Abstract
Objective
Th17 cells and interleukin (IL)-17 cytokine family members are implicated in the pathogenesis of many rheumatic diseases. Most studies of mouse models of inflammatory arthritis have demonstrated a key role for the pro-inflammatory cytokine IL-17A and its receptor, the IL-17 receptor (IL-17R) A/C heterodimer. Using a rigorous genetic approach, we evaluated the contribution of Th17 cells and IL-17 in the autoantibody-dependent, KRN T cell receptor (TCR) transgenic mouse model of arthritis.
Methods
We bred KRN mice expressing the MHCII molecule Ag7 (K/B/g7) and genetically lacking the related cytokines IL-17A and IL-17F or their critical receptor subunit, IL-17RA. Using bone marrow transplantation, we generated mice in which K/B/g7 hematopoietic donor cells lacked the key Th17-differentiating transcription factor, Rorγt.
Results
K/B/g7 mice lacking both IL-17A and IL-17F produced normal titers of pathogenic autoantibodies and developed arthritis normally. Similarly, IL-17RA was not required for disease development in this model, nor was Rorγt expression by hematopoietic cells.
Conclusions
Despite prior reports suggesting that Th17 cells and IL-17A are crucially involved in the pathogenesis of arthritis in K/BxN mice, the results presented here provide genetic evidence that IL-17A and IL-17F, IL-17RA, and hematopoietic cell Rorγt expression are dispensable for normal arthritis progression in the K/B/g7 model system. We discuss potential explanations for the discrepancies between these two highly similar model systems. These findings plus those from other mouse models of arthritis provide insight regarding why biologic therapeutics targeting the Th17/IL-17 axis are beneficial in some human rheumatic diseases but not others.
Many lines of evidence from both animal models and human studies suggest that interleukin-17 produced by Th17 and other cells is a key driver of inflammatory arthritis and other autoimmune diseases. Indeed, biologic therapeutic agents targeting the IL-17 pathway have shown great promise for the treatment of psoriasis, psoriatic arthritis (PsA), and spondyloarthropathies. In contrast, trials of these same agents in rheumatoid arthritis (RA) and Crohn’s disease have shown only modest or no benefit (1, 2). These discrepant outcomes suggest that the importance of Th17 cells and IL-17 cytokine family members to disease pathogenesis may vary among even clinically similar diseases.
The focus of the present study is on the closely-related IL-17 family members IL-17A (often termed simply IL-17) and IL-17F. Three biologically-active forms of these cytokines exist: A/A and F/F homodimers and A/F heterodimers. Each of these cytokine dimers signals through the IL-17 receptor comprising IL-17RA and IL-17RC subunits (3). IL-17A and IL-17RA are critical for the development of arthritis in some commonly-used mouse models of arthritis. A recent report documented elevated serum levels of IL-17A, IL-17F, and the IL-17AF heterodimer in mice with collagen-induced arthritis (CIA), and showed that blocking IL-17A reduced arthritis severity, whereas IL-17F blockade had no effect (4). Genetic deficiency of IL-17A or both IL-17A and IL-17F significantly reduced the severity of CIA (5), whereas IL-17RA deficiency provided complete protection (6). Similarly, arthritis due to the lack of IL-1 receptor antagonist (Il1rn) was also less severe in the absence of IL-17A or both IL-17A and IL-17F (5).
We sought to investigate the contribution of IL-17A, IL-17F, and IL-17RA in the widely-used K/BxN T cell receptor (TCR) transgenic mouse model of arthritis. Arthritis in K/BxN mice develops as a result of combined T- and B-cell autoreactivity against the autoantigen, glucose-6-phosphate isomerase (GPI), resulting in the production of pathogenic GPI-specific autoantibodies.
