Abstract
Objective
The recruitment of IL-17-producing T helper (Th17) cells to inflammatory sites has been implicated in the development of organ damage in inflammatory and autoimmune diseases including systemic lupus erythematosus (SLE). To define the mechanism of CaMK4 activation on Th17-cell recruitment to target tissues, we performed anti-glomerular basement membrane antibody-induced glomerulonephritis (AIGN) experiments in mice and studied samples from patients with SLE.
Methods
We induced experimental AIGN in Camk4-sufficient or -deficient mice and compared histology, Th17-related chemokine expression and numbers of IL-17-producing cells in the kidneys. We also evaluated the efficacy of the CaMK4 antagonist KN-93 in alter AIGN induced kidney disease. The expression of CCR6 in memory CD4 T cells before AIGN induction was analyzed by flow cytometry. We investigated the correlation between CCR6 expression in the peripheral blood and the severity of glomerulonephritis in patients with SLE.
Results
Camk4-deficient mice displayed less glomerular injury after the induction of AIGN. Kidney infiltration by IL-17-producing CD4 T cells along with CCR6 and CCL20 expression were significantly decreased in Camk4-deficient mice. Similarly, the KN-93 treatment of mice prior to exposure to AIGN improved the clinical and pathological finings. The expression and function of CCR6 in memory CD4 T cells in the peripheral blood was decreased in the Camk4-deficient mice. The expression of CCR6 correlated positively with the severity of organ damage in SLE patients.
Conclusion
Our results indicate that CaMK4 represents a novel therapeutic strategy for the treatment of Th17 cell-mediated tissue damage in inflammatory diseases.
INTRODUCTION
The recruitment of interleukin (IL)-17-producing T helper (Th17) cells to inflammatory sites has been implicated in the development of organ damage in inflammatory and autoimmune diseases, including systemic lupus erythematosus (SLE) (1–3). Chemokine receptors and their ligands also play critical roles in the recruitment of T cells into sites of inflammation (4, 5). We and another group have reported that IL-17-mediated signaling is important in the expression of anti-glomerular basement membrane antibody-induced glomerulonephritis (AIGN) (6, 7). Of note, our group has demonstrated that IL-17-producing T cells infiltrate the kidneys of lupus-prone mice (8) and the kidneys of patients with lupus nephritis (LN) (9). It has been suggested that the pathogenic Th17 population expressing the protein CCR6 (chemokine [C-C Motif] receptor 6) plays a critical role in accelerating tissue damage in animal models of kidney injury (4, 10) and arthritis (11). More recently, Zhou et al. reported a genetic association between CCR6 variants and susceptibility to LN (12).
Calcium/calmodulin-dependent protein kinase IV (CaMK4) is a multifunctional serine/threonine kinase that activates several transcription factors to regulate gene expression (13). Our group has proposed that CaMK4 promotes the differentiation of Th17 cells and plays a central role in determining the balance between Th17 cells and Tregs (14). We have also shown that the inhibition of CaMK4 ameliorates experimental autoimmune encephalomyelitis (EAE) and autoimmunity in lupus-prone mice (15). However, the precise molecular mechanism whereby CaMK4 affects the recruitment of pathogenic T cells to target tissues in inflammatory settings has not been identified.
Here we investigated the effect of gene ablation and of pharmacological inhibition of CaMK4 on the glomerular damage and cells infiltrating the inflamed kidney induced by AIGN. Our findings demonstrate that CaMK4 accelerates the glomerular injury by enhancing CCR6 and CCL20 in the inflamed kidney. Similarly, we obtained evidence that CCR6 expression in effector memory CD4 T cells from SLE patients is associated with the severity of organ damage. Our findings suggest that the CaMK4/CCR6/CCL20 axis represents a possible therapeutic target for the treatment of Th17 cell-mediated tissue damage in inflammatory diseases.
MATERIALS AND METHODS
Mice
Female B6.129X1-Camk4tm1Tch/J and C57BL/6J (B6) mice were purchased from The Jackson Laboratory. Mice were maintained in an SPF animal facility at Beth Israel Deaconess Medical Center (Boston, MA). The experiments were approved by the Institutional Animal Care Committee (IACUC) of Beth Israel Deaconess Medical Center.
