Abstract
Purpose
Unintentional weight loss is important and predicts long-term outcomes in rheumatoid arthritis (RA). This study assessed how primary RA therapies influence changes in body mass index (BMI) in a large administrative database.
Methods
Unique dispensing episodes of methotrexate, prednisone, leflunomide and tumor necrosis factor inhibitors (TNFi) administered to RA patients were identified from national Veterans Affairs pharmacy databases. The closest values for C-reactive protein (CRP) and BMI within 30 days of the course start date and follow-up time-points were linked. Missing laboratory values were imputed. Weight loss was defined as a decrease in BMI of >1 kg/m2. Regression models evaluated changes in BMI with use of each drug compared to methotrexate. Propensity scores were incorporated in analyses using matched-weighting techniques to assess the impact of confounding by indication.
Results
There were 52,662 treatment courses in 32,859 patients. Weight gain was seen at 6 months among users of methotrexate, prednisone, and TNFi. On average, prednisone-treated patients had significantly more weight gain, while leflunomide-treated patients demonstrated weight loss. In multivariable models, there was more weight loss among leflunomide users [β: −0.41 kg/m2 (95% CI −0.46, −0.36) p<0.001] compared to methotrexate and a greater risk of weight loss [OR 1.73 (95% CI 1.55, 1.79) p<0.001]. Prednisone was associated with greater weight gain [β: 0.072 kg/m2 (95% CI 0.042, 0.10) p<0.001]. These associations persisted with propensity-adjustment and in sensitivity analyses.
Conclusions
Leflunomide is associated with significant but modest weight loss compared to other RA therapies, while prednisone is associated with greater weight gain.
Keywords: leflunomide, prednisone, rheumatoid arthritis, body mass index
Low body mass index (BMI) is associated with adverse long-term outcomes in rheumatoid arthritis (RA) (1–6). Associations between BMI and long-term risks may be partially explained by disease-related changes in weight (ie. weight loss) over time among patients with severe RA (6, 7). Disease modifying treatments (DMARDs) utilized for the treatment of RA might also influence changes in weight.
For more than a decade there has been debate regarding whether leflunomide is causally associated with weight loss in RA (8–11). Initial phase II trials demonstrated more weight loss in leflunomide-treated subjects, though in subsequent trials weight loss was not identified as an adverse event (8, 9). A case series also suggested that leflunomide could result in weight loss among a subset of patients without an identifiable cause (11). Previous studies have not comprehensively studied weight change as a continuous outcome in a large real-world setting. Anecdotal observations of greater weight loss among subjects treated with leflunomide may also be confounded by the use of the drug as a salvage therapy, particularly among patients with comorbid conditions such as chronic lung disease.
High-dose prednisone has also been widely accepted as a cause of weight gain. However, less evidence supports weight gain among RA patients initiating prednisone, typically used in low doses, compared to the initiation of other therapies. One study demonstrated that weight gain associated with low-dose prednisone use was primarily seen among those who achieved improved disease activity (12). Tumor necrosis factor-α inhibition (TNFi) has been associated with weight gain in some studies (14–16). However, these previous studies have not directly compared weight change observed with TNFi to that seen with other treatments and comprehensively considering the impact of disease activity and other concurrent treatments.
Therefore, to address these knowledge gaps related to these particular drug classes, we evaluated weight change over 3, 6, and 12 months among subjects initiating leflunomide, prednisone, and TNFi compared to subjects initiating methotrexate from a large national database of patients with RA from the Veterans Affairs (VA) health system. We sought to compare weight change among treatments before and after adjusting for potential confounding factors associated with weight change. We also assessed the impact on drug discontinuation and 3-year mortality.
Methods
Study Setting
To establish an analytic dataset, we used 3 national administrative VA databases: The Corporate Data Warehouse (CDW), the Decision Support System (DSS) National Pharmacy Extract, and the Pharmacy Benefits Management (PBM) database between 2000 and 2014. These databases were accessed and linked via the VA Informatics and Computing Infrastructure (VINCI) (17). Algorithms were used to integrate the information from these 3 sources and to define each dispensing episode of each drug for each patient as previously described (18, 19). Treatment courses that did not represent the initial exposure to the drug or that were missing BMI at baseline or at 6-months were excluded. Details regarding the selection of this population are outlined in the Supplementary Figure 1.
DMARD Exposures
For this study, we focused on incident courses of 4 common disease-modifying therapies, namely methotrexate, prednisone, leflunomide, and TNFi, among patients with at least one diagnosis code for RA in the preceding 12 months (International Classification of Diseases, 9th edition, 714.xx). Similar definitions of RA have demonstrated an 81–97% positive predictive value (20). Each prescription fill of a drug was defined as a dispensing episode and a unique observation (18). For each episode, the amount of the drug dispensed and the expected duration of the treatment episode were determined. The expected days of supply were determined based on the dosing instructions. A drug course was defined as a period of continuous treatment consisting of 1 or more dispensing episodes without a gap of ≥90 days between the expected end of the days of supply for that episode and the start of the subsequent dispensing episode. Duration of treatment was calculated as the time from the date of first treatment until the date of the expected end of the last dispensing episode. The primary exposure for each prescription fill was the type of drug with comparison to methotrexate as the reference. Concurrent overlapping treatment courses were identified to assess the impact of combination therapy with multiple drugs.
