Highlights
-
•
First report of renal infarct via patent foramen ovale following bariatric surgery.
-
•
Up to date recommendations on work-up and management of patent foramen ovale discussed.
-
•
Presentation includes radiologic images.
Keywords: Bariatric, Case report, Renal infarct, PFO, Embolism, Complication
Abstract
50-year-old female presented with abdominal pain 9 days post sleeve gastrectomy and was found to have acute renal infarction caused by paradoxical emboli through patent foramen ovale (PFO) as a cause of the renal infarction. Renal infarctions caused by paradoxical embolism are rare and have not been previously reported following surgery, bariatric surgery in particular. This report describes presentation, work up and management of a patient with renal infarct following bariatric surgery.
1. Introduction
Left sided abdominal pain in the early post-operative period after sleeve gastrectomy is concerning for staple line leak or subphrenic abscess. Other differentials include acute pancreatits, acute paraoesophageal hernia, splenic injury, cholelithiasis, port hernia, constipation, gastro-oesophageal reflux disease (GORD); and rarely portal vein thrombosis or gastro-bronchial fistula [1], [2]. Renal infarction by paradoxical embolism has never been reported after surgery, particularly following bariatric surgery.
2. Presentation of case
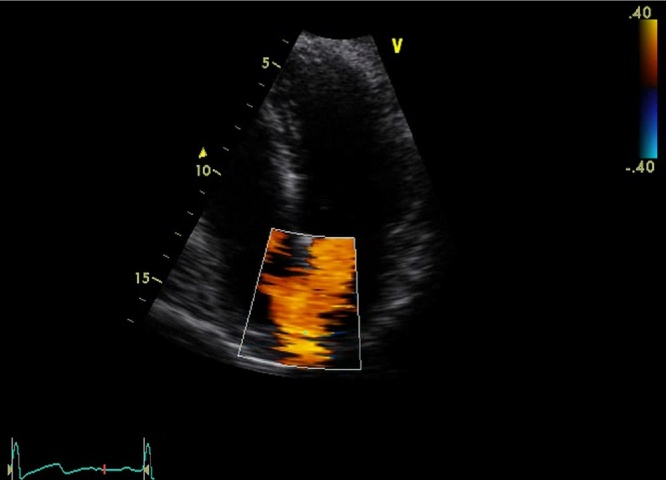
A 50-year-old female presented with acute left-sided abdominal pain 9 days after laparoscopic sleeve gastrectomy for morbid obesity BMI 41. Her procedure was uncomplicated and she received standard deep vein thrombosis (DVT) prophylaxis which included peri-operative calf compressors, post-operative compression stockings (worn for the duration of hospital stay and given to the patient on discharge) and prophylactic dose of 40 mg enoxaparin daily commenced on the day of surgery and continued during inpatient stay (3 days total). She had no significant medical history, normal postoperative recovery and was discharged home on day two. She was walking on treadmill on day 9 when she developed the pain. On examination blood pressure 140/80 mmHg, heart rate 68 bpm, respiratory rate 17/min, saturation 98%, temperature of 36C with localised left-sided and epigastric tenderness and no peritonism. Pathology showed WCC 5.5 × 109/L, Hb 130 g/L, CRP 6 mg/L with normal liver and renal function. CT of abdomen and pelvis revealed an anterior-inferior left renal wedge infarction and incidental bilateral renal and hepatic cysts (Fig. 1). Renal Doppler showed normal arterial and venous flow without evidence of renal thrombus. ECG has revealed sinus rhythm and a 24-h Holter monitor showed sinus rhythm with rare ventricular ectopic beats. Thrombophilia screen (coagulation profile, ESR, protein-C, protein-S, antithrombin-III, nuclear and cardiolipin antibodies) was normal. Factor VIII was mildly elevated at 235% (normal high range 220%) but not clinically significant. Bilateral lower and upper limb Doppler ultrasound showed good flow in deep venous system and no thromboembolism. Transthoracic echocardiogram (TTE) showed good contractility and no evidence of thrombus or valvular disease. Colour flow echocardiogram revealed left-to-right jet. Qp:Qs was 1.25 indicating a small shunt through patent foramen ovale (Fig. 2). She was discharged home on warfarin (target INR 2–3) with minimum duration of therapy of 6 months and was assessed by a cardiologist as an outpatient for the ongoing management of PFO.
Fig. 1.
CT of abdomen and pelvis showing an aterior-inferior renal wedge infarction.
Fig. 2.
Colour flow echocardiogram reveals small jet via PFO.
3. Discussion
Renal infarction is rare and caused by either thromboembolic phenomenon associated with atrial fibrillation (AF) or endocarditis; or renal artery thrombosis usually due to spontaneous renal artery dissection; or fibromuscular dysplasia and hypercoagulable state such as hereditary thrombophilia and antiphospholipid syndrome [3], [4]. Workup for suspected renal infarction includes full blood count with differential, creatinine, lactate dehydrogenase, urinalysis and urine culture; transthoracic or transoesophageal echocardiogram to exclude endocarditis, valvular or cardiac thrombus and septal defects; CT KUB to exclude renal calculi and assess extent of infarction, renal Doppler ultrasound to exclude renal artery thrombus but can be normal in small infarcts, ECG and 24-h Holter monitor for paroxysmal AF [3], [4], [5].
