Abstract
Background
Lung cancer screening (LCS) with low dose radiation computed tomography saves lives. Despite recent US Preventative Services Task Force draft endorsement of LCS, a minority of patients eligible is screened. Meaningful use is a set of standards for Electronic Health Records (EHR) established by the Centers for Medicare and Medicaid Services and includes reporting of smoking status. We sought to improve rates of LCS among patients treated at our institution by identifying eligible patients using augmented smoking-related meaningful use criteria.
Methods
We launched a LCS program at our institution, an NCCN cancer center, in January 2013. We developed a “Tobacco Screen”, administered by clinic staff to all adult outpatients every 6 months and entered into the EHR. This contained smoking-related meaningful use criteria, as well as a pack-year calculation and quit date, if applicable. Weekly electronic reports of patients who met eligibility criteria for LCS were generated, and EHR review excluded patients who had a chest CT within 12 months or who were undergoing cancer treatment. We then contacted those patients to review eligibility for LCS and communicated with the primary treating physician regarding the plan for LCS.
Results
During the first 3 months of the program, 4 patients were enrolled, 2 by physician-referral and 2 by self-referral. We then began to utilize the Tobacco Screen reports and identified 418 patients potentially eligible for LCS. Over the next 7 months, we enrolled a total of 110 patients. 58 (53%) were identified from the Tobacco Screen, 32 (29%) were self-referred, and 20 (18%) were physician referrals. Three stage I lung cancers were detected and treated. The tobacco screen was easily implemented by clinic staff and took a median time of 2 minutes to enter for current and former smokers. Lack of response to attempts at telephone contact and objection to paying out-of-pocket costs were the most common reasons for failing to screen eligible patients.
Conclusions
Use of augmented meaningful use criteria containing detailed tobacco exposure history is feasible and allows for identification of patients eligible for LCS in a medical center. Barriers to LCS include lack of insurance coverage and lack of systematic referral of patients at high risk.
Keywords: Lung cancer, Computed tomography, CAT scans, Health provider
Introduction
Lung cancer screening (LCS) with low dose radiation computed tomography scanning (LDCT) decreases lung cancer specific and overall mortality. With just three annual screens, the National Lung Screening Trial (NLST) detected a 20% reduction in lung cancer mortality and a 7% decrease in overall mortality.[1] It is estimated that screening eligible patients with yearly LDCT may decrease lung cancer deaths by up to 80% in that population.[2] Lung cancer screening is recommended by a number of professional societies, including the Society of Thoracic Surgeons (STS), American College of Chest Physicians (ACCP), National Comprehensive Cancer Network (NCCN), American Cancer Society (ACS), and American Society for Clinical Oncology (ASCO). Recently, the U.S. Preventative Task Force (USPSTF) issued a recommendation supporting the use of LDCT for lung cancer screening in patients ages 55–80 who have a 30 pack-year history of smoking and are currently smoking or have quit within the past 15 years.[3] Despite this recommendation, relatively few eligible patients are currently screened. While there are no estimates available of the proportion of eligible patients who are screened, patient volumes are reportedly low at most lung cancer screening programs. According to one survey of lung cancer screening programs reported in 2013, the median number of patients screened per program annually was 10.[4] Barriers to screening patients for lung cancer include the lack of coverage for LCS by many carriers, the lack of referral by primary care physicians, and the lack of public awareness on the benefits of LCS. On the other hand, there is great interest in starting LCS programs on the part of lung cancer clinicians and some radiologists. According to the Oncology Roundtable survey, the number of LCS programs doubled in 2012, the year after the NLST results were published, compared with 2011.[4]
The Health Information Technology for Economic and Clinical Health Act of 2009 earmarked funds for incentive payments to physicians to encourage meaningful use of electronic health records (EHR).[5] In order to receive these incentives, physicians must attest to meeting a set of meaningful use criteria that have been defined in three stages, with the goal of providing high-quality and efficient patient care using the EHR. In stage 1 of implementation (2011–2017), providers and hospitals must attest to ascertaining tobacco use, including whether a patient is a current or former smoker and providing a tobacco cessation intervention.[6] In stage 2 of implementation (2014–2020), additional information is gathered on the number of cigarettes smoked daily, and a larger percentage of patients with documented tobacco history is required than in stage 1.