AIGN model
AIGN was induced as described (6). Briefly, 10–12-week-old female mice were pre-immunized subcutaneously with 50 μg of rabbit IgG in complete Freund’s adjuvant (CFA) 3 days prior to an intravenous injection of 50 μl of rabbit experimental nephrotoxic serum (NTS). Mice induced AIGN were initiated the CaMK4 inhibitor KN-93 (Calbiochem) just after the NTS injection by intraperitoneal injections at three different amounts (4, 16 and 160 μg/week), then treated three times a week. Spot urine samples were collected at the indicated time points after the NTS injection. At 3 weeks after the NTS injection, kidneys from euthanized mice were harvested for the histological analysis, flow cytometry, and polymerase chain reaction (PCR) analysis.
Enzyme-linked immunosorbent assay (ELISA)
We used a mouse albumin ELISA quantitation set (Bethyl Laboratories, Montgomery, TX, USA) and Creatinine Assay Kit (Cayman Chemicals, Ann Arbor, MI) to determine the urine albumin concentrations and creatinine levels in the urine of the mice, respectively.
Histology
The 10% formalin-fixed kidneys were processed for periodic acid-Schiff (PAS) and hematoxylin and eosin (H&E) staining. Kidney sections were evaluated blindly as described (8).
Isolation of single cells
To obtain single-cell suspensions, we excised the spleens from the euthanized mice and teased them through a nylon mesh. The isolation of single cells from the kidneys was performed as described (16).
RNA isolation and real-time PCR analysis
We isolated total mRNA from spleen and kidney CD4 T cells using the RNeasy Mini Kit (Qiagen, Hilden, Germany), and then we synthesized cDNA by using cDNA EcoDry Premix (Clontech, Mountain View, CA) for PCR amplification. All primers and probes were from Applied Biosystems (Foster City, CA): Il17a (Mm00439619_m1), Ccr6 (Mm01700300_g1), Ccl20 (Mm01268754_m1) and Gapdh (Mm99999915_g1). Gene expressions were assessed by the comparative CT method.
Flow cytometry
Isolated cells were stained for flow cytometry with antibodies against CD3e (17A2, eBioscience, San Diego, CA), CD4 (GK1.5, BioLegend, San Diego, CA), CD8a (53-6.7, eBioscience), CD44 (IM7, BioLegend), CD45RA (RA-3-6B2, BioLegend), CD62L (MEL-14, BioLegend), CCR6 (29-2L17, G034E3, BioLegend) and CCR7 (G043H7, BioLegend) for 30 min at 4°C. Intracellular cytokine staining was performed as described (15). All antibodies to cytokines (anti-IFN-γ, XMG1.2, and anti-IL-17A; JC11-18H10.1) were from BioLegend.
Migration assay
A migration assay was performed in 24-well plates (Costar, San Diego, CA) carrying Transwell-permeable supports with a 3-μm polycarbonate membrane for T cells. Recombinant CCL20 was placed on the lower chamber (0 or 10 ng/ml). Memory CD4 T cells from B6 wild-type (WT) or B6 Camk−/− mice were sorted by magnetic beads as described (15). Then, 5 × 105 memory T cells placed on the upper wells of the Transwell membranes were stimulated with anti-CD3 antibody and with anti-CD28 antibody at 37°C for 6 hr. The number of cells that had migrated to the lower chamber was determined by flow cytometry.
Human SLE T cells
Sixteen patients fulfilling at least four of the 11 revised criteria of the American College of Rheumatology for the classification of SLE were enrolled in this study (17). Disease activity was assessed with the SLE Disease Activity Index (SLEDAI) (18). Among these patients, five had a histologically confirmed diagnosis of LN (according to the International Society of Nephrology and Renal Pathology Society classification [ISN/RPS]; Class II: n=0, Class III: n=2, Class IV: n=2, Class V: n=1). Among patients with LN, all patients had moderate to severe glomerular involvement and 2 patients had mild tubular involvement. Three patients were diagnosed with neuropsychiatric SLE (NPSLE) based on the ACR criteria (19). The protocol was approved by the Institutional Review Board of Nagasaki University Hospital. The patients’ peripheral venous blood was collected in heparin-lithium tubes, and human CD4 T cells from the blood were purified by the RosetteSep™ human CD4+ T cell enrichment cocktail (Stemcell, Vancouver, BC, Canada).
