Abstract
Neonatal innate immunity is distinct from that of adults, which may contribute to increased susceptibility to infection and limit vaccine responses. B cells play critical roles in protection from infection and detect PAMPs via TLRs, that, when co-activated with CD40, can drive B cell proliferation and antibody production. We characterized the expression of TLRs in circulating B cells from newborns and adults, and evaluated TLR- and CD40-mediated naïve B cell class-switch recombination (CSR) and cytokine production. Gene expression levels of most TLRs was similar between newborn and adult B cells, except newborn naïve B cells expressed more TLR9 than adult naïve B cells. Neonatal naïve B cells demonstrated impaired TLR2- and TLR7- but enhanced TLR9-mediated cytokine production. Significantly fewer newborn naïve B cells underwent CSR to produce IgG, an impairment also noted with IL-21 stimulation. Additionally, co-stimulation via CD40 and TLRs induced greater cytokine production in adult B cells. Thus, while newborn naïve B cells demonstrate adult-level expression of TLRs and CD40, the responses to stimulation of these receptors are distinct. Relatively high expression of TLR9 and impaired CD40-mediated Ig secretion contributes to distinct innate and adaptive immunity of human newborns and may inform novel approaches to early life immunization.
Keywords: B lymphocyte, TLR, cytokine, neonatal, class-switch recombination
Introduction
B cells play a critical role in protection from infectious diseases. While they are best known for their contribution to adaptive immunity via production of soluble immunoglobulins (antibodies, Igs or Abs), innate responses of B cells also impact the subsequent adaptive immune response.1 B cells express TLRs,2 a family (10 members in humans) of innate pattern-recognition receptors (PRRs) that recognize conserved microbial molecular patterns and initiate MyD88-dependent and -independent signaling events,3 and stimulation of B cell TLRs can induce cytokine production and influence their proliferation and activation.4 In particular, B cells express TLR9, which is responsive to CpG motif-rich bacterial DNA and to synthetic CpG DNA.5
While maternal Abs provide some protection from infection at birth, endogenous neonatal B cell responses to immunization and infection are often inadequate and as circulating levels of maternal IgG diminish with age (half-life ~21–30 days) this contributes to a general susceptibility to infection.6 Overall, innate immune responses of murine and human neonatal leukocytes such as monocytes and dendritic cells are different from adult counterparts,7, 8 with characteristic impaired inflammatory/Th1-polarizing cytokine (e.g., TNF and IL-12p70) production while anti-inflammatory cytokines, such as IL-10, are elevated.9–11
Multiple studies have described the ontogeny of murine B cell function.6 For example, murine neonatal B cell production of IL-10 suppresses pro-inflammatory responses of dendritic cells thereby inhibiting Th1-polarizing immune responses.12, 13 Immune responses can be species-specific, and relatively little is known regarding human neonatal B cell-mediated innate immune responses. Only limited data is available regarding the expression of TLRs on human neonatal B cells, and regarding the responsiveness of human neonatal B cells to TLR agonists. Two studies have reported that human cord blood B cells respond to CpG stimulation by producing cytokines and up-regulating genes that are involved in plasma cell differentiation.14, 15 Because TLR agonists may be used as vaccine adjuvants and characterization of age-specific TLR function may inform adjuvanted vaccine development, we sought to more completely characterize the expression of TLRs in human circulating B cells and B cell subsets from both newborn and adult subjects, and to evaluate TLR- mediated cytokine production and Ig class-switch recombination by circulating newborn (cord blood) and adult (peripheral blood) B cells. To characterize B cell ontogeny, our approach entailed comparing newborn and adult total naïve B cells, as this subset represents the vast majority of all neonatal B cells,16 and has been the subject of prior studies in this area.17–19 We have found that neonatal naïve B cells exhibit distinct functional expression of TLRs with relatively high TLR9 expression and TLR9-mediated cytokine induction and impaired CD40-mediated responses, including Ig production and class switching, providing fresh insights into the ontogeny and innate function of this key leukocyte population.
Materials and Methods
Blood collection
Peripheral blood was collected after informed consent from healthy adult volunteers according to Boston Children’s Hospital Institutional Review Board-approved protocols (Boston, MA, USA; mean age 32.4 years) and newborn cord blood (mean gestational age 39.1 weeks) was collected immediately after elective cesarean section delivery (epidural anesthesia) of the placenta. Births to HIV-positive mothers were excluded. Human experimentation guidelines of the US Department of Health and Human Services, the Brigham and Women’s Hospital, Beth Israel Medical Center, and Boston Children’s Hospital were observed, following protocols approved by the local institutional review boards. Number of repeats (n) indicates the number of independent experiments. No subject was studied more than once in each of the different experiments. Blood was collected into syringes containing a final concentration of 20 U/ml pyrogen-free heparin (Sagent Pharmaceuticals, Schaumberg, IL, USA), and processed within 2 hours of collection.
Fluorescence-activated Cell Sorting
Fluorescence-activated cell sorting (FACS) was utilized to acquire highly pure B cell subsets for analysis of TLR mRNA expression. Mononuclear cells were stained with antibodies targeting CD19 (APC-Cy7), CD24 (PE-Cy7), CD27 (PerCP-Cy5.5), CD38 (BV-605), and IgD (BV-421). Cells were sorted using a FACSAria II cell sorter (BD Biosciences). The gating strategy has been previously described 16. In brief, B cells were CD19+, and subsets were naïve B cells (IgD+, CD27−), early memory (IgD+, CD27+), class-switched memory (IgD−, CD27+, CD38+/−), and plasmablasts (IgD−, CD27+, CD38++). B cell subpopulations were sorted into tubes containing RPMI 1640 media (Invitrogen; Carlsbad, CA, USA) supplemented with 10% Fetal Bovine Serum (FBS, HyClone, VWR; Radnor, PA, USA), centrifuged at 500 g for 10 minutes, and cell pellets were resuspended in Buffer RLT (Qiagen GmbH; Hilden, Germany) for immediate RNA isolation. We seldom recovered sufficient adult plasmablast cells or any subset in newborn samples (save naïve) for subsequent workup, and they were not included in this analysis.