We set out to expand upon tobacco-related meaningful use questions so as to document detailed tobacco use history in the EHR on all outpatients at our medical center. We developed reports based on these data to identify patients eligible for LCS. We sought to determine if this approach was feasible, and whether utilizing this information to identify eligible patients would result in increased patient volume in a LCS program.
Patients and Methods
Development of the LCS Program and Tobacco Screen
We launched an integrated LCS and tobacco cessation program at our institution, an NCCN cancer center in January 2013. The program was staffed by a nurse practitioner who is a certified tobacco dependency counselor. Our institution offered LCS at a discounted price of $150 when insurance did not provide coverage for LCS. There was minimal advertising for the program outside of the institution. We developed a “Tobacco Screen” administered by clinic staff to all adult outpatients every 6 months and entered into the EHR (Figure 1). This screen included meaningful use questions as well as a more extensive tobacco use history including a pack-year calculator and determination of number of years quit for former smokers. The screen also gauges the interest and motivation of the patient in quitting smoking, which is useful in initiating discussion on tobacco cessation. This information was entered into the EHR by the outpatient clinic staff. We asked a sample of clinic staff working in the ambulatory clinics to complete a brief survey which included questions on the average time it took to complete the tobacco screen in current and former smokers, and staff was asked to agree or disagree to three statements on a scale from 1–5 (1=strongly agree, 2=agree, 3=neutral, 4=disagree, and 5=strongly disagree):
Figure 1.
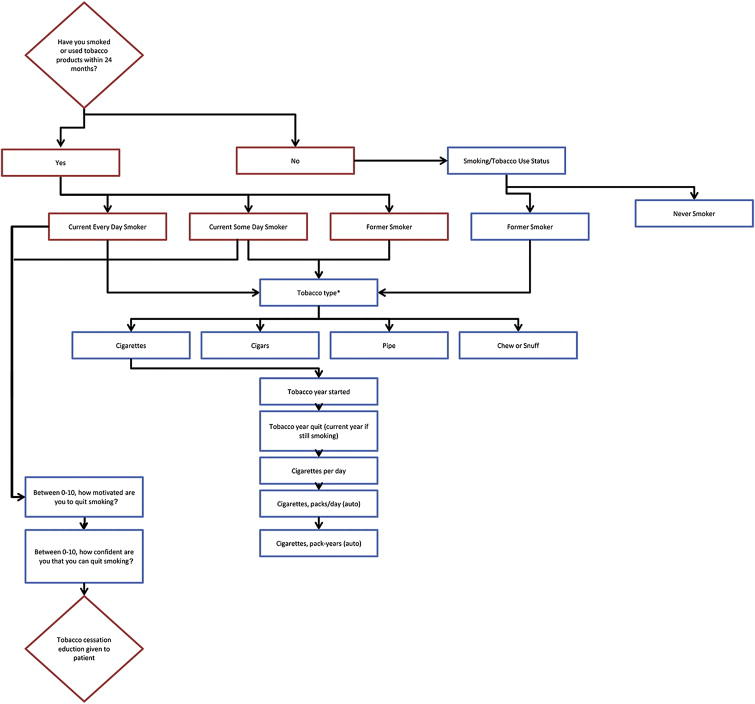
Flow chart of tobacco screen. Meaningful use questions are in red boxes. The screen was administered to all adult ambulatory care patients every six months.
*Decision tree for cigarettes shown here. Other decision trees for cigar, pipe, chew, or snuff.
The tobacco screen did not add a significant amount of time to my interaction with patients.
The tobacco screen did not interfere with patient flow in clinic.
Patients were willing to participate in the tobacco screen.