There are no reported cases of paradoxical renal infarction causing acute pain post-operatively. Post-operative pain from renal infarction has been recently reported, but in this case arose from thrombus formation in a resected pulmonary vein stump and hence, not a true paradoxical emboli [6]. Only a limited number of paradoxical renal emboli have been reported and usually present as spontaneous, not post-operative, abdominal pain [7], [8], [9]. PFO occurs in 25–35% of the population and is usually asymptomatic but can cause cryptogenic stroke, migraine, platypnea-orthodeoxia syndrome and air embolism during decompression sickness in divers [10]. Diagnosis is usually made by transthoracic and/or transoesophageal echocardiogram. Incidental PFO does not require treatment. Treatment for symptomatic patients or those with complications of PFO is anticoagulation (warfarin or aspirin or both) or surgery (percutaneous closure most common) [10]. Three trials comparing percutaneous closure with medical treatment of PFO did not show significant benefit of percutaneous closure, although all 3 had short follow up period (2–2.6 years) [11]. Optimal management of PFO remains uncertain. Decision to manage PFO medically with antiplatelet or vitamin K antagonist as opposed to percutaneous closure should be made on case-by-case basis. Surgical treatment seems more appropriate in cases of recurrent paradoxical embolic events, especially whilst receiving medical treatment, recurrent DVT or high risk for DVT recurrence and in patients with contraindications to anticoagulation [12].
4. Conclusion
In conclusion we would recommend having low threshold on performing transthoracic or transoesophageal echocardiogram as a part of workup of an ischaemic event with no known risk factors to exclude of confirm diagnosis of paradoxical emboli. Obesity is a risk factor for venous thrombosis and the complications of such is often overlooked. With increased obesity amongst surgical population, effective thromboembolism prophylaxis as well as high index of suspicion for complications of venous thromboembolism is crucial.
Conflict of interest
No conflict of interest exists for this case report.
Funding
No funding was received in preparation of this case report.
Ethical approval
Ethical approval was not required as this is a case report.
Consent
No consent is required as current submission is a case report. However, the unique nature of the case was discussed with the patient and the intention of publishing it stated.
Author contribution
Oleksandr Khoma was the main author of the case report. Collected and systemised available information.
Aravind Suppiah was second author, contributed reviewing the report, modifying the structure of the information presented.
David Martin is the attending physician for the patient in the report. He established the unique nature of the problem discussed. Revised the final version and approved for submission.
Guarantor
Oleksandr Khoma is the guarantor for this publication.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.ijscr.2016.06.047.
Contributor Information
Oleksandr Khoma, Email: khoma.a@gmail.com.
Aravind Suppiah, Email: aravindsuppiah@hotmail.com.
David Martin, Email: davidmartin72@hotmail.com.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Alharbi S.R. Gastrobronchial fistula a rare complication post laparoscopic sleeve gastrectomy. Ann. Thorac. Med. 2013;8(3):179–180. doi: 10.4103/1817-1737.114285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarkhosh K., Birch D.W., Sharma A., Karmali S. Complications associated with laparoscopic sleeve gastrectomy for morbid obesity: a surgeon’s guide. Can. J. Surg. 2013;56(5):347–352. doi: 10.1503/cjs.033511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antopolsky M., Simanovsky N., Stalnikowicz R., Salameh S., Hiller N. Renal infarction in the ED: 10-year experience and review of the literature. Am. J. Emerg. Med. 2012;30(7):1055–1060. doi: 10.1016/j.ajem.2011.06.041. [DOI] [PubMed] [Google Scholar]
- 4.Bourgault M., Grimbert P., Verret C., Pourrat J., Herody M., Halimi J.M. Acute renal infarction: a case series. Clin. J. Am. Soc. Nephrol. 2013;8(3):392–398. doi: 10.2215/CJN.05570612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai S., Ouyang Y.S., Li J.C., Dai Q., Tan L., Xia Y. Evaluation of acute renal artery thrombosis or embolism with color Doppler sonography. Clin. Imaging. 2008;32(5):367–371. doi: 10.1016/j.clinimag.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 6.Manabe S., Oshima Y., Nakano M., Fujii T., Maehara T., Nitta K. Renal infarction in a patient with pulmonary vein thrombosis after left upper lobectomy. Case Rep. Nephrol. Dial. 2014;4(2):103–108. doi: 10.1159/000363224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwasaki M., Joki N., Tanaka Y., Hara H., Suzuki M., Hase H. A suspected case of paradoxical renal embolism through the patent foramen ovale. Clin. Exp. Nephrol. 2011;15(1):147–150. doi: 10.1007/s10157-010-0354-4. [DOI] [PubMed] [Google Scholar]
- 8.Jeong H., Woo Lee H., Young Joung J., Young Cho Y., Je D., Huh K. Renal infarction caused by paradoxical embolism through a patent foramen ovale. Kidney Res. Clin. Pract. 2012;31(3):196–199. doi: 10.1016/j.krcp.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nara M., Komatsuda A., Fujishima M., Fujishima N., Nara M., Iino T. Renal paradoxical embolism in a hypertensive young adult without acute ischemic symptoms. Clin. Exp. Nephrol. 2011;15(4):582–585. doi: 10.1007/s10157-011-0436-y. [DOI] [PubMed] [Google Scholar]
- 10.Kedia G., Tobis J., Lee M.S. Patent foramen ovale: clinical manifestations and treatment. Rev. Cardiovasc. Med. 2008;9(3):168–173. [PubMed] [Google Scholar]
- 11.Spencer F.A., Lopes L.C., Kennedy S.A., Guyatt G. Systematic review of percutaneous closure versus medical therapy in patients with cryptogenic stroke and patent foramen ovale. BMJ Open. 2014;4(3):e004282. doi: 10.1136/bmjopen-2013-004282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pizzino F., Khandheria B., Carerj S., Oreto G., Cusma-Piccione M., Todaro M.C. PFO: Button me up, but wait … Comprehensive evaluation of the patient. J. Cardiol. 2016;67(6):485–492. doi: 10.1016/j.jjcc.2016.01.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.