Abstract
Aim
Hepatic sarcoidosis is a rare indication for orthotopic liver transplantation (OLT). Hence, studies evaluating these patients are scarce. We present a single center experience with OLT for hepatic sarcoidosis in a case–control study.
Methods
A retrospective chart review was performed on 970 patients with OLT at our center, and 13 patients (1.3%) were identified who underwent 14 OLTs for hepatic sarcoidosis. For each case, two controls matched for etiology of liver disease, recipient age (±5 years), and duration since transplant (within 5 years) were selected.
Results
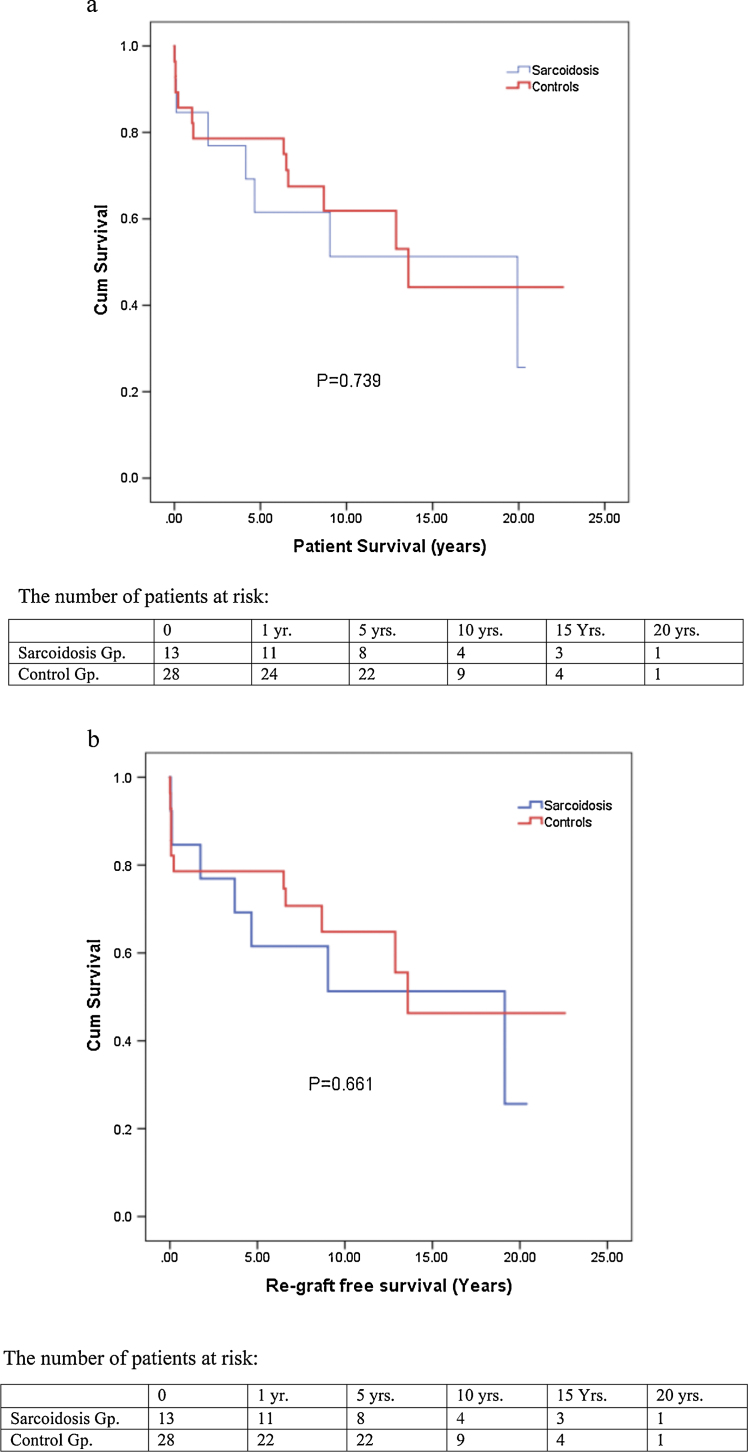
For the 13 patients transplanted for sarcoidosis, the median age was 46 years. The majority were women (62%) and African-American (85%). Cholestatic liver disease was the primary manifestation. Portal hypertensive complications were present in 11 patients (84%). The median MELD score at transplantation was 19. Extra-hepatic manifestations were present in ten patients (77%). All patients received whole deceased 14 donor allografts. Six patients remain alive with a median post-OLT follow-up of 8.4 years. The 1-, 3-, 5-, and 10-year patient survival rates were 84.6%, 76.9%, 61.1%, and 51.3%, respectively for the sarcoidosis group and 82.1%, 78.6%, 78.6%, and 61.9%, respectively for the matched PSC/PBC group (P = 0.739). Re-graft free survival for sarcoidosis patients was 84.6%, 76.9%, 61.5%, and 51.3% for 1-, 3-, 5-, and 10-years and for the matched control group re-graft free survival was 78.6% at 1-, 3-, 5-years, and 64.8% at 10-years (P = 0.661). Recurrence of hepatic sarcoidosis was found in 4 patients at 11 days, 112 days, 222 days, and 6.6 years.
Conclusions
Our study depicts the long-term benefit of liver transplantation in patients with end stage liver disease secondary to sarcoidosis. It shows statistically comparable graft and patient survival for such patients when compared to other cholestatic diseases. Disease recurrence, although possible, has not been shown to cause allograft dysfunction.