Statistical analyses
We used the two-tailed Student’s t-test and Mann-Whitney tests for analyzing the differences between two groups. For time-series experiments, paired t test or two-way ANOVA with Bonferroni’s posttest was used. Differences between three data sets were analyzed by one-way ANOVA. Pearson’s correlation coefficients with 2-tailed p-values were determined in the analysis of correlations. All statistical analyses were performed by JMP Pro 11.2 and GraphPad Prism 6.0 software. A p-value <0.05 was considered significant.
RESULTS
Camk4 deficiency ameliorates AIGN
To evaluate the relevance of CaMK4 in antibody-antigen complex-mediated inflammation, we induced AIGN in Camk4-sufficient and -deficient mice by immunizing them with rabid IgG in CFA followed by an injection of NTS.
The proteinuria at day 21 after immunization was significantly decreased in the Camk4-deficient mice (Fig. 1A). Study of kidney histology showed that the glomerulonephritis seen in wild-type mice was very severe in contrast to that of the Camk4-deficient mice (Fig. 1B). This finding suggests that CaMK4 deficiency ameliorates AIGN.
Figure 1. Camk4 deficiency ameliorated AIGN.

(A) Disease severity was quantified as proteinuria (urinary albumin/creatinine ratio; mg/gCr (gCr = grams creatinine)) at the indicated time points. (B) Representative images of PAS-stained sections. WT: B6 Camk4+/+, KO: B6 Camk4−/− mice. Scale bars, 100 μm. Data are cumulative results of two independent experiments, including a total of 7 mice per group.
Camk4 deficiency limited the recruitment of IL-17-producing CD4 T cells and decreased CCR6 and CCL20 expression in kidneys
AIGN is associated with inflammatory cell infiltration in the kidney as a result of antibody deposition, followed by interstitial leukocyte accumulation and damage to the glomerulus (20, 21). To determine the local function of CaMK4 in injured tissue, we examined the recruitment of IL-17-producing CD4 T cells in kidneys by flow cytometry, and we found that the percentage of IL-17-producing CD4 T cells was lower in the Camk4-deficient mice compared to the WT mice (Fig. 2A,B). Similarly, the levels of IL-17 in the serum on day 21 after the NTS injection was decreased in Camk4-deficient mice (Fig. 2C).
Figure 2. Camk4 deficiency inhibited the recruitment of IL-17-producing CD4 T cells and decreased the CCR6 and CCL20 expression in kidneys.

(A) Representative flow cytometric staining profile for IL-17-producing CD4 T cells in the kidneys on day 21 after the NTS injection. (B) Percentage of IL-17-producing CD4 T cells in kidneys. (*p<0.05; mean ± SD; WT: n=6, KO: n=7). (C) The concentration of IL-17 in the serum on day 21 after the NTS injection (*p<0.05; mean ± SD; n=7, KO: n=7). (D) The expressions of Il17a, Ccr6 and Ccl20 in infiltrated cells from kidneys of Camk4-sufficient or -deficient mice determined by qPCR. (*p<0.05; mean ± SEM; WT: n=6, KO: n=7). Data are cumulative results of two independent experiments, including a total of 6–7 mice per group.
It has been reported that injection of NTS in mice results in increased expressions of CCR6 and CCL20, followed by T-cell recruitment (4, 10). To determine the role of CaMK4 in the migration of Th17 cells to inflamed tissues, we collected infiltrated cells from kidneys and investigated the expressions of CCR6 and CCL20 in the kidneys by qPCR. As expected, the expression of both Ccr6 and Ccl20 was decreased in the Camk4-deficient mice, as was the Il17a expression (Fig. 2D).
Collectively, these data indicate that CaMK4 facilitates inflammatory cell infiltration by up-regulating IL-17-producing CD4 T cells in inflamed tissues.