Clear evidence highlights the importance of the gut microbiota in generating intestinal Th17 cells (7). In the K/BxN mouse model, a seminal study suggested that gut-residing segmented filamentous bacteria (SFB) promoted autoantibody-mediated arthritis via induction of IL-17-producing Th17 cells (8). K/BxN mice raised in germfree conditions had lower anti-GPI autoantibody titers and less severe arthritis, effects attributed to reduced Th17 cell generation by the demonstration that in the presence of KRN TCR transgenic T cells, IL-17RA-deficient B cells participated inefficiently in germinal center reactions relative to wildtype B cells (8). We used a rigorous genetic approach to explore further the contribution of IL-17A, IL-17F, IL-17RA, and Th17 cells in the closely-related K/B/g7 mouse model of arthritis.
Materials and Methods
Mice
KRN TCR transgenic mice on the C57BL/6 (B6) background and B6 mice congenic for H-2g7 (B6.g7) were gifts from Diane Mathis and Christophe Benoist (Harvard Medical School, Boston, MA, USA and the Institut de Génétique et de Biologie Moléculaire et Cellulaire, Strasbourg, France) (9, 10). Mice with a targeted deletion of both Il17a and Il17f (henceforth termed Il17af) have been previously described (11); the genes encoding IL-17A and IL-17F are adjacent on chromosome 1, and the 46bp deletion encompasses exons 2 and 3 of both Il17a and Il17f. The Il17ra-deficient mice were obtained from Amgen (Thousand Oaks, CA) and have been previously described (12). Rorγt-deficient mice on the B6 background which express green fluorescent protein driven by the Rorγt promoter (Rorctm2Litt, stock number 007572) (13), Rag1-deficient B6 mice, and NOD mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice with the described targeted alleles were interbred with KRN mice and B6.g7 congenic mice to generate most mice described in the study; these mice are termed “K/B/g7”. For some experiments, KRN mice were intercrossed with NOD mice to generate K/BxN mice. The mice used for each experiment are clearly indicated. Genotype was determined by PCR for all and by analysis of protein expression in some cases (See Supplementary Figure 1). Mice were bred in our specific-pathogen-free colonies and maintained under Institutional Animal Care and Use Committee-approved protocols at the University of Minnesota (protocols 1207A17481,1506-32700A, and 1303-30457A).
Antibodies
Antibodies used for flow cytometry included B220 (RA3-6B2, Biolegend), CD3 (clone 145-2C11, BD Biosciences), CD4 (RM4-5, Biolegend), CD8 (53-6.7, Biolegend), CD11b (M1/70, eBioscience), CD11c (N418, eBioscience), CD38 (90, eBioscience), CD44 (IM7, eBioscience), F4/80 (BM8, eBioscience), GL7 (GL7, BD Biosciences), Gr-1 (RB6, eBioscience), IgM (eB121-15F9, eBioscience), IL-17A (TC11-18H10, BD Biosciences), IL-17F (18F10, eBioscience), and IL-17RA (PAJ-17R, eBioscience).
Administration of IL-17A blocking antibodies
Monoclonal antibody recognizing mouse IL-17A (clone 17F3) was purchased from BioXCell (West Lebanon, NH, USA). Control mouse polyclonal IgG antibody was from Jackson ImmunoResearch (West Grove, PA, USA). Antibodies (200 micrograms/dose) were injected intraperitonally twice weekly for 5 weeks, beginning at 4 weeks of age.
Flow cytometry
Flow cytometry was performed using an LRII or an LSRFortessa (BD Biosciences), and cells were analyzed using FlowJo version 8.8.7 software (TreeStar). The gating scheme for all experiments included first using forward and side scatter identify singlet lymphocytes. IL-17A- and IL-17F-producing Th cells were identified by first gating CD3+ B220−, CD11b−, CD11c− cells, then gating on CD4+ CD8− cells, followed by analysis of intracellular IL-17A and IL-17F expression performed according to the instructions of the manufacturer (eBioscience). Briefly, 1 x 10e6 cells were stimulated with 50 ng/mL PMA and 1 μg/mL ionomycin in the presence of GolgiStop (BD Biosciences) for 4 hours. Germinal center B cells were identified by first gating CD3-, B220+, IgM-low cells, then enumerating CD38-, GL7+ cells (14).