Ascertainment of Body Mass Index
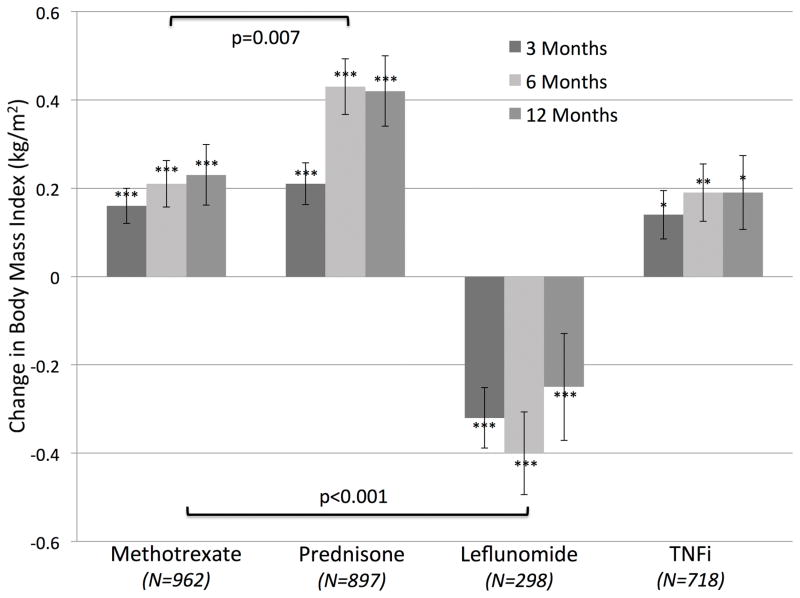
The primary outcome was the change in BMI at 6 months from the date of the fill. Height and weight (as measured and recorded during routine clinical encounters) were extracted from electronic medical records. Missing height values were imputed by carrying the last observation forward. BMI was determined (weight/height2) and values were linked if they fell within 30 days of the date of interest. In this study, weight change was operationalized as the difference in BMI between the follow-up and baseline time-point. Based on previous publications, we defined weight loss as a negative change in BMI of greater than 1 kg/m2 (6, 13, 21, 22). A loss of 1 kg/m2 of BMI has been associated with death in RA, and other populations(6, 7, 21). For an average man or woman in the US (1.77 and 1.62 meters tall, respectively), this change represents approximately 3.1 and 2.6 kg (6.8 or 5.7 pounds), respectively. Six-month changes were considered the primary endpoint for further analyses given that changes in BMI tended to plateau after 6-months (Figure 1).
Figure 1.
Unadjusted changes in BMI over 12 months in treatment courses of methotrexate, prednisone, leflunomide, and TNFi.
Other Study Covariables
Results of testing for C-reactive protein (CRP, mg/dL) were extracted from the electronic medical record. The closest values for CRP within 30 days of the course start date were used as baseline values. CRP values 180 days from the course start date were also linked for each treatment course. CRP was log-adjusted to approximate a normal distribution (lnCRP). The change in lnCRP was defined as the difference in ln(CRP) between the 6-month and baseline time-point. The most recent positive or negative results at any time for testing for anti-cyclic citrullinated peptide (CCP) antibodies and rheumatoid factor (RF) were recorded. Comorbid conditions of interest were identified at the start of the treatment course or any time prior. Coding of comorbidities was based on previously validated algorithms(23, 24), or algorithms appearing in peer-reviewed publications (25–29). Specific cancer types of interest were identified, consistent with Surveillance, Epidemiology, and End Results (SEER) coding algorithms. Our “Comorbidity Score” was the Rheumatology Disease Comorbidity Index, a validated quantitative measure of comorbid illness in RA (30).
Current smoking was considered to be present when documentation of current smoking was identified as a health factor up to 3 years prior to the course start date. Disease duration was estimated by searching for the earliest diagnosis code in the medical record. Disease duration of greater than 5 years was considered to be present if the first diagnosis code occurred more than 5 years prior. If the diagnosis code occurred less than 5 years prior and more than 1 year after the first data in the VA system, this was considered to reflect disease duration of less than 5 years. Other scenarios were considered missing/unknown. Deaths and date of death were identified through the VA’s electronic medical record throughout the period of January 2005 to July 2014 (1). The use of national data was approved by the University of Utah Institutional Review Board.
Statistical Analysis
Differences in patient characteristics across treatments were tested using Analysis of Variance (ANOVA) or Kruskal-Wallis tests for non-parametric data. Changes in BMI over 3, 6, and 12 months were illustrated among a subgroup of subjects without missing BMI data at any of these three time-points. In subsequent analyses, weight change was examined as the absolute change in BMI at 6-months and as a decrease in BMI of more than 1 kg/m2.
Multiple imputation (10 imputations) was performed to replace missing values for CRP at baseline and 6-months in addition to baseline CCP antibody status, RF status, and disease duration (> or ≤5 years). Robust marginal regression models using generalized estimating equations (GEE) to allow clustering on study subject evaluated changes in weight at 6 months in different treatment groups compared to methotrexate as the reference. Hypothesized confounders included demographics, baseline BMI, seropositivity, systemic inflammation (baseline CRP), response to therapy (change in CRP), current smoking, comorbidity score, disease duration, as well as a-priori hypothesized comorbidities including interstitial lung disease (ILD), other lung disease, congestive heart failure (CHF), history of myocardial infarction (MI), diabetes, chronic kidney disease (CKD), history of any malignancy, lung cancer, colon cancer, and prostate cancer.
The propensity to receive prednisone, leflunomide, or TNFi compared to methotrexate was determined for each treatment course using logistic regression with the following independent variables as predictors: course start date, age, sex, race, BMI, ln(CRP), comorbidity score, diabetes, ILD, other lung disease, any malignancy, lung cancer, colon cancer, prostate cancer, CHF, history of MI, HTN, CKD, concurrent RA therapies (methotrexate, leflunomide, TNFi, prednisone, hydroxychloroquine, sulfasalazine), CCP and RF seropositivity, disease duration greater than 5 years, and smoking. Linear and logistic regression models were adjusted for propensity using matched-weighting techniques as described (31). For these analyses, clustering on study subject was not performed since only ~1% of subjects contributed multiple observations. The standardized difference between treatment groups was illustrated over all variables before and after matched weighting to determine assess for adequate balance. Variables that were not balanced were included in multivariable models (see Supplementary Figures 2a–2c).
Sensitivity analyses assessed changes in estimates with the adjustment for concurrent medications and with the exclusion of subjects with overlapping use of methotrexate. Additional sensitivity analyses were performed by excluding subjects receiving concurrent treatment within the methotrexate group (ie. to compare TNFi users who did not take methotrexate to methotrexate users who did not take TNFi). Additional sensitivity analyses were performed by excluding subjects whose treatment course did not last the entire 6-months, those whose weights were not stable prior to initiation of drug, and excluding those without data greater than 6-months prior to the course start date (to exclude those recently entering the VA).
The association between weight loss and discontinuation of therapy by 6-months was also assessed and effect modification by treatment was tested using multiplicative interaction terms (ie to assess if the association between weight loss and drug discontinuation differed by drug). Associations between treatments, weight loss, and 3-year mortality were also assessed. All analyses were performed using Stata 12.0 software (StataCorp, LP, College Station, TX) within VINCI.