KN-93 protected against glomerular basement membrane (GBM)-induced renal damage
To determine whether the pharmacological inhibition of CaMK4 could affect the severity of renal damage in AIGN, we treated B6 mice with three different amounts of the CaMK4 inhibitor KN-93 (4, 16 and 160 μg/week), and then we measured the level of proteinuria every week. We also evaluated the levels of IL-17 in the serum and renal tissues in these mice at day 21 after the NTS induction. We observed a significant reduction of proteinuria (Fig. 3A) along with a decreased levels of IL-17 in the serum (Fig. 3B) in the group of mice treated with the highest dose of this CaMK4 inhibitor. Consistent with these observations, the severity of the glomerulonephritis as well as the IL-17 production by CD4 T cells in the kidneys were notably decreased in the group treated with the 160 μg/week dose of KN-93 (Fig. 3C, D).
Figure 3. KN-93 protected against GBM-induced renal damage.
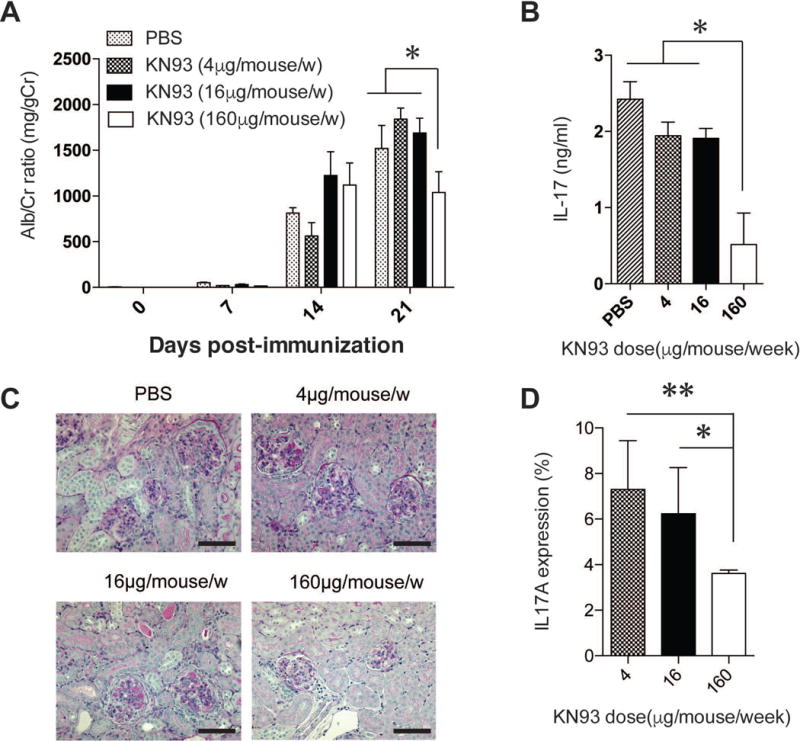
The level of proteinuria every week (A), and the level of IL-17 in the serum on day 21 (B). Representative images of PAS-stained sections (C). Percentage of IL-17-producing CD4 T cells in kidneys (D) in B6 mice treated with KN-93 (4 μg/week, 16 μg/week and 160 μg/week). (*p<0.05, **p<0.01; mean ± SEM; n=4–6). Data are representative of two experiments, each with ≥4 mice per group.
These data indicate that KN-93 protects against glomerular basement membrane (GBM)-induced renal damage through regulating aberrant production of IL-17 in kidneys.
Camk4-deficient mice showed decreased CCR6 expression and migrating capacity in circulating memory CD4 T cells
It was reported that chemokines promoted the recruitment of immune cells from the blood into the inflamed organ to promote inflammatory responses (22). To determine the expression of CCR6 in memory T cells before AIGN induction, we investigated the percentage of CCR6-positive cells among the CD4 memory subset in the peripheral blood of the mice. Importantly, the percentages of CCR6-positive central memory CD4 T cells (CD4+CD44+CD62LhighCCR6high) and effector memory CD4 T cells (CD4+CD44+CD62LlowCCR6high) were reduced in the Camk4-deficient mice at 11 weeks of age (Fig. 4A).
Figure 4. Camk4-deficient mice showed decreased CCR6 expression and migrating capacity of circulating memory CD4 T cells.

