Abstract
Background
High body mass index (BMI) and low physical fitness are risk factors for stroke, but their interactive effects are unknown. Elucidation of interactions between these modifiable risk factors can help inform preventive interventions in susceptible subgroups.
Methods
National cohort study of all 1,547,294 military conscripts in Sweden during 1969-1997 (97-98% of all 18-year-old males). Standardized aerobic capacity, muscular strength, and BMI measurements were examined in relation to stroke identified from inpatient and outpatient diagnoses through 2012 (maximum age 62 years).
Results
16,979 men were diagnosed with stroke in 39.7 million person-years of follow-up. High BMI, low aerobic fitness, and (less strongly) low muscular fitness were associated with higher risk of any stroke, ischemic stroke, and intracerebral hemorrhage, independently of family history and sociodemographic factors. High BMI (overweight/obese vs. normal) and low aerobic capacity (lowest vs. highest tertile) had similar effect magnitudes, and their combination was associated with highest stroke risk (incidence rate ratio, 2.36; 95% CI, 2.14-2.60; P<0.001). Aerobic capacity and muscular strength had a positive additive and multiplicative interaction (P<0.001), indicating that low aerobic capacity accounted for more strokes among men with low compared with high muscular strength.
Conclusions
High BMI and low aerobic capacity in late adolescence are associated with increased risk of stroke in adulthood. Low aerobic capacity and low muscular strength also have a synergistic effect on stroke risk. These findings suggest that preventive interventions should include weight control and aerobic fitness early in life, and muscular fitness especially among those with low aerobic capacity.
Keywords: body mass index, interactions, physical fitness, stroke
INTRODUCTION
Stroke is the second leading cause of death worldwide (1). Despite declines in mortality in the US over the past 4 decades, stroke still accounts for 130,000 deaths and $34 billion in health care and economic costs in the US annually (2). Because of its high incidence, long-term disability, and mortality, better primary prevention of stroke is a major public health priority in the US and globally. High body mass index (BMI) (3-9), low physical activity (10-12), and low physical fitness (13-17) are established risk factors for stroke. Physical fitness (particularly aerobic capacity) has been found to be a stronger predictor of cardiovascular risk factors (18) and disease (19) than physical activity, and a better indicator of habitual physical activity than self-reported activity (20). Although the separate effects of BMI (3-9) and physical fitness (13-17) on stroke risk have been examined, their interactive effects remain unknown. For example, it is unknown whether low physical fitness has a stronger effect on stroke risk among persons with high BMI, or to what extent improvements in fitness may offset risk in this group. A better understanding of these common modifiable risk factors, including their combined and interactive effects, is needed to inform more effective preventive interventions in susceptible subgroups.
We conducted the first study to examine the interactive effects between physical fitness (including both aerobic capacity and muscular strength) and BMI in late adolescence in relation to the risk of stroke and its major subtypes in adulthood. Aerobic capacity, muscular strength, and BMI were assessed using standardized measurements in ~1.5 million 18-year-old male military conscripts in Sweden who were followed up to a maximum age of 62 years. Our aims were to examine potential additive and multiplicative interactions among aerobic capacity, muscular strength, and BMI in relation to stroke risk in a large national cohort, which may help inform more effective primary prevention.
METHODS
Study Population
We identified all 1,547,478 males (age 18 years) who underwent a military conscription examination in Sweden during 1969-1997. This examination was compulsory for all 18-year-old males nationwide each year except for 2-3% who either were incarcerated or had severe chronic medical conditions or disabilities documented by a physician. We excluded 184 (0.01%) individuals who had a prior stroke identified from hospital discharge diagnoses. A total of 1,547,294 (>99.9% of the original cohort) remained for inclusion in the study. This study was approved by the Regional Ethics Committee of Lund University in Sweden (No. 2010/476).
Aerobic Capacity, Muscular Strength, and BMI Ascertainment
Aerobic capacity, muscular strength, and BMI measurements were obtained from the Swedish Military Conscription Registry, which contains information from a 2-day standardized physical and psychological examination required for all conscripts starting in 1969. Aerobic capacity was measured as the maximal aerobic workload in Watts, using a standard well-validated electrically-braked stationary bicycle ergometer test, as previously described (21). Maximal aerobic workload is highly correlated with maximal oxygen uptake (VO2 max) (correlation ~0.9) (22), and its measurement using this bicycle ergometer test is highly reproducible, with a test-retest correlation of 0.95 (23). Muscular strength was measured as the weighted sum of maximal knee extension (weighted × 1.3), elbow flexion (weighted × 0.8), and hand grip (weighted × 1.7), each measured in Newtons, using standard well-validated isometric dynamometer tests (24). Each dynamometer test was performed three times and the maximum value was recorded for analysis, except when the last value was highest, in which case testing was repeated until strength values stopped increasing. BMI was determined using standardized height and weight measurements and calculated as: [weight in kg]/[height in m]2. All testing equipment was calibrated daily (21, 24).
In the present study, aerobic capacity and muscular strength were modeled alternatively as continuous variables or categorical variables in tertiles (aerobic capacity in Watts: low [<240], medium [240-288], high [≥289]; muscular strength in Newtons: low [<1900], medium [1900-2170], high [≥2171]). BMI was modeled alternatively as a continuous or categorical variable using Centers for Disease Control and Prevention (CDC) definitions for children and adolescents aged 2 to 19 years to facilitate comparability with US studies: “overweight or obesity” is defined as ≥85th percentile on the CDC's 2000 sex-specific BMI-for-age growth charts, which corresponds to BMI ≥25.6 for 18-year-old males (25).
Stroke Ascertainment
The study cohort was followed up through December 31, 2012 for stroke and its major subtypes (ischemic stroke, intracerebral hemorrhage, and subarachnoid hemorrage), which were identified using International Classification of Diseases (ICD) diagnosis codes in the Swedish Hospital Registry and Swedish Outpatient Registry (any stroke: ICD-8 430-434; ICD-9 430-431, 433-434, 436; ICD-10 I60-I61, I63-I64; ischemic stroke: ICD-8 433-434; ICD-9 434; ICD-10 I63; intracerebral hemorrhage: ICD-8/9 431; ICD-10 I61; subarachnoid hemorrhage: ICD-8/9 430; ICD-10 I60). The Swedish Hospital Registry contains all primary and secondary hospital discharge diagnoses from six populous counties in southern Sweden starting in 1964, and with nationwide coverage starting in 1987; and the Swedish Outpatient Registry contains outpatient diagnoses from all specialty clinics nationwide starting in 2001.