Candida albicans skin infection
Mice were infected with C. albicans as previously described (15). Briefly, growth of C. albicans strain SC5314 occurred after inoculation of a colony at 30°C in YPAD (yeast extract-peptone-dextrose medium + adenine) overnight and, the next day, diluted 1:10 and cultured in 30°C in YPAD until OD600 reached 1.5 and then washed and re-suspended at 4x109 CFU/ml in PBS. Mice were anesthetized with a mixture of ketamine and xylazine (100/10 mg/kg body weight), shaved on the back with electric clipper, and chemically depilated with Nair hair remover (Church & Dwight, Princeton, NJ) per the manufacturer’s instructions. The stratum corneum was removed with 10 strokes with 220 grit sandpaper (3M, St Paul, MN). After washing with sterile PBS, 2x108 C. albicans in 50 μl of sterile PBS was applied on to the skin. Seven days later, lymph nodes (axillary, brachial, inguinal and cervical) and spleen were harvested and smashed over a 40 μm filter to get single cell suspensions. Intracellular cytokine expression was then determined as described in the preceding section.
K/BxN serum-transferred arthritis
Serum-transferred arthritis was induced by injection of 150 μL of serum from K/BxN mice on day 0 and day 3. Mice were assessed for the development of arthritis as described in the next section.
Assessment of arthritis, anti-GPI titers, and histology
Arthritis was assessed and serum anti-GPI IgG titers and IgG subtypes were measured as previously described (9). Briefly, for the arthritis score, each paw is assessed a score of from 0 (no arthritis) to 3 (maximum arthritis); the maximum total score is 12. Ankle tissues were first fixed in 10% formalin for 24 hours, then decalcified in a 1:1 solution of 8N formic acid/1N sodium formate for 48 hours and dehydrated in 70% ethanol, after which they were embedded in paraffin and sectioned at a thickness of 5 μm. Tissues were stained with hematoxylin and eosin (H&E) using standard protocols. Slides were viewed on an Olympus BX51 microscope equipped with a digital camera and DP-BSW software (Olympus, Center Valley, PA, USA).
Bone marrow transplantation
Rag1-deficient 6-to-12-week-old recipient mice were irradiated with 900 Rad. Four hours after irradiation, 1 x 106 donor bone marrow cells were injected intravenously.
Quantification of SFB
Fresh fecal samples were collected from mice, and genomic DNA was extracted using the Power-Soil DNA extraction kit (Mo Bio, Carlsbad, CA) according to manufacturer’s instructions. Relative quantification of bacteria by real-time quantitative PCR was performed using Fast SYBR Green Master Mix (Applied Biosystems, Foster City, CA) and primers targeting either mouse SFB or total 16S rDNA using comparative Ct method (16). Fecal samples from mice obtained from The Jackson Laboratory and from Taconic Farms were used as negative and positive control, respectively (17). Melting curves were generated and PCR products were visualized by 4% agarose gel electrophoresis to assess for primer dimers.
Statistics
The non-parametric Mann-Whitney U test was used for continuous data that were not normally distributed. Arthritis severity scores were compared using repeated measures ANOVA. Statistical analysis was performed using GraphPad Prism software (La Jolla, CA). A p-value <0.05 was considered significant.