Mononuclear Cell Isolation and Magnetic Bead naïve B cell Isolation
Peripheral blood mononuclear cells (PBMC) or cord blood mononuclear cells (CBMC) were isolated from heparinized whole blood by Ficoll density gradient centrifugation. Naïve B cells were isolated by negative selection. Non-naïve B cells (CD27+ B cells, T cells, NK cells, monocytes, dendritic cells, granulocytes, platelets, and erythroid cells) were labeled with a cocktail of biotinylated CD2, CD14, CD16, CD27, CD36, CD43, and CD235a Abs and magnetically labeled with Anti-Biotin MicroBeads for depletion (Naïve B Cell Isolation Kit II, human, Miltenyi Biotec, Auburn, CA). To improve purity, the isolated naïve B cell fraction was subsequently labeled with CD19 microbeads (Miltenyi Biotec) for positive selection, resulting in highly pure naïve B cell populations. Only samples with naïve B cell purities of >90% were used for assays (average purity CD20+ IgD+ was 96% for adults and 95% for cord blood isolates), and cell isolates were also evaluated by flow cytometry (LSR Fortessa) to evaluate for specific impurities (CD27+ IgD+ cells <0.2%, CD27+ IgD− <0.04%, CD3+ and CD14+ both <0.6%, see flow cytometry methods section for more details). Flow cytometry analysis of a representative sample is shown in Supplementary Figure 1. Panel A indicates the percentage of CD20+ events, panel B the low frequencies of CD14+ and CD3+ events, panel C shows the percentage of CD20+ cells that are IgD+ and CD27-. For select experiments total CD19+ B cells were isolated from adult PBMC fractions utilizing CD19 microbeads (Miltenyi Biotec), for which the purity (CD20+) was always >90%, and the average specific subsets or impurities were: CD3+ 1.5% and CD14+ 0.7%.
Flow Cytometry
For analysis of TLR expression on B cell subsets, mononuclear cells were stained with CD19 (APC-Cy7), CD27 (PerCP-Cy5.5), CD38 (BV-605), IgD (BV-421) and TLR4 (FITC). For staining of intracellular TLRs, cells were subsequently fixated with 4% paraformaldehyde (Alfa Aesar; Ward Hill, MA, USA) in PBS for 30 minutes at 4°C. Fixated cells were washed in 1X BD Perm/Wash (BD Biosciences, Frederick, MD, USA) and stained with TLR7 (PE) and TLR9 (APC) in 1X Perm/Wash. Stained cells were washed with Perm/Wash and subsequently with PBS before measurement of fluorescent intensity on an LSR Fortessa (BD Biosciences; Frederick, MD, USA). For purity analysis of B cells isolated using magnetic beads, cells were stained with CD20 (PE-Cy7), CD3 (FITC), CD14 (APC-Cy7), CD27 (PerCP-Cy5.5), CD40 (APC), and IgD (BV-421) specific Abs, and subsequently resuspended in PBS with 1% paraformaldehyde before measurement of fluorescent intensity on an LSR Fortessa. Fluorescent intensities were analyzed using Flowjo software version 10 (Tree Star Inc; Ashland, OR, USA).
Cell Culture
Isolated B cell populations were plated in round-bottom 96-well plates (Corning, Tewksbury, MA, USA) in 100 μL of RPMI 1640 media (Invitrogen; Carlsbad, CA, USA) supplemented with 10% FBS (HyClone, VWR), and stimulated with indicated reagents prepared in an additional 100 μL of media to achieve the described reagent concentrations, and were incubated at 37°C in a 5% CO2 incubator for the indicated assay-specific amount of time before utilization.
RNA purification and cDNA synthesis
Total RNA was isolated from sorted B cell subpopulations using the RNeasy Mini Kit with RNase-free DNase treatment (Qiagen). Up to 500 ng of mRNA was reverse-transcribed to cDNA using the RT2 First-strand Kit (SABiosciences; Frederick, MD, USA), according to the manufacturer’s instructions.
qRT-PCR
Expression levels of selected genes were assessed by qRT-PCR analysis using an ABI 7300 real-time PCR system machine and software (Applied Biosystems; Foster City, CA, USA). The baseline adjustment method of the ABI 7300 software was used to determine the cycle threshold (Ct) in each reaction. A melting curve was constructed for each primer pair to verify the presence of one amplicon-specific peak and the absence of primer dimerization. All samples were amplified in triplicates and the mean was used for further analysis. Relative expression of TLR mRNA was compared to that of the “housekeeping” genes hypoxanthine phosphoribosyltransferase1 (HPRT1) and β-actin (ACTB) using the ΔΔ-Ct method. Relative comparison between populations was similar for each housekeeping gene, and results shown here are for comparison with HPRT1, the expression levels of which were more similar to the expression levels of TLR mRNA.
Reagents
The following commercially available reagents were used at the concentrations indicated in the figure legends: Heat-killed Listeria monocytogenes (HKLM, TLR2) R848 (TLR7/8), CpG (ODN 2395, TLR9) (InvivoGen; San Diego, CA), IL-2 (R&D Systems, Minneapolis; MN, USA), human recombinant CD40L (MegaCD40L, Enzo Life Sciences; Farmingdale, NY, USA), and recombinant human IL-21 (Cell Signaling; Danver, MA, USA).
B cell IgM and IgG ELISpot
At the end of the incubation period 96 well membrane plates (Cat# MSIPS4W, Millipore, Billerica, MA) were coated with Anti-human IgG or IgM Abs diluted in PBS (Cat# SELB002 – IgG, SELB003 – IgM, R&D systems, Minneapolis, MN). After 24 hour incubation at 4°C, the plates were blocked at room temperature with PBS and 1% BSA for 2 hours. Plates were washed with PBS and 104 (newborn IgM, adult IgM and adult IgG) or 105 (newborn IgG and adult IgG) B cells were added to duplicate wells and incubated at 37°C overnight (10–14 hours). Plates were subsequently washed 3 times with PBS/0.5%Tween and then 3 times with PBS. Kit Isotype-specific detection Abs were added and further incubated at 4°C for 24 hours. Plates were washed 3 times with PBS and streptavidin HRP (Invitrogen; Carlsbad, CA, USA) was added for 30 minutes at room temperature. After washing plates with PBS, 50 μL TMB (Cat# 3651-10, Mabtech, Cincinnati, OH, USA) substrate was added. When spots were clearly developed (15 seconds to 30 seconds), plates were washed quickly with distilled water. Spots were counted using an ELISpot plate reader (CTL, Shaker Heights, OH, USA). When spot counts were below the limits of detection, a value of ½ the lower limit of detection was assigned.
Cytokine measurement by multi-analyte fluorescent bead-based array
The cytokine profile of B cell culture supernatants was analyzed using multi-analyte bead array (Millipore; Billerica, MA, USA). Results were obtained with a MAGPIX system with Luminex xPONENT software (both from Luminex Corp.; Austin, TX, USA). Cytokine concentrations were determined using Milliplex Analyst (version 3.5.5.0, Millipore) software. When cytokines were below the limits of detection, a value of ½ the lower limit of detection for that particular cytokine was assigned.
Statistical analysis
The indicated statistical tests, as described in the Figure legends, were performed using GraphPad Prism version 5.0b for Mac.