Adjustment Variables
Other variables that may be associated with stroke were obtained from the Swedish Military Conscription Registry and national census data, which were linked using an anonymous personal identification number. The following were used as adjustment variables: year of the military conscription examination (modeled simultaneously as a continuous and categorical [1969-1979, 1980-1989, 1990-1997] variable to account for follow-up time and attained age); family history of stroke in a parent or sibling (yes or no, identified from diagnoses in the Swedish Hospital Registry during 1964-2012 and the Swedish Outpatient Registry during 2001-2012, using the same diagnosis codes noted above, plus 330-332 in ICD-7); highest education level attained during the study period (<12, 12-14, ≥15 years); and neighborhood socioeconomic status (SES) at baseline (included because neighborhood characteristics have been associated with stroke (26, 27) and with physical activity (28) and obesity (29); composed of an index that includes low education level, low income, unemployment, and social welfare receipt, as previously described (30); categorized as low [>1 SD below the mean], medium [within 1 SD from the mean], or high [>1 SD above the mean]).
As alternatives to BMI, we also examined height and weight simultaneously in a separate model, and modeled them alternatively as continuous or categorical (height: <175, 175-184, ≥185 cm; weight: <60, 60-79, ≥80 kg) variables. Blood pressure may be in the causal pathway between high BMI or low physical fitness and stroke, and therefore is not considered a confounder. However, in a secondary analysis, we examined the effect on risk estimates of further adjusting for systolic and diastolic blood pressure at baseline (measured in mm Hg using a standard protocol at the military conscription examination) as continuous variables.
Missing data for each variable were imputed using a standard multiple imputation procedure based on the variable's relationship with all other covariates and the outcome (stroke) (31). Missing data were relatively infrequent for aerobic capacity (5.7%), muscular strength (5.0%), height (7.2%), weight (7.3%), education level (0.4%), neighborhood SES (9.1%), systolic blood pressure (8.6%), and diastolic blood pressure (8.7%). As an alternative to multiple imputation, sensitivity analyses were performed after restricting to individuals with complete data for all variables (N=1,303,705; 84.2%).
Statistical Analysis
Poisson regression with robust standard errors was used to compute incidence rate ratios (IRRs) and 95% confidence intervals (CIs) for associations between aerobic capacity, muscular strength, or BMI and subsequent risk of stroke (32). In the main analyses, two different adjusted models were performed: the first was adjusted for year of the military conscription examination (to account for follow-up time and attained age), and the second was additionally adjusted for aerobic capacity, muscular strength, BMI, family history of stroke, education level, and neighborhood SES (each as a categorical variable as defined above). In a secondary analysis, we adjusted additionally for systolic and diastolic blood pressure (as continuous variables). Poisson model goodness-of-fit was assessed using deviance and Pearson chi-squared tests, which showed a good fit in all models.
Interactions among aerobic capacity, muscular strength, and BMI on either the additive or multiplicative scale were examined in relation to stroke risk. Additive interactions were assessed using the “relative excess risk due to interaction” (RERI), which is computed for binary variables as: RERIIRR = IRR11 – IRR10 – IRR01 + 1 (33, 34). Multiplicative interactions were assessed using the ratio of IRRs: IRR11 / (IRR10 × IRR01) (33). All statistical tests were 2-sided and used an α-level of 0.05. All analyses were conducted using Stata version 14.0 (35).
RESULTS
Among the 1,547,294 men in this cohort, 16,979 (1.1%) were subsequently diagnosed with stroke in 39.7 million person-years of follow-up (mean follow-up, 25.7 years). Among all strokes, 10,467 (61.6%) were ischemic, 3,425 (20.2%) were intracerebral hemorrhages, and 3,087 (18.2%) were subarachnoid hemorrhages. The median age at the end of follow-up was 47.2 years (mean 47.4, SD 7.9, range 23.2 to 62.0), and median age at any stroke diagnosis was 48.0 years (mean 46.1, SD 9.4, range 18.5 to 61.9). The median BMI among men diagnosed with any stroke was 21.1 (mean 21.6, SD 3.0, range 15.3 to 39.3), and among those without stroke was 21.1 (mean 21.7, SD 2.8, range 15.6 to 37.8); median aerobic capacity (in Watts) among those diagnosed with any stroke was 231.4 (mean 234.9, SD 49.3, range 110.8 to 429.0), and among those without stroke was 262.0 (mean 267.3, SD 54.4, range 114.8 to 455.0); and median muscular strength (in Newtons) among those diagnosed with any stroke was 1,996 (mean 1,997, SD 343, range 550 to 3,175), and among those without stroke was 2,020 (mean 1,985, SD 454, range 428 to 3,199). These distributions were similar among all stroke subtypes (data not shown).
Main Effects of Aerobic Capacity, Muscular Strength, and BMI
Low aerobic capacity was associated with a subsequent increased risk of any stroke, after adjusting for BMI and other factors (Table 1, adjusted model 2: IRR for lowest vs. highest tertile, 1.64; 95% CI, 1.55-1.73; P<0.001), including a strong inverse trend across the full range of aerobic capacity (adjusted model 2: IRR for trend test per 100 Watts, 0.61; 95% CI, 0.59-0.64; P<0.001). Low muscular strength was associated with a modestly increased risk of stroke (adjusted model 2: IRR for lowest vs. highest tertile, 1.10; 95% CI, 1.05-1.14; P<0.001).
Table 1.
