Highlights
-
•
Cancer stem cells (CSCs) resist chemotherapy, thereby causing relapse of the disease.
-
•
A new chemotherapy drug response assay (ChemoID), which measures the sensitivity of CSCs to a variety of chemotherapy agents has been developed.
-
•
The ChemoID assay may assist an oncologist in making treatment decisions.
-
•
The ChemoID procedure may lower treatment costs by eliminating ineffective chemotherapies and unnecessary toxicity.
Keywords: Drug response assay, Chemosensitivity assay, ChemoID assay, Cancer stem cells, Oral cancer
Abstract
Introduction
Administration of ineffective anticancer therapy is associated with unnecessary toxicity and development of resistant clones. Cancer stem cells (CSCs) resist chemotherapy, thereby causing relapse of the disease. Thus, development of a test that identifies the most effective chemotherapy management offers great promise for individualized anticancer treatments. We have developed an ex vivo chemotherapy drug response assay (ChemoID®), which measures the sensitivity of CSCs as well as the bulk of tumor cells to a variety of chemotherapy agents to assist an oncologist in making treatment decisions.
Methods
Three patients affected by oral cancer were referred.
Results
Biopsy showed a well-differentiated squamous cell carcinoma (G1) in case 1, a G2 adenocarcinoma in case 2 and a G3 squamous cell carcinoma in case 3. In all of the three cases, after clinical inspection and suspicion of a diagnosis of cancer, a double biopsy was performed. One specimen was sent to the ChemoID laboratory for chemosensitivity assay and the other for histological analysis. Chemotherapy dose response curves were generated, and grouped in 3 categories: 1. No response (less than 30% cell kill), Intermediate (30–60% cell kill), and 3. Sensitive (60% cell kill or above).
Conclusions
This procedure may be useful in helping physicians choose an effective chemotherapy regimen for head and neck cancer patients and lower treatment costs by eliminating ineffective chemotherapies and unnecessary toxicity particularly in elderly patients.
1. Introduction
Because of difficulties in treatment of head and neck malignant tumors, investigation and development of novel strategies and integrated therapies are required to find more effective treatments for these malignant tumors [1], [2].
Patients with the same stage and grade of cancer may vary considerably in their clinical response to chemotherapy [3]. Ineffective anticancer therapy can result in unnecessary toxicity and the development of resistant clones. The surviving cancer cells are often more resistant to therapy [4]. Many attempts have been made over the years to develop an ex-vivo anti-cancer test that could help in discerning the best treatment options for each individual patient while minimizing toxicity [4], [5].
Animal xenograft models have shown that only a subset of cancer cells within each tumor is capable of initiating tumor growth. This biological behavior, first observed in AML, has been extended to a multitude of solid tumors, including breast, glioblastoma, colorectal, ovarian, head & neck, and pancreatic cancers [6], [7], [8], [9].
This pool of cancer cells is operationally defined as the “Cancer Stem Cell” (CSC) subset. According to the “cancer stem cell” theory, tumors are a complex, growing population of abnormal cells originating from a minority of CSCs [6], [7], [8], [9]. These cells maintain stem-like characteristics in that they proliferate very slowly and have an inherent capacity to self-renew and differentiate into phenotypically heterogeneous, aberrant progeny [9], [10], [11].
Unlike the bulk of tumor cells, CSCs resist chemotherapy and radiation therapy and are responsible for tumor relapse and metastasis [4], [6], [7], [10], [11], [12], [13], [14]. Targeting CSCs in addition to the bulk of other cancer cells within a tumor is a new paradigm in cancer treatment [15].
Our recent studies show that selectively enriched CSCs from primary cancer cell cultures can be used in a chemosensitivity assay (ChemoID®) [4], [11].
ChemoID® is a proprietary drug response assay, which measures an individual's malignant tumor response to arrange a standard-of-care anticancer drugs therapy under consideration by a physician [4], [12]. ChemoID® uses one of the most advanced technologies to identify chemotherapies that can eliminate the CSCs that are the root of the cancer behavior and relapse [4], [12]. ChemoID® is a test that quantifies an individual cancer patient's tumor response to various chemotherapeutic drugs against cancer stem cells and bulk of tumor cells providing both sensitivity and resistance information that can help the Oncologists to tailor the best chemotherapy cocktail directed against the patient’s cancer cells. This equates to “individualized” chemotherapy based on the specific patient's own tumor characteristics to give patients an edge against cancer [4], [5], [12], [13], [14], [15]. ChemoID® can be used for selecting the first, second or third line chemotherapies, whether the aim of chemotherapy is cure or palliation.