Results
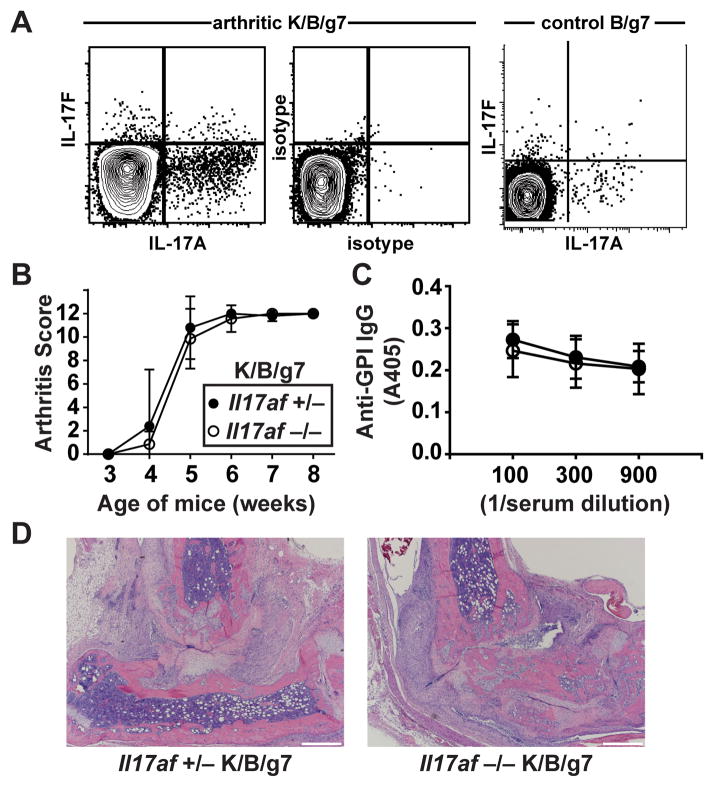
CD4+ T cells producing IL-17A, IL-17F, or both were readily identified in the spleens of arthritic K/B/g7 mice (Figure 1A). To determine if IL-17A or IL-17F are required for arthritogenesis in this model, we bred K/B/g7 mice genetically lacking both cytokines. We verified that the IL-17AF-deficient mice indeed could not produce IL-17A or IL-17F by using a Candida skin infection model (See Supplementary Figure 1A). The absence of both IL-17A and IL-17F in K/B/g7 mice impacted neither arthritis development (Figure 1B) nor anti-GPI IgG antibody formation (Figure 1C). Serum levels of GPI-specific IgG1, IgG2b, IgG2c, and IgG3 subtypes also were not different between the IL-17AF-sufficient and –deficient K/B/g7 mice (see Supplementary Figure 2A). As has been previously demonstrated (8), antibody-mediated blockade of IL-17A led to a modest reduction of arthritis severity in K/BxN mice but in our experiment did not affect anti-GPI autoantibody titers (see Supplementary Figure 3).
Figure 1. Arthritis develops normally in IL-17AF-deficient K/B/g7 mice.
A) IL-17A and IL-17F expression were determined among CD3+ CD4+ splenocytes obtained from arthritic K/B/g7 mice (left panel) or control B/g7 mice (right panel). The middle panel shows staining of K/B/g7 splenocytes with isotype control antibodies. B) Arthritis severity in Il17af +/− and IL17af −/− K/B/g7 mice was assessed at the indicated ages and is depicted as a qualitative score. C) Serum anti-GPI IgG antibody titers were determined by enzyme-linked immunosorbent assay at the indicated dilutions. Closed circles indicate Il17af +/− mice, and open circles indicate Il17af −/− mice. For B and C, Plotted values are means ± SD; n=5–7 mice/group. D) Representative photomicrographs of ankle sections from mice of the indicated genotypes (objective 4x; scale bars indicate 500 microns).
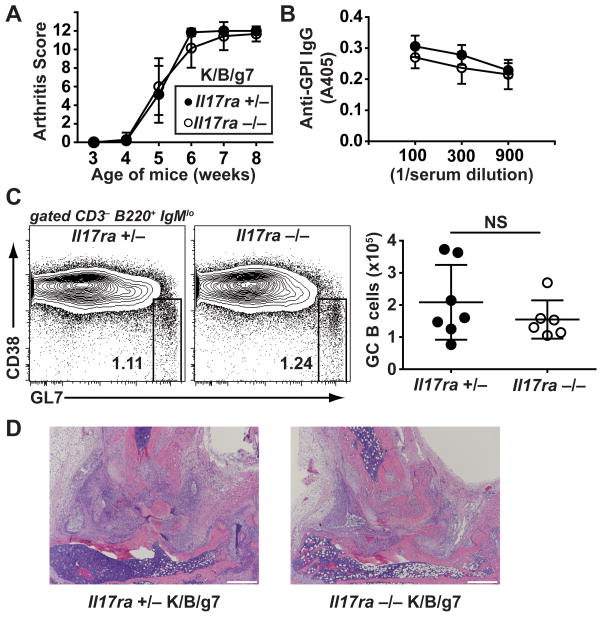
We next bred K/B/g7 mice lacking IL-17RA, a critical subunit of the IL-17A and F receptor. We verified the lack of IL-17RA expression in these mice by flow cytometry (see Supplementary Figure 1B). IL-17RA-deficient K/B/g7 mice also developed arthritis equivalently to littermate control mice, with preserved anti-GPI IgG autoantibody formation (Figure 2A and 2B). Serum levels of GPI-specific IgG1, IgG2b, IgG2c, and IgG3 subtypes also were not different between the IL-17RA-sufficient and –deficient K/B/g7 mice (see Supplementary Figure 2B). B cells are known to express the IL-17RA and respond to IL-17A signaling, and a prior report indicated that B cells lacking IL-17RA enter germinal center reactions less efficiently than do wildtype B cells when placed in competition in the presence of KRN T cells (8). However, we found that the frequency and number of splenic germinal center B cells was not appreciably reduced in IL-17RA-deficient K/B/g7 mice relative to littermate controls (Figure 2C). We therefore conclude that IL-17A, IL-17F, and their shared receptor subunit IL-17RA are dispensable for autoantibody production and arthritis development in K/B/g7 mice.