Abbreviations: OLT, orthotopic liver transplantation; ACE, angiotensin converting enzyme; PBC, primary biliary cirrhosis; PSC, primary sclerosing cholangitis; CTP, Child–Turcotte–Pugh; MELD, Model for End Stage Liver Disease; HCV, hepatitis C virus; DM, diabetes mellitus
Keywords: sarcoidosis, liver transplantation, cirrhosis, portal hypertension
Sarcoidosis is a multisystem disease characterized by non-caseating granulomas in the affected organs. The most commonly involved organs are lungs, skin, and eyes although any organ can be affected. Liver involvement is seen by biopsy or autopsy in 50–79% of sarcoidosis patients.1, 2, 3, 4 Patients with sarcoidosis are infrequently symptomatic due to liver disease and may present with pruritus, abdominal pain, and fever.5 Hepatomegaly is found in about 21–50% of the patients.6, 7, 8 Liver involvement may occur without lung involvement in up to 47% of the patients.9
Abnormal liver chemistries are noted in only 35% of patients.9, 10 In hepatic sarcoidosis, the histologic abnormalities include non-caseating granulomas, chronic intrahepatic cholestasis, progressive diminution in the number of interlobular bile ducts, peri-portal fibrosis, and micronodular biliary cirrhosis.11 Jaundice is rare and may be due to intrahepatic cholestasis, hemolysis, hepatocellular dysfunction, or obstruction of the extra-hepatic bile ducts by granulomatous hepatic hilar lymph nodes.6 Progressive liver disease due to sarcoidosis may lead to the development of portal hypertension.12, 13 Development of cirrhosis is not a pre-requisite for portal hypertension.14 Severe liver dysfunction and jaundice are uncommon.12 Other vascular complications include portal vein thrombosis because of stasis from obliteration of small portal veins12 and Budd-Chiari syndrome because of extrinsic compression of hepatic veins by sarcoid granulomas, causing narrowing of venous vessels, venous stasis, and subsequent thrombosis.15
Considering its rarity as an etiology for end stage liver disease, limited information is available on the long-term outcome in recipients with liver transplantation for sarcoidosis. Our aim is to evaluate the long-term outcomes in recipients with orthotopic liver transplantation (OLT) for hepatic sarcoidosis at our center.