(A) Representative flow cytometric staining profile for CCR6-positive cells among central memory CD4 T cells (CD4+CD44+CD62LhighCCR6high) and effector memory CD4 T cell s(CD4+CD44+CD62LlowCCR6high) in the peripheral blood. (B) The migrated memory T cells (B6 Camk4+/+ and B6 Camk4−/− mice) after stimulation with anti-CD3 and anti-CD28 in the presence or absence of recombinant CCL20. (*p<0.05; mean ± SEM; n=4–6). Data are representative of two experiments, each with ≥4 mice per group.
To explore the direct effect of CaMK4 on the migratory capacity of memory T cells, we performed a migration assay in vitro. As shown in Figure 4B, the numbers of memory T cells which migrated after stimulation by anti-CD3 and anti-CD28 antibody in the presence or absence of recombinant CCL20 were comparable between the Camk4-sufficient and -deficient B6 mice in the absence of CCL20. In contrast, the memory T cells that migrated toward CCL20 were significantly reduced in the Camk4-deficient mice (Fig. 4B).
Taken together, these results suggest that the number and the migratory capacity of CCR6-expressing circulating memory T cells may be involved in the degree of organ damage in this AIGN model.
CCR6 in memory CD4 T cells correlated with the severity of organ involvement in the SLE patients
Since we observed that the inhibition of CaMK4 mitigated the severity of glomerulonephritis as well as the CCR6/CCL20 expression in the AIGN model, we asked whether CCR6-mediated organ damage is relevant to patients with SLE. The characteristics of the studied patients are shown in Table 1. We measured the expression of CCR6 in the memory CD4 T cells, and we investigated the relationship between its expression and organ involvement in the patients (including the LN or NPSLE patients). As shown in Figure 5A, the expression of CCR6 in effector memory T cells (CD3+CD4+CD45RA−CCR7−) was significantly higher in the SLE patients with organ involvement compared to those without organ involvement. In contrast, there was no significant difference in central memory T cells (CD3+CD4+CD45RA−CCR7+) between these two groups of patients (Fig. 5B). There was no significant correlation between memory T cells and the SLE Disease Activity Index (SLEDAI) (Fig 5C and 5D).
Table 1.
The patients’ demographic profile*
| Variables | All SLE patients (n=16) | organ involvement
|
p-value | |
|---|---|---|---|---|
| − (n=8) | + (n=8) | |||
| Age | 44 (27–51) | 47 (36–53) | 34 (23–47) | 0.34 |
| Female | 15 (94) | 8 (100) | 7 (88) | 0.30 |
| SLEDAI | 8 (2–16) | 3 (2–14) | 9 (6–19) | 0.081 |
| Anti-dsDNA antibody (U/ml) | 25 (3–350) | 10 (1–38) | 194 (7–372) | 0.14 |
| C3 (mg/dL) | 64 (43–79) | 76 (46–94) | 52 (43–75) | 0.34 |
| C4 (mg/dL) | 10 (5–15) | 11 (5–27) | 9 (5–15) | 0.83 |
| LN | 5 (31) | |||
| Glomerular involvement | 5/5 (100) | |||
| Tubular involvement | 2/5 (40) | |||
| NPSLE | 3 (19) | |||
| Treatment | ||||
| Untreated | 6 (38) | 4 (50) | 2 (25) | 0.61 |
| Prednisolone | 10 (63) | 4 (50) | 6 (75) | 0.61 |
| Tacrolimus | 4 (25) | 2 (50) | 2 (50) | 1.00 |
Median (interquartile range) or number (percentages) is shown. P-values were established using Fisher’s exact test or the Mann-Whitney U-test. SLEDAI: the SLE Disease Activity Index, LN: lupus nephritis, NPSLE: neuropsychiatric SLE. Organ involvement: patients with LN (n=5) or NPSLE (n=3).
Figure 5. CCR6 in effector memory CD4 T cells was correlated with the severity of organ involvement in SLE patients.