The aim of this study was to demonstrate the feasibility and efficacy of the ChemoID® chemo predictive assay, which is performed in a CLIA accredited laboratory in the United States for patients affected by extensive lesions of the oral and maxillofacial area who are located and treated in Europe, thus opening new prospective treatment of extensive cancer lesions where a technology such as ChemoID® is not available yet, by just performing two specimen biopsies immediately at the beginning of the treatment.
2. Material and methods
2.1. Chemotherapy agents
Bevacizumab (Avastin), Cisplatin, Methotrexate, 5-Fluorouracil, Docetaxel, Bleomycin, Epirubicin, Gemcitabine, Paclitaxel, Carboplatin, Cetuximab were acquired as clinical grade chemotherapy agents.
2.2. Methods for collecting biopsy specimen from oral cavity and shipping modalities
We have developed a specimen collection procedure that involves a thorough disinfection of the oral cavity before the biopsy, followed by a subsequent disinfection of the collected specimen with a Betadine solution, followed by a wash of the specimen with a sterile saline solution before placing in the transportation medium. Fresh biopsy specimens were shipped using courier services in a specially designed temperature controlled styrofoam container packaging. Biopsy specimens were received at the ChemoID laboratory in the United States within 18–36 h from Europe.
Samples were transported in sterile test tubes containing RPMI-1640 culture media with 2% Penicillin/Streptomycin. The professionally designed cardboard packaging followed international standards for impact durability and safety for the shipment of biological materials.
For international shipments, a lead sheet of 2.5 mm thickness was wrapped around the biopsy collection vial to shield from radiation exposure of airport x-ray scanners. The radiation shielding lead sheet ensured biopsy viability for recovering optimum live cells for the assay.
Another innovative aspect of the collection process that we developed is the implementation of a methodical aseptic procedure at the time of biopsy collection, particularly for oral cancers. Biopsy specimens taken from the oral cavity are usually very high in bacterial and fungal content even after several chlorhexidine rinses. To minimize contamination problems, we developed a decontamination procedure that consists of thorough disinfection of the oral cavity prior to the biopsy, followed by a subsequent disinfection of the specimen with a Betadine solution. This was followed by a meticulous wash of the specimen with a sterile saline solution to remove the Betadine disinfectant.
2.3. Patients
The study was conducted in accordance with the ethical principles provided by the Declaration of Helsinki and the principles of good clinical practice, under the IRB protocol number 695141. Case 1 was a 55 years old woman, heavy smoker, obese, without any history for other pathology, who consulted our the Department of Medicine and Surgery, Unit of Maxillofacial Surgery, University of Salerno, Salerno, Italy for a neoplastic ulcer of the left buccal mucosa of about 6 cm in diameter, infiltrating the muscles of the cheek. Clinical examination revealed pain in the involved area.
The patient didn't suffer from human immunodeficiency virus (HIV-1) infection. She did not complain of bleeding, and no other significant complaints were present in her clinical history. On Computerized Tomography (CT) exam there was clear presence of the tumor and other suspicious lymph nodes at the level of the submandibular and hyoid bone in the absence of distant metastases, with a staging of T4, N2, M0, and a G1 grade carcinoma at the pathological exam.
Case 2 was an 83 years old woman with an adenocarcinoma (G2) of the left submandibular gland with mandibular bone involvement and spontaneous bleeding, and without distant metastases who was staged as T4 N2 M0.
Case 3 was a 53 years old man with a recurrent lower lip carcinoma and submandibular lymph nodes involvement. He was operated for the first time in Bulgaria for tumor resection without laterocervical neck dissection. Some months later he was operated in Italy two more times for recurrence of the tumor and then he was referred to our Department where he was staged as an inoperable T4 N2 M0 because of carotid infiltration at CT scan imaging. A biopsy was performed resulting in the diagnosis of a G3 squamous cell carcinoma.