Methods
A retrospective chart review was performed on 970 patients who underwent OLT at our center from October 1993 to February 2016. Thirteen patients (1.3%) were identified who underwent 14 OLTs with hepatic sarcoidosis as the indication. The diagnosis of hepatic sarcoidosis was established by a combination of characteristic clinical findings (lung/skin/eye involvement, high serum ACE levels, etc.), presence of non-caseating granulomas in the pre-transplant liver biopsy specimen (4 patients, biopsies were not done on all patients and on a few records could not be obtained) or the explant (all patients) at the time of transplantation, and the absence of other etiologies.
The OLT recipients for sarcoidosis were compared to matched controls that received OLT for an indication of cholestatic liver disease, primary biliary cirrhosis (PBC) or primary sclerosing cholangitis (PSC). Two controls were selected in a random fashion after they were matched for recipient age (±5 years) and duration since transplant (within 5 years). Twenty-eight PBC or PSC recipients as controls were matched to the study group.
Retrospective chart review was completed to obtain demographic data, viral serologies, titers of autoantibodies, co-morbid conditions, serum transaminases, alkaline phosphatase, total bilirubin, albumin, INR, prothrombin time, Child–Turcotte–Pugh (CTP) score, Model for End Stage Liver Disease (MELD) score, creatinine, immunosuppression, complications, liver biopsies, and transplant-related outcomes. Liver biopsies were performed at one year post-transplant or for liver dysfunction identified by laboratory studies, and disease recurrence was defined by evidence of non-caseating granulomas in the liver biopsy specimen and absence of other etiologies of hepatic granulomas. Categorical data were compared with Fisher's exact test with Yates correction as appropriate. Continuous variables were compared as medians using Mann–Whitney U test. Patient and graft survival rates were estimated using Kaplan–Meier curves with comparison by log-rank test. Statistical significance was set a priori at P < 0.05. SPSS 23.0 (IBM Corporation, Armonk, NY) was used for analysis.
Results
Pre-transplant Characteristics
Pre-transplant characteristics are summarized in Table 1. For the 13 patients transplanted for sarcoidosis, the median age was 46 years (range: 30–61 years). The majority were women (62%) and African-American (85%). The indication for OLT in all of our patients was sarcoidosis, but 2 patients had concomitant chronic hepatitis C virus infection. The median duration of disease from diagnosis to OLT was 7.6 years (range: 1–16 years). Five patients (38%) had disease limited to liver only. Extra-hepatic manifestations were present in ten patients (77%): all with pulmonary disease, 2 with hypercalcemia, 1 with cutaneous manifestation, 1 with uveitis, and 1 with arteritis. Three patients had diabetes mellitus preceding OLT (27%). Cholestatic liver disease was the primary manifestation with a median serum total bilirubin of 8.9 mg/dL (range: 0.6–18.0 mg/dL) and median serum alkaline phosphatase of 353 U/dL (range: 85–1100 U/dL). Portal hypertensive complications were present in 11 patients (84%): 7 with ascites, 11 with symptomatic esophageal varices, and 4 with hepatic encephalopathy. The median MELD score at transplantation was 19 (range: 9–29).
Table 1.
Pre-transplant Characteristics of Cases and Controls.
| Characteristics | Cases (n = 13, 14 OLTs) | Controls (n = 28) | P-value |
|---|---|---|---|
| Demographics | |||
| Age at LT (yrs) ± SD (range) | 45.5 ± 8.7 (30–61) | 46.32 ± 9.32 (25–64) | 0.8 |
| Male/female | 5/8 (females 62%) | 16/12 (females 43%) | 0.5 |
| Race (Caucasian/African-American/Other) | 2/11 (African-American 85%) | 22/5/1 (African-American 18%) | <.0001 |
| Laboratory data | |||
| Serum total bilirubin (mg/dL) | 8.9 (0.6–18) | 5 (1–33) | .102 |
| Serum alkaline phosphatase (IU/L) | 353 (85–1100) | 297 (77–1100) | .91 |
| AST (IU/L) | 88 (39–209) | 154 (33–1600) | .30 |
| ALT (IU/L) | 71 (15–172) | 96 (13–1200) | .72 |
| Serum albumin (g/dL) | 2.6 (1.7–4.2) | 3.0 (1.4–4.1) | .44 |
| MELD | 19 (9–29) | 19 (7–37) | .94 |
| Serum creatinine (mg/dL) | 1.1 (0.6–2.6) | 1.0 (.6–4.5) | .74 |
| INR | 1.4 (1.0–2.4) | 1.3 (0.9–2.0) | .83 |
Data are reported as median (range) except gender and race that are expressed as numbers.