Results
TLR expression by newborn and adult circulating B cells
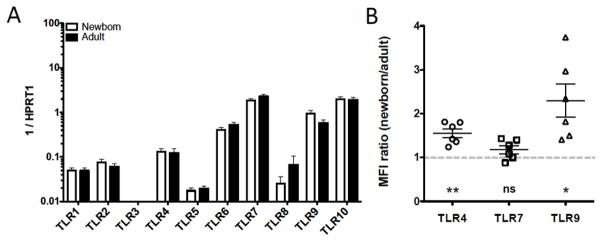
mRNAs encoding TLRs 1, 2, and 4–10 were detected in circulating naïve B cells, isolated by fluorescence-activated cell sorting, from both adult and newborn subjects – only TLR3 was not detected (Figure 1A). The highest levels of transcription were detected for TLRs -6, -7, -9 and -10, with moderate levels of TLRs -1, -2, and -4, and low levels of TLR5 and TLR8. There were no statistically significant differences in mRNA expression of any individual TLR between populations (newborn and adult), although there was a trend towards elevated TLR9 in neonatal naïve B cells compared to adult counterparts. Protein expression of select TLRs (4, 7, and 9) was also evaluated by flow cytometry, which indicated modestly elevated expression of TLR4 and TLR9 on newborn naïve B cells relative to adult counterparts (Figure 1B). Comparisons were made within each experiment (at least 2 adults each run) due to variable mean fluorescent intensity between experiments.
Figure 1.
TLR expression by adult and newborn circulating B cells. (A) naïve B cell TLR mRNA expression is similar in newborn subjects (N=7) relative to adults (N=9), no statistical significance, one-way ANOVA with Bonferroni post-test correction. (B) Flow cytometry evaluation of naïve B cell expression relative to adult subjects from the same experimental run (mean fluorescent intensity, N=6 each population) demonstrated greater TLR4 and TLR9, but similar TLR7 expression, in the newborn. Paired Student’s t-tests, * p<0.05, ** p<0.01, *** p<0.001.
Adult B cells were also sorted into subpopulations, and TLR mRNA expression evaluated revealing statistically significant increases in TLR4, TLR6, and TLR9 with maturation from naïve B cells to class-switched (IgD−) memory B cells (Supplementary Figure 2A). Protein expression of TLRs -4, -7, and -9, was also evaluated by flow cytometry on these subpopulations, which indicated increased expression for each with progression to IgD+ memory cells and IgD− memory cells (Supplementary Figure 2B).
TLR-mediated B cell cytokine production
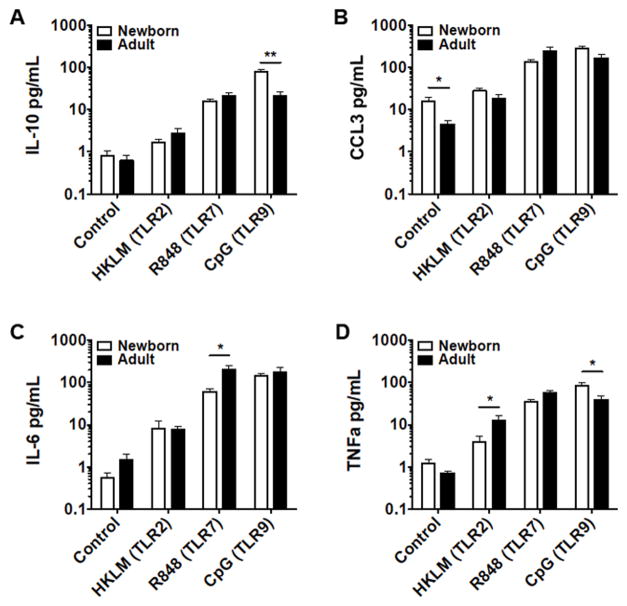
Newborn and adult naïve B cells and adult total CD19+ B cells isolated by magnetic bead assay were stimulated with agonists for TLR2 (HKLM), TLR7/8 (R848), TLR9 (CpG), a combination of R848 and CD40L, or vehicle control, and cultured for 24 hours in a CO2 incubator at 37° C before evaluation of culture supernatants for a panel of cytokines utilizing a multiplex assay. Results from naïve cells for IL-10, CCL3 (also known as MIP-1α), IL-6, and TNFα are shown in figure 2, while data for all measured cytokines under these conditions, including CXCL8 (IL-8), IP-10, GM-CSF, IFNα2, IFNγ, MCP-1, IL-12p40, IL-12p70 and IL-1β, are shown in Supplementary Tables 1–2. Following stimulation of TLR2 or TLR7, neonatal naïve B cells produced generally lower or similar levels of cytokines compared to adult counterparts, with significantly lower levels of IL-6 following TLR7 activation and lower levels of TNFα following TLR2 activation. In contrast, treatment with CpG (TLR9) elicited significantly higher levels of IL-10 and TNFα from newborn naïve B cells than from adult naïve B cells (Figure 2). Total CD19+ B cells (adult) secreted considerably higher levels of some cytokines (IL-6, IL-8, MCP-1, CCL3, and TNFα) than naïve B cells from the same subjects (supplementary tables 1–2).
Figure 2.
Distinct TLR-mediated cytokine production by adult and newborn circulating B cells. Naïve B cells were stimulated with HKLM (109 cells/mL), R848 (10 μM), CpG (1 μM), or vehicle control for 24 hours before evaluation of supernatant cytokines by multiplex assay. Neonatal B cells demonstrated impaired TLR2- and 7- but enhanced TLR9-mediated responses. N=6-8, Student’s t-tests, * p<0.05, ** p<0.01.
CD40 stimulation modulates B cell cytokine production
CD40 signaling plays a critical role in B cell biology, particularly for induction of class-switch recombination. Accordingly, we next evaluated how CD40 stimulation influenced TLR-mediated cytokine production from naïve B cells. CD40L caused significantly enhanced production of IL-10, IL-6, and TNFα during TLR7/8 stimulation with R848 (Figure 3), although to a lesser extent in newborn naïve B cells than in adult naïve B cells, suggesting differences in CD40 signaling between these populations.
Figure 3.