Lung cancer screening clinical flow
We used the most recent NCCN LCS eligibility criteria for our program.[7] NCCN criteria differs from NLST and USPTF criteria in part because it also allows for a 20 pack-year history of smoking if there is another risk factor other than second-hand smoke, including a prior cancer history. In addition, we do not adhere to an absolute upper age limit for LCS. The institution’s information technology department identified patients who were older than 50 years and had at least a 20 pack-year history of smoking then generated automated weekly electronic reports. The program nurse practitioner then searched the EHR to ensure that the patient was not undergoing active treatment for a malignancy and that no CT scan of the chest or PET/CT had been done over the last 12 months. The patient was contacted to review eligibility and interest in participating in screening. If the patient’s insurance did not cover LCS, the patient was notified regarding out of pocket expenses involved. An email was then sent to the patient’s primary treating physician informing them of enrollment of the patient in the LCS program and requesting communication if the physician disagreed with participation. Once screened, patients were notified of CT scan results by telephone and mail. If results were negative, a provider did not see the patient and a repeat study was arranged for 12 months. If results were positive, as defined by our institution’s NCCN-based nodule management protocol, most patients had a follow-up LDCT scan at 3 months with a visit from the program NP on the day of the repeat scan. If the repeat CT scan showed nodule growth, a thoracic surgeon or pulmonary medicine physician also saw the patient to determine further evaluation.
Data Collection and Statistics
The total number of patients enrolled in the LCS program and the source of the referral to the program between January 1 2013 and October 31, 2013 were recorded. We collected information on whether patients who were deemed potentially eligible for LCS based on the tobacco screen did not undergo LCS because they could not be reached by telephone, because they refused to pay out of pocket expenses, because they did not in fact meet criteria for LCS, or because of other reasons.
Demographics of patients who underwent LCS after being identified by the tobacco screened were compared with those who were screened after physician or self-referral. Mean age was compared using a two sample t-test. Proportions were compared using a two sample test of proportions. Race/ethnicity categories were compared for using non-parametric test of trend of categories. All statistics were calculated using STATA 11.0 (College Park, TX).
This study protocol was reviewed and approved by the Institutional Review Board at City of Hope Medical Center.
Results
Twenty seven clinic staff responded to surveys on the tobacco screens. The median score for question 1 (The tobacco screen did not add a significant amount of time to my interaction with patients) was 2 (range 1–5), for question 2 (the tobacco screen did not interfere with patient flow in clinic) was 2 (range 1–5), and for question 3 (patients were willing to participate in the tobacco screen) was 2 (range 1–4).
The median self-reported time to complete a tobacco screen for current or former smokers was 2 minutes (range 1–7 minutes).
During the first 3 months of the program, before implementation of the tobacco screen, four patients were enrolled in the LCS program. Two were referred by physicians and two by self-referral. After implementing the tobacco screen and utilizing reports identifying patients potentially eligible for LCS, the EHR was reviewed to exclude patients who were actively being treated for an invasive cancer or who had had a chest CT or PET/CT in our system over the prior year. Of 418 potentially eligible patients, we enrolled a total of 58. Reasons for not participating in screening are listed in table 1. 190 (45%) could not be reached by telephone, 10 (2%) refused to pay the out of pocket expense for screening, and 160 (38%) had a recent chest CT scan elsewhere, did not meet eligibility upon further question, or other reasons. Of the 110 patients enrolled, 58 (53%) were identified from the tobacco screen, 32 (29%) were self-referred, and 20 (18%) were physician referrals.
Table 1.