Given the long period of the study, post-transplant immunosuppression changed over the course of the study. For patients in either group transplanted prior to 1994 (2 patients), steroids, cyclosporine, and azathioprine were used. From 1994 to early 1998, immunosuppression was steroids, tacrolimus, and azathioprine (2 patients). In early 1998 when mycophenolate was approved, it was substituted for azathioprine (4 patients). Beginning in 2006, steroid-free anti-thymocyte globulin was used for induction with tacrolimus and mycophenolate for maintenance immunosuppression (6 patients).
Post-transplant Characteristics
All patients received whole deceased donor liver allografts with one patient also receiving a simultaneous kidney allograft due to hepatorenal syndrome. Six of the thirteen OLT recipients with an indication of sarcoidosis remain alive with a median post-OLT follow-up of 8.4 years. Three sarcoidosis patients (23%) required re-OLT. The indications for re-OLT were recurrent sarcoidosis/HCV at 3.7 years (with kidney), autoimmune hepatitis/severe rejection at 1.7 years, and idiopathic hepatoportal sclerosis at 19.1 years (with kidney). Kaplan–Meier estimates were done of overall patient as well as re-graft free survival. The 1-, 3-, 5-, and 10-year patient survival rates were 84.6%, 76.9%, 61.1%, and 51.3%, respectively for the sarcoidosis group and 82.1%, 78.6%, 78.6%, and 61.9%, respectively for the matched PSC/PBC group (Figure 1A, P = 0.739). Re-graft free survival for sarcoidosis patients was 84.6%, 76.9%, 61.5%, and 51.3% for 1-, 3-, 5-, and 10-years (Figure 1B) and for the matched control group re-graft free survival was 78.6% at 1-, 3-, 5-years, and 64.8% at 10-years (P = 0.661). Five patients died from sepsis, including the three patients with re-OLT. The two other deaths were respiratory failure at 18 days and cardiac arrest at 9 years (Table 2).
Figure 1.
(A) Cumulative overall patient survival. (B) Cumulative re-graft free survival.
Table 2.
Demographics and Clinical Profile of the Patients With Sarcoidosis and Summary of Their Post-transplant Course.
| Patient profile | MELD | Bil | ALT | AP | Varices | EHS | Time to recurrence | Re-LT | Graft surv. (yrs.) | Patient surv. (yrs.) | Etiology of death |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 46 yr. old AAF | 19 | 1.5 | 41 | 768 | Yes | Lungs | – | No | 7.70 | 7.70 | – |
| 43 yr. old AAF | 20 | 6.8 | 75 | 515 | Yes | Lungs/↑Ca | – | No | 19.00 | 19.00 | – |
| 57 yr. old AAF | 15 | 2.4 | 23 | 174 | Yes | Lungs | – | No | 7.90 | 7.90 | – |
| 49 yr. old AAM | 19 | 10.4 | 102 | 297 | Yes | 222 days | No | 4.60 | 4.60 | Sepsis | |
| 47 yr. old CM | 20 | 18.0 | 141 | 479 | Yes | Lungs, skin and retina | – | No | 9.00 | 9.00 | Cardiac |
| 30 yr. old AAF | 13 | 11.0 | 95 | 1063 | No | ↑Ca | 6.6 years | Yes | 19.1 | 19.30 | Sepsis |
| 48 yr. old AAF | 9 | 0.6 | 19 | 375 | Yes | Lungs | – | No | 11.20 | 11.20 | – |
| 40 yr. old AAF | 12 | 13.8 | 138 | 192 | Yes | – | No | 20.30 | 20.30 | – | |
| 42 yr. old AAMa | 20 | 7.3 | 150 | 449 | Yes | Lungs | 112 days | Yes | 3.7 | 4.10 | Sepsis |
| 47 yr. old AAMa | 29 | 7.6 | 15 | 190 | Yes | Lungs | – | No | 0.40 | 4.10 | Sepsis |
| 32 yr. old AAM | 17 | 12.0 | 172 | 331 | Yes | Lungs | – | No | 0.11 | 0.11 | Sepsis |
| 61 yr. old CF | 23 | 14.0 | 31 | 222 | Yes | Lungs | – | No | 9.70 | 9.70 | – |
| 40 yr. old AAF | 16 | 10.2 | 121 | 778 | No | – | Yes | 1.7 | 1.90 | Sepsis (mycotic aneurysm) | |