Results
Out of 347,373 total incident treatment courses, there were 52,662 in 32,859 unique RA patients in which BMI values at baseline and at 6-months were available. Basic characteristics of patients receiving courses of treatment with methotrexate, prednisone, leflunomide, and TNFi are presented in Table 1. Overall, patients receiving courses of leflunomide were more likely to be Caucasian, to be seropositive, to receive concurrent prednisone, and were less likely to receive concurrent methotrexate. Patients receiving prednisone or leflunomide had higher CRP levels at baseline, lower baseline BMI, greater comorbidity, and were more likely to have been diagnosed with lung disease and CHF compared to those receiving methotrexate or TNFi. Patients receiving TNFi were younger, were less likely to be taking concurrent prednisone and more likely to be taking concurrent methotrexate. TNFi users were also less likely to have been diagnosed with CHF or any malignancy. TNFi and leflunomide users were more likely to have had disease for greater than 5 years. Courses of prednisone were more likely to be ended prior to the 6-month time-point.
Table 1.
Baseline characteristics of dispensing episodes for different disease modifying therapies (N=.52,662 incident treatment courses identified in 32,859 patients).
| Methotrexate (Obs=17287) | Prednisone (Obs=20354) | Leflunomide (Obs=4970) | TNFi (Obs=10051) | |
|---|---|---|---|---|
| DEMOGRAPHICS | ||||
| Age (years) | 62.7 (11.5) | 62.7 (11.6) | 63.4 (10.6) | 60.3 (11.0) |
| Male, % | 88% | 88% | 88% | 88% |
| Race | ||||
| White | 74% | 74% | 78% | 78% |
| Black | 14% | 15% | 11% | 12% |
| BMI, (kg/m2) | 29.4 (5.8) | 29.0 (5.9) | 29.1 (5.8) | 29.6 (5.9) |
| Current Smoking | 14% | 14% | 16% | 15% |
| DISEASE FEATURES | ||||
| CRP, mg/dL | 1.9 (0.3, 4.5) | 2.0 (0.2, 4.7) | 1.7 (0.2, 4.4) | 1.5 (0.1, 4.1) |
| CCP Positive, % | 60% | 57% | 70% | 66% |
| RF Positive, % | 64% | 63% | 72% | 67% |
| Disease >5 years | 24% | 40% | 42% | 33% |
| COMORBID CONDITIONS | ||||
| Interstitial Lung Dis, N (%) | 7% | 10% | 17% | 10% |
| Other Lung Dis, N (%) | 32% | 37% | 244% | 32% |
| Diabetes, N (%) | 28% | 27% | 31% | 28% |
| History of MI, N (%) | 6% | 6% | 8% | 5% |
| Any Malignancy, N (%) | 13% | 16% | 17% | 10% |
| Lung Cancer | 1.1% | 1.7% | 2.2% | 0.9% |
| Colon Cancer | 1.0% | 1.2% | 1.5% | 0.8% |
| Prostate Cancer | 5.9% | 6.1% | 6.3% | 4.0% |
| CHF, N (%) | 8%) | 10% | 12% | 7% |
| Hypertension, N (%) | 68% | 69% | 72% | 67% |
| CKD, N (%) | 2% | 3% | 4% | 2% |
| Comorbidity Score | 1.73 (1.38) | 1.86 (1.44) | 2.01 (1.47) | 1.68 (1.36) |
| CONCURRENT MEDICATIONS | ||||
| Methotrexate | -- | 23% | 27% | 41% |
| Prednisone | 36% | -- | 41% | 33% |
| Leflunomide | 2% | 4% | -- | 9% |
| TNFi | 9% | 10%) | 17% | -- |
| Hydroxychloroquine | 14% | 12% | 20% | 16% |
| Sulfasalazine | 4% | 4% | 8% | 9% |
| 6 MONTH OUTCOMES | ||||
| Change in BMI (kg/m2) | 0.10 (1.63) | 0.18 (1.83) | −0.37 (1.73) | 0.13 (1.68) |
| Weight Loss, N (%) | 19% | 20% | 31% | 19% |
| Drug Continued, N (%) | 59% | 33% | 52% | 64% |
| Died at 3 Years, N(%) | 6% | 9% | 10% | 6% |
All variables were significantly different across groups (p<0.001) with the exception of gender (p=0.61)
Abbreviations: TNFi= Tumor Necrosis Factor Inhibitor; BMI= Body Mass Index; CRP= C-Reactive Protein; CCP= Cyclic Citrullinated Peptide Antibody; RF= Rheumatoid Factor; dis= Disease; MI=Myocardial Infarction; CKD=Chronic Kidney Disease; CHF= Congestive Heart Failure; MI= Myocardial Infarction; RDCI= Rheumatology Disease Comorbidity Index
In unadjusted analyses among the sub-cohort with all BMI data available, there was a significant increase in BMI from baseline over 3, 6, and 12 months with the use of methotrexate (p < 0.001), prednisone (p < 0.001), and TNFi (p<0.01) (Figure 1). In contrast, there was a decrease in BMI among leflunomide users (p<0.001). There was greater weight gain at 6 months among those who initiated prednisone compared to those initiating methotrexate (p<0.001) (Table 1). Weight loss seen with leflunomide was significantly different from the weight gain demonstrated for other drugs (all p<0.001). A greater proportion of patients taking leflunomide (30%) lost >1 kg/m2 compared to other groups including methotrexate (19%) (p<0.001).
Leflunomide
In unadjusted models, leflunomide was associated with greater loss of BMI [β: −0.47 kg/m2 (95% CI −0.53, −0.42 kg/m2) p<0.001] and a greater risk of weight loss [OR 1.86 (95% CI 1.71, 1.97) p<0.001] compared to methotrexate. In multivariable models the association between leflunomide and greater weight loss was not attenuated (Table 2). Among treatment courses of other drugs, concurrent leflunomide use (compared to no use) was also associated with greater weight loss [β: −0.089 kg/m2 (95% CI −0.17, −0.0094 kg/m2) p=0.03] (full model not shown). Finally, leflunomide was associated with an increased risk of weight loss after multivariable adjustment [OR 1.73 (95% CI 1.55, 1.79) p<0.001] (Table 3). Among women, leflunomide was associated with a similar decrease in weight [β: −0.37 kg/m2 (95% CI −0.55, −0.20) p<0.001] and odds of weight loss [OR 1.73 (95% CI 1.41, 2.14) p<0.001] (p for interaction all >0.53). The effect of leflunomide on weight was also similar among those who had more improvement in CRP (greater than the median) [β: −0.42 kg/m2 (95% CI −0.52, −0.32) p<0.001] and among those whose CRP changed to a lesser degree [β: −0.41 kg/m2 (95% CI −0.54, −0.30) p<0.001] (p for interaction = 0.93). The magnitude of the association was also similar when stratifying by weight loss (>1 kg/m2) and no weight loss (stable or increased BMI) in the preceding 12 months among a subset with available data [Total N=32,400 (62% of observations), Leflunomide users N=3489 (70%)] (p<0.001). There was no evidence of an interaction by race (all p=0.89)
Table 2.
