Abstract
BACKGROUND
Alzheimer’s disease is a neurodegenerative brain disease that causes cognitive impairment and dementia. Within the United States, Alzheimer’s disease is the most common form of dementia in the elderly, affecting 1 in 10 people over the age of 65. Sleep disturbance has been called a ‘public health epidemic’ and, like depression, is a prodromal symptom of AD but may also contribute to the risk of developing AD. It was hypothesized that sleep disturbance, depression, and the apolipoprotein E (APOE) genotype increase the likelihood of Alzheimer’s disease.
METHODS
Utilizing data from the National Alzheimer’s Coordinating Center, information from evaluations of 11,453 cognitively asymptomatic participants was analyzed. Survival analysis was used to explore the independent relationships between depression, sleep disturbance, and APOE genotypes with eventual Alzheimer’s disease diagnosis. . Cox proportional hazard models were utilized to explore the main effects and synergistic effects of psychosocial factors as moderated by APOE genotypes.
RESULTS
This study reinforced the association between APOE and Alzheimer’s disease. The hazard of developing Alzheimer’s disease was eight times higher for those with recent depression and the ε4 homozygote (HR=8.15 [3.70-17.95]). Among ε4 carriers with clinician-verified depression, the hazard was ten times that of the reference group (HR=10.11 [4.43-23.09]). The hazard for ε4 carriers reporting sleep disturbance was almost 7 times greater than the reference group (HR=6.79 [2.38-19.37]).
CONCLUSION
Findings suggest that sleep disturbance, depression, and APOE ε4 genotype are associated with Alzheimer’s disease during follow-up evaluations among a group of initially cognitively asymptomatic participants. This study contributes to the literature base exploring an increased hazard or risk of Alzheimer’s disease due to potential modifiable risk factors as well as genetic biomarkers, such as APOE.
Keywords: Alzheimer’s disease, dementia, depression, sleep disturbance, apolipoprotein e
Alzheimer’s disease dementia (AD) is a neurodegenerative brain disease that causes cognitive impairment and dementia. The neurodegeneration in the brain eventually manifests as clinically evident deficits in memory, thinking, and behavior (Meng & D’Arcy, 2013). While clinical symptoms may become apparent as the disease progresses, research indicates that AD lacks a clear time of onset; it is therefore conceptualized more aptly as a “pathophysiological disease progression” (Sperling et al., 2011, p. 282). Perhaps 20% to 30% of older adults who are clinically asymptomatic may possess pathophysiological features of AD as much as 20 years before cognitive symptom onset (Bateman et al., 2012). Additionally, many who are eventually diagnosed with AD dementia encounter a stage of mild cognitive impairment prior to receiving a diagnosis of dementia due to AD pathology (McKhann et al., 2011).
Alzheimer’s disease dementia currently affects more than 39.9 million people worldwide. In 2015, it is expected that 473,000 people will be diagnosed with AD in the United States (Alzheimer’s Association, 2015); that number is expected to triple over the next 40 years (Barnes & Yaffe, 2011). In the United States, there were 5.4 million cases of AD in 2013, and 10 percent of those cases were in people over the age of 65 (Donix, Small, & Bookheimer, 2012). In addition, 32% of Americans 85 and older are diagnosed with AD dementia (CDC, 2013). Currently, there is no cure for AD, and while the mortality rates in other disease groups from 2000 to 2010 have decreased, such as heart disease by 16%, stroke by 23%, and prostate cancer by 8%, AD mortality has increased by 68% (Thies & Bleiler, 2013).
Age is the strongest predictor of AD dementia, irrespective of one’s ethnic background (Fortune, Lang, Cook, & Byrd, 2013). Similarly, there are differences in prevalence based on ethnic background: non-Hispanic Whites have higher mortality rates than non-Hispanic African Americans and those of Hispanic descent. (CDC, 2013). A predisposition to vascular disease has been posited as one possible explanation of the disparity by race. Vascular risk factors, such as diabetes mellitus, hypertension and high cholesterol have been hypothesized to act as independent and aggregate risk factors for AD dementia in epidemiologic studies (Reitz et al., 2010; Qiu, Xu, Winblad, and Fratiglioni, 2010). Accounts regarding the exact vascular risk for AD development in the literature remain conflicted (Chui, Zheng, Reed, Vinters, and Mack, 2012). Lifetime risk of AD due to dementia is also strongly predicted by sex; women have a 30% higher risk of dying from AD than men, owing in part to the longer life span of women (CDC, 2013).