| 55 yr. old AAMa | 22 | 3.5 | 23 | 85 | Yes | Lungs | 11 days | Yes | 0.05 | 0.05 | Respiratory failure |
ReLT, re-transplant; Bil, bilirubin; MELD, Model for End Stage Liver Disease score; AAF, African American female; AAM, African American male; CM, Caucasian male; CF, Caucasian female; EHS, extra hepatic sarcoidosis.
* Explant pathology in all patients showed features of granulomatous hepatitis and cirrhosis of the liver.
Concomitant HCV infection.
Other post-transplant complications were noted in 4 patients. Three patients developed chronic renal failure requiring hemodialysis. The cause of renal failure was chronic exposure to calcineurin inhibitors in one patient, hypercalcemia along with chronic exposure to calcineurin inhibitors in one patient, and hepatorenal syndrome in one patient. The patient with hypercalcemia also developed pancreatitis. One patient developed severe autoimmune hepatitis resulting in graft failure.
Recurrence of hepatic sarcoidosis, defined by reappearance of noncaseating granulomas in the liver, was found by biopsy in 4 patients at 11 days, 112 days, 222 days, and 6.6 years. The patient which had the liver biopsy showing recurrence at 112 days did require re-OLT for recurrent sarcoidosis after 3.7 yrs. Additionally, the patient that had re-OLT for autoimmune hepatitis/severe rejection had a few scattered non-caseating granulomas in the explanted allograft.
Discussion
End stage liver disease due to hepatic sarcoidosis requiring transplantation is extremely rare. Consequently, data on the outcomes of liver transplantation for hepatic sarcoidosis are scarce. We report our experience with 13 sarcoidosis patients, which is the largest number of patients reported from a single center. In our cohort of cases and control, the median age was similar around 46 years. In the sarcoidosis group, the majority were women and African-American in contrast to controls where women and African-American were the minority in this group. This is not surprising, as female preponderance of sarcoidosis is known across all ethnicities and races. Additionally, the incidence of sarcoidosis in African-American is about 3 times higher than other races.16 As expected, the liver function test abnormalities were cholestatic in pattern for both cases and controls as depicted in Table 1. The median MELD score was similar for both groups.
Although sarcoidosis can involve any organ, more than 90% cases will have involvement of lungs, eyes, and skin in some combination.17 About 10% of patients with sarcoidosis will have some form of abnormality in their transaminases or alkaline phosphatase and only about 1% will develop portal hypertension progressing to cirrhosis and liver failure.18, 19 In our group of patients with sarcoidosis, 23% had disease limited to liver only. In other studies, disease limited to liver has been reported in up to 47% of patients seen in a liver clinic.10 Extra-hepatic manifestations were present in 10 of 13 and pulmonary disease was present in 9 of the patients (69%). The ACCESS study showed that around 90% of patients with sarcoidosis have pulmonary disease.17 However, the principal investigator in this study was a pulmonologist and extensive pulmonary evaluation was undertaken including CXR, spirometry, and dyspnea scores. Obviously, our patients were referred for their liver disease alone, and the relatively less involvement of lungs is not surprising. Additionally, we may not have screened them for pulmonary disease aggressively in absence of pulmonary symptoms.