Adjusted incidence rate ratios for associations between physical fitness, BMI, or other factors among 18-year-old males and subsequent risk of stroke.
| Any Stroke | Adjusted Model 1a | Adjusted Model 2b | ||||||
|---|---|---|---|---|---|---|---|---|
| No (N=1,530,315) | Yes (N=16,979) | IRR | 95% CI | P | IRR | 95% CI | P | |
| Aerobic capacity (tertiles) | ||||||||
| Low | 501,504 (32.8) | 9,802 (57.7) | 1.80 | 1.71, 1.90 | <0.001 | 1.64 | 1.55, 1.73 | <0.001 |
| Medium | 515,394 (33.7) | 5,092 (30.0) | 1.28 | 1.21, 1.35 | <0.001 | 1.21 | 1.14, 1.28 | <0.001 |
| High | 513,417 (33.5) | 2,085 (12.3) | 1.00 | 1.00 | ||||
| Per 100 Watts (trend test) | 0.59 | 0.56, 0.61 | <0.001 | 0.61 | 0.59, 0.64 | <0.001 | ||
| Muscular strength (tertiles) | ||||||||
| Low | 504,896 (33.0) | 5,936 (35.0) | 1.19 | 1.15, 1.24 | <0.001 | 1.10 | 1.05, 1.14 | <0.001 |
| Medium | 517,164 (33.8) | 6,348 (37.4) | 1.07 | 1.04, 1.12 | <0.001 | 1.04 | 1.00, 1.08 | 0.05 |
| High | 508,255 (33.2) | 4,695 (27.6) | 1.00 | 1.00 | ||||
| Per 1000 Newtons (trend test) | 0.79 | 0.75, 0.82 | <0.001 | 0.87 | 0.83, 0.91 | <0.001 | ||
| Body mass indexc | ||||||||
| Normal | 1,411,897 (92.3) | 15,448 (91.0) | 1.00 | 1.00 | ||||
| Overweight or obese | 118,418 (7.7) | 1,531 (9.0) | 1.53 | 1.46, 1.62 | <0.001 | 1.58 | 1.50, 1.67 | <0.001 |
| Per 1 BMI unit (trend test) | 1.03 | 1.02, 1.04 | <0.001 | 1.05 | 1.04, 1.06 | <0.001 | ||
| Height (cm) | ||||||||
| <175 (5 ft. 9 in.) | 342,623 (22.4) | 4,207 (24.8) | 1.08 | 1.05, 1.12 | <0.001 | 1.07 | 1.03, 1.11 | <0.001 |
| 175-184 | 903,405 (59.0) | 10,438 (61.5) | 1.00 | 1.00 | ||||
| ≥185 (6 ft. 1 in.) | 284,287 (18.6) | 2,334 (13.7) | 0.79 | 0.76, 0.83 | <0.001 | 0.83 | 0.80, 0.87 | <0.001 |
| Per 5 cm (trend test) | 0.91 | 0.90, 0.92 | <0.001 | 0.92 | 0.91, 0.93 | <0.001 | ||
| Weight (kg) | ||||||||
| <60 (132 lbs.) | 189,260 (12.4) | 2,479 (14.6) | 1.07 | 1.02, 1.11 | 0.004 | 0.94 | 0.90, 0.98 | <0.001 |
| 60-79 | 1,136,314 (74.2) | 12,339 (72.7) | 1.00 | 1.00 | ||||
| ≥80 (176 lbs.) | 204,741 (13.4) | 2,161 (12.7) | 1.23 | 1.17, 1.28 | <0.001 | 1.44 | 1.37, 1.51 | <0.001 |
| Per 5 kg (trend test) | 1.04 | 1.03, 1.05 | <0.001 | 1.09 | 1.08, 1.10 | <0.001 | ||
| Family history of stroke | ||||||||
| No | 1,250,181 (81.7) | 12,209 (71.9) | 1.00 | 1.00 | ||||
| Yes | 280,134 (18.3) | 4,770 (28.1) | 1.24 | 1.20, 1.28 | <0.001 | 1.24 | 1.20, 1.28 | <0.001 |
| Education (years) | ||||||||
| <12 | 232,414 (15.2) | 4,403 (25.9) | 1.18 | 1.14, 1.23 | <0.001 | 1.14 | 1.09, 1.18 | <0.001 |
| 12-14 | 676,202 (44.2) | 7,333 (43.2) | 1.00 | 1.00 | ||||
| ≥15 | 621,699 (40.6) | 5,243 (30.9) | 0.71 | 0.69, 0.74 | <0.001 | 0.77 | 0.74, 0.80 | <0.001 |
| Per higher category (trend) | 0.77 | 0.76, 0.79 | <0.001 | 0.82 | 0.80, 0.84 | <0.001 | ||
| Neighborhood SES | ||||||||
| Low | 236,332 (15.4) | 3,034 (17.9) | 1.05 | 1.00, 1.09 | 0.03 | 1.01 | 0.98, 1.06 | 0.47 |
| Medium | 1,010,311 (66.0) | 11,813 (69.6) | 1.00 | 1.00 | ||||
| High | 283,672 (18.5) | 2,132 (12.6) | 0.81 | 0.77, 0.85 | <0.001 | 0.89 | 0.85, 0.93 | <0.001 |
| Per higher category (trend) | 0.89 | 0.87, 0.91 | <0.001 | 0.94 | 0.92, 0.97 | <0.001 | ||
Adjusted for year of military conscription examination (to account for follow-up time and attained age).
Adjusted for year of military conscription examination, aerobic capacity, muscular strength, BMI, family history of stroke, education, and neighborhood SES. Height and weight were modeled simultaneously as an alternative to BMI in a separate model. The reference category for all variables is indicated by an IRR of 1.00.
BMI was categorized using CDC definitions for children and adolescents aged 2 to 19 years: “overweight or obese” is defined as ≥85th percentile from the CDC's 2000 sex-specific BMI-for-age growth charts, which corresponds to BMI ≥25.6 for 18-year-old males.
BMI = body mass index, IRR = incidence rate ratio, SES = socioeconomic status.
High BMI also was an independent risk factor for stroke. Overweight or obese males (≥85th percentile on the CDC's 2000 sex-specific BMI-for-age growth chart) had more than a 1.5-fold risk of stroke relative to those with normal BMI (Table 1, adjusted model 2: IRR, 1.58; 95% CI, 1.50-1.67, P<0.001). When both height and weight were included in the model as an alternative to BMI, low height and high weight were each independently associated with increased stroke risk (see Table 1, adjusted model 2), although high weight was a much stronger risk factor (Pheterogeneity<0.001).