Figure 2. Arthritis develops normally in IL-17RA-deficient K/B/g7 mice.
A) Arthritis severity in Il17ra +/− and IL17ra −/− K/B/g7 mice was assessed at the indicated ages and is depicted as a qualitative score. Plotted values are means ± SD. B) Serum anti-GPI IgG antibody titers were determined by enzyme-linked immunosorbent assay at the indicated dilutions. Closed circles indicate Il17ra +/− mice, and open circles indicate Il17ra −/− mice. Plotted values are means ± SD. C) Germinal center B cells (CD3−, B220+, IgMlo, CD38−, GL7+) were identified among splenocytes from the indicated mice by flow cytometry. The number indicates the percentage of cells within the gate. The plot to the right indicates the number of germinal center B cells; each point represents one mouse; lines represent means ± SD. For all panels, n=6–7 mice/group. D) Representative photomicrographs of ankle sections from mice of the indicated genotypes (objective 4x; scale bars indicate 500 microns).
Although we primarily are focused here on the contribution of Th17 cells and IL-17 to the initiation of spontaneous autoimmunity in TCR transgenic K/B/g7 mice, the question of whether IL-17 participates in the effector phase of arthritogenesis is also of interest. The K/BxN serum transfer arthritis model can be used to interrogate the effector pathways downstream of autoantibody production. Prior reports have demonstrated reduced severity of K/BxN serum-transferred arthritis in IL-17A-deficient mice (18) and in IL-17RA-deficient mice (19). We repeated this latter experiment, and similarly observed a very modest reduction in arthritis severity (see Supplementary Figure 4).
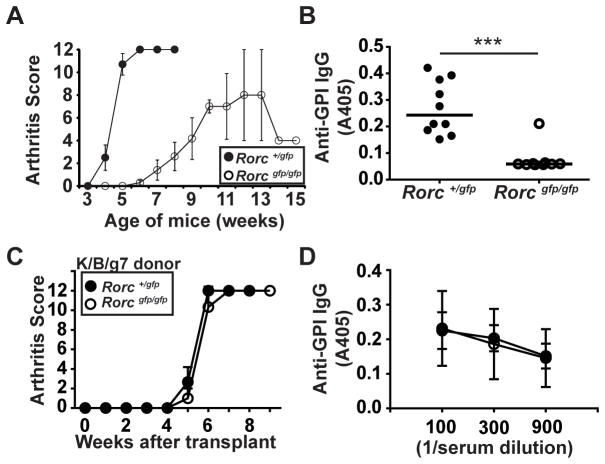
Based on the prior report that gut SFB can promote Th17 cell differentiation in K/BxN mice (8), we sought to evaluate whether Th17 cell differentiation was necessary for arthritis development in K/B/g7 mice. The transcription factor Rorγt is essential for Th17 cell differentiation (13). Germline deficiency of Rorγt in mice prevents the formation of peripheral lymph nodes, severely impairing T-B cell interactions (20). Not surprisingly, we found that germline deficiency of Rorγt in K/B/g7 mice impaired the development of arthritis, most likely due to a drastic inhibition of anti-GPI autoantibody formation (Figure 3A, B). To circumvent this difficulty engendered by the lack of lymph nodes in Rorγt-deficient mice, we turned to bone marrow transplantation to determine if Rorγt expression by K/B/g7 hematopoietic cells was necessary for arthritis development. We found that transplantation of either Rorγt-sufficient or Rorγt-deficient K/B/g7 bone marrow into Rag1-deficient hosts resulted in equivalent development of arthritis and of anti-GPI IgG autoantibodies (Figure 3C, D). This result is consistent with a prior report demonstrating that adoptive transfer of Rorγt-deficient KRN T cells into T-cell-deficient hosts results in normal anti-GPI antibody production and arthritogenesis (21). These findings demonstrate that the Rorγt-dependent Th17 transcriptional program is not required for the pathogenesis of autoantibody-dependent arthritis in K/B/g7 mice.