Patients Contacted From Tobacco Screen
| n=418 | |
|---|---|
| Enrolled in Screening | 58 (14%) |
| Could not be reached by phone | 190 (45%) |
| Did not meet eligibility/other reason | 160 (38%) |
| Refused out of pocket expense | 10 (2%) |
102 of 110 patients enrolled had screening completed at the time this study was completed. Mean age of screened patients was 65 years. 70% were non-Hispanic whites, 11% were Asian, 10% were Latino, 4% were blacks, and 6% were other or non-disclosed. For reference, our regional population (San Gabriel Valley, with a population of 1.43 million in the 2000 US Census) is 45% Latino, 26% Asian, 25% non-Hispanic white, 2% Black, and 2% other.[8] Our hospital population is 25% Latino, 13% Asian, and 5% Black. 46% of screened patients were current smokers, compared with 53% former smokers. 60% of patients screened had a history of a prior cancer, with breast and prostate cancer the most common primary sites. Of patients screened, 32% had a positive scan. Of patients with positive scans, three patients underwent transthoracic core needle biopsies of the lung (9%), and all three had non-small cell lung cancer (NSCLC). There were no complications from any of the three needle biopsies. All three had clinical stage I lung cancer. All three were treated with stereotactic body radiation therapy (SBRT) after mediastinal staging with endobronchial ultrasound (EBUS). One patient had severe COPD and was on home oxygen and was not a surgical candidate. The other two patients were at high operative risk because of emphysema and other medical conditions and elected to undergo SBRT instead of surgical resection. One patient with screen-detected lung cancer had a history of laryngeal cancer and another had a history of breast cancer. An additional patient had a thyroid needle biopsy performed because workup of a lung nodule included a PET-CT scan which identified an FDG-avid thyroid nodule. The thyroid nodule was benign.
Comparing patients who were identified by tobacco screen and those who were self or physician-referred, tobacco screen patients were more likely to have a prior cancer history (82% vs 32%, p<0.001), and less likely to be of Asian ethnicity (3% vs 19%, test of trend for all race/ethnicity p=0.93).
Comment
In this study, we found that identification of patients eligible for LCS using augmented meaningful use tobacco-related questions resulted in increased enrollment in our LCS program. More than half of our LCS program patients enrolled because they were contacted as a result of being identified as eligible through the tobacco screen. Once implemented, we found that adding these additional questions did not seem to burden the clinic staff or interfere with the flow of patients.
As part of phase 2 of meaningful use, additional information regarding the amount of tobacco use will be required. Documenting tobacco use in a detailed and systematic format, similar to how most clinicians would take a smoking history, can be used to fulfill meaningful use requirements as well as to identify patients eligible for lung cancer screening and tobacco cessation. Collecting detailed information on tobacco history in an easily accessible electronic format also facilitates future clinical research on lung cancer and other tobacco related diseases, since detailed tobacco history is not currently recorded in cancer registries.
In this study, we found that patients identified through meaningful use questions were more likely to be cancer survivors and less likely to be Asians. Because our institution is a cancer center, a large majority of patients treated have a history of cancer and is the primary reason why patients identified using the tobacco screen were more likely to have a cancer history than those self-referred or referred by another physician. This patient population offers unique challenges and opportunities for a LCS program. First, there is a higher incidence of lung cancer among patients with a prior malignancy, including lung cancer, head and neck cancer, breast cancer, and lymphomas.[9–12] NCCN LCS guidelines recognize this, and call for LCS for patients with a history of a prior malignancy and a 20 pack-year history of smoking, rather than the 30-pack year history of smoking recommended by the USPTF. The challenge lies in using the same nodule criteria for patients with a history of cancer so as to avoid missing a new lung metastasis. While we use the NCCN recommended 6mm nodule threshold for patients without a history of an invasive cancer, we repeat studies on patients with a history of a prior malignancy within 5 years of screening when the nodule is 3mm or larger. This, and a high prevalence of granulomatous disease in our geographic location, likely accounts for the higher percentage of positives in our screening population than we expected.