A first-degree family history of stroke was associated with a ~1.2-fold risk of stroke (Table 1). Education level and neighborhood SES were inversely related to stroke risk (i.e., high education level and high neighborhood SES were modestly protective) (Table 1; Ptrend<0.001). There was no evidence of statistical or biologically meaningful interaction between neighborhood SES and other factors in relation to stroke risk (P>0.05 for each). In a secondary analysis, further adjustment for systolic and diastolic blood pressure had a negligible effect on any of the risk estimates (data not shown). In sensitivity analyses that were restricted to individuals with no missing data, all risk estimates were very similar to the main results (data not shown).
All main effects for stroke subtypes were similar to those for strokes overall (Supplemental Tables 1a, 1b, and 1c), except that high BMI was not a significant risk factor for subarachnoid hemorrhage (fully adjusted IRR, 1.11; 95% CI, 0.97-1.28; P=0.13; Supplemental Table 1c).
Interactions Among Aerobic Capacity, Muscular Strength, and BMI
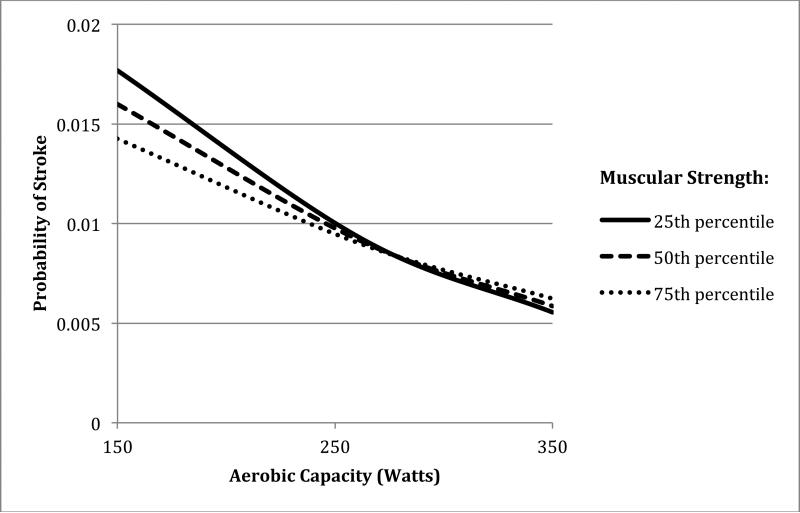
The interactive effects of aerobic capacity and muscular strength on risk of stroke are shown in Table 2. Low aerobic capacity was associated with increased risk of stroke irrespective of muscular strength level (Table 2, right-most column), whereas low muscular strength was associated with a (modestly) increased risk of stroke only among men with low aerobic capacity. The combination of low aerobic capacity and low muscular strength was associated with highest stroke risk (IRR, 1.75; 95% CI, 1.64-1.88; P<0.001), relative to the reference group of men with high aerobic capacity and normal BMI. Low aerobic capacity and low muscular strength had a significant positive interaction on both the additive and multiplicative scale (P<0.001) (i.e., their combined effect on stroke risk exceeded the sum or product of their separate effects). The positive additive interaction indicates that low aerobic capacity accounts for more strokes among men with low compared with high muscular strength. Figure 1 shows the probability of stroke for the 25th, 50th, and 75th percentiles of muscular strength across the full distribution of aerobic capacity, from the fully adjusted model. The non-parallel curves, particularly at the lower range of aerobic capacity, reflect the positive interaction in relation to stroke risk.
Table 2.
Interactions between aerobic capacity and muscular strength among 18-year-old males in relation to subsequent risk of any stroke.a
| Aerobic capacity (tertiles) | IRRs for medium aerobic capacity within strata of muscular strength | IRRs for low aerobic capacity within strata of muscular strength | ||||||
|---|---|---|---|---|---|---|---|---|
| High | Medium | Low | ||||||
| No. cases/total | IRR (95% CI) | No. cases/total | IRR (95% CI) | No. cases/total | IRR (95% CI) | |||
| Muscular strength (tertiles) | ||||||||
| High | 1,163/250,825 | 1.00 | 2,038/177,441 | 1.19 (1.11, 1.28); P<0.001 | 1,494/84,684 | 1.42 (1.31, 1.54); P<0.001 | 1.19 (1.11, 1.28); P<0.001 | 1.42 (1.31, 1.54); P<0.001 |
| Medium | 589/140,515 | 0.95 (0.86, 1.05); P=0.32 | 1,818/175,246 | 1.20 (1.11, 1.29); P<0.001 | 3,941/207,751 | 1.62 (1.51, 1.74); P<0.001 | 1.26 (1.14, 1.38); P<0.001 | 1.71 (1.55, 1.86); P<0.001 |
| Low | 333/124,162 | 0.91 (0.81, 1.03); P=0.15 | 1,236/167,799 | 1.18 (1.09, 1.28); P<0.001 | 4,367/218,871 | 1.75 (1.64, 1.88); P<0.001 | 1.30 (1.14, 1.46); P<0.001 | 1.92 (1.70, 2.15); P<0.001 |
| IRRs (95% CI) for medium muscular strength within strata of aerobic capacity | 0.95 (0.86, 1.05); P=0.32 | 1.00 (0.94, 1.07); P=0.89 | 1.14 (1.07, 1.21); P<0.001 | |||||
| IRRs (95% CI) for low muscular strength within strata of aerobic capacity | 0.91 (0.81, 1.03); P=0.15 | 0.99 (0.92, 1.07); P=0.88 | 1.23 (1.16, 1.30); P<0.001 | |||||
| Interaction on additive scale for low vs. high tertiles: RERI (95% CI) | 0.42 (0.28, 0.55); P<0.001 | |||||||
| Interaction on multiplicative scale for low vs. high tertiles: Ratio of IRRs (95% CI) | 1.35 (1.17, 1.53); P<0.001 | |||||||
IRRs are adjusted for year of military conscription examination, BMI, family history of stroke, education, and neighborhood SES.