Multivariable linear regression model utilizing GEE (clustered on patient) to assess independent associations with the change in BMI at 6-months. Beta coefficient reflects relative change in BMI at 6 months (kg/m2).
| Change in BMI at 6 Months N=31,175, Obs=45,264 |
||
|---|---|---|
|
| ||
| β (Δ in kg/m2) (95% CI) | p | |
|
| ||
| Methotrexate | (Reference) | N/A |
| Prednisone | 0.072 (0.042, 0.10) | <0.001 |
| Leflunomide | −0.41 (−0.46, −0.36) | <0.001 |
| TNFi | 0.0052 (−0.033, 0.043) | 0.78 |
| Age (per 1 year) | −0.014 (−0.015, −0.012) | <0.001 |
| Male | 0.010 (−0.048, 0.069) | 0.74 |
| Caucasian | −0.0040 (−0.043, 0.035) | 0.84 |
| Baseline BMI, (kg/m2) | −0.050 (−0.054, −0.046) | <0.001 |
| Current Smoker | 0.022 (−0.025, 0.071) | 0.39 |
| Ln(CRP, mg/dL) | −0.0079 (−0.022, 0.0064) | 0.26 |
| Change in lnCRP | −0.040 (−0.053, −0.027) | <0.001 |
| Anti-CCP Positive | −0.027 (−0.080, 0.026) | 0.32 |
| RF Positive | 0.024 (−0.022, 0.074) | 0.31 |
| Disease > 5 years | −0.11 (−0.15, −0.075) | <0.001 |
| Interstitial Lung Disease | 0.0025 (−0.028, 0.033) | 0.88 |
| Other Lung Disease | −0.045 (−0.083, −0.0069) | 0.02 |
| Diabetes | 0.034 (−0.0089, 0.077) | 0.12 |
| History of MI | 0.017 (−0.057, 0.091) | 0.65 |
| Any Malignancy | −0.12 (−0.20, −0.046) | 0.002 |
| Lung Cancer | 0.018 (−0.15, 0.18) | 0.83 |
| Colon Cancer | 0.083 (−0.11, 0.28) | 0.41 |
| Prostate Cancer | 0.10 (−0.0057, 0.20) | 0.04 |
| CHF | 0.043 (−0.043, 0.11) | 0.39 |
| Hypertension | 0.076 (0.033, 0.12) | <0.001 |
| CKD | −0.089 (−0.21, 0.034) | 0.16 |
| Comorbidity Score (per unit) | 0.019 (0.0023, 0.035) | 0.03 |
| Concurrent Hydroxychloroquine | 0.035 (−0.0087, 0.080) | 0.12 |
| Concurrent Sulfasalazine | 0.074 (0.0016, 0.15) | 0.05 |
Abbreviations: BMI= Body Mass Index; 95% CI=95% Confidence Interval; TNFi= Tumor Necrosis Factor Inhibitors; CRP= C-Reactive Protein; CCP= Cyclic Citrullinated Peptide; RF= Rheumatoid Factor; MI=Myocardial Infarction; CKD=Chronic Kidney Disease; CHF= Congestive Heart Failure; MI= Myocardial Infarction; RDCI= Rheumatology Disease Comorbidity Index
Table 3.
Multivariable logistic regression model utilizing GEE (clustered on patient) to assess independent associations with the risk of weight loss at 6-months.
| Odds of Weight Loss >1kg/m2 N=31175, Obs=45264 |
||
|---|---|---|
|
| ||
| OR (95% CI) | p | |
|
|
||
| Methotrexate | (Reference) | N/A |
| Prednisone | 1.01 (0.96, 1.05) | 0.76 |
| Leflunomide | 1.73 (1.61, 1.86) | <0.001 |
| TNFi | 1.01 (0.95, 1.08) | 0.67 |
| Age (yrs) | 1.01 (1.01, 1.01) | <0.001 |
| Male | 0.89 (0.82, 0.96) | <0.001 |
| Caucasian | 1.02 (0.96, 1.08) | 0.95 |
| BMI, (kg/m2) | 1.07 (1.06, 1.07) | <0.001 |
| Current Smoker | 1.10 (1.03, 1.18) | 0.005 |
| Ln(CRP, mg/dL) | 1.07 (1.05, 1.09) | <0.001 |
| Change in lnCRP | 1.06 (1.03, 1.08) | 0.001 |
| Anti-CCP Positive | 1.04 (0.98, 1.10) | 0.19 |
| RF Positive | 0.99 (0.92, 1.07) | 0.90 |
| Disease > 5 years | 1.07 (1.01, 1.13) | 0.02 |
| Interstitial Lung Disease | 1.05 (0.97, 1.14) | 0.20 |
| Other Lung Disease | 1.06 (1.00, 1.11) | 0.04 |
| Diabetes | 1.01 (0.99, 1.07) | 0.86 |
| History of MI | 0.98 (0.88, 1.09) | 0.73 |
| Any Malignancy | 1.16 (1.05, 1.27) | 0.003 |
| Lung Cancer | 0.89 (0.72, 1.31) | 0.33 |
| Colon Cancer | 1.05 (0.83, 1.33) | 0.69 |
| Prostate Cancer | 0.88 (0.77, 1.01) | 0.07 |
| CHF | 1.05 (0.97, 1.17) | 0.26 |
| Hypertension | 0.89 (0.83, 0.95) | <0.001 |
| CKD | 1.19 (1.04, 1.38) | 0.01 |
| Comorbidity Score (per 1 unit) | 1.03 (1.01, 1.05) | 0.007 |
| Concurrent Hydroxychloroquine | 0.98 (0.91, 1.04) | 0.46 |
| Concurrent Sulfasalazine | 0.93 (0.84, 1.03) | 0.15 |
Abbreviations: OR= Odds Ratio; 95% CI= 95% Confidence Interval; TNFi= Tumor Necrosis Factor Inhibitors; BMI= Body Mass Index; CRP= C-Reactive Protein; CHF= Congestive Heart Failure; CCP= Cyclic Citrullinated Peptide; RF= Rheumatoid Factor; MI= Myocardial Infarction; CKD= Chronic Kidney Disease; CHF= Congestive Heart Failure; RCDI= Rheumatology Disease Comorbidity Index
The association between leflunomide and greater weight loss was not attenuated in weighted analyses considering the propensity for the drug (Table 4). Furthermore, in sensitivity analyses exploring the exclusion of treatment courses where concurrent medications were used, the magnitude and significance of the association with change in weight was similar (Table 4). Leflunomide was also associated with an increased risk of weight loss (>1 kg/m2) that persisted with propensity adjustment and in sensitivity analyses (Table 5).