2.4. ChemoID® assay
The ChemoID® assay is a second-generation functional drug response assay that targets cancer stem cells and bulk of tumor cells. It is a clinical laboratory-developed test, which is performed in a CLIA certified and CAP accredited clinical laboratory at the Cabell Huntington Hospital and Edwards Cancer Center in West Virginia, USA.
Sensitivity to chemotherapy was assessed using a viability assay (MTT) on both CSCs and bulk of tumor cells plated in 5 replicas into 96-well plates. Briefly, equal number of bulk of tumor cells and CSCs, were grown, counted and seeded separately in 96-well dishes and incubated at 37 °C for 24-h as previously described [4].
The cells were then challenged for a 1-h pulse with a panel of anticancer drugs as chosen by the oncologist to mimic the average clinical chemotherapy infusion schedule. All anticancer drugs were each tested in a range of doses including the clinically relevant dose. An MTT assay was performed 48-h following chemotherapy treatment to assess cell viability as previously described [4]. A dose response chart was developed in which samples were scored as responsive (0–30% cell survival), intermediate (30–60% cell survival), and non-responsive (60–100% cell survival).
3. Results
For the first case (Table 1) the ChemoID® assay indicated that the CSC and bulk of tumor cells of this patient were sensitive to Paclitaxel and Carboplatin (70% and 85% cell kill, respectively), but not to other chemotherapies tested such as Cisplatin, 5-Fluorouracil, Docetaxel, Bleomycin, Epirubicin, Methotrexate and Cetuximab (less than 10%). Patient was operated with a resection of the tumor and a neck dissection, followed by reconstruction using a forehead flap.
Table 1.
Data is reported as percentage of cell kill. Chemotherapies are indicated as: Responsive if 60%–100% cell kill is observed. Intermediate Response if 30%–60% cell kill is observed. Non-responsive if <10%–30% cell kill is observed.
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Responsive 60%-100% Cell Kill | Paclitaxel 80 mg/m2 (70%) | Epirubicin 100 mg/m2 (60%) | Cisplatin 100 mg/m2 (70%) |
| Carboplatin 70 mg/m2 (85%) | Cisplatin 60 mg/m2 + 5-Fluorouracil 800 mg/m2 (50%) | ||
| Intermediate response 30%-60% Cell Kill | |||
| Non-Responsive < 10%–30% Cell Kill | Cisplatin 60 mg/m2 (20%) | Methotrexate 40 mg/m2 (15%) | Methotrexate 40 mg/m2 (15%) |
| 5-Fluorouracil 1000 mg/m2 (15%) | Cisplatin 100 mg/m2 (<10%) | 5-Fluorouracil 1000 mg/m2 (<10%) | |
| Docetaxel 70 mg/m2 (<10%) | Cetuximab 250 mg/m2 + Carboplatin 100 mg/m2 (<10%) | Carboplatin 25 mg/m2 (<10%) | |
| Bleomycin 15 mg/m2 (<10%) | 5-Fluorouracil 500 mg/m2 (<10%) | Paclitaxel 80 mg/m2 (<10%) | |
| Epirubicin 100 mg/m2 (<10%) | Carboplatin 100 mg/m2 (<10%) | Docetaxel 70 mg/m2 (<10%) | |
| Methotrexate 40 mg/m2 (<10%) | Paclitaxel 175 mg/m2 (<10%) | ||
| Cetuximab 400 mg/m2 (<10%) | Docetaxel 75 mg/m2 (<10%) | ||
In the second case ChemoID® assay indicated that the CSC and bulk of tumor cells of this patient were sensitive to Epirubicin (60% cell kill), but not to the other chemotherapies tested such as Methotrexate, Cisplatin, combination of Cetuximab and Carboplatin, 5-Fluorouracil, Carboplatin, Paclitaxel and Docetaxel (between 30 and 10%). Unfortunately both patients 1 and 2 refused both radiation and chemotherapy treatment options and were lost at follow-up.