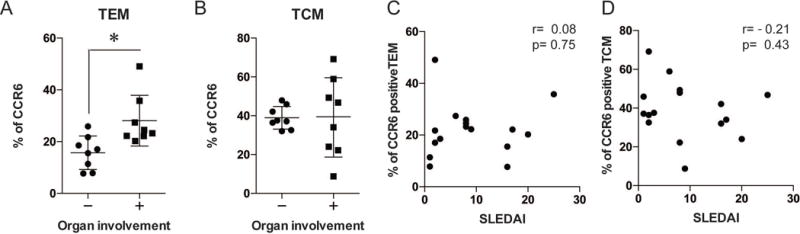
The expression of CCR6 in the effector memory CD4 T cells (TEM; CD3+CD4+CD45RA−CCR7−) (A) and central memory T cells (TCM; CD3+CD4+CD45RA−CCR7+) (B) were measured in SLE patients with or without organ involvement including LN and NPSLE patients. (*p<0.05, mean ± SD; n=8) The association between the CCR6 expression in memory CD4 T cells (C; TEM, D; TCM) and the SLE Disease Activity Index (SLEDAI).
These data indicate that CCR6 expression in effector memory T cells is important in the autoimmune-mediated tissue damage in human SLE.
DISCUSSION
Recent findings have revealed that the chemotaxis of inflammatory cells including neutrophils, macrophages and T cells contributes to the tissue injury, and recent data highlight the importance of Th17 cell infiltration in immune-mediated inflammatory diseases (4, 22, 23). Since IL-17 stimulates the production of CCL20, which can attract CCR6 positive Th17 cells at inflamed tissue (24, 25), disruption of this positive feedback loop may be possible strategy to prevent tissue injury. In the present study, we observed a novel role of CaMK4 in Th17 cell recruitment into a target organ: CaMK4 acted by facilitating the CCR6/CCL20 axis in the AIGN model. Our work identifies CaMK4 as a critical molecule involved in Th17 cell-mediated tissue damage in inflammatory diseases. Of note, the CCR6 expression in effector memory T cells from SLE patients was positively correlated with organ involvement.
We have reported that the genetic or pharmacologic inhibition of CaMK4 in MRL/lpr mice results in decreased autoantibody production and the improvement of lupus-related pathology including glomerulonephritis (14, 26). However, it is not yet known how CaMK4 regulates tissue injury downstream of autoantibody production. Our present findings demonstrate that the inhibition of CaMK4 ameliorates AIGN, suggesting that CaMK4 signaling is important for not only autoantibody production but also autoantibody-induced tissue injury.
We observed decreased IL-17 production in the serum of Camk4-deficient mice and the mice treated with a CaMK4 inhibitor. Based on our observation that the Il17a gene expression of infiltrated cells from kidneys was reduced following CaMK4 inhibition, we speculate that it contributes to the recruitments of neutrophils and macrophages at inflamed kidneys. Further studies are needed to identify the specific IL-17-producing cells other than CD4 T cells at the kidneys in this and other experimental settings.
Although the treatment with high dose of KN-93 inhibited the IL-17-producing CD4 T cells in kidneys and decreased the severity of histological findings, the level of proteinuria at day 21 was less improved than expected. The reason of the discrepancy between the degree of proteinuria and the histology finding is that CaMK4 is involved in limiting the infiltration of inflammatory cells including Th17 cells and that CaMK4 may not have a direct effect on correcting proteinuria in this model.
Not only did the inhibition of CaMK4 lead to a reduced number of CCR6-expressing memory T cells before NTS induction, it also resulted in a significant reduction of chemotactic capacity in the presence of CCL20 in in vitro. Accordingly, we speculate that CaMK4 facilitates the increase in the pathogenic memory CD4 T-cell population and induces the aberrant migration of CCR6-expressing CD4 T cells directly into the inflamed tissue. This observation also implies that CaMK4 signaling is critical for chemokine-mediated autoantibody-induced tissue injury.
The recruitment of inflammatory cells including activated T cells and autoantibody-induced tissue injury are characteristic features of tissue injury in LN and NPSLE (27–29). Previous studies revealed that locally secreted chemokines and their receptors contribute to renal damage at the initiation and the progression phase of LN (30). In line with the present data from our AIGN experiments, CCR6 expression by CD4 effector memory T cells was associated with organ involvement in patients with SLE, indicating the relevance of CaMK4-mediated chemotaxis in the organ damage of human SLE.