Table 4.
Propensity-adjusted changes in body mass index (BMI) at 6 months compared to methotrexate and considering concurrent medication use.
| Unadjusted | Matched Weighting (Propensity-Adjusted) | |||
|---|---|---|---|---|
|
| ||||
| Excluding Concurrent MTX
|
||||
| Compared to MTX Alone
|
||||
| Prednisone (N=37,641) | 0.075*** (0.042, 0.11 | 0.17 *** (0.13, 0.20) | 0.16 *** (0.12, 0.20) | 0.16 *** (0.12, 0.20) |
| Leflunomide (N=22,257) | −0.47 *** (−0.53, −0.42) | −0.43*** (−0.49, −0.37) | −0.42 *** (−0.49, −0.35) | −0.42 *** (−0.49, −0.35) |
| TNFi (N=27,337) | 0.024 (−0.018, 0.067) | 0.025 (−0.019, 0.070) | −0.016 (−0.070, 0.038) | −0.016 (−0.070, 0.037) |
p<0.05,
p<0.01,
p<0.001
Table 5.
Propensity-adjusted risk of weight loss (> 1 kg/m2) at 6 months compared to methotrexate and considering concurrent medication use.
| Unadjusted | Matched Weighting (Propensity-Adjusted | |||
|---|---|---|---|---|
|
| ||||
| Excluding Concurrent MTX
|
||||
| Compared to MTX Alone
|
||||
| Prednisone (N=37,641) | 1.03 (0.98, 1.08) | 0.95 (0.89, 1.01) | 0.97 (0.91, 1.03) | 0.97 (0.91, 1.03) |
| Leflunomide (N=22,257) | 1.86 *** (1.71, 1.97) | 1.77 *** (1.63, 1.91) | 1.79 *** (1.63, 1.95) | 1.79 *** (1.63, 1.95) |
| TNFi (N=27,337) | 1.00 (0.94, 1.06) | 1.00 (0.94, 1.07) | 1.04 (0.96, 1.13) | 1.04 (0.96, 1.12) |
p<0.05,
p<0.01,
p<0.001
Prednisone and TNFI
In unadjusted analyses, prednisone was associated with modestly greater gains in BMI at 6 months compared to methotrexate [β: 0.078 kg/m2 (95% CI 0.048, 0.11 kg/m2) p<0.001] and this persisted in multivariable models (Table 2). Similarly, among methotrexate, leflunomide, and TNFi users, concurrent prednisone (ie. compared to no use) was associated with greater weight gain [β: 0.21 kg/m2 (95% CI 0.17, 0.26 kg/m2) p<0.001] (full model not shown). The increase in weight associated with prednisone did not significantly differ by sex or race (not shown, p>0.18), but was more significant among those who had more substantial improvement in CRP [β: 0.12 kg/m2 (95% CI 0.067, 0.18 kg/m2) p<0.001] compared to those whose CRP changed to a lesser degree [β: 0.014 kg/m2 (95% CI −0.045, 0.072 kg/m2) p=0.64] (p for interaction = 0.006). Prednisone initiation remained associated with weight gain after stratifying by weight loss in the preceding 12 months in a subset with available data [Prednisone users N=12,682 (62%)] [β: 0.042 kg/m2 (95% CI 0.0031, 0.082 kg/m2) p=0.04]. Prednisone use was not associated with weight loss (Table 3). TNFi use was not associated with weight change or weight loss in similar multivariable models and no significant nteractions were noted with age, sex, or change in CRP (all p >0.17).
In weighted analyses considering the propensity for the drug, there remained a significant association between prednisone and weight gain. In a sensitivity analysis exploring the adjustment for, and exclusion of concurrent medications, the magnitude and significance of the association with change in weight was even greater when compared to methotrexate users who were not using concurrent prednisone (Table 4). In unadjusted analyses, TNF use was associated with similar weight change compared to methotrexate. This observation was consistent with propensity-adjustment and in sensitivity analyses (Tables 4 and 5).
Factors Associated with Weight Loss
Factors associated with greater weight loss in this study included older age, greater baseline CRP, less improvement in CRP at 6 months, greater baseline BMI, CCP seropositivity, disease duration >5 years, and the presence of a history of malignancy or lung disease (Table 2). In similar models, more weight gain with initiation of therapy was observed among those who had lost weight in the past year [β: 0.64 kg/m2 (95% CI 0.59, 0.69 kg/m2) p<0.001]. Factors associated with weight loss >1 kg/m2 were similar. However, males were less likely to lose weight. Active smoking and higher comorbidity score were also associated with an increased risk of weight loss (Table 3). Predictors of weight loss among leflunomide and prednisone users were similar to the overall study population. Concurrent methotrexate or TNFi use was not associated with weight change among leflunomide users (not shown) while concurrent prednisone was associated with weight gain [β: 0.14 kg/m2 (95% CI 0.041, 0.24 kg/m2) p=0.005]. Concurrent use of sulfasalazine was associated with modest weight gain (compared to no use) among all treatment courses [β: 0.074 kg/m2 (95% CI 0.0016, 0.15) p=0.05].