BMI = body mass index, IRR = incidence rate ratio, RERI = relative excess risk due to interaction.
Figure 1.
Probability of stroke by aerobic capacity and muscular strength in 18-year-old males with average follow-up of 25.7 years (maximum age 62 years).
The interactive effects of aerobic capacity and BMI on risk of stroke are shown in Table 3. Both low aerobic capacity and high BMI were independent risk factors with similar effect magnitudes. The combination of low aerobic capacity and high BMI was associated with highest stroke risk, which was ~2.3-fold relative to the reference group of men with high aerobic capacity and normal BMI, but with a negative multiplicative interaction (ratio of IRRs, 0.86; P=0.03) (i.e., their combined effect was less than the product of their separate effects).
Table 3.
Interactions between aerobic capacity and BMI among 18-year-old males in relation to subsequent risk of any stroke.a
| Aerobic capacity (tertiles) | IRRs (95% CI) for medium aerobic capacity within strata of BMI | IRRs (95% CI) for low aerobic capacity within strata of BMI | ||||||
|---|---|---|---|---|---|---|---|---|
| High | Medium | Low | ||||||
| No. cases/total | IRR (95% CI) | No. cases/total | IRR (95% CI) | No. cases/total | IRR (95% CI) | |||
| BMI | ||||||||
| Normal | 1,747/457,912 | 1.00 | 4,436/479,732 | 1.20 (1.14, 1.28); P<0.001 | 9,265/489,701 | 1.66 (1.56, 1.76); P<0.001 | 1.20 (1.14, 1.28); P<0.001 | 1.66 (1.56, 1.76); P<0.001 |
| Overweight or obese | 338/57,590 | 1.65 (1.46, 1.85); P<0.001 | 656/40,754 | 2.07 (1.89, 2.27); P<0.001 | 537/21,605 | 2.36 (2.14, 2.60); P<0.001 | 1.26 (1.09, 1.43); P=0.002 | 1.43 (1.23, 1.63); P<0.001 |
| IRRs (95% CI) for BMI within strata of aerobic capacity | 1.65 (1.46, 1.85); P<0.001 | 1.72 (1.58, 1.86); P<0.001 | 1.42 (1.30, 1.55); P<0.001 | |||||
| Interaction on additive scale: RERI (95% CI) | 0.22 (−0.02, 0.47); P=0.07 | 0.05 (−0.22, 0.32); P=0.70 | ||||||
| Interaction on multiplicative scale: IRR ratio (95% CI) | 1.05 (0.90, 1.20); P=0.53 | 0.86 (0.74, 0.99); P=0.03 | ||||||
IRRs are adjusted for year of military conscription examination, muscular strength, family history of stroke, education, and neighborhood SES.
BMI = body mass index, IRR = incidence rate ratio, RERI = relative excess risk due to interaction.
The interactive effects of muscular strength and BMI on risk of stroke are shown in Table 3. High BMI was a strong risk factor irrespective of muscular strength level, whereas low muscular strength was associated with a modestly increased risk of stroke only among men with normal BMI (Table 4, right-most column). Low muscular strength and high BMI had a negative interaction on both the additive (P=0.02) and multiplicative (P<0.001) scale, suggesting that high BMI accounted for more strokes among men with high compared with low muscular strength.
Table 4.
Interactions between muscular strength and BMI among 18-year-old males in relation to subsequent risk of any stroke.a
| Muscular strength (tertiles) | IRRs (95% CI) for medium muscular strength within strata of BMI | IRRs (95% CI) for low muscular strength within strata of BMI | ||||||
|---|---|---|---|---|---|---|---|---|
| High | Medium | Low | ||||||
| No. cases/total | IRR (95% CI) | No. cases/total | IRR (95% CI) | No. cases/total | IRR (95% CI) | |||
| BMI | ||||||||
| Normal | 3,778/443,031 | 1.00 | 5,948/498,131 | 1.05 (1.01, 1.10); P=0.02 | 5,722/486,183 | 1.12 (1.07, 1.17); P<0.001 | 1.05 (1.01, 1.10); P=0.02 | 1.12 (1.07, 1.17); P<0.001 |
| Overweight or obese | 917/69,919 | 1.67 (1.56, 1.80); P<0.001 | 400/25,381 | 1.64 (1.48, 1.82); P<0.001 | 214/24,649 | 1.51 (1.31, 1.73); P<0.001 | 0.98 (0.87, 1.09); P=0.73 | 0.90 (0.77, 1.03); P=0.14 |
| IRRs (95% CI) for BMI within strata of muscular strength | 1.67 (1.56, 1.80); P<0.001 | 1.56 (1.41, 1.72); P<0.001 | 1.35 (1.17, 1.53); P<0.001 | |||||
| Interaction on additive scale: RERI (95% CI) | −0.08 (−0.28, 0.11); P=0.40 | −0.29 (−0.52, −0.05); P=0.02 | ||||||
| Interaction on multiplicative scale: IRR ratio (95% CI) | 0.93 (0.82, 1.05); P=0.24 | 0.81 (0.68, 0.93); P<0.001 | ||||||
IRRs are adjusted for year of military conscription examination, aerobic capacity, family history of stroke, education, and neighborhood SES.
BMI = body mass index, IRR = incidence rate ratio, RERI = relative excess risk due to interaction.
Compared with strokes overall, all major subtypes (ischemic stroke, intracerebral hemorrhage, and subarachnoid hemorrhage) had very similar interactive effects between aerobic capacity and muscular strength (Supplemental Tables 2a, 2b, and 2c), aerobic capacity and BMI (Supplemental Tables 3a, 3b, and 3c), and muscular strength and BMI (Supplemental Tables 4a, 4b, and 4c). Relatively few men with high BMI were diagnosed with subarachnoid hemorrhage (n=218), which limited statistical power for detecting interactions involving BMI in relation to this outcome.