Figure 3. Hematopoietic cell Rorγt expression is not required for K/B/g7 arthritis.
A) Arthritis was assessed in K/B/g7 mice expressing non-functional alleles encoding Rorγt (Rorcgfp) in heterozygosity (Rorc+/gfp) or homozygosity (Rorcgfp/gfp) at the indicated ages; n=10/group, plotted values are means ± SD. B) Serum anti-GPI IgG titers from the mice shown in panel A, measured at 8 weeks of age at 1:300 dilution. Each point represents one mouse, means ± SD, *** p<0.001. C and D) Bone marrow from K/B/g7 mice lacking Rorγt expression (Rorcgfp/gfp) or expressing one wildtype allele (Rorc+/gfp) was transplanted into irradiated, Rag1-deficient C57BL/6 mice. C) The mice were followed for the development of arthritis. D) Serum anti-GPI IgG antibody titers were determined by enzyme-linked immunosorbent assay at the indicated dilutions. Closed circles indicate Rorc+/gfp bone marrow donor mice, and open circles indicate Rorcgfp/gfp bone marrow donor mice. For C and D, plotted values are means ± SD; n=3 mice/group.
Discussion
There is great clinical interest in understanding how Th17 cells and IL-17 contribute to the pathogenesis of numerous human inflammatory diseases. Clinical trials in rheumatic diseases have had mixed results. Biologic therapeutic agents targeting IL-17A (ixekizumab and secukinumab) or IL-17RA (brodalumab) have shown efficacy in psoriasis, PsA, and ankylosing spondylitis. In contrast, results of trials of these agents in RA have been less impressive. In phase II trials, ixekizumab reached its primary endpoints, whereas secukinumab and brodalumab did not. However, long-term therapy of initial responders to secukinumab resulted in continued improvement, justifying ongoing phase III trials of secukinumab in patients with inadequate responses to TNF inhibitors (NURTURE: NCT01350804 and REASSURE: NCT01377012) (reviewed in (1)). These mixed results are puzzling and suggest that our understanding of how the IL-17 family of cytokines contributes to the pathogenesis of the most common rheumatic diseases remains incomplete.
The mixed results of IL-17 blocking agents in different types of human arthritis are mirrored by reports from animal models. We report here that IL-17A and IL-17F, IL-17RA, and Th17 cell differentiation are dispensable for arthritis in the K/B/g7 mouse model. In stark contrast, deficiency of IL-17A led to partial protection from CIA, and IL-17RA deficiency resulted in complete protection (5, 6). In the latter study, the absence of IL-17RA was associated with increased numbers of IL-4-producing CD4+ T cells, increased titers of IgG1 and lower titers of pathogenic IgG2c anti-collagen antibodies (6). In contrast to the CIA model in which IL-4 and IgG1 are associated with protection from disease, arthritis in K/BxN mice is driven by IL-4 and by pathogenic IgG1 anti-GPI autoantibodies (22). Thus, it is possible in K/B/g7 mice lacking IL-17 or its receptor, IL-4 was further upregulated, preserving anti-GPI autoantibody production and permitting normal arthritogenesis. However, we did not detect any difference in IgG subtype usage in K/B/g7 mice sufficient or deficient for IL-17A and IL-17F or for IL-17RA.