Drug Continuation
In propensity-adjusted models, drug continuation for the entire 6-month follow-up period was less likely for prednisone [OR 0.37 (95% CI 0.35, 0.38) p<0.001] and leflunomide [OR 0.73 (95% CI 0.68, 0.78) p<0.001] compared to methotrexate. TNFi was more likely to be continued [OR 1.25 (95% CI 1.18, 1.32) p<0.001] at 6-months compared to methotrexate. Overall, weight loss at 6 months was independently associated with a lower likelihood of continuing the treatment at 6-months [OR 0.85 (95% CI 0.81, 0.89) p<0.001]. In contrast, among leflunomide users, weight loss was associated with a greater likelihood of completing the treatment course [OR 1.27 (95% CI 1.12, 1.44) p<0.001] (p for interaction<0.001).
In sensitivity analysis excluding patients who discontinued their treatment prior to 6 months, leflunomide was associated with even greater weight loss on average [β: −0.62 kg/m2 (95% CI −0.69, −0.55 kg/m2) p<0.001] and a greater risk of weight loss [OR 2.15 (95% CI 1.95, 2.38) p<0.001] compared to methotrexate. In this sensitivity analysis, prednisone was also associated with more substantial weight gain [β: 0.16 kg/m2 (95% CI 0.12, 0.21 kg/m2) p<0.001].
Weight Loss and Risk of Death
In multivariable models adjusting for the factors in Tables 2 and 3, weight loss at 6-months was independently associated with a greater risk of death at 3-years [OR 1.56 (1.45, 1.67) p<0.001] and this association did not vary by study drug (no significant interaction). In multivariable models, compared to methotrexate, 3-year mortality was greater among those using prednisone [OR 1.10 (1.05, 1.15) p<0.001], and leflunomide [OR 1.23 (1.14, 1.33) p<0.001], and similar for TNFi [OR 1.06 (0.99, 1.12) p=0.09]. Further adjustment for weight loss slightly attenuated associations with 3-year mortality for leflunomide [OR 1.18 (1.09, 1.27) p<0.001] compared to methotrexate. These associations were largely unchanged in sensitivity analyses.
Discussion
This study is the first large-scale observational study to demonstrate a significant and relevant increase in the risk of weight loss among patients treated with leflunomide. The strength of this association, lack of attenuation with multivariable and propensity adjustment, as well as the biologic plausibility suggest that this is observation is not likely to be fully explained by confounding by indication and channeling bias., Sensitivity analyses performed here suggest that the association was even more robust when excluding those who discontinued the drug early; suggesting that the observed association is not simply due to poor response and resulting treatment failure. While there were significant differences in the change in BMI among leflunomide users, the changes were modest on average, with a less than 2-fold increase in odds of losing more than 1 kg/m2 at 6 months.
The underlying reasons for weight loss among leflunomide users are unclear. Diarrhea, gastrointestinal upset, and decreased appetite due to the drug may contribute to weight loss, though this remains speculative (10, 11, 32). Leflunomide may also inhibit oxidative phosphorylation in the mitochondria to increase metabolic requirements (33, 34). Predictors of weight loss were similar to the overall population. Our study is limited in the assessment of the underlying mechanisms.
How to approach weight loss among leflunomide users is unclear (35). It remains unknown whether weight loss is a worrisome event, or whether leflunomide can be safely continued. While weight loss was a predictor of drug discontinuation at 6-months in the overall study, weight loss among leflunomide users was not associated with drug discontinuation. Weight loss has been associated with greater mortality in RA (7). In this study, weight loss within the first 6-months after drug initiation was again associated with 3-year risk of death. This association was not altered among leflunomide users and therefore leflunomide-related weight loss is not necessarily of lesser concern with regard to mortality.
This study also identified significant, though modest, weight gain with prednisone compared to methotrexate. Previous studies have shown that increases in weight observed with low-dose prednisone use might be potentially explained by improved disease control (12). The current study suggests that the modest increase in weight occurring in the prednisone group is greater than that seen with the use of other DMARDs and that this weight gain is independent of improvements in CRP. However, compared to methotrexate, prednisone-related weight gain was most pronounced among patients with the greatest improvements in systemic inflammation, suggesting that influence of prednisone may be most notable in this group.
In this study, TNFi use was not consistently associated with weight gain or weight loss when compared to methotrexate. Overall, initiation of new DMARD therapy, whether methotrexate or TNFi, appears to result in modest weight gain, perhaps related to the reduction in inflammation and resting energy expenditure. The data presented here do not support a direct and causal effect of TNFi on weight gain..
Predictors of weight loss among leflunomide users were similar to those identified in the overall population. The data presented here support a previous study demonstrating that age, baseline CRP, improvement in CRP, baseline BMI, and smoking are associated with weight change. These associations should be interpreted with caution since the observation periods in this study were selected based on the initiation of new therapies. However, the consistency with previous observations and biologic plausibility(10, 11, 33, 34) suggest that these are meaningful predictors. Additional factors identified in this study included chronic lung disease, any malignancy, CCP seropositivity, and longer disease duration (>5 years). Greater comorbidity was associated with weight gain on average, however those with higher comorbidity were also at greater risk for weight loss (>1kg/m2), suggesting that a subset is at risk for weight loss. Finally, those with recent weight loss were most likely to gain weight with initiation of therapy, perhaps suggesting that individuals that have experienced weight loss may return toward their baseline weight with the initiation of treatments.
Weight loss in this study was associated with 3-year mortality, confirming previous studies (7). In addition, both prednisone and leflunomide use were associated with greater 3-year mortality compared to methotrexate. However, the adjustment for propensity partially attenuated these associations, suggesting that residual confounding by indication might be present. It is therefore difficult to draw any strong conclusions about mortality risk directly related to the drugs themselves. It is important to emphasize that the risk estimates do not reflect the risk compared to no treatment, but rather compared to treatment with methotrexate, the use of which is known to be associated with lower mortality in RA.(1, 36)
There are several limitations of the current study. While the VA setting was ideal for many aspects of the study, the proportion of men is high. Although observations in the VA setting may not be entirely generalizable and should be confirmed in other study populations, it is worth noting that we found no interactions with gender or race in the primary analyses. The selection of subjects on the basis of the availability of weight measurements could have resulted in bias. Subjects included were similar in demographics to those who were missing BMI, but were slightly younger in age (62.3 v. 63.1 years), more likely to be female (12.0% v. 11.1%), and more likely to use leflunomide (9.4% v. 8.2%). The use of administrative data limits the assessment of more granular features of the disease phenotypes. For example, we did not have access to joint counts or functional assessments, which may help to clarify the role of RA disease severity., We previously observed, however, that joint counts, functional disability, and patient/evaluator scores were not associated with weight change in a similar population of RA patients from the VARA registry (13). Finally, BMI may not be an ideal metabolic indicator in disease states associated with changes in body composition such as in RA.