Investigations are ongoing regarding the role of depression in AD development. Depression is a neuropsychiatric disorder; with symptoms including depressed mood, loss of pleasure in usual activities, weight and appetite fluctuations, sleep disturbance, psychomotor changes, and fatigue (American Psychiatric Association, 2013). In the search for a genetic basis for depression, López-León et al. (2008) discovered that APOE ε2 was the most strongly correlated with depression out of the genes explored in their meta-analysis. Although APOE ε2 is associated with depression (López-León et al., 2008), this gene is considered to be protective with respect to AD (Talbot et al., 1994). The relationship between depression and AD dementia is complicated by questions of reverse causation. It remains unclear whether depression is an early expression of AD pathology or whether depression beginning early in adult life is a risk factor for AD pathology (Marques, Oliveira, Outeiro, & Pereira, 2010).
Like depression, the contribution of sleep disturbance to AD risk is continuing. Sleep disturbance is a hallmark symptom of depression (American Psychiatric Association, 2013), but sleep issues also increase one’s risk for depression (Kumar & Chanana, 2014). Correlations between mild, moderate, and major depressive disorder and insomnia have been found with regard to AD risk (Pistacchi, Gioulis, Contin, Sanson, & Marsala, 2014). Recent sleep research has shown that the sleep process removes neurotoxins from the brain by flushing out interstitial proteins. One such interstitial protein is β-amyloid, a peptide derived from the amyloid precursor protein and a main factor in the deposits found in the brains of those diagnosed with AD (Mendelsohn & Larrick, 2013). In mice, β-amyloid cleared twice as fast in sleeping mice compared to those that were awake (Xie et al., 2013). Disrupted sleep has been related to increased levels of β-amyloid in the brains of older adults. Though the direction of causation is still unresolved, empirical studies indicate that sleep disturbance may subsequently result in neurodegeneration. One investigation found that mice subjected to sleep deprivation possessed an increase in β-amyloid plaques (compared to controls), indicating that the disruption of circadian rhythms may increase risk of AD (Kumar & Chanana, 2014). Conversely, in APOE ε4 carriers, sleeping without disruption may decrease the risk of developing AD (Lim et al., 2013).
Sleep disturbance may be an issue for those diagnosed with AD (Yesavage et al., 2004), especially sleep fragmentation (Kumar & Chanana, 2014). At least half of community-dwelling adults over the age of 65 report a sleep problem. Among those with dementia, 25 to 50% suffer from sundowning or chronic nighttime unsettled sleep (Anderson & Bradley, 2013). Of those diagnosed with AD, 25% experience a circadian disturbance: a disorder in the 24-hour sleep cycle (Weldemichael & Grossberg, 2010).
Extended sleep length has been noted to be significant risk factor for AD. Those who slept less than seven hours a day or more than eight hours a day at baseline had lower scores on two measures of cognitive function compared to subjects who slept between seven and eight hours a day (Virta et al., 2013).
A focus on neurodegenerative disease development requires attention to both the psychosocial factors aforementioned, as well as hereditary and genetic factors. First-degree relatives have a considerably increased risk of developing AD. Twin studies suggest that heritability may reach or exceed 70%, indicating that genetic transmission accounts for a large portion of the variance associated with AD. Due to the role of genetic and environmental effects, family history has been referred to as a “composite factor” (Donix, Small, & Bookheimer, 2012, p. 299) because this variable may include risks related to heritability, apolipoprotein (APOE) ε4, and lifestyle or environmental factors that are transmitted from generation to generation (Donix, Small, & Bookheimer, 2012). Researchers continue to hypothesize about the role of genetic factors in AD development.
Gene-environment Interaction
Gene-environment interaction (Belsky, Moffitt, & Caspi, 2013) offers a framework from which an examination of susceptibility genes and environmental influences can be situated. This model posits that different genotypes may produce differing responses to environmental factors, resulting in the possibility for greater disease risk in some people versus others.
Major susceptibility genes have been identified, such as APOE, which has been associated with sporadic late-onset AD cases (Corder et al., 1993; Farrer et al., 1997; Saunders et al., 1993). This study describes the association of sleep disturbance, depression, and APOE genotypes among cognitively asymptomatic older adults with a subsequent diagnosis of AD dementia. Psychological conditions are investigated as a product of environment and their influence on eventual AD dementia development is investigated independently and in interaction with genetic factors. It is theorized that psychological factors increase risk and certain apolipoprotein genotypes can moderate that risk, either increasing or decreasing the risk of AD development.