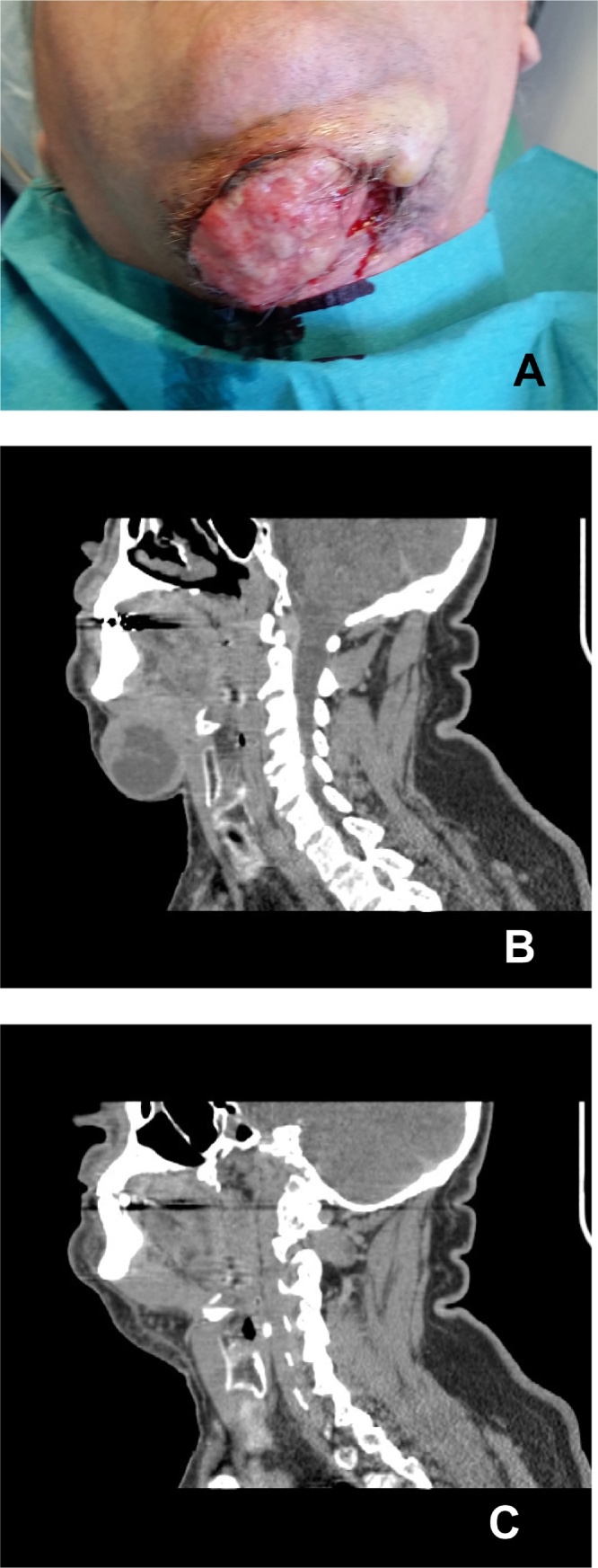
For the third case the ChemoID® assay indicated that the CSC and bulk of tumor cells of this patient were sensitive to Cisplatin and a combination of Cisplatin and 5-Fluorouracil (70% and 50% cell kill, respectively), but not to the other chemotherapies tested such as 5-Fluorouracil, Carboplatin, Methotrexate, Paclitaxel and Docetaxel (between 30 and 10%). Patient #3 was not a surgical candidate because of a massive infiltration of the carotid artery by the tumor. Patient was treated by chemotherapy according to the ChemoID® assay results and a good outcome was recorded at 6 months with significant tumor reduction measured by CT scan (Fig. 1).
Fig. 1.
Clinical presentation and CT scans of case a recurrent lower lip carcinoma infiltrating the submandibular area. (A) Patient view showing a large relapse of a lower lip carcinoma infiltrating the submandibular area. (B) CT scan of the head and neck showing a large infiltrating mass in the submandibular area. (C) 6 months follow-up CT scan of the head and neck post chemotherapy according to the ChemoID assay showing significant tumor reduction.
