1987 Aug;105(2):957–963. doi: 10.1083/jcb.105.2.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawdey M. S., Loskutoff D. J. Regulation of murine type 1 plasminogen activator inhibitor gene expression in vivo. Tissue specificity and induction by lipopolysaccharide, tumor necrosis factor-alpha, and transforming growth factor-beta. J Clin Invest. 1991 Oct;88(4):1346–1353. doi: 10.1172/JCI115440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawdey M., Podor T. J., Loskutoff D. J. Regulation of type 1 plasminogen activator inhibitor gene expression in cultured bovine aortic endothelial cells. Induction by transforming growth factor-beta, lipopolysaccharide, and tumor necrosis factor-alpha. J Biol Chem. 1989 Jun 25;264(18):10396–10401. [PubMed] [Google Scholar]
- Schleef R. R., Bevilacqua M. P., Sawdey M., Gimbrone M. A., Jr, Loskutoff D. J. Cytokine activation of vascular endothelium. Effects on tissue-type plasminogen activator and type 1 plasminogen activator inhibitor. J Biol Chem. 1988 Apr 25;263(12):5797–5803. [PubMed] [Google Scholar]
- Schleef R. R., Higgins D. L., Pillemer E., Levitt L. J. Bleeding diathesis due to decreased functional activity of type 1 plasminogen activator inhibitor. J Clin Invest. 1989 May;83(5):1747–1752. doi: 10.1172/JCI114076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz C. J., Valente A. J., Kelley J. L., Sprague E. A., Edwards E. H. Thrombosis and the development of atherosclerosis: Rokitansky revisited. Semin Thromb Hemost. 1988 Apr;14(2):189–195. doi: 10.1055/s-2007-1002775. [DOI] [PubMed] [Google Scholar]
- Suffredini A. F., Harpel P. C., Parrillo J. E. Promotion and subsequent inhibition of plasminogen activation after administration of intravenous endotoxin to normal subjects. N Engl J Med. 1989 May 4;320(18):1165–1172. doi: 10.1056/NEJM198905043201802. [DOI] [PubMed] [Google Scholar]
- Vague P., Juhan-Vague I., Aillaud M. F., Badier C., Viard R., Alessi M. C., Collen D. Correlation between blood fibrinolytic activity, plasminogen activator inhibitor level, plasma insulin level, and relative body weight in normal and obese subjects. Metabolism. 1986 Mar;35(3):250–253. doi: 10.1016/0026-0495(86)90209-x. [DOI] [PubMed] [Google Scholar]
- Wilcox J. N., Smith K. M., Schwartz S. M., Gordon D. Localization of tissue factor in the normal vessel wall and in the atherosclerotic plaque. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2839–2843. doi: 10.1073/pnas.86.8.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox J. N., Smith K. M., Williams L. T., Schwartz S. M., Gordon D. Platelet-derived growth factor mRNA detection in human atherosclerotic plaques by in situ hybridization. J Clin Invest. 1988 Sep;82(3):1134–1143. doi: 10.1172/JCI113671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiman B., Chmielewska J. A novel fast inhibitor to tissue plasminogen activator in plasma, which may be of great pathophysiological significance. Scand J Clin Lab Invest Suppl. 1985;177:43–47. [PubMed] [Google Scholar]