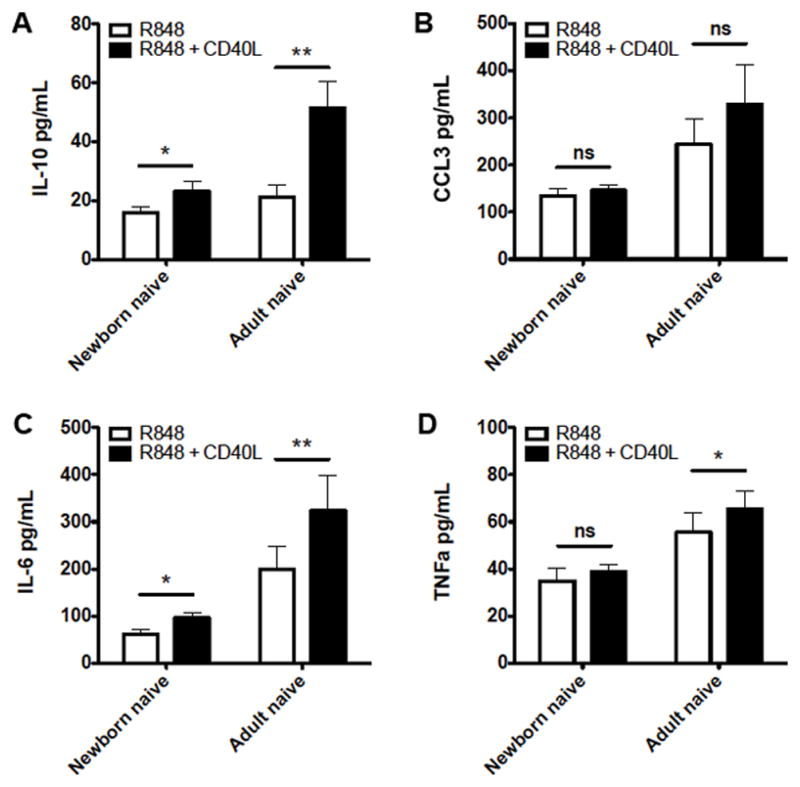
Impaired CD40 enhancement of cytokine production in newborn circulating B cells. Naïve newborn and adult B cells were stimulated with R848 (10 μM), or R848 plus recombinant CD40L (20 ng/mL), for 24 hours before evaluation of supernatant cytokines by multiplex assay. N=6-8, Student’s t-tests, * p<0.05, ** p<0.01.
Circulating newborn naïve B cells are impaired in CD40/TLR-mediated CSR
TLR stimulation, in combination with CD40 activation, can lead to B cell Ig CSR, even in the absence of B cell receptor (BCR) triggering.20–22 Naïve B cells from both newborn cord blood and adult peripheral blood were stimulated with soluble CD40L and IL-2 (to simulate T-cell help) in conjunction with select TLR agonists in culture for 80 hours, after which supernatants were evaluated for cytokine production (by multiplex assay) and cells were re-plated for ELISpot assay to enumerate IgM and IgG secreting cells. The profile of cytokine production following stimulation of both CD40 and TLRs for several days in culture was similar to that observed with only TLR stimulation, with the exceptions that stimulation of CD40 in conjunction with TLR9 stimulation promoted IL-10 production in adult naïve B cells to levels similar to that observed in newborn naïve B cells (Figure 4), and that for some of these conditions we detected production of additional cytokines, including IFNγ, IL-12p40, and IL-12p70 (Supplementary Tables 1–2). Data for other cytokines produced under these conditions are shown in Supplementary Tables 1–2, including data for both newborn and adult naïve B cell isolates and adult total CD19+ B cell isolates. Supplementary Figure 3 provides an overview of the cytokine secretion patterns as a fold-increase over control conditions.
Figure 4.
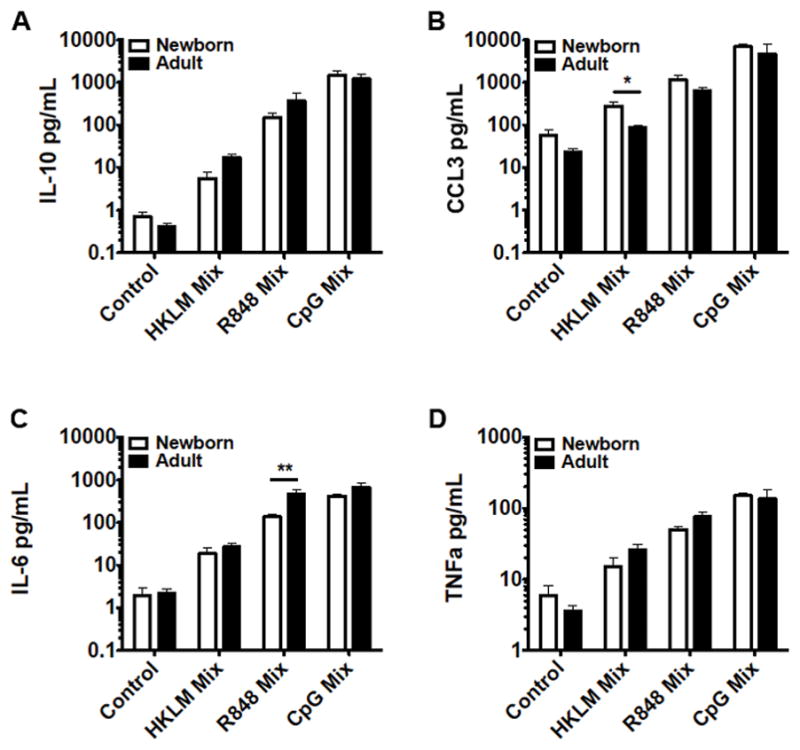
Neonatal naïve B cells demonstrate distinct TLR/CD40-mediated cytokine production. Naïve B cells were stimulated with the following: control condition, IL-2 (30 ng/mL), HKLM condition, IL-2, CD40L (20 ng/mL), and HKLM (109 cells/mL), R848 condition, IL-2, CD40L, and R848 (10 μM), CpG condition, IL-2, CD40L, and CpG (1 μM). Cells were cultured with stimulation for 80 hours before evaluation of supernatant cytokines by multiplex assay. N=3-5 for HKLM condition, N=6-8 for control, R848, and CpG conditions. Student’s t-tests, * p<0.05, ** p<0.01.
CSR to yield IgG secreting B cells with dual CD40 and TLR stimulation was significantly impaired in neonatal naïve B cells, as compared to adult naïve B cells (Figure 5). The percentage of cells secreting IgG was significantly lower for newborn naïve B cells relative to adult counterparts for both TLR7/8/CD40-stimulating and TLR9/CD40-stimulating conditions. Newborn naïve B cells exhibited significantly greater IgG CSR in response to TLR9/CD40 stimulation than for TLR7/8/CD40 stimulation. Dual CD40 and TLR stimulation induced similar numbers of IgM-secreting cells in both populations, with the exception that a higher percentage of newborn naïve B cells secreted IgM in response to TLR2/CD40 stimulation. As expected, adult total CD19+ B cells, which include CD27+ memory B cells, yielded a higher percentage of IgG-secreting cells under all conditions. Because this population was not included for newborn samples, the adult total B cell data were not compared to other populations in a statistical analysis.
Figure 5.