Strengths of this study include the use of VA electronic medical records, allowing for the reliable ascertainment of multiple BMI measures over time to support robust and comprehensive analyses in a very large cohort of patients. A number of sensitivity analyses were also performed that consistently corroborated initial observations.
In conclusion, in this large-scale observational study, leflunomide use was associated with weight loss at 6 months compared to methotrexate while prednisone use was associated with weight gain. Greater age, baseline CRP, less improvement in CRP, greater baseline BMI, active smoking, CCP seropositivity, longer disease duration, a history of lung disease, malignancy, or CHF, and greater overall comorbidity were independently associated with a greater risk of weight loss at 6 months.
Supplementary Material
Supplementary Figure 1: Flow chart illustrating the selection of subjects into the final analysis cohort.
Supplementary Figure 2: Standardized difference for study variables between treatment groups compared to methotrexate unadjusted (diamonds) and adjusted using matched weighting based on the propensity score (squares). Separate figures are presented for a) leflunomide, b) prednisone, and c) TNFi.
Acknowledgments
Funding: Dr. Baker is funded by a Veterans Affairs Clinical Science Research & Development Career Development Award (IK2 CX000955). Dr. Ibrahim is supported by the National Institutes of Musculoskeletal and Skin Disorders (award 1K24AR055259-01). Dr. Mikuls is funded by a Veterans Affairs Merit Award.
Dr. Baker would like to acknowledge funding through a Veterans Affairs Clinical Science Research & Development Career Development Award (IK2 CX000955). Dr. Ibrahim is supported by a K24 Mid-Career Development Award from the National Institutes of Musculoskeletal and Skin Disorders (1K24AR055259-01).
Footnotes
Conflicts of Interest: The authors have no conflicts to disclose.
The contents of this work do not represent the views of the Department of the Veterans Affairs or the United States Government.
References
- 1.Mikuls TR, Fay BT, Michaud K, Sayles H, Thiele GM, Caplan L, et al. Associations of disease activity and treatments with mortality in men with rheumatoid arthritis: results from the VARA registry. Rheumatology (Oxford) 2011;50(1):101–9. doi: 10.1093/rheumatology/keq232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaufmann J, Kielstein V, Kilian S, Stein G, Hein G. Relation between body mass index and radiological progression in patients with rheumatoid arthritis. J Rheumatol. 2003;30(11):2350–5. [PubMed] [Google Scholar]
- 3.van der Helm-van Mil AH, van der Kooij SM, Allaart CF, Toes RE, Huizinga TW. A high body mass index has a protective effect on the amount of joint destruction in small joints in early rheumatoid arthritis. Ann Rheum Dis. 2008;67(6):769–74. doi: 10.1136/ard.2007.078832. [DOI] [PubMed] [Google Scholar]
- 4.Westhoff G, Rau R, Zink A. Radiographic joint damage in early rheumatoid arthritis is highly dependent on body mass index. Arthritis Rheum. 2007;56(11):3575–82. doi: 10.1002/art.23033. [DOI] [PubMed] [Google Scholar]
- 5.Escalante A, Haas RW, del Rincon I. Paradoxical effect of body mass index on survival in rheumatoid arthritis: role of comorbidity and systemic inflammation. Arch Intern Med. 2005;165(14):1624–9. doi: 10.1001/archinte.165.14.1624. [DOI] [PubMed] [Google Scholar]
- 6.Myrskyla M, Chang VW. Weight change, initial BMI, and mortality among middle- and older-aged adults. Epidemiology. 2009;20(6):840–8. doi: 10.1097/EDE.0b013e3181b5f520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker JF, Billig E, Michaud K, Ibrahim S, Caplan L, Cannon GW, et al. Weight loss, the obesity paradox, and the risk of death in rheumatoid arthritis. Arthritis Rheumatol. 2015 doi: 10.1002/art.39136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osiri M, Shea B, Robinson V, Suarez-Almazor M, Strand V, Tugwell P, et al. Leflunomide for the treatment of rheumatoid arthritis: a systematic review and metaanalysis. J Rheumatol. 2003;30(6):1182–90. [PubMed] [Google Scholar]
- 9.Osiri M, Shea B, Robinson V, Suarez-Almazor M, Strand V, Tugwell P, et al. Leflunomide for treating rheumatoid arthritis. Cochrane Database Syst Rev. 2003;(1):CD002047. doi: 10.1002/14651858.CD002047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alcorn N, Saunders S, Madhok R. Benefit-risk assessment of leflunomide: an appraisal of leflunomide in rheumatoid arthritis 10 years after licensing. Drug Saf. 2009;32(12):1123–34. doi: 10.2165/11316650-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 11.Coblyn JS, Shadick N, Helfgott S. Leflunomide-associated weight loss in rheumatoid arthritis. Arthritis Rheum. 2001;44(5):1048–51. doi: 10.1002/1529-0131(200105)44:5<1048::AID-ANR184>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 12.Jurgens MS, Jacobs JW, Geenen R, Bossema ER, Bakker MF, Bijlsma JW, et al. Increase of body mass index in a tight controlled methotrexate-based strategy with prednisone in early rheumatoid arthritis (CAMERA-II): Side-effect of the prednisone or better control of disease activity? Arthritis Care Res (Hoboken) 2012 doi: 10.1002/acr.21797. [DOI] [PubMed] [Google Scholar]
- 13.Baker JF, Cannon GW, Ibrahim S, Haroldsen C, Caplan L, Mikuls TR. Predictors of Longterm Changes in Body Mass Index in Rheumatoid Arthritis. J Rheumatol. 2015 doi: 10.3899/jrheum.141363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toussirot E, Mourot L, Dehecq B, Wendling D, Grandclement E, Dumoulin G, et al. TNFalpha blockade for inflammatory rheumatic diseases is associated with a significant gain in android fat mass and has varying effects on adipokines: a 2-year prospective study. Eur J Nutr. 2014;53(3):951–61. doi: 10.1007/s00394-013-0599-2. [DOI] [PubMed] [Google Scholar]
- 15.Tan E, Baker C, Foley P. Weight gain and tumour necrosis factor-alpha inhibitors in patients with psoriasis. Australas J Dermatol. 2013;54(4):259–63. doi: 10.1111/ajd.12044. [DOI] [PubMed] [Google Scholar]
- 16.Brown RA, Spina D, Butt S, Summers GD. Long-term effects of anti-tumour necrosis factor therapy on weight in patients with rheumatoid arthritis. Clin Rheumatol. 2012;31(3):455–61. doi: 10.1007/s10067-011-1863-6. [DOI] [PubMed] [Google Scholar]
- 17.United States Department of Veterans Affiars. VA Informatics and Computing Infrastructure. 2014 [cited 2014; Available from: http://www.hsrd.research.va.gov/for_researchers/vinci/cdw.cfm.