In conclusion, the results of the present study demonstrate that Camk4-deficient mice as well as mice treated with a CaMK4 inhibitor displayed less glomerular injury after the induction of AIGN. These effects are mediated by the suppression of the CCR6/CCL20 axis at the inflamed sites. Our data suggest the therapeutic potential of a CaMK4 inhibitor to prevent the chemotaxis of inflammatory cells into target organs in patients with Th17-driven diseases, including SLE.
Acknowledgments
Financial Support:
This work was supported by a U.S. National Institutes of Health Grant R01AR064350 (to G.C.T.) and by a Grant in Aid for Scientific Research, Japan Society for the Promotion of Science 26893198 (to T.K.).
Footnotes
Conflicts of Interest
The authors declare that no conflicts of interest exist concerning this study.
Author contributions
Drs. Koga and Tsokos had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study design: T. Koga, G.C. Tsokos
Acquisition of data: T. Koga, K. Otomo, M. Mizui, N. Yoshida, M. Umeda, K. Ichinose, A. Kawakami
Analysis and interpretation of data: Tomohiro Koga, Masayuki Mizui, Nobuya Yoshida and G.C. Tsokos
Manuscript preparation: T. Koga, G.C. Tsokos
Statistical analysis: T. Koga
References
- 1.Lech M, Anders HJ. The pathogenesis of lupus nephritis. J Am Soc Nephrol. 2013;24(9):1357–66. doi: 10.1681/ASN.2013010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365(22):2110–21. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 3.Lourenco EV, La Cava A. Cytokines in systemic lupus erythematosus. Curr Mol Med. 2009;9(3):242–54. doi: 10.2174/156652409787847263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paust HJ, Turner JE, Riedel JH, Disteldorf E, Peters A, Schmidt T, et al. Chemokines play a critical role in the cross-regulation of Th1 and Th17 immune responses in murine crescentic glomerulonephritis. Kidney Int. 2012;82(1):72–83. doi: 10.1038/ki.2012.101. [DOI] [PubMed] [Google Scholar]
- 5.Liu Z, Davidson A. Taming lupus-a new understanding of pathogenesis is leading to clinical advances. Nat Med. 2012;18(6):871–82. doi: 10.1038/nm.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crispin JC, Apostolidis SA, Rosetti F, Keszei M, Wang N, Terhorst C, et al. Cutting edge: protein phosphatase 2A confers susceptibility to autoimmune disease through an IL-17-dependent mechanism. J Immunol. 2012;188(8):3567–71. doi: 10.4049/jimmunol.1200143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pisitkun P, Ha HL, Wang H, Claudio E, Tivy CC, Zhou H, et al. Interleukin-17 cytokines are critical in development of fatal lupus glomerulonephritis. Immunity. 2012;37(6):1104–15. doi: 10.1016/j.immuni.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mizui M, Koga T, Lieberman LA, Beltran J, Yoshida N, Johnson MC, et al. IL-2 protects lupus-prone mice from multiple end-organ damage by limiting CD4-CD8- IL-17-producing T cells. J Immunol. 2014;193(5):2168–77. doi: 10.4049/jimmunol.1400977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crispin JC, Oukka M, Bayliss G, Cohen RA, Van Beek CA, Stillman IE, et al. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J Immunol. 2008;181(12):8761–6. doi: 10.4049/jimmunol.181.12.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turner JE, Paust HJ, Steinmetz OM, Peters A, Riedel JH, Erhardt A, et al. CCR6 recruits regulatory T cells and Th17 cells to the kidney in glomerulonephritis. J Am Soc Nephrol. 2010;21(6):974–85. doi: 10.1681/ASN.2009070741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204(12):2803–12. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou XJ, Mu R, Li C, Nath SK, Zhang YM, Qi YY, et al. Association of variants in CCR6 with susceptibility to lupus nephritis in Chinese. Arthritis Rheumatol. 2015;67(11):3091–3. doi: 10.1002/art.39268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Racioppi L, Means AR. Calcium/calmodulin-dependent kinase IV in immune and inflammatory responses: novel routes for an ancient traveller. Trends Immunol. 2008;29(12):600–7. doi: 10.1016/j.it.