Background
Alopecia areata (AA) is a highly prevalent autoimmune disease in the US with a lifetime risk of 1.7%. However, AA remains a significant unmet need in dermatology and treatments are lacking. Janus kinase inhibitors are emerging as potential therapies for many autoimmune conditions, including most recently AA (1,2). We report a patient who was treated with oral tofacitinib citrate, a preferential JAK3/JAK1 inhibitor, for AA, resulting in significant hair regrowth and concurrent skin and blood biomarker changes.
Questions addressed
We hypothesized that effective tofacitinib treatment of alopecia areata would be accompanied by changes in expression of AA-associated genes in skin as well as circulating serum CXCL10 levels.
Experimental Design
Punch biopsies were taken at baseline and after four weeks of treatment. Total RNA was extracted, reverse transcribed, amplified, biotinylated, and then hybridized to Human U133 Plus 2.0 gene chips (Affymetrix, Santa Clara, CA) as previously described (3). ALADIN scores were calculated as previously described relative to healthy controls at baseline (3).
Serum from blood draws taken prior to the initiation of tofacitinib treatment and after four weeks of treatment were assayed for CXCL10 levels using the Human IP-10/CXCL10 ELISA kit (Sigma-Aldrich, St. Louis, MO) according to the manufacturer’s instructions.
Results
A 40-year-old Caucasian woman with persistent moderate/severe AA was enrolled in our open-label pilot study to test the efficacy of oral tofacitinib for AA (https://clinicaltrials.gov/ct2/show/NCT02299297). Her AA began on her scalp five years prior to enrollment, and resolved completely within one year in the setting of pregnancy. A few months after delivery, her AA recurred as patchy disease. Treatment with topical corticosteroids, anthralin cream and intralesional corticosteroids were of limited benefit. Her AA progressed to involve all extremities, eyelashes and eyebrows with patchy scalp involvement, and remained stable until her enrollment into the clinical trial (Figure 1). Her past medical history was unremarkable and she denied a family history of AA.
Figure 1.
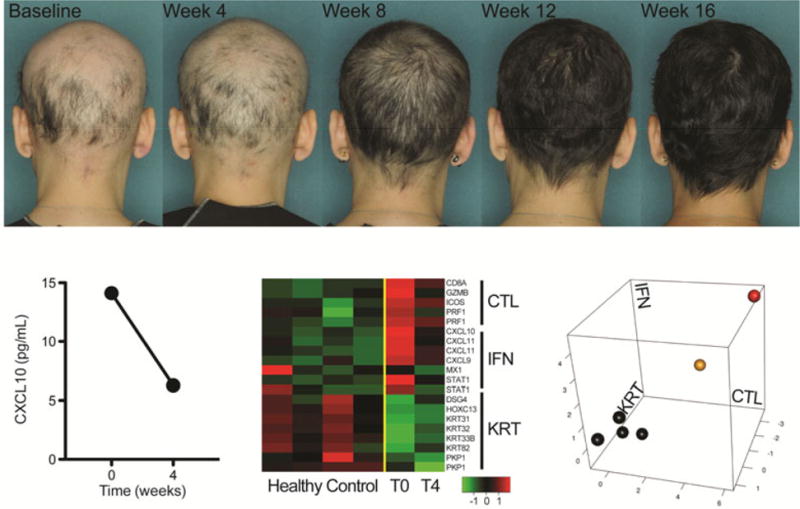
Clinical photographs of and serum CXCL10 and ALADIN profile of skin biopsy samples from an AA patient treated with tofacitinib. Top panel, Photographs were taken of the posterior scalp over 16 weeks of treatment with tofacitinib 5 mg twice daily. Bottom left panel, Blood and skin samples were taken at baseline and after 4 weeks of treatment with tofacitinib. CXCL10 ELISA was performed. Bottom middle panel, Heat map of ALADIN genes from skin samples taken from healthy control patients (normal) and the AA patient at baseline (T0) and after 4 weeks of treatment (T4). Bottom right panel, ALADIN plot of skin samples taken from healthy control patients (black) and the AA patient at baseline (red) and after 4 weeks of treatment (yellow).