4. Discussion
The ChemoID® drug sensitivity assay used in this study, measures the survival of CSCs and bulk of tumor cells cultured from human cancer biopsies following chemotherapy [4]. The ChemoID® drug sensitivity assay has been tested and it is currently used to measure sensitivity of cancer stem cells to different chemotherapies in several solid malignancies including lung, breast, brain, colon, ovarian carcinoma, and advanced prostate, renal and pancreatic carcinoma, and metastatic melanoma.
The advantage of the ChemoID® assay is to aid the oncologists in selecting the most appropriate chemotherapy regimen on an individual basis especially when a number of equivalent options are available [4], [15], [16], [17], [18]. The ChemoID® assay allows various available chemotherapy drugs, which are part of standard-of-care to be tested, for efficacy against the cancer stem cells as well as the bulk of tumor cells [4].
The ChemoID® assay reports are usually released within 21 days from the acceptance of a viable biopsy at the ChemoID lab, therefore we obtained two biopsies. One specimen was submitted to our local Department of Pathology for histological diagnosis and one specimen was shipped to the ChemoID lab for chemotherapy sensitivity assessment. In this way the treating physician can receive the result of the drug response assay against the individual cancer stem cells and bulk of tumor cells prior to initiating chemotherapy. ChemoID assay can be particularly useful to help guide chemotherapy selection both in cases of post surgical cancer relapse and in non-surgically operable tumors because of poor health conditions of the patient [19]. In those cases of post-surgical large tumor recurrence in which the patient cannot be subjected to a second surgery for poor health conditions or for the infiltration and extension of the tumor to vital structures, the utility of a ChemoID® assay to avoid resistant drugs is even more compelling [20]. In fact this is particularly important because this new diagnostic test may decrease unnecessary toxicity for patients already proven and with a bad performance status derived by ineffective chemotherapies. The effectiveness of this procedure also dwells in its safety because the same biopsy procedure can be used to obtain the second specimen for ChemoID purposes at the time in which a biopsy is performed for histological diagnosis without causing additional stress to the patient.
Hypothesis of future prospective use of the ChemoID assay could be the treatment of extensive cancer lesions of the maxillofacial area combining surgery and specific chemotherapy against CSCs.
The ChemoID assay has the additional advantage that it can be repeated after a few cycles of chemotherapy if needed, because it has been previously demonstrated [4] that chemotherapy may become ineffective following some cycles of its administration due to the selection of resistant clones in the CSC populations [14]. In these cases the ChemoID assay could be used to further analyze the sensitivity of the CSCs from a relapsed tumor against a larger panel of drugs.
Unfortunately, in some of the very advanced cases, the use of the ChemoID assay may not be indicated because patients cannot wait 21 days before initiating therapy, however this test could be more useful if performed as a routine examination at earlier stages in case of the need to replace the surgical therapeutic step with chemotherapy [21].
Although generally chemotherapy is not widely used in head and neck tumors, the use of this novel drug response assay, could increase the adoption of chemotherapy to treat this type of cancer as an alternative therapeutic step in those cases in which surgery or radiation are not an option.
The present communication is the first report of a successful technique to ensure the shipment of viable live biological samples from Europe to the United States, which allowed cancer cells and cancer stem cells to be cultured and the ChemoID test to be performed. In our small cohort of patients affected by head & neck cancer we tested, unfortunately patients 1 and 2 refused radiation and/or chemotherapy treatment options and were lost at follow-up. However patient 3 affected by an advanced cancer of the oral pavement infiltrating the surrounding tissues and vital organs was successfully treated, thereby providing further evidence that personalized therapy can be useful for the management of therapy refractory and inoperable head & neck cancers.
5. Conclusions
ChemoID® results from these three cases of head and neck cancer we conducted showed the technical feasibility of this laboratory procedure even for patients hospitalized in other countries at transcontinental distances from the ChemoID lab. Larger studies using head and neck tumors will be needed to gain more objective data about drugs effectiveness on various head and neck tumors in order to avoid useless toxicity for patients and high costs for the health care system. The selective and specific analysis for chemosensitivity offered by a test such as the ChemoID® assay will be useful for the development of new therapies and for testing new chemotherapy drugs in clinical management of head and neck cancer.
Competing interest
Authors declare no competing interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
The study was conducted in accordance with the ethical principles provided by the Declaration of Helsinki and the principles of good clinical practice, under the IRB protocol number 695141 at Marshall University.