We found a low rate of screening among Latinos, which was surprising given that our region’s population is 45% Latino and our medical center population is 25% Latino. Unfortunately we do not have data on the ethnic makeup of patients who were eligible for screening but did not undergo screening to confirm if there were race/ethnicity differences among those who do and do not get screened. We are currently prospectively evaluating this. Ours is currently the only LCS center within the San Gabriel Valley section of Los Angeles county, yet it is possible that because of insurance contracts and referral patterns that eligible Latinos are being screened elsewhere. It is also possible that the out of pocket expense is burdensome to patients of low socioeconomic background, who are disproportionately Latino in our region. Similarly, Asians, and in particular Chinese-Americans, who are populous in our region and have a high rate of tobacco use, were underrepresented in our screening program although similar to our hospital population. Our program is currently making outreach to our region’s at-risk Latino and Chinese-speaking population a high priority.
There were several limitations to our approach. Almost half of patients could not be contacted or did not return phone calls. Additional outreach such as tracking of these patients with mailing of letters to patients and primary physicians encouraging screening might have increased participation further. Determining eligibility for LCS and distributing literature on screening at the time of tobacco screen administration may be an alternative. An additional limitation is that the approach we used is resource intensive. The screening coordinator must evaluate reports, briefly review the medical record, determine eligibility, and contact patients, sometimes more than once. This effort might eventually obviated by development of automated preventative care alerts to clinicians indicating that a patient is eligible for LCS, and suggesting consultation to the lung cancer screening program. We are currently working on such an automated alert system for eligible patients at our institution whereby the primary physician would be notified of eligibility for LCS. In addition, a small number of patients refused to pay the $150 out of pocket expense our institution, but all of these patients indicated that they would be interested in screening in the future once LCS becomes a covered benefit. We expect that once LCS becomes a covered benefit universally patient participation will increase. Finally, we found that the tobacco screen underestimated the true tobacco exposure history of patients. Despite training clinic staff on how to ask tobacco use questions, a common error was to list the current number of cigarettes smoked which were often substantially less than the highest or average number smoked in the past. This likely led to missing some patients who would have been eligible for LCS.
A broader question is how LCS patients will be identified and referred for screening, now that the USPTF has issued guidelines recommending LCS. Ideally, patients will be referred by primary care providers (PCP) to screening sites for LCS and tobacco cessation counseling. PCPs may also identify patients eligible for LCS through expanding meaningful use tobacco-related questions as we have done. Increasingly automated preventative care alerts are built-in to EHR systems, and eligibility for LCS can and should be included. Identification of patients eligible for LCS outside of the PCP may still be very important, as it is unclear how quickly LCS will be adopted as by primary care providers. LCS should be done as part of a program that incorporates using low-dose radiation scanning, an evidence-based nodule management protocol, tobacco cessation, multidisciplinary review of positive findings, and timely evaluation and communication of results.[13, 14] Communication with PCPs regarding screening results is of high importance, especially when subclinical emphysema and coronary calcifications are detected. These tobacco-related manifestations are often detected on LCS and require follow-up with primary care, pulmonary medicine, or cardiology, for treatment and further preventative care.
Our LCS and tobacco cessation programs are both run by a nurse practitioner. In our opinion, an advanced practice nurse led LCS program has several advantages compared to a program relying on individual providers to act on results. First, this ensures that evidence based nodule management protocol are followed. Next, it ensures that care is coordinated and non-compliance with future screening is kept to a minimum. Finally, integrating LCS with tobacco cessation is critical in patients who are still smoking. While it is our opinion that an advanced practice nurse improves the quality of a LCS program, we do not have data on the financial viability of a LCS program. In high volume LCS centers, an advanced practice nurse may be cost effective by allowing pulmonologists or thoracic surgeons to be more clinically productive.
In summary, the systematic documentation of detailed tobacco use history allows for identification of patients who are eligible for LCS. Such methods of identifying at risk patients are important, especially when physician referrals for LCS and public awareness about LCS with LDCT seem to be low.
Table 2.