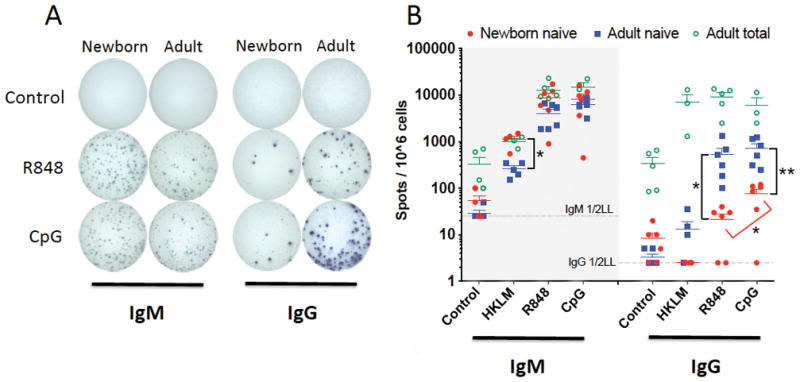
Deficient TLR/CD40-mediated antibody class-switch recombination in newborn naïve B cells tested in vitro. Naïve B cells, or total B cells (adult) were stimulated with the following; control condition, IL-2 (30 ng/mL), HKLM condition, IL-2, CD40L (20 ng/mL), and HKLM (109 cells/mL), R848 condition, IL-2, CD40L, and R848 (10 μM), CpG condition, IL-2, CD40L, and CpG (1 μM). Cells were cultured with stimulation for 80 hours before cells were transferred to ELISpot assay plates (104 cells per well for IgM, 105 for IgG). (A) IgG and IgM spots from naïve B cells for 1 assay representative of 6, with stimulation conditions as indicated above. (B) Quantification of IgM (left) and IgG (right) producing cells. N=3-5 for adult total B cells, N=3-5 for naïve B cell HKLM condition, N=6 for naïve B cell control, R848, and CpG conditions. 2-tailed, unpaired Student’s t-tests for comparison between populations (adult/newborn), paired tests for different stimuli within population, * p<0.05, ** p<0.01.
As production of IL-21 by T follicular helper (Tfh) cells can enhance CSR and plasma cell differentiation during T cell-dependent B cell responses,23, 24 we evaluated the effect of addition of IL-21 in vitro. Addition of recombinant human IL-21 appeared to further enhance CSR in response to TLR9/CD40 or TLR7/8/CD40 stimulation in both newborns and adults. However, even in the presence of IL-21, the percentage of cells secreting IgG was significantly lower for newborn naïve B cells relative to adult counterparts (p <0.01). Stimulation of newborn naïve B cells with TLR agonists also resulted in less up-regulation of activation markers CD80 and CD86, especially in the presence of IL-21.
Discussion
Herein we compared for the first time the functional expression of TLRs on subsets of circulating CD27− naïve and CD27+ memory B cells of human newborns and adults. Although expression of most TLRs was quite similar for naïve B cells from newborns and adults, TLR9 expression was greater in newborn naïve B cells. Moreover, we noted significant differences with age related to TLR-mediated cytokine induction and the capacity to transition to IgG secretion.
TLRs are critical regulators of B cell function, and TLR ligation can serve as a co-stimulatory signal in B cell activation.25 TLR expression has been evaluated by qRT-PCR of total B cells from human adult peripheral blood isolated by indirect magnetic bead isolation,26 and by PCR of flow-sorted adult peripheral blood B cell subsets with qPCR of TLRs 2, 4, 7, and 9.2 These studies came to distinct conclusions, with the latter finding “low to undetectable” expression of all of the TLRs in naïve B cells.2 Our study has the advantages of utilizing flow-sorted cells (based on IgD, CD27, and CD38 expression levels) for greater purity and subset analysis, qRT-PCR for quantification of TLR expression on B cell subsets, and a relatively higher number of study participants (N=9 per age group). While TLR expression on cord blood T cells and monocytes has been characterized,27 expression of TLRs1-10 in circulating B cells from cord blood has not previously been evaluated. In this study, we characterized TLR mRNA expression for newborn cord naïve B cells and circulating adult B cell subsets, including naïve, early memory and class-switched memory B cells. TLRs 6, 7, 9, and 10 were highly expressed on newborn and adult naïve B cells, TLRs 1, 2, and 4 showed moderate expression and very low levels of TLR5 and 8 expression were observed. Of note, while heat-killed Listeria monocytogenes activates via TLR2, live L. monocytogenes expresses a ligand of TLR10,28 which is one of the mostly highly expressed TLRs on human B cells. On the protein level, newborn naïve B cells display greater TLR4 and TLR9 expression compared to adults. Adult memory B cells subsets demonstrated elevated mRNA for TLR7 and elevated mRNA and protein TLR9 expression compared to adult naïve B cells. Elevated TLR9 expression in adult memory B cell subsets is in agreement with prior studies.2 Overall, differences in TLR expression likely do not account for all of the distinct responsiveness to TLR stimulation between adult and newborn circulating naïve B cells. Additional features newborn B cells that may contribute to their distinct response to TLR stimulation includes impairment in purine salvage due to diminished levels of CD73.29
We also evaluated TLR-mediated cytokine production by B cells in this study, both for B cells stimulated with single TLR agonists for 24 hours (Supplementary Table 1) and for B cells treated with a combination of TLR agonists, CD40L and IL-2 for 80 hours (Supplementary Table 2). Newborn naïve B cells produced significantly greater levels of IL-10 following stimulation with CpG (TLR9) than adult naïve B cells, while producing similar levels following stimulation of TLR2 or TLR4. IL-10 production was enhanced by dual stimulation of TLR7/8 and CD40 in both newborn and adult naïve B cells, although to a greater degree in adult cells than newborn. IL-10 production by B cells can suppress inflammation,12, 13 and murine B cell IL-10 production regulates neonatal dendritic cell function,13 an activity that has earned them the name B regulatory cells 30. Of note, naïve adult B cells demonstrate greater IL-10 production in response to CD40 stimulation than memory cells. CCL3 (formerly known as “MIP-1α”) is a T cell chemoattractant that modulates activity of a variety of leukocytes, and is produced by B cells following BCR engagement, but not following CD40 stimulation.31 CCL3 concentrations were moderately up-regulated following TLR7 or TLR9 stimulation in our assay, and were higher in control naïve B cell supernatants from newborns relative to adults.