A recent report demonstrated that K/BxN mice kept in germ-free conditions were protected from arthritis, an effect correlated with fewer splenic Th17 cells and lower anti-GPI antibody titers. Gut repopulation with SFB restored splenic Th17 cells, anti-GPI autoantibody production, and arthritis development. Furthermore, antibody-blockade of IL-17A in K/BxN mice kept in specific pathogen free conditions (not germ-free) resulted in less severe arthritis, lower anti-GPI IgG titers, and impaired germinal center B cell formation (8). These results strongly suggested a role for Th17 cells and IL-17A in the K/BxN system. Although we similarly observed a slight reduction in arthritis severity among K/BxN mice given anti-IL17A blocking antibody, we observed no reduction in anti-GPI antibody formation (see Supplementary Figure 3). Thus, even in mice with intact IL-17 production, we found that blockade of this pathway had minimal impact.
It is challenging to square the results of that prior study with the results reported here. Our findings provide conclusive genetic evidence in K/B/g7 mice that IL-17A and IL-17F, IL-17RA and Th17 cells are not required for autoantibody production or arthritis development. It is possible that the genetic background differences between K/BxN mice (an F1 cross of C57BL/6 and NOD strains) and K/B/g7 mice (C57BL/6 strain with congenic expression of Ag7) are responsible, either directly or through strain-dependent differences in the commensal microbiota. Of note, both K/BxN and K/B/g7 mice in our colony develop severe arthritis despite lacking detectable SFB colonization of the gut. As suggested by Wu et al, however, it is possible that other species of intestinal (or other anatomic site) microbiota could fulfill this arthritis-promoting role (8). A more likely explanation for the difference between the present report and the prior one (8) is that in the genetic absence of IL-17A and IL-17F or of IL-17RA, expression of another pro-inflammatory cytokine increases in a compensatory fashion to drive germinal center B cell formation and autoantibody production. For instance, another Th17 cell-derived cytokine such as IL-22 could be involved. However, we also have reported that hematopoietic cell expression of Rorγt is not necessary for arthritogenesis, demonstrating that Th17 cells are dispensable in the K/B/g7 system. Based on the available data, it is fair to speculate that the arthritis-inducing activity of gut-residing SFB reported in the prior study (8) could be mediated by cell types and cytokines in addition to or other than Th17 cells, IL-17A, and IL-17F, and that other microbial species could exert similar effects.
How do our findings inform current understanding of the role of Th17 cells and IL-17 in human rheumatic diseases? There are clear differences in the immunopathogenesis of PsA and RA, as there are between the CIA and K/BxN mouse models of inflammatory arthritis. In particular, IL-17A and IL-17RA appear to be critical drivers of PsA in humans and CIA in mice (1, 6). The mixed results of biologic therapeutic blocking studies suggest a less prominent role for IL-17A and IL-17RA in RA (or perhaps in subgroups of RA), and our findings demonstrate that in the K/B/g7 mouse model these molecules are completely dispensable. The predominance of IL-4-mediated generation of pathogenic IgG1 autoantibodies may explain the lack of a requirement for IL-17A /F and IL-17RA in our present study. Ongoing efforts seek to define further the non-IL-17-dependent pathways that serve an analogous role in human RA.
In sum, we have demonstrated that IL-17A and IL-17F, IL-17RA, and Th17 cells are not required for arthritis to develop in K/B/g7 mice. These “negative” findings may provide important insight regarding why clinical trials of IL-17 pathway blocking agents in human RA have shown only modest or no benefit, and help to broaden the search for better therapeutic targets.
Supplementary Material
Acknowledgments
Funding Sources:
This work was supported by NIH R01 HL121093 to B.A.B.
Non-standard abbreviations
- GPI
glucose-6-phosphate isomerase
- SFB
segmented filamentous bacteria
Footnotes
Disclosures
Il17ra-deficient mice were obtained from Amgen.