DISCUSSION
In this large national cohort study, we found that high BMI, low aerobic fitness, and (less strongly) low muscular fitness were associated with higher risk of stroke, independently of family history and sociodemographic factors. Second, high BMI and low aerobic capacity had similar effect magnitudes, and their combination was associated with highest stroke risk, but with a negative multiplicative interaction (i.e., their combined effect was less than the product of their separate effects). Third, low aerobic capacity and low muscular strength had a synergistic effect on stroke risk (i.e., significant super-additive and multiplicative interactions), indicating that low aerobic capacity accounted for more strokes among men with low compared with high muscular strength. All interactions were similar for ischemic stroke, intracerebral hemorrhage, and subarachnoid hemorrhage. If these associations are causal, they suggest that preventive interventions should begin early in life and include both weight control and aerobic fitness, as well as muscular fitness especially among those with low aerobic capacity.
To our knowledge, this large cohort study is the first to examine not only the independent effects of physical fitness and BMI on stroke risk, but also potential multiplicative and additive interactions. A better understanding of the interactive effects of these common modifiable factors can help inform primary prevention in susceptible subgroups. Additive interactions are often unexamined despite being more informative about public health impact (36, 37). Previous studies have reported similar main effects for BMI (3-9), aerobic fitness (13-17), or self-reported physical activity (10-12) in relation to stroke, without examining their interactions. In the present study, we found that the combination of low aerobic capacity and high BMI in late adolescence was associated with highest stroke risk in adulthood. Furthermore, low aerobic capacity and low muscular strength had a synergistic effect on the risk of stroke and all major subtypes.
These findings provide additional evidence that both “fatness” and “fitness” are important factors affecting the long-term risk of stroke. In this cohort, high BMI was associated with more than a 1.5-fold risk of any stroke (and ischemic stroke or intracerebral hemorrhage subtypes), but no significantly increased risk of subarachnoid hemorrhage. This is consistent with previous findings for total or ischemic stroke (3-9), whereas associations with hemorrhagic stroke have been less consistent (5-9), but have seldom distinguished between intracerebral vs. subarachnoid hemorrhage. Our findings also suggest that obesity and low aerobic fitness have similar magnitudes of effect on stroke risk, and that each of these factors is associated with increased risk even in the absence of the other factor. In contrast, low muscular strength was associated with only a modestly increased risk of stroke.
The mechanisms by which obesity and low physical fitness contribute to the development of stroke are highly complex. Both increased adiposity and reduced physical fitness are known to have adverse effects on insulin sensitivity, lipid metabolism, autonomic tone, fibrinolysis and inflammation, which contribute to endothelial dysfunction and atherosclerosis (38, 39). Obesity and physical inactivity are associated with higher levels of prothrombotic and inflammatory markers (e.g., plasminogen activator inhibitor-1 [PAI-1] antigen, fibrinogen, and C-reactive protein) (40-43), which are associated with ischemic stroke (44, 45). In addition, physical activity may promote vascular upregulation of endothelial nitric oxide synthase (eNOS), which is associated with better cerebral blood flow, reduced brain injury during ischemia, and lower stroke risk (46).
Strengths of this study include its large national cohort design with prospective ascertainment of aerobic capacity, muscular strength, BMI, and stroke. The national cohort design prevented selection bias, and the use of registry data with prospectively measured exposures prevented bias that may result from self-reporting. We examined objective, well-validated measures of aerobic capacity and muscular strength, which are likely better indicators of habitual physical activity than self-reported activity (20). We were able to adjust for several other risk factors for stroke, including family history and both individual and neighborhood-level socioeconomic factors, which also were prospectively ascertained and not self-reported.
Limitations include a lack of information on smoking, which is another known risk factor for stroke. Aerobic capacity (VO2 max) has been reported to be only 7% lower in 18-year-old male smokers compared with non-smokers (47), thus smoking is unlikely to account for more than a small proportion of our observed association between aerobic capacity and stroke risk (16). Physical fitness and BMI were measured at only one age (~18 years), and hence we were unable to examine changes in these factors over time. Because this study was based on Swedish military conscripts, the cohort consisted entirely of men. Other studies have reported similar main effects for high BMI (3-8) or low physical fitness (15) in relation to stroke among women. In addition, this study cohort was relatively young, with a median age of 47 years (maximum 62) at the end of follow-up. Additional follow-up will be needed to examine these relationships at older ages when stroke is more common.
In summary, this large national cohort study is the first to examine potential interactions between physical fitness and BMI early in life in relation to stroke risk in adulthood. We found that high BMI, low aerobic fitness, and (less strongly) low muscular fitness in late adolescence were associated with higher risk of stroke in adulthood. Low aerobic capacity and low muscular strength had a synergistic effect on the risk of stroke and all major subtypes. These findings suggest that the risk of stroke in adulthood may be reduced by early lifestyle interventions, including weight control and aerobic fitness, as well as muscular fitness especially among those with low aerobic capacity. Additional studies with longitudinal measurements of fitness will be needed to delineate the most important windows of susceptibility and further inform interventions.
Supplementary Material
ACKNOWLEDGMENTS
Funding: This work was supported by the National Heart, Lung, and Blood Institute at the National Institutes of Health (R01 HL116381); the Swedish Research Council; and ALF project grant, Region Skåne/Lund University, Sweden. The funding agencies had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Author contributions: JS had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: CC, JS, MW, KS.
Acquisition of data: JS, KS.
Analysis and interpretation of data: CC, JS, MW, KS.
Drafting of the manuscript: CC.
Critical revision of the manuscript for important intellectual content: CC, JS, MW, KS.
Statistical analysis: CC, JS.
Obtained funding: JS, KS.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Contributor Information
Casey Crump, Icahn School of Medicine at Mount Sinai, Departments of Family Medicine and Community Health and of Population Health Science and Policy, One Gustave L. Levy Place, Box 1077, New York, NY 10029, USA.