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Koga T, Ichinose K, Mizui M, Crispin JC, Tsokos GC. Calcium/Calmodulin-Dependent Protein Kinase IV Suppresses IL-2 Production and Regulatory T Cell Activity in Lupus. J Immunol. 2012;189(7):3490–6. doi: 10.4049/jimmunol.1201785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koga T, Hedrich CM, Mizui M, Yoshida N, Otomo K, Lieberman LA, et al. CaMK4-dependent activation of AKT/mTOR and CREM-alpha underlies autoimmunity-associated Th17 imbalance. J Clin Invest. 2014;124(5):2234–45. doi: 10.1172/JCI73411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koga T, Mizui M, Yoshida N, Otomo K, Lieberman LA, Crispin JC, et al. KN-93, an inhibitor of calcium/calmodulin-dependent protein kinase IV, promotes generation and function of Foxp3(+) regulatory T cells in MRL/lpr mice. Autoimmunity. 2014;47(7):445–50. doi: 10.3109/08916934.2014.915954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25(11):1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 18.Petri M. Disease activity assessment in SLE: do we have the right instruments? Ann Rheum Dis. 2007;66(Suppl 3):iii61–4. doi: 10.1136/ard.2007.078477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum. 1999;42(4):599–608. doi: 10.1002/1529-0131(199904)42:4<599::AID-ANR2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 20.Fries JW, Mendrick DL, Rennke HG. Determinants of immune complex-mediated glomerulonephritis. Kidney Int. 1988;34(3):333–45. doi: 10.1038/ki.1988.186. [DOI] [PubMed] [Google Scholar]
- 21.Vielhauer V, Stavrakis G, Mayadas TN. Renal cell-expressed TNF receptor 2, not receptor 1, is essential for the development of glomerulonephritis. J Clin Invest. 2005;115(5):1199–209. doi: 10.1172/JCI23348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oo YH, Banz V, Kavanagh D, Liaskou E, Withers DR, Humphreys E, et al. CXCR3-dependent recruitment and CCR6-mediated positioning of Th-17 cells in the inflamed liver. J Hepatol. 2012;57(5):1044–51. doi: 10.1016/j.jhep.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang W, Nie L, Wang Y, Wang XP, Zhao H, Dongol S, et al. CCL20 Secretion from the Nucleus Pulposus Improves the Recruitment of CCR6-Expressing Th17 Cells to Degenerated IVD Tissues. PLoS One. 2013;8(6):e66286. doi: 10.1371/journal.pone.0066286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harper EG, Guo C, Rizzo H, Lillis JV, Kurtz SE, Skorcheva I, et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol. 2009;129(9):2175–83. doi: 10.1038/jid.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu Y, Shen F, Crellin NK, Ouyang W. The IL-17 pathway as a major therapeutic target in autoimmune diseases. Ann N Y Acad Sci. 2011;1217:60–76. doi: 10.1111/j.1749-6632.2010.05825.x. [DOI] [PubMed] [Google Scholar]
- 26.Ichinose K, Juang YT, Crispin JC, Kis-Toth K, Tsokos GC. Suppression of autoimmunity and organ pathology in lupus-prone mice upon inhibition of calcium/calmodulin-dependent protein kinase type IV. Arthritis Rheum. 2011;63(2):523–9. doi: 10.1002/art.30085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mondal TK, Saha SK, Miller VM, Seegal RF, Lawrence DA. Autoantibody-mediated neuroinflammation: pathogenesis of neuropsychiatric systemic lupus erythematosus in the NZM88 murine model. Brain Behav Immun. 2008;22(6):949–59. doi: 10.1016/j.bbi.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 28.Jeltsch-David H, Muller S. Neuropsychiatric systemic lupus erythematosus: pathogenesis and biomarkers. Nat Rev Neurol. 2014;10(10):579–96. doi: 10.1038/nrneurol.2014.148. [DOI] [PubMed] [Google Scholar]
- 29.Dai C, Deng Y, Quinlan A, Gaskin F, Tsao BP, Fu SM. Genetics of systemic lupus erythematosus: immune responses and end organ resistance to damage. Curr Opin Immunol. 2014;31:87–96. doi: 10.1016/j.coi.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rovin BH. The chemokine network in systemic lupus erythematous nephritis. Front Biosci. 2008;13:904–22. doi: 10.2741/2731. [DOI] [PubMed] [Google Scholar]