TNFα production by murine B cells plays a critical role in maturation of follicular dendritic cells,32 and is involved in murine B cell mediated upregulation of IFNγ from T cells.33 We detected TNFα production by human B cells, which varied between newborn and adult populations and in a TLR-specific manner. Neonatal naïve B cells demonstrated less TLR1-2 (Pam3CSK4)-mediated TNFα but higher TLR9 (CpG)-mediated TNFα compared to adult counterparts. IFNγ, IL-12p40 and IL-12p70 production were detected only at low levels with 24 hours of TLR stimulation, but were increased after 80 hours of culture with stimulation of both TLRs and CD40. Production of IL-12p40 by human B cells is enhanced by CD40 ligation.34, 35 The relatively high neonatal B cell expression of TLR9 coupled with greater TLR9-mediated cytokine production is notable, particularly in relation to studies of newborn mice wherein TLR9 agonists such as CpG markedly enhanced responses to vaccinal antigens such as HBV.36
We noted a marked impairment in the ability of neonatal B cells to class-switch to IgG in response to combined TLR and CD40 signaling. Newborn naïve B cells demonstrated an equal if not greater ability to secrete IgM, but significantly impaired capacity to undergo CSR and secrete IgG. Impurity of CD27+ cells in these assays is unlikely to account for the differences observed, as the level of CD27+ B cells was slightly higher for the newborn B cell isolates, and did not correlate with the percentage of IgG-secreting cells. Previous reports have demonstrated impaired IgG and IgA secretion in vitro by newborn total B cells relative to adult total B cells following CpG stimulation 37, or dual pokeweed mitogen (PWM)/donor CD4 T cell stimulation,38 although comparisons were among total B cell fractions, and adult samples would have contained significantly more memory B cells. We detected no differences in CD40 expression between newborn and adult B cells (data not shown), in agreement with other studies.39 Despite adult-level CD40 expression, neonatal B cells demonstrated diminished CD40 responsiveness, including limited ability of CD40L to enhance newborn B cell cytokine production, as previously noted 40 Of note, neonatal T cells express very low levels of CD40L,40, 41 suggesting another mechanism whereby capacity to undergo CSR may be impaired in vivo.
Although we provide novel human data with fresh insights into ontogeny and potential translational implications, our study does have limitations. Firstly, as vaccine adjuvants are administered together with a target antigen, further studies will be required to characterize neonatal B cells responses to candidate adjuvants in the context of vaccinal antigen. Secondly, naive B cells from newborns contain more immature or transitional (CD24hi/CD38hi) B cells than adult naïve B cells,16 such that differences would be expected when directly comparing newborn and adult naïve B cells. Our approach was selected in order to further characterize neonatal B cells, including functional TLR expression, in the context of prior work,14, 15, 42 and to model neonatal naïve B cell responses to TLR agonists that may serve as vaccine adjuvants,43, 44 as any of the neonatal naïve B cell subsets may respond, and indeed may affect one another. Indeed, the differences we report, including robust neonatal naïve B cell functional expression of TLR9, were not predictable from the relative proportion of immature B cells.
While the development of new vaccine adjuvants is a growing field,45 less attention has been given to human B cell responses to adjuvants. Of note, the magnitude and persistence of Ab responses against pandemic H1N1 influenza in Rhesus macaques may involve direct triggering of TLRs on B cells and dendritic cells, as well as on T-cell help.46 Additionally, components of subunit vaccines, such as pneumococcal conjugate vaccine polysaccharides,47 while directly interacting with B cells, may lack the adjuvant activity to induce and optimally shape an immune response in early or later life. Indeed, most infant vaccines require multiple doses to achieve protective Ab titers. Accordingly, increased appreciation of B cell immune ontogeny may inform development of rationally designed age-specific vaccine formulations, which may include adjuvants that more effectively enhance B cell responses in early life potentially providing dose-sparing effects or possibly even single shot protection. TLR9 stimulation enhances humoral immune responses in both neonatal and adult mice,36, 48, 49 as well as in human clinical trials.50, 51 TLR7/8 agonists may also be effective at enhancing Ab responses indirectly through activation of other antigen-presenting cells that subsequently influence B cell activation.46 To the extent that our in vitro studies of human newborn B cells reflect their potential responses in vivo, our current work suggests that TLR9 agonists may be particularly effective in activating human newborn and adult B cells for vaccination strategies wherein enhanced Ab production is desirable.
Supplementary Material
Figure 6.
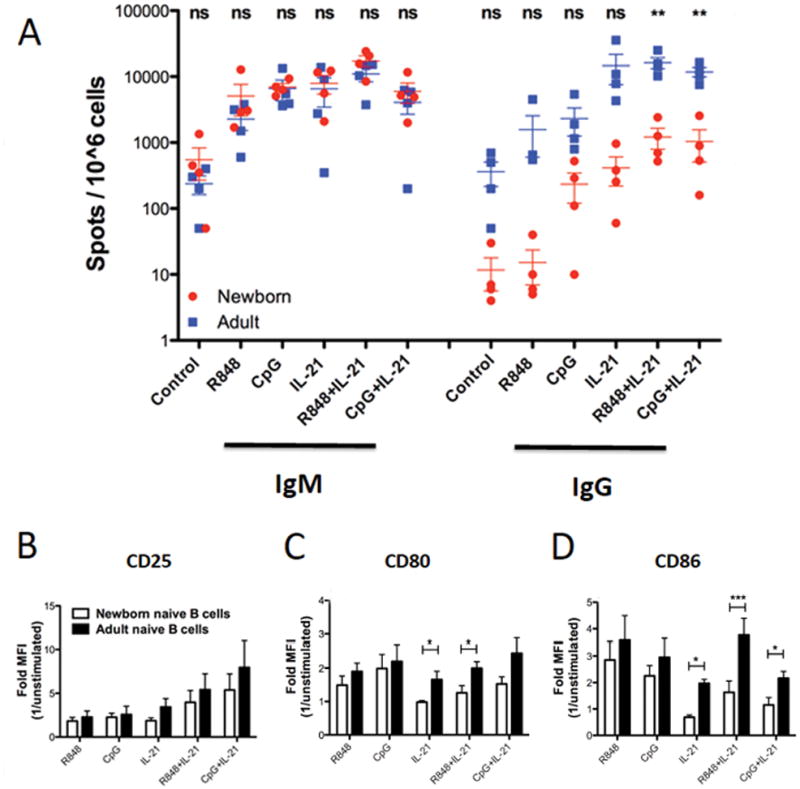
Neonatal naïve B cells demonstrate impaired IL-21 induced class-switching and expression of co-stimulatory receptors CD80 and CD86. Naïve B cells were stimulated with IL-2 (30 ng/mL) and CD40L (20 ng/mL) alone, or in combination with R848 (10 μM), CpG (1 μM) and/or IL-21 (50 ng/ml). Cells were cultured with stimulation for 80 hours before cells were transferred to ELISpot assay plates (104 cells per well for IgM, 105 for IgG). (A) IgG and IgM spots from naïve B cells, with stimulation conditions as indicated above. (B-D) Quantification of surface expression of CD25, CD80 and CD86 by flow cytometry. Fluorescence intensities are shown as MFI fold increase over the control condition. N=4 for all conditions. 2-tailed, unpaired Student’s t-tests for comparison between adults and newborns is indicated. * p<0.05, ** p<0.01, *** p<0.001
Acknowledgments
OL’s laboratory is supported by an internal Boston Children’s Hospital award to the Precision Vaccines Program, Global Health (OPPGH5284) and Grand Challenges Explorations (OPP1035192) awards from the Bill & Melinda Gates Foundation and by NIH grants 1R01AI100135-01, 3R01AI067353-05S1, U01AI124284-01 as well as National Institute of Allergy & Infectious Diseases Adjuvant Discovery Program, Contract No. HHSN272201400052C. MP was supported by NIH Training Grant T32 HD055148. SvH was supported by an Early career Award from the Thrasher Research Fund. We thank the Labor and Delivery staff at The Brigham & Women’s Hospital, Boston, MA and Beth Israel Deaconess Medical Center Boston, MA for their kind assistance with sample acquisition. Drs. Michael Wessels and Gary Fleisher are thanked for their support of the Precision Vaccines Program.