There are three forms of APOE: ε2, ε3, and ε4 (Schipper, 2011). The possible combinations of APOE in humans are ε2, ε2; ε2, ε3; ε2, ε4; ε3, ε3; ε3, ε4; and ε4, ε4. The presence of ε2 is considered to be neuroprotective with respect to developing AD (Talbot et al., 1994), while the presence of ε3 and ε4 confer greater risks (Schipper, 2011). Some have argued that ε3 may be neuroprotective relative to ε4 (Aboud, Mrak, Boop, & Griffin, 2012). Compared to non-APOE ε4 carriers, the risk is two to four times greater in those with one ε4 allele and 12 times greater in those with two ε4 alleles (Hollingworth, Harold, Jones, Owen, & Williams, 2011). The debate on the exact mechanism of APOE ε4 influence continues. One study found APOE to be significantly associated with late-onset AD (Ghebranious et al., 2011). APOE ε4 has been associated with earlier onset of AD than non-carriers (de Oliveira, Bertolucci, Chen, & Smith, 2014). Two studies (Brainerd et al., 2013; Brainerd, Reyna, Petersen, Smith, & Taub, 2011) confirmed the role of APOE ε4 in leading to mild cognitive impairment (MCI) for those with normal cognition at baseline, but did not support the theory that APOE is associated with a transition to AD dementia development among those with MCI or other types of cognitive impairment.
This study focused on three hypotheses. First, it was hypothesized that the presence of sleep disturbance, depression, and APOE genotype, as individual factors, increase the likelihood of meeting the criteria for diagnosis with AD. Second, it was hypothesized that the additive effect of the psychosocial factors in the study in concert with APOE genotype, especially genotypes containing ε4, will increase the hazard of AD development. Thirdly, APOE is expected to moderate the multiplicative effect of some psychosocial conditions.
Methods
This study examined risk factors for sporadic, late-onset AD through a secondary data analysis of the NACC UDS. A prospective cohort design was utilized with individuals who were cognitively asymptomatic at the time of their initial visit (n = 11,453). The analysis sought to determine who among those initially unaffected demonstrated clinical signs of AD dementia by their last visit.
Participants
The National Alzheimer’s Coordinating Center began in 1999 as a repository for the standardized longitudinal clinical evaluations that are collected in the Alzheimer’s Disease Centers program. The original goal of the program was to study the spectrum from normal cognition (controls), mild cognitive impairment, to AD dementia. NACC has now expanded to include subtypes of dementia beyond AD. Containing the evaluations of over 33,000 participants as of September 2015, the UDS specifically captures prospective and longitudinal assessment information. Participants generally present to an ADC yearly and provide information on a total of 918 variables (Weintraub et al., 2009). The sample in the current study was drawn from the UDS (September 2014; n = 29,765). The data used for this analysis was collected from 34 AD Centers (ADCs) from visits conducted between September 2005 and September 2014. Subjects with dementia, mild cognitive impairment, and cognitively intact subjects were recruited by individual ADCs throughout the United States. Though it remains the largest sample of its kind in the United States, the UDS is not a nationally representative sample of the United States population with respect to AD and dementia, as these subjects voluntarily present for an examination at one of the participating NIH/NIA sponsored Alzheimer’s Disease Centers. Participants may be required by individual ADCs to agree to an autopsy before acceptance as a research participant. Written informed consent was obtained from all subjects and self-designated informants (NACC, 2010).
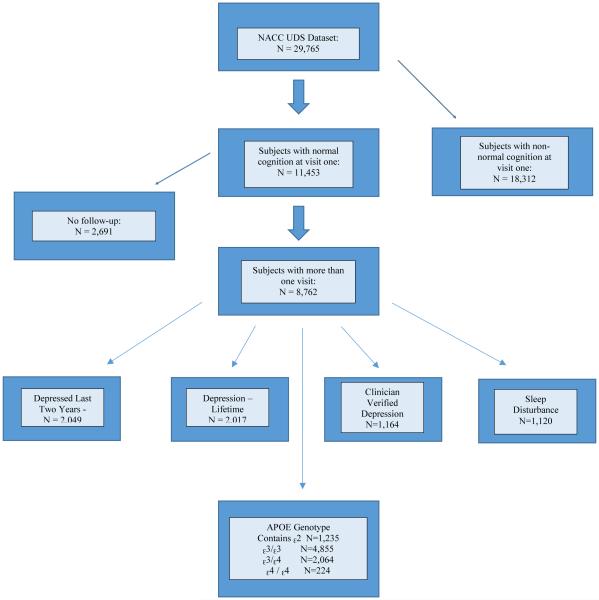
Participants eligible for this study were cognitively asymptomatic at visit one (n = 11,453). Of this initial sample, 8,762 participants were eligible for analysis as a result of their participation in at least one follow-up visit. Participants with more than one dementia-type diagnosis were excluded. Figure 1 provides a visual depiction of the sample and the flow of this research design. The mean age of subjects with normal cognition at visit one was 71.2 (SD: 10.89; Mdn: 72 years). At visit one, 80.7% of the sample population were White, 13.2% were African American, and 5.9% were from other ethnic groups. Six percent of the sample reported Hispanic origin. Almost 35% of subjects reported that their mother