According to our review of literature, two previous retrospective studies have been published evaluating hepatic sarcoidosis patients following OLT. Casavilla et al.20 did a small retrospective study involving 9 patients who underwent OLT for hepatic sarcoidosis in 1993. Post-OLT survival of graft and patients at 5 years was reported as 70% and 90% and was similar to control groups including patients with cholestatic and parenchymal disease. No recurrence of hepatic sarcoidosis was reported. Post-OLT sarcoidosis activity was reported to be in remission while on immunosuppression, which at that time primarily included cyclosporine. In our study, patients were thoroughly screened for other causes of liver disease. Although 2 patients did have concomitant HCV infection, explanted liver pathology was dominated by non-caseating granulomas consistent with active hepatic sarcoidosis. One of these 2 patients died within 1 month due to post-operative complications. The second patient with concomitant HCV infection developed cirrhosis and complications of liver disease related to hepatic sarcoidosis with HCV. Retransplantation was performed after 3.75, which was complicated by infections leading to the patient's death shortly following the retransplantation procedure. The second study was done by Lipson et al.,21 which included 7 carefully selected patients. However, the control arm included only 14 patients and most of them had parenchymal disease and only one had cholestatic disease, whereas we have selected controls with similar cholestatic disease pattern. They detected a statistically significant increase in the frequency of diabetes mellitus preceding liver transplantation in sarcoid cases. However, in our study, pre-OLT incidence of DM was noted to be 23% (3/13 patients) and 18% (4/22) for cases and controls respectively which is statistically insignificant. Presence of pre-OLT DM begs the questions whether these patients had developed cirrhosis due to underlying non-alcoholic steatohepatitis (NASH) rather than sarcoidosis. However, explant pathology in all patients only showed negligible steatosis without any suggestive findings of NASH, such as ballooning, or Mallory-Denk bodies that would suggest progression from underlying NASH, but rather florid evidence of granulomatous liver disease with frank cirrhosis was noted in all included patients. Additionally, presence of multiple non-caseating granulomas is not typical of NASH. Disease recurrence in this study was noted in 1 of 7 patients at round 5.6 yrs post-OLT.
Recurrence of sarcoid hepatic granulomas has been previously reported but most of these have been case reports or solitary case in a retrospective study.21, 22, 23, 24 In our study, 4 of 13 patients (31%) developed sarcoidosis recurrence in the allograft defined by development of non-caseating granulomas. One of them developed recurrence in 11 days after the transplant which is earliest such case ever reported. Before this, the earliest recurrence in the allograft was reported at 8 months after the transplant.24 This patient had a donor recovery liver biopsy, which was normal and did not show any evidence of granulomas. We are surprised by such an early recurrence, but sampling error must be considered, and the possibility of undiagnosed donor granulomatous disease cannot be entirely ruled out. This patient with recurrence died on day 18 from respiratory complications and unfortunately could not have longer follow-up. Other 3 patients died in about 4.5 and 19 years post-OLT as detailed in Table 2. Other studies have not shown any impact of recurrence especially on graft or patient survival and we had similar results.21 We have studied the maximum number of sarcoidosis patients (13) followed over the maximum duration (median 8.4 years).
In our study, the 1-, 3-, 5-, and 10-yr graft and patient survivals were statistically similar between cases and controls but numerically worse for sarcoidosis patients. This adds to our recent publication on this subject when we found statistically worse outcomes (both graft and patient survival) for sarcoidosis patients when compared with other cholestatic diseases like PSC and PBC.25 Previous studies, however, showed similar outcomes for these patient groups but these were small and selection bias remains a concern.20, 21 One possible reason for relatively poorer outcome of liver transplantation for these patients could be that sarcoidosis is a multi-organ disease with special predilection for vital organs. Such involvement could have affected the patient before or after OLT.