Consent
Consent was obtained under IRB 695141.
Author contribution
Antonio Cortese operated patients and collected and interepreted the data, and writing the paper.
Giuseppe Pantaleo operated patients and collected and interepreted the data, and writing the paper.
Massimo Amato operated patients and collected and interepreted the data.
Logan Lawrence performed the assay, collected the data.
Veronica Mayes performed the assay, collected the data.
Linda Brown participated in the study concept, design, data interpretation, writing the paper.
Maria Rosaria Sarno participated in the data interpretation, writing the paper.
Jagan Valluri participated in the study concept, design, data interpretation, writing the paper.
Pier Paolo Claudio participated in the study concept, design, data interpretation, writing the paper.
Guarantor
Pier Paolo Claudio.
Contributor Information
Antonio Cortese, Email: ancortese@unisa.it.
Giuseppe Pantaleo, Email: giuseppepantaleo88@gmail.com.
Massimo Amato, Email: mamato@unisa.it.
Logan Lawrence, Email: loganlawrence38@gmail.com.
Veronica Mayes, Email: Veronica.Mayes@chhi.org.
Linda Brown, Email: lbrown@marshall.edu.
Maria Rosaria Sarno, Email: mrsarno@yahoo.com.
Jagan Valluri, Email: valluri@marshall.edu.
Pier Paolo Claudio, Email: pclaudio@olemiss.edu, pclaudio@umc.edu.
References
- 1.Furness S., Glenny A.M., Worthington H.V., Pavitt S., Oliver R., Clarkson J.E., Macluskey M., Chan K.K., Conway D.I. CSROC Expert Panel. Interventions for the treatment of oral cavity and oropharyngeal cancer: chemotherapy. Cochrane Database Syst. Rev. 2010;8:CD006386. doi: 10.1002/14651858.CD006386.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Mirabile A., Numico G., Russi E.G., Bossi P., Crippa F., Bacigalupo A., De Sanctis V., Musso S., Merlotti A., Ghi M.G., Merlano M.C., Licitra L., Moretto F., Denaro N., Caspiani O., Buglione M., Pergolizzi S., Cascio A., Bernier J., Raber-Durlacher J., Vermorken J.B., Murphy B., Ranieri M.V., Dellinger R.P. Sepsis in head and neck cancer patients treated with chemotherapy and radiation: literature review and consensus. Crit. Rev. Oncol. Hematol. 2015;95:191–213. doi: 10.1016/j.critrevonc.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Cortese A., Pantaleo G., Ferrara I., Vatrella A., Cozzolino I., Di Crescenzo V., Amato M. Bone and soft tissue non-Hodgkin lymphoma of the maxillofacial area: report of two cases, literature review and new therapeutic strategies. Int. J. Surg. 2014;12:S23–8. doi: 10.1016/j.ijsu.2014.08.388. [DOI] [PubMed] [Google Scholar]
- 4.Mathis S.E., Alberico A., Nande R., Neto W., Lawrence L., McCallister D.R., Denvir J., Kimmey G.A., Mogul M., Oakley G., 3rd, Denning K.L., Dougherty T., Valluri J.V., Claudio P.P. Chemo-predictive assay for targeting cancer stem-like cells in patients affected by brain tumors. PLoS One. 2014;9:e105710. doi: 10.1371/journal.pone.0105710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hatok J., Babusikova E., Matakova T., Mistuna D., Dobrota D., Racay P. In vitro assays for the evaluation of drug resistance in tumor cells. Clin. Exp. Med. 2009;9(March (1)):1–7. doi: 10.1007/s10238-008-0011-3. Epub 2008 Sep 26. [DOI] [PubMed] [Google Scholar]
- 6.Williams S.A., Anderson W.C., Santaguida M.T., Dylla S.J. Patient-derived xenografts, the cancer stem cell paradigm, and cancer pathobiology in the 21 st century. Lab. Invest. 2013;93(September (9)):970–982. doi: 10.1038/labinvest.2013.92. Epub 2013 Aug 5. [DOI] [PubMed] [Google Scholar]