Jan Sundquist, Lund University, Center for Primary Health Care Research, Clinical Research Centre (CRC), building 28, floor 11, Jan Waldenströms gata 35, Skåne University Hospital, SE-205 02 Malmö, Sweden. jan.sundquist@med.lu.se
Marilyn A. Winkleby, Stanford University, Stanford Prevention Research Center, Medical School Office Building, 251 Campus Drive, Room X318, Stanford, California 94305-5411, USA. winkleby@stanford.edu
Kristina Sundquist, Lund University, Center for Primary Health Care Research, Clinical Research Centre (CRC), building 28, floor 11, Jan Waldenströms gata 35, Skåne University Hospital, SE-205 02 Malmö, Sweden. Kristina.Sundquist@med.lu.se
REFERENCES
- 1.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC. Stroke Facts. Centers for Disease Control and Prevention website; 2015. [September 15, 2015]. [updated 2015]; Available from: http://www.cdc.gov/stroke/facts.htm. [Google Scholar]
- 3.Mitchell AB, Cole JW, McArdle PF, et al. Obesity increases risk of ischemic stroke in young adults. Stroke; a journal of cerebral circulation. 2015;46(6):1690–2. doi: 10.1161/STROKEAHA.115.008940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yatsuya H, Folsom AR, Yamagishi K, et al. Race- and sex-specific associations of obesity measures with ischemic stroke incidence in the Atherosclerosis Risk in Communities (ARIC) study. Stroke; a journal of cerebral circulation. 2010;41(3):417–25. doi: 10.1161/STROKEAHA.109.566299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bazzano LA, Gu D, Whelton MR, et al. Body mass index and risk of stroke among Chinese men and women. Annals of neurology. 2010;67(1):11–20. doi: 10.1002/ana.21950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurth T, Gaziano JM, Rexrode KM, et al. Prospective study of body mass index and risk of stroke in apparently healthy women. Circulation. 2005;111(15):1992–8. doi: 10.1161/01.CIR.0000161822.83163.B6. [DOI] [PubMed] [Google Scholar]
- 7.Hu G, Tuomilehto J, Silventoinen K, Sarti C, Mannisto S, Jousilahti P. Body mass index, waist circumference, and waist-hip ratio on the risk of total and type-specific stroke. Archives of internal medicine. 2007;167(13):1420–7. doi: 10.1001/archinte.167.13.1420. [DOI] [PubMed] [Google Scholar]
- 8.Rexrode KM, Hennekens CH, Willett WC, et al. A prospective study of body mass index, weight change, and risk of stroke in women. JAMA : the journal of the American Medical Association. 1997;277(19):1539–45. doi: 10.1001/jama.1997.03540430051032. [DOI] [PubMed] [Google Scholar]
- 9.Kurth T, Gaziano JM, Berger K, et al. Body mass index and the risk of stroke in men. Archives of internal medicine. 2002;162(22):2557–62. doi: 10.1001/archinte.162.22.2557. [DOI] [PubMed] [Google Scholar]
- 10.Wendel-Vos GC, Schuit AJ, Feskens EJ, et al. Physical activity and stroke. A meta-analysis of observational data. International journal of epidemiology. 2004;33(4):787–98. doi: 10.1093/ije/dyh168. [DOI] [PubMed] [Google Scholar]
- 11.Lee CD, Folsom AR, Blair SN. Physical activity and stroke risk: a meta-analysis. Stroke; a journal of cerebral circulation. 2003;34(10):2475–81. doi: 10.1161/01.STR.0000091843.02517.9D. [DOI] [PubMed] [Google Scholar]
- 12.Alevizos A, Lentzas J, Kokkoris S, Mariolis A, Korantzopoulos P. Physical activity and stroke risk. International journal of clinical practice. 2005;59(8):922–30. doi: 10.1111/j.1742-1241.2005.00536.x. [DOI] [PubMed] [Google Scholar]
- 13.Lee CD, Blair SN. Cardiorespiratory fitness and stroke mortality in men. Medicine and science in sports and exercise. 2002;34(4):592–5. doi: 10.1097/00005768-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Kurl S, Laukkanen JA, Niskanen L, et al. Cardiac power during exercise and the risk of stroke in men. Stroke; a journal of cerebral circulation. 2005;36(4):820–4. doi: 10.1161/01.STR.0000157592.82198.28. [DOI] [PubMed] [Google Scholar]
- 15.Hooker SP, Sui X, Colabianchi N, et al. Cardiorespiratory fitness as a predictor of fatal and nonfatal stroke in asymptomatic women and men. Stroke; a journal of cerebral circulation. 2008;39(11):2950–7. doi: 10.1161/STROKEAHA.107.495275. [DOI] [PubMed] [Google Scholar]
- 16.Aberg ND, Kuhn HG, Nyberg J, et al. Influence of Cardiovascular Fitness and Muscle Strength in Early Adulthood on Long-Term Risk of Stroke in Swedish Men. Stroke; a journal of cerebral circulation. 2015;46(7):1769–76. doi: 10.1161/STROKEAHA.115.009008. [DOI] [PubMed] [Google Scholar]
- 17.Sieverdes JC, Sui X, Lee DC, Lee IM, Hooker SP, Blair SN. Independent and joint associations of physical activity and fitness on stroke in men. The Physician and sportsmedicine. 2011;39(2):119–26. doi: 10.3810/psm.2011.05.1902. [DOI] [PubMed] [Google Scholar]
- 18.Lochen ML, Rasmussen K. The Tromso study: physical fitness, self reported physical activity, and their relationship to other coronary risk factors. Journal of epidemiology and community health. 1992;46(2):103–7. doi: 10.1136/jech.46.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams PT. Physical fitness and activity as separate heart disease risk factors: a meta-analysis. Medicine and science in sports and exercise. 2001;33(5):754–61. doi: 10.1097/00005768-200105000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swift DL, Lavie CJ, Johannsen NM, et al. Physical activity, cardiorespiratory fitness, and exercise training in primary and secondary coronary prevention. Circulation journal : official journal of the Japanese Circulation Society. 2013;77(2):281–92. doi: 10.1253/circj.cj-13-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nordesjo L, Schele R. Validity of an ergometer cycle test and measures of isometric muscle strength when prediction some aspects of military performance. Swedish J Defence Med. 1974;10:11–23. [Google Scholar]