Abbreviations
- ACTB
β-actin
- BSA
bovine serum albumin
- CBMC
cord blood mononuclear cell
- CpG
bacterial unmethylated C-phosphodiester-G DNA motif
- CSR
class-switch recombination
- Ct
cycle threshold
- FACS
fluorescence-activated cell sorting
- HIV
human immunodeficiency virus
- HKLM
heat-killed Listeria monocytogenes
- HPRT1
hypoxanthine phosphoribosyltransferase 1
- HRP
horseradish peroxidase
- IL-10
interleukin 10
- IL-12
interleukin 12
- ODN
oligodeoxynucleotide
- TMB
3,3′,5,5′-Tetramethylbenzidine
- TNFSF
tumor necrosis factor super family
- qRT-PCR
quantitative real-time polymerase chain reaction
Footnotes
Authorship
MP designed the study, MP, IB, and SvH carried out the experiments and wrote the manuscript. JJ and LN assisted with experiments and helped write the manuscript. DD and GF provided intellectual input and helped write the manuscript. OL assisted in design of the study, reviewed and helped interpret the data, and helped write the manuscript.
Conflict of Interest Disclosure
OL’s laboratory has received sponsored research support from Crucell (Johnson & Johnson), MedImmune and 3M Drug Delivery Systems, companies that develop adjuvants and/or vaccines.
References
- 1.Lund FE. Cytokine-producing B lymphocytes-key regulators of immunity. Curr Opin Immunol. 2008;20:332–8. doi: 10.1016/j.coi.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernasconi NL, Onai N, Lanzavecchia A. A role for Toll-like receptors in acquired immunity: up-regulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood. 2003;101:4500–4. doi: 10.1182/blood-2002-11-3569. [DOI] [PubMed] [Google Scholar]
- 3.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–84. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 4.Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438:364–8. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 5.He B, Qiao X, Cerutti A. CpG DNA induces IgG class switch DNA recombination by activating human B cells through an innate pathway that requires TLR9 and cooperates with IL-10. J Immunol. 2004;173:4479–91. doi: 10.4049/jimmunol.173.7.4479. [DOI] [PubMed] [Google Scholar]
- 6.Siegrist CA, Aspinall R. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol. 2009;9:185–94. doi: 10.1038/nri2508. [DOI] [PubMed] [Google Scholar]
- 7.Dowling DJ, Levy O. Ontogeny of early life immunity. Trends Immunol. 2014;35:299–310. doi: 10.1016/j.it.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7:379–90. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 9.Belderbos ME, Levy O, Stalpers F, Kimpen JL, Meyaard L, Bont L. Neonatal plasma polarizes TLR4-mediated cytokine responses towards low IL-12p70 and high IL-10 production via distinct factors. PLoS One. 2012;7:e33419. doi: 10.1371/journal.pone.0033419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levy O, Coughlin M, Cronstein BN, Roy RM, Desai A, Wessels MR. The adenosine system selectively inhibits TLR-mediated TNF-alpha production in the human newborn. J Immunol. 2006;177:1956–66. doi: 10.4049/jimmunol.177.3.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levy O, Suter EE, Miller RL, Wessels MR. Unique efficacy of Toll-like receptor 8 agonists in activating human neonatal antigen-presenting cells. Blood. 2006;108:1284–90. doi: 10.1182/blood-2005-12-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker WE, Goldstein DR. Neonatal B cells suppress innate toll-like receptor immune responses and modulate alloimmunity. J Immunol. 2007;179:1700–10. doi: 10.4049/jimmunol.179.3.1700. [DOI] [PubMed] [Google Scholar]
- 13.Sun CM, Deriaud E, Leclerc C, Lo-Man R. Upon TLR9 signaling, CD5+ B cells control the IL-12-dependent Th1-priming capacity of neonatal DCs. Immunity. 2005;22:467–77. doi: 10.1016/j.immuni.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Capolunghi F, Cascioli S, Giorda E, et al. CpG drives human transitional B cells to terminal differentiation and production of natural antibodies. J Immunol. 2008;180:800–8. doi: 10.4049/jimmunol.180.2.800. [DOI] [PubMed] [Google Scholar]
- 15.Tasker L, Marshall-Clarke S. Functional responses of human neonatal B lymphocytes to antigen receptor cross-linking and CpG DNA. Clin Exp Immunol. 2003;134:409–19. doi: 10.1111/j.1365-2249.2003.02318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morbach H, Eichhorn EM, Liese JG, Girschick HJ. Reference values for B cell subpopulations from infancy to adulthood. Clin Exp Immunol. 2010;162:271–9. doi: 10.1111/j.1365-2249.2010.04206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu YC, Kipling D, Dunn-Walters DK. The relationship between CD27 negative and positive B cell populations in human peripheral blood. Front Immunol. 2011;2:81. doi: 10.3389/fimmu.2011.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huggins J, Pellegrin T, Felgar RE, et al. CpG DNA activation and plasma-cell differentiation of CD27- naive human B cells. Blood. 2007;109:1611–9. doi: 10.1182/blood-2006-03-008441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bastian JF, Ruedi JM, MacPherson GA, Golembesky HE, O’Connor RD, Thompson LF. Lymphocyte ecto-5′-nucleotidase activity in infancy: increasing activity in peripheral blood B cells precedes their ability to synthesize IgG in vitro. J Immunol. 1984;132:1767–72. [PubMed] [Google Scholar]
- 20.Jain S, Chodisetti SB, Agrewala JN. CD40 signaling synergizes with TLR-2 in the BCR independent activation of resting B cells. PLoS One. 2011;6:e20651. doi: 10.1371/journal.pone.0020651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozcan E, Rauter I, Garibyan L, Dillon SR, Geha RS. Toll-like receptor 9, transmembrane activator and calcium-modulating cyclophilin ligand interactor, and CD40 synergize in causing B-cell activation. J Allergy Clin Immunol. 2011;128:601–9. e1–4. doi: 10.1016/j.jaci.2011.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pone EJ, Lou Z, Lam T, et al. B cell TLR1/2, TLR4, TLR7 and TLR9 interact in induction of class switch DNA recombination: Modulation by BCR and CD40, and relevance to T-independent antibody responses. Autoimmunity. 2015;48:1–12. doi: 10.3109/08916934.2014.993027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ettinger R, Sims GP, Fairhurst AM, et al. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J Immunol. 2005;175:7867–79. doi: 10.4049/jimmunol.175.12.7867. [DOI] [PubMed] [Google Scholar]