- 7.Reya T., Morrison S.J., Clarke M.F. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 8.Rastogi P. Emergence of cancer stem cells in head and neck squamous cell carcinoma: a therapeutic insight with literature review. Dent. Res. J. (Isfahan) 2012;9:239–244. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.O’Brien C.A., Kreso A., Jamieson C.H. Cancer stem cells and self-renewal. Clin. Cancer Res. 2010;16:3113–3120. doi: 10.1158/1078-0432.CCR-09-2824. [DOI] [PubMed] [Google Scholar]
- 10.De Carlo F., Witte T.R., Hardman W.E., Claudio P.P. Omega-3 eicosapentaenoic acid decreases CD133 colon cancer stem-like cell marker expression while increasing sensitivity to chemotherapy. PLoS One. 2013;8(July (7)):e69760. doi: 10.1371/journal.pone.0069760. Print 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aimola P., Desiderio V., Graziano A., Claudio P.P. Stem cells in cancer therapy: from their role in pathogenesis to their use as therapeutic agents. Drug News Perspect. 2010;23:175–183. doi: 10.1358/dnp.2010.23.3.1489979. [DOI] [PubMed] [Google Scholar]
- 12.Kelly S.E., Di Benedetto A., Greco A., Howard C.M., Sollars V.E., Primerano D.A., Valluri J.V., Claudio P.P. Rapid selection and proliferation of CD133 + cells from cancer cell lines: chemotherapeutic implications. PLoS One. 2010;5:e10035. doi: 10.1371/journal.pone.0010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malik B., Nie D. Cancer stem cells and resistance to chemo and radiotherapy. Front. Biosci. (Elite Ed.) 2012;1:2142–2149. doi: 10.2741/531. [DOI] [PubMed] [Google Scholar]
- 14.Yu Y., Ramena G., Elble R.C. The role of cancer stem cells in relapse of solid tumors. Front. Biosci. (Elite Ed.) 2012;4:1528–1541. doi: 10.2741/e478. [DOI] [PubMed] [Google Scholar]
- 15.Korkaya H., Wicha M.S. Selective targeting of cancer stem cells: a new concept in cancer therapeutics. BioDrugs. 2007;21:299–310. doi: 10.2165/00063030-200721050-00002. [DOI] [PubMed] [Google Scholar]
- 16.Hagemeister F.B. Treatment of relapsed aggressive lymphomas: regimens with and without high-dose therapy and stem cell rescue. Cancer Chemother. Pharmacol. 2002;49:S13–20. doi: 10.1007/s00280-002-0447-1. [DOI] [PubMed] [Google Scholar]
- 17.Suntharalingam M., Haas M.L., Van Echo D.A., Haddad R., Jacobs M.C., Levy S., Gray W.C., Ord R.A., Conley B.A. Predictors of response and survival after concurrent chemotherapy and radiation for locally advanced squamous cell carcinomas of the head and neck. Cancer. 2001;91:548–554. doi: 10.1002/1097-0142(20010201)91:3<548::aid-cncr1033>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 18.Cortese A., Pantaleo G., Amato M., Claudio P.P. A patient with a rare single mandibular localization of non-hodgkin B-cell lymphoma: early differential diagnosis with dental lesions. J. Craniofac. Surg. 2015;26:e365–6. doi: 10.1097/SCS.0000000000001797. [DOI] [PubMed] [Google Scholar]
- 19.Qian X., Ma C., Nie X., Lu J., Lenarz M., Kaufmann A.M., Albers A.E. Biology and immunology of cancer stem(-like) cells in head and neck cancer. Crit. Rev. Oncol. Hematol. 2015;95:337–345. doi: 10.1016/j.critrevonc.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 20.William W.N., Jr., El-Naggar A.K. A novel target for oral cancer chemoprevention? Notch quite yet. Cancer Prev. Res. (Phila.) 2015;8:262–265. doi: 10.1158/1940-6207.CAPR-15-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cripps C., Winquist E., Devries M.C., Stys-Norman D., Gilbert R. Head and Neck Cancer Disease Site Group. Epidermal growth factor receptor targeted therapy in stages III and IV head and neck cancer. Curr. Oncol. 2010;17:37–48. doi: 10.3747/co.v17i3.520. [DOI] [PMC free article] [PubMed] [Google Scholar]