The patient began treatment with tofacitinib 5 mg twice daily. Patchy regrowth was noted at month 1. After two and three months of treatment, she had scalp hair regrowth of 62.5% and 94%, respectively. Significant regrowth of her eyebrows and eyelashes was noted. Scalp hair regrowth was nearly complete 4 months after initiating treatment (Figure 1). There were no adverse events reported and no laboratory abnormalities in her complete blood count, complete metabolic panel, or lipid profile. Cessation of treatment with tofacitinib resulted in near-complete hair loss (Supplemental Figure 1).
Punch skin biopsies (Supplemental Figure 2) and blood draws were performed at baseline and after four weeks of treatment to monitor gene expression and biomarker changes. Serum levels of CXCL10, an interferon (IFN)-induced chemokine found at high levels in AA skin (3), decreased after 4 weeks of treatment (Figure 2). In addition, microarray analysis was performed on the skin biopsy samples. Based on the AA Disease Activity Index (ALADIN) (3), the patient exhibited high IFN and cytotoxic T lymphocyte (CTL) signatures at baseline that decreased by 4 weeks of treatment, although not to the level of normal controls (Figure 2).
Conclusion
AA is an autoimmune disease with strong associations with genetic loci in close proximity to genes with immune functions (4). Targeting candidate immune pathways that may be contributing to disease pathogenesis is an active area of investigation (5), and JAK inhibitors target multiple immune signaling pathways involved in AA. We have previously shown systemic and topical tofacitinib to be effective in preventing the development of AA, as well as reversing established AA, in the graft model of AA in C3H/HeJ mice (3). We report here effective treatment of a human subject with persistent patchy AA, correlating with a diminished ALADIN profile compared to baseline. Larger clinical studies are needed to assess the safety, efficacy, and durability of tofacitinib and other JAK inhibitors in the treatment of AA and to correlate response to biomarkers identified for AA. JAK inhibitors are a promising class of drugs for treatment of AA, however, further work is needed to determine optimal dose and administration regimens to generate durable responses.
Supplementary Material
Footnotes
Author Contributions
A Jabbari, N Nguyen, J Cerise, G Ulerio, A de Jong, and J Mackay-Wiggan performed the research. A Jabbari, N Nguyen, J Cerise, G Ulerio, A de Jong, J Mackay-Wiggan, R Clynes, and A Christiano designed the research study. A Jabbari, N Nguyen, J Cerise, G Ulerio, A de Jong, and J Mackay-Wiggan analyzed the data and A Jabbari, N Nguyen, and J Mackay-Wiggan wrote the manuscript.
Conflicts of Interest:
The authors have declared no conflicting interests.
References
- 1.Craiglow BG, King BA. Killing Two Birds with One Stone: Oral Tofacitinib Reverses Alopecia Universalis in a Patient with Plaque Psoriasis. Journal of Investigative Dermatology. 2014;134:2988–2990. doi: 10.1038/jid.2014.260. [DOI] [PubMed] [Google Scholar]
- 2.Jabbari A, Dai Z, Xing L, et al. Reversal of Alopecia Areata Following Treatment With the JAK1/2 Inhibitor Baricitinib. EBioMedicine. 2015;2:351–355. doi: 10.1016/j.ebiom.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xing L, Dai Z, Jabbari A, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20:1043–1049. doi: 10.1038/nm.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Betz RC, Petukhova L, Ripke S, et al. Genome-wide meta-analysis in alopecia areata resolves HLA associations and reveals two new susceptibility loci. Nat Commun. 2015;6:5966. doi: 10.1038/ncomms6966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jabbari A, Petukhova L, Cabral RM, et al. Genetic basis of alopecia areata: a roadmap for translational research. Dermatol Clin. 2013;31:109–117. doi: 10.1016/j.det.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