- 18.Cannon GW, Mikuls TR, Hayden CL, Ying J, Curtis JR, Reimold AM, et al. Merging Veterans Affairs rheumatoid arthritis registry and pharmacy data to assess methotrexate adherence and disease activity in clinical practice. Arthritis Care Res (Hoboken) 2011;63(12):1680–90. doi: 10.1002/acr.20629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cannon GW, DuVall SL, Haroldsen CL, Caplan L, Curtis JR, Michaud K, et al. Persistence and dose escalation of tumor necrosis factor inhibitors in US veterans with rheumatoid arthritis. J Rheumatol. 2014;41(10):1935–43. doi: 10.3899/jrheum.140164. [DOI] [PubMed] [Google Scholar]
- 20.Singh JA, Holmgren AR, Noorbaloochi S. Accuracy of Veterans Administration databases for a diagnosis of rheumatoid arthritis. Arthritis Rheum. 2004;51(6):952–7. doi: 10.1002/art.20827. [DOI] [PubMed] [Google Scholar]
- 21.Prescott E, Almdal T, Mikkelsen KL, Tofteng CL, Vestbo J, Lange P. Prognostic value of weight change in chronic obstructive pulmonary disease: results from the Copenhagen City Heart Study. Eur Respir J. 2002;20(3):539–44. doi: 10.1183/09031936.02.00532002. [DOI] [PubMed] [Google Scholar]
- 22.Bodegard J, Sundstrom J, Svennblad B, Ostgren CJ, Nilsson PM, Johansson G. Changes in body mass index following newly diagnosed type 2 diabetes and risk of cardiovascular mortality: a cohort study of 8486 primary-care patients. Diabetes Metab. 2013;39(4):306–13. doi: 10.1016/j.diabet.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–9. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 24.Birman-Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care. 2005;43(5):480–5. doi: 10.1097/01.mlr.0000160417.39497.a9. [DOI] [PubMed] [Google Scholar]
- 25.Szeto HC, Coleman RK, Gholami P, Hoffman BB, Goldstein MK. Accuracy of computerized outpatient diagnoses in a Veterans Affairs general medicine clinic. Am J Manag Care. 2002;8(1):37–43. [PubMed] [Google Scholar]
- 26.Davis LA, Cannon GW, Pointer LF, Haverhals LM, Wolff RK, Mikuls TR, et al. Cardiovascular events are not associated with MTHFR polymorphisms, but are associated with methotrexate use and traditional risk factors in US veterans with rheumatoid arthritis. J Rheumatol. 2013;40(6):809–17. doi: 10.3899/jrheum.121012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michaud K, Wolfe F. The Development of a Rheumatic Disease Research Comorbidity Index for Use in Outpatients with RA, OA, SLE, and Fibromyalgia (FMS) Arthritis & Rheumatology, Supplement. 2007 [Google Scholar]
- 28.Wolfe F, Michaud K, Li T, Katz RS. Chronic conditions and health problems in rheumatic diseases: comparisons with rheumatoid arthritis, noninflammatory rheumatic disorders, systemic lupus erythematosus, and fibromyalgia. J Rheumatol. 2010;37(2):305–15. doi: 10.3899/jrheum.090781. [DOI] [PubMed] [Google Scholar]
- 29.Mulla ZD, Simons FE. Concomitant chronic pulmonary diseases and their association with hospital outcomes in patients with anaphylaxis and other allergic conditions: a cohort study. BMJ Open. 2013;3(7) doi: 10.1136/bmjopen-2013-003197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.England BR, Sayles H, Mikuls TR, Johnson DS, Michaud K. Validation of the Rheumatic Disease Comorbidity Index. Arthritis Care Res (Hoboken) 2014 doi: 10.1002/acr.22456. [DOI] [PubMed] [Google Scholar]
- 31.Li L, Greene T. A weighting analogue to pair matching in propensity score analysis. Int J Biostat. 2013;9(2):215–34. doi: 10.1515/ijb-2012-0030. [DOI] [PubMed] [Google Scholar]
- 32.Strand V, Cohen S, Schiff M, Weaver A, Fleischmann R, Cannon G, et al. Treatment of active rheumatoid arthritis with leflunomide compared with placebo and methotrexate. Leflunomide Rheumatoid Arthritis Investigators Group. Arch Intern Med. 1999;159(21):2542–50. doi: 10.1001/archinte.159.21.2542. [DOI] [PubMed] [Google Scholar]
- 33.Silva HT, Jr, Cao W, Shorthouse RA, Loffler M, Morris RE. In vitro and in vivo effects of leflunomide, brequinar, and cyclosporine on pyrimidine biosynthesis. Transplant Proc. 1997;29(1–2):1292–3. doi: 10.1016/s0041-1345(96)00523-4. [DOI] [PubMed] [Google Scholar]
- 34.Silva HT, Jr, Morris RE. Leflunomide and malononitriloamides. Expert Opin Investig Drugs. 1997;6(1):51–64. doi: 10.1517/13543784.6.1.51. [DOI] [PubMed] [Google Scholar]
- 35.Kremer JM. What I would like to know about leflunomide. J Rheumatol. 2004;31(6):1029–31. [PubMed] [Google Scholar]
- 36.Choi HK, Hernan MA, Seeger JD, Robins JM, Wolfe F. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet. 2002;359(9313):1173–7. doi: 10.1016/S0140-6736(02)08213-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Flow chart illustrating the selection of subjects into the final analysis cohort.
Supplementary Figure 2: Standardized difference for study variables between treatment groups compared to methotrexate unadjusted (diamonds) and adjusted using matched weighting based on the propensity score (squares). Separate figures are presented for a) leflunomide, b) prednisone, and c) TNFi.