- 22.Patton JF, Vogel JA, Mello RP. Evaluation of a maximal predictive cycle ergometer test of aerobic power. European journal of applied physiology and occupational physiology. 1982;49(1):131–40. doi: 10.1007/BF00428971. [DOI] [PubMed] [Google Scholar]
- 23.Andersen LB. A maximal cycle exercise protocol to predict maximal oxygen uptake. Scandinavian journal of medicine & science in sports. 1995;5(3):143–6. doi: 10.1111/j.1600-0838.1995.tb00027.x. [DOI] [PubMed] [Google Scholar]
- 24.Hook O, Tornvall G. Apparatus and method for determination of isometric muscle strength in man. Scand J Rehabil Med. 1969;1:139–42. [PubMed] [Google Scholar]
- 25.Ogden CL, Flegal KM. Changes in terminology for childhood overweight and obesity. National health statistics reports. 2010;(25):1–5. [PubMed] [Google Scholar]
- 26.Hamano T, Kawakami N, Li X, Sundquist K. Neighbourhood environment and stroke: a follow-up study in Sweden. PloS one. 2013;8(2):e56680. doi: 10.1371/journal.pone.0056680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lisabeth LD, Diez Roux AV, Escobar JD, Smith MA, Morgenstern LB. Neighborhood environment and risk of ischemic stroke: the brain attack surveillance in Corpus Christi (BASIC) Project. American journal of epidemiology. 2007;165(3):279–87. doi: 10.1093/aje/kwk005. [DOI] [PubMed] [Google Scholar]
- 28.Ding D, Sallis JF, Kerr J, Lee S, Rosenberg DE. Neighborhood environment and physical activity among youth a review. American journal of preventive medicine. 2011;41(4):442–55. doi: 10.1016/j.amepre.2011.06.036. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Memarian E, Sundquist J, Zoller B, Sundquist K. Neighbourhood deprivation, individual-level familial and socio-demographic factors and diagnosed childhood obesity: a nationwide multilevel study from Sweden. Obesity facts. 2014;7(4):253–63. doi: 10.1159/000365955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crump C, Sundquist K, Sundquist J, Winkleby MA. Neighborhood deprivation and psychiatric medication prescription: a Swedish national multilevel study. Annals of epidemiology. 2011;21(4):231–7. doi: 10.1016/j.annepidem.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubin DB. Multiple Imputation for Nonresponse in Surveys. Wiley; New York: 1987. [Google Scholar]
- 32.Zou G. A modified poisson regression approach to prospective studies with binary data. American journal of epidemiology. 2004;159(7):702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 33.VanderWeele TJ, Knol MJ. A tutorial on interaction. Epidemiol Methods. 2014;3(1):33–72. [Google Scholar]
- 34.Li R, Chambless L. Test for additive interaction in proportional hazards models. Annals of epidemiology. 2007;17(3):227–36. doi: 10.1016/j.annepidem.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 35.StataCorp . Stata Statistical Software: Release 14. StataCorp LP; College Station, TX: 2014. [Google Scholar]
- 36.Knol MJ, Egger M, Scott P, Geerlings MI, Vandenbroucke JP. When one depends on the other: reporting of interaction in case-control and cohort studies. Epidemiology. 2009;20(2):161–6. doi: 10.1097/EDE.0b013e31818f6651. [DOI] [PubMed] [Google Scholar]
- 37.Greenland S. Interactions in epidemiology: relevance, identification, and estimation. Epidemiology. 2009;20(1):14–7. doi: 10.1097/EDE.0b013e318193e7b5. [DOI] [PubMed] [Google Scholar]
- 38.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444(7121):875–80. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 39.Sandvik L, Erikssen J, Thaulow E, Erikssen G, Mundal R, Rodahl K. Physical fitness as a predictor of mortality among healthy, middle-aged Norwegian men. The New England journal of medicine. 1993;328(8):533–7. doi: 10.1056/NEJM199302253280803. [DOI] [PubMed] [Google Scholar]
- 40.De Pergola G, De Mitrio V, Giorgino F, et al. Increase in both pro-thrombotic and anti-thrombotic factors in obese premenopausal women: relationship with body fat distribution. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 1997;21(7):527–35. doi: 10.1038/sj.ijo.0800435. [DOI] [PubMed] [Google Scholar]
- 41.Juhan-Vague I, Alessi MC, Morange PE. Hypofibrinolysis and increased PAI-1 are linked to atherothrombosis via insulin resistance and obesity. Annals of medicine. 2000;32(Suppl 1):78–84. [PubMed] [Google Scholar]
- 42.Morange PE, Alessi MC, Verdier M, Casanova D, Magalon G, Juhan-Vague I. PAI-1 produced ex vivo by human adipose tissue is relevant to PAI-1 blood level. Arteriosclerosis, thrombosis, and vascular biology. 1999;19(5):1361–5. doi: 10.1161/01.atv.19.5.1361. [DOI] [PubMed] [Google Scholar]
- 43.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA : the journal of the American Medical Association. 1999;282(22):2131–5. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 44.Di Napoli M, Papa F, Bocola V. C-reactive protein in ischemic stroke: an independent prognostic factor. Stroke; a journal of cerebral circulation. 2001;32(4):917–24. doi: 10.1161/01.str.32.4.917. [DOI] [PubMed] [Google Scholar]
- 45.Rost NS, Wolf PA, Kase CS, et al. Plasma concentration of C-reactive protein and risk of ischemic stroke and transient ischemic attack: the Framingham study. Stroke; a journal of cerebral circulation. 2001;32(11):2575–9. doi: 10.1161/hs1101.098151. [DOI] [PubMed] [Google Scholar]
- 46.Endres M, Gertz K, Lindauer U, et al. Mechanisms of stroke protection by physical activity. Annals of neurology. 2003;54(5):582–90. doi: 10.1002/ana.10722. [DOI] [PubMed] [Google Scholar]
- 47.Dyrstad SM, Aandstad A, Hallen J. Aerobic fitness in young Norwegian men: a comparison between 1980 and 2002. Scandinavian journal of medicine & science in sports. 2005;15(5):298–303. doi: 10.1111/j.1600-0838.2005.00432.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.