Patient demographics stratified by means of patient referral
| Tobacco screen | Physician or self | p value | |
|---|---|---|---|
| Age (Mean Years+ SD)1 | 64.4+8.9 | 65.2+7.9 | 0.62 |
| Sex (Male, %)2 | 44% | 40% | 0.74 |
| Race/Ethnicity3 | |||
| Non-Hispanic White | 75% | 64% | 0.93 |
| Asian | 3% | 19% | |
| Latino | 11% | 9% | |
| Black | 5% | 2% | |
| Other/ Non-disclosed | 6% | 5% | |
| Smoking status2 | |||
| Current (%) | 51% | 45% | 0.53 |
| Former (%) | 49% | 55% | |
| Prior cancer history2 | 82% | 32% | <0.001 |
| Positive scan2 | 33% | 34% | 0.88 |
Two sample t-test
Two-sample test of proportion
Non-parametric test of trend across categories
Acknowledgments
The authors would like to acknowledge Annie Shum, CPHIT and Kathleen Dorsey, RN for their assistance with this manuscript.
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award number NIH 5K12CA001727-20.
Footnotes
Presented at annual meeting of The Society of Thoracic Surgeons, Orlando, FL January 25–29, 2014
The content is solely the responsibility of the Dr. Raz and does not necessarily represent the official views of the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Lung Screening Trial Research, T., et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Early Lung Cancer Action Program, I., et al. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med. 2006;355(17):1763–71. doi: 10.1056/NEJMoa060476. [DOI] [PubMed] [Google Scholar]
- 3.Moyer VA. Screening for Lung Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2013 doi: 10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- 4.Conway L. Oncology Roundtable. 2013. 2013 Lung Cancer Screening Quick Poll. [Google Scholar]
- 5.Blumenthal D. Implementation of the federal health information technology initiative. N Engl J Med. 2011;365(25):2426–31. doi: 10.1056/NEJMsr1112158. [DOI] [PubMed] [Google Scholar]
- 6.Services, C.f.M.M. Medicare & Medicaid EHR Incentive Program: Meaningful Use Stage 1 Requirements Overview. 2010 Available from: www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms/downloads/MU_Stage1_ReqOverview.pdf.
- 7.Oncology, N.C.P.G.i. Lung Cancer Screening. 2014. [Google Scholar]
- 8.Times, L.A. . The San Gabriel Valley. Mapping L.A. [cited 2014 1/21/2014]; Available from: http://maps.latimes.com/neighborhoods/region/san-gabriel-valley/
- 9.Swerdlow AJ, et al. Second cancer risk after chemotherapy for Hodgkin’s lymphoma: a collaborative British cohort study. J Clin Oncol. 2011;29(31):4096–104. doi: 10.1200/JCO.2011.34.8268. [DOI] [PubMed] [Google Scholar]
- 10.Morris LG, et al. Anatomic sites at elevated risk of second primary cancer after an index head and neck cancer. Cancer Causes Control. 2011;22(5):671–9. doi: 10.1007/s10552-011-9739-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berrington de Gonzalez A, et al. Second solid cancers after radiotherapy for breast cancer in SEER cancer registries. Br J Cancer. 2010;102(1):220–6. doi: 10.1038/sj.bjc.6605435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duchateau CS, Stokkel MP. Second primary tumors involving non-small cell lung cancer: prevalence and its influence on survival. Chest. 2005;127(4):1152–8. doi: 10.1378/chest.127.4.1152. [DOI] [PubMed] [Google Scholar]
- 13.Field JK, et al. The International Association Study Lung Cancer (IASLC) Strategic Screening Advisory Committee (SSAC) Response to the USPSTF Recommendations. J Thorac Oncol. 2014;9(2):141–3. doi: 10.1097/JTO.0000000000000060. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson FL, et al. Development of The American Association for Thoracic Surgery guidelines for low-dose computed tomography scans to screen for lung cancer in North America: recommendations of The American Association for Thoracic Surgery Task Force for Lung Cancer Screening and Surveillance. J Thorac Cardiovasc Surg. 2012;144(1):25–32. doi: 10.1016/j.jtcvs.2012.05.059. [DOI] [PubMed] [Google Scholar]