References
- 1.Lubberts E. The IL-23-IL-17 axis in inflammatory arthritis. Nat Rev Rheumatol. 2015;11:415–29. doi: 10.1038/nrrheum.2015.53. [DOI] [PubMed] [Google Scholar]
- 2.Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol. 2014;14:585–600. doi: 10.1038/nri3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9:556–67. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarkar S, Justa S, Brucks M, Endres J, Fox DA, Zhou X, et al. Interleukin (IL)-17A, F and AF in inflammation: a study in collagen-induced arthritis and rheumatoid arthritis. Clin Exp Immunol. 2014;177:652–61. doi: 10.1111/cei.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30:108–19. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Corneth OB, Mus AM, Asmawidjaja PS, Klein Wolterink RG, van Nimwegen M, Brem MD, et al. Absence of interleukin-17 receptor a signaling prevents autoimmune inflammation of the joint and leads to a Th2-like phenotype in collagen-induced arthritis. Arthritis Rheumatol. 2014;66:340–9. doi: 10.1002/art.38229. [DOI] [PubMed] [Google Scholar]
- 7.Yang Y, Torchinsky MB, Gobert M, Xiong H, Xu M, Linehan JL, et al. Focused specificity of intestinal TH17 cells towards commensal bacterial antigens. Nature. 2014;510:152–6. doi: 10.1038/nature13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–27. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Binstadt BA, Hebert JL, Ortiz-Lopez A, Bronson R, Benoist C, Mathis D. The same systemic autoimmune disease provokes arthritis and endocarditis via distinct mechanisms. Proc Natl Acad Sci U S A. 2009;106:16758–63. doi: 10.1073/pnas.0909132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kouskoff V, Korganow AS, Duchatelle V, Degott C, Benoist C, Mathis D. Organ-specific disease provoked by systemic autoimmunity. Cell. 1996;87:811–22. doi: 10.1016/s0092-8674(00)81989-3. [DOI] [PubMed] [Google Scholar]
- 11.Haas JD, Ravens S, Duber S, Sandrock I, Oberdorfer L, Kashani E, et al. Development of interleukin-17-producing gammadelta T cells is restricted to a functional embryonic wave. Immunity. 2012;37:48–59. doi: 10.1016/j.immuni.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–27. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 14.Pape KA, Taylor JJ, Maul RW, Gearhart PJ, Jenkins MK. Different B cell populations mediate early and late memory during an endogenous immune response. Science. 2011;331:1203–7. doi: 10.1126/science.1201730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kashem SW, Riedl MS, Yao C, Honda CN, Vulchanova L, Kaplan DH. Nociceptive Sensory Fibers Drive Interleukin-23 Production from CD301b+ Dermal Dendritic Cells and Drive Protective Cutaneous Immunity. Immunity. 2015;43:515–26. doi: 10.1016/j.immuni.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barman M, Unold D, Shifley K, Amir E, Hung K, Bos N, et al. Enteric salmonellosis disrupts the microbial ecology of the murine gastrointestinal tract. Infect Immun. 2008;76:907–15. doi: 10.1128/IAI.01432-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–98. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katayama M, Ohmura K, Yukawa N, Terao C, Hashimoto M, Yoshifuji H, et al. Neutrophils are essential as a source of IL-17 in the effector phase of arthritis. PLoS One. 2013;8:e62231. doi: 10.1371/journal.pone.0062231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sadik CD, Kim ND, Alekseeva E, Luster AD. IL-17RA signaling amplifies antibody-induced arthritis. PLoS One. 2011;6:e26342. doi: 10.1371/journal.pone.0026342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- 21.Block KE, Huang H. The Cellular Source and Target of IL-21 in K/BxN Autoimmune Arthritis. J Immunol. 2013;191:2948–55. doi: 10.4049/jimmunol.1301173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohmura K, Nguyen LT, Locksley RM, Mathis D, Benoist C. Interleukin-4 can be a key positive regulator of inflammatory arthritis. Arthritis Rheum. 2005;52:1866–75. doi: 10.1002/art.21104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.