Our sarcoidosis patients did very well on our post-OLT immunosuppression regimen, which primarily includes steroids, tacrolimus, and mycophenolate. Beginning in 2006, we have switched to a steroid-free regimen using rabbit anti-thymocyte globulin for induction with tacrolimus and mycophenolate. Despite steroid avoidance, none of our patients has showed signs of decompensated sarcoidosis. This lends a unique perspective in terms of sarcoidosis management post-OLT where a steroid-free-regimen could also be a viable option.
The limitations of the current study are those inherent to any retrospective, single-center study. Donor selection criteria and recipient selection criteria, although integral to the study design, are center-specific and introduce bias that may limit applicability of these results to other centers. On the other hand, single-center analyses do provide some clarity to certain variables by permitting observations with consistent donor and recipient selection, operative experience, and post-operative management, or by identifying and testing variables not available in registries. Additionally, small samples have limited statistical power but permit meaningful observations that can be used to develop future areas of investigation. However, given the rarity of liver transplantation for hepatic sarcoidosis, center-specific studies may afford the best alternative to building the knowledge that defines the best management of these patients. Additionally, although we evaluated patients for extra-hepatic disease prior to transplant, we did not precisely track the effect of OLT on extra-hepatic disease unless it impacted patient survival.
Our study depicts the long-term benefit of liver transplantation in patients with end stage liver disease secondary to sarcoidosis. It shows statistically comparable (although slightly numerically worse) graft and patient survival for such patients when compared to other cholestatic diseases. Disease recurrence, although possible, has not been shown to cause allograft dysfunction, particularly when patients survive the immediate post-OLT period.
Conflicts of Interest
The authors have none to declare.
References
- 1.Ricker W., Clark M. Sarcoidosis; a clinicopathologic review of 300 cases, including 22 autopsies. Am J Clin Pathol. 1949;19:725–749. doi: 10.1093/ajcp/19.8.725. [DOI] [PubMed] [Google Scholar]
- 2.Hercules H.D., Bethlem N.M. Value of liver biopsy in sarcoidosis. Arch Pathol Lab Med. 1984;108:831–834. [PubMed] [Google Scholar]
- 3.Irani S.K., Dobbins W.O., 3rd Hepatic granulomas: review of 73 patients from one hospital and survey of the literature. J Clin Gastroenterol. 1979;1:131–143. [PubMed] [Google Scholar]
- 4.Iwai K., Oka H. Sarcoidosis report of ten autopsy cases in Japan. Am Rev Respir Dis. 1964;90:612–622. doi: 10.1164/arrd.1964.90.4.612. [DOI] [PubMed] [Google Scholar]
- 5.Nolan J.P., Klatskin G. The fever of sarcoidosis. Ann Intern Med. 1964;61:455–461. doi: 10.7326/0003-4819-61-3-455. [DOI] [PubMed] [Google Scholar]
- 6.Branson J.H., Park J.H. Sarcoidosishepatic involvement: presentation of a case with fatal liver involvement; including autopsy findings and review of the evidence for sarcoid involvement of the liver as found in the literature. Ann Intern Med. 1954;40:111–145. doi: 10.7326/0003-4819-40-1-111. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg J.C. Portal hypertension complicating hepatic sarcoidosis. Surgery. 1971;69:294–299. [PubMed] [Google Scholar]
- 8.Warshauer D.M., Molina P.L., Hamman S.M. Nodular sarcoidosis of the liver and spleen: analysis of 32 cases. Radiology. 1995;195:757–762. doi: 10.1148/radiology.195.3.7754007. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy P.T., Zakaria N., Modawi S.B. Natural history of hepatic sarcoidosis and its response to treatment. Eur J Gastroenterol Hepatol. 2006;18:721–726. doi: 10.1097/01.meg.0000223911.85739.38. [DOI] [PubMed] [Google Scholar]