- 24.Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annual review of immunology. 2008;26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- 25.Bekeredjian-Ding I, Jego G. Toll-like receptors--sentries in the B-cell response. Immunology. 2009;128:311–23. doi: 10.1111/j.1365-2567.2009.03173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hornung V, Rothenfusser S, Britsch S, et al. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–7. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 27.Dasari P, Zola H, Nicholson IC. Expression of Toll-like receptors by neonatal leukocytes. Pediatr Allergy Immunol. 2011;22:221–8. doi: 10.1111/j.1399-3038.2010.01091.x. [DOI] [PubMed] [Google Scholar]
- 28.Regan T, Nally K, Carmody R, et al. Identification of TLR10 as a key mediator of the inflammatory response to Listeria monocytogenes in intestinal epithelial cells and macrophages. J Immunol. 2013;191:6084–92. doi: 10.4049/jimmunol.1203245. [DOI] [PubMed] [Google Scholar]
- 29.Pettengill MA, Levy O. Circulating Human Neonatal Naive B Cells are Deficient in CD73 Impairing Purine Salvage. Front Immunol. 2016;7:121. doi: 10.3389/fimmu.2016.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lo-Man R. Regulatory B cells control dendritic cell functions. Immunotherapy. 2011;3:19–20. doi: 10.2217/imt.11.34. [DOI] [PubMed] [Google Scholar]
- 31.Krzysiek R, Lefevre EA, Zou W, et al. Antigen receptor engagement selectively induces macrophage inflammatory protein-1 alpha (MIP-1 alpha) and MIP-1 beta chemokine production in human B cells. J Immunol. 1999;162:4455–63. [PubMed] [Google Scholar]
- 32.Endres R, Alimzhanov MB, Plitz T, et al. Mature follicular dendritic cell networks depend on expression of lymphotoxin beta receptor by radioresistant stromal cells and of lymphotoxin beta and tumor necrosis factor by B cells. J Exp Med. 1999;189:159–68. doi: 10.1084/jem.189.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menard LC, Minns LA, Darche S, et al. B cells amplify IFN-gamma production by T cells via a TNF-alpha-mediated mechanism. J Immunol. 2007;179:4857–66. doi: 10.4049/jimmunol.179.7.4857. [DOI] [PubMed] [Google Scholar]
- 34.Gagro A, Servis D, Cepika AM, et al. Type I cytokine profiles of human naive and memory B lymphocytes: a potential for memory cells to impact polarization. Immunology. 2006;118:66–77. doi: 10.1111/j.1365-2567.2006.02342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schultze JL, Michalak S, Lowne J, et al. Human non-germinal center B cell interleukin (IL)-12 production is primarily regulated by T cell signals CD40 ligand, interferon gamma, and IL-10: role of B cells in the maintenance of T cell responses. J Exp Med. 1999;189:1–12. doi: 10.1084/jem.189.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brazolot Millan CL, Weeratna R, Krieg AM, Siegrist CA, Davis HL. CpG DNA can induce strong Th1 humoral and cell-mediated immune responses against hepatitis B surface antigen in young mice. Proc Natl Acad Sci U S A. 1998;95:15553–8. doi: 10.1073/pnas.95.26.15553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Landers CD, Bondada S. CpG oligodeoxynucleotides stimulate cord blood mononuclear cells to produce immunoglobulins. Clin Immunol. 2005;116:236–45. doi: 10.1016/j.clim.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 38.Tsuji T, Nibu R, Iwai K, et al. Efficient induction of immunoglobulin production in neonatal naive B cells by memory CD4+ T cell subset expressing homing receptor L-selectin. J Immunol. 1994;152:4417–24. [PubMed] [Google Scholar]
- 39.Elliott SR, Roberton DM, Zola H, Macardle PJ. Expression of the costimulator molecules, CD40 and CD154, on lymphocytes from neonates and young children. Hum Immunol. 2000;61:378–88. doi: 10.1016/s0198-8859(99)00189-5. [DOI] [PubMed] [Google Scholar]
- 40.Durandy A, De Saint Basile G, Lisowska-Grospierre B, et al. Undetectable CD40 ligand expression on T cells and low B cell responses to CD40 binding agonists in human newborns. J Immunol. 1995;154:1560–8. [PubMed] [Google Scholar]
- 41.Brugnoni D, Airo P, Graf D, et al. Ineffective expression of CD40 ligand on cord blood T cells may contribute to poor immunoglobulin production in the newborn. Eur J Immunol. 1994;24:1919–24. doi: 10.1002/eji.1830240831. [DOI] [PubMed] [Google Scholar]
- 42.Landers CD, Bondada S. CpG oligodeoxynucleotides stimulate cord blood mononuclear cells to produce immunoglobulins. Clin Immunol. 2005;116:236–45. doi: 10.1016/j.clim.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 43.Seder R, Reed SG, O’Hagan D, et al. Gaps in knowledge and prospects for research of adjuvanted vaccines. Vaccine. 2015;33(Suppl 2):B40–3. doi: 10.1016/j.vaccine.2015.03.057. [DOI] [PubMed] [Google Scholar]
- 44.Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat Med. 2013;19:1597–608. doi: 10.1038/nm.3409. [DOI] [PubMed] [Google Scholar]
- 45.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kasturi SP, Skountzou I, Albrecht RA, et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011;470:543–7. doi: 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Avci FY, Li X, Tsuji M, Kasper DL. A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nat Med. 2011;17:1602–9. doi: 10.1038/nm.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kovarik J, Bozzotti P, Love-Homan L, et al. CpG oligodeoxynucleotides can circumvent the Th2 polarization of neonatal responses to vaccines but may fail to fully redirect Th2 responses established by neonatal priming. J Immunol. 1999;162:1611–7. [PubMed] [Google Scholar]
- 49.Weeratna RD, Brazolot Millan CL, McCluskie MJ, Siegrist CA, Davis HL. Priming of immune responses to hepatitis B surface antigen in young mice immunized in the presence of maternally derived antibodies. FEMS Immunol Med Microbiol. 2001;30:241–7. doi: 10.1111/j.1574-695X.2001.tb01577.x. [DOI] [PubMed] [Google Scholar]
- 50.Cooper CL, Angel JB, Seguin I, Davis HL, Cameron DW. CPG 7909 adjuvant plus hepatitis B virus vaccination in HIV-infected adults achieves long-term seroprotection for up to 5 years. Clin Infect Dis. 2008;46:1310–4. doi: 10.1086/533467. [DOI] [PubMed] [Google Scholar]
- 51.Cooper CL, Davis HL, Morris ML, et al. CPG 7909, an immunostimulatory TLR9 agonist oligodeoxynucleotide, as adjuvant to Engerix-B HBV vaccine in healthy adults: a double-blind phase I/II study. J Clin Immunol. 2004;24:693–701. doi: 10.1007/s10875-004-6244-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.