- 10.Vatti R., Sharma O.P. Course of asymptomatic liver involvement in sarcoidosis: role of therapy in selected cases. Sarcoidosis Vasc Diffuse Lung Dis. 1997;14:73–76. [PubMed] [Google Scholar]
- 11.Rudzki C., Ishak K.G., Zimmerman H.J. Chronic intrahepatic cholestasis of sarcoidosis. Am J Med. 1975;59:373–387. doi: 10.1016/0002-9343(75)90396-4. [DOI] [PubMed] [Google Scholar]
- 12.Moreno-Merlo F., Wanless I.R., Shimamatsu K. The role of granulomatous phlebitis and thrombosis in the pathogenesis of cirrhosis and portal hypertension in sarcoidosis. Hepatology. 1997;26:554–560. doi: 10.1002/hep.510260304. [DOI] [PubMed] [Google Scholar]
- 13.Maddrey W.C., Johns C.J., Boitnott J.K. Sarcoidosis and chronic hepatic disease: a clinical and pathologic study of 20 patients. Medicine. 1970;49:375–395. doi: 10.1097/00005792-197009000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Valla D., Pessegueiro-Miranda H., Degott C. Hepatic sarcoidosis with portal hypertension. A report of seven cases with a review of the literature. Q J Med. 1987;63:531–544. [PubMed] [Google Scholar]
- 15.Russi E.W., Bansky G., Pfaltz M. Budd-Chiari syndrome in sarcoidosis. Am J Gastroenterol. 1986;81:71–75. [PubMed] [Google Scholar]
- 16.Rybicki B.A., Major M., Popovich J., Jr. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145:234–241. doi: 10.1093/oxfordjournals.aje.a009096. [DOI] [PubMed] [Google Scholar]
- 17.Iannuzzi M.C., Rybicki B.A., Teirstein A.S. Sarcoidosis. N Engl J Med. 2007;357:2153–2165. doi: 10.1056/NEJMra071714. [DOI] [PubMed] [Google Scholar]
- 18.Baughman R.P., Teirstein A.S., Judson M.A. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164:1885–1889. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- 19.Harder H., Buchler M.W., Frohlich B. Extrapulmonary sarcoidosis of liver and pancreas: a case report and review of literature. World J Gastroenterol. 2007;13:2504–2509. doi: 10.3748/wjg.v13.i17.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casavilla F.A., Gordon R., Wright H.I. Clinical course after liver transplantation in patients with sarcoidosis. Ann Intern Med. 1993;118:865–866. doi: 10.7326/0003-4819-118-11-199306010-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lipson E.J., Fiel M.I., Florman S.S. Patient and graft outcomes following liver transplantation for sarcoidosis. Clin Transplant. 2005;19:487–491. doi: 10.1111/j.1399-0012.2005.00372.x. [DOI] [PubMed] [Google Scholar]
- 22.Fidler H.M., Hadziyannis S.J., Dhillon A.P. Recurrent hepatic sarcoidosis following liver transplantation. Transplant Proc. 1997;29:2509–2510. doi: 10.1016/s0041-1345(97)00488-0. [DOI] [PubMed] [Google Scholar]
- 23.Hunt J., Gordon F.D., Jenkins R.L. Sarcoidosis with selective involvement of a second liver allograft: report of a case and review of the literature. Mod Pathol. 1999;12:325–328. [PubMed] [Google Scholar]
- 24.Cengiz C., Rodriguez-Davalos M., deBoccardo G. Recurrent hepatic sarcoidosis post-liver transplantation manifesting with severe hypercalcemia: a case report and review of the literature. Liver Transplant. 2005;11:1611–1614. doi: 10.1002/lt.20626. [DOI] [PubMed] [Google Scholar]
- 25.Vanatta J.M., Modanlou K.A., Dean A.G. Outcomes of orthotopic liver transplantation for hepatic sarcoidosis: an analysis of the United Network for Organ Sharing/Organ Procurement and Transplantation Network data files for a comparative study with cholestatic liver diseases. Liver Transplant. 2011;17:1027–1034. doi: 10.1002/lt.22339. [DOI] [PubMed] [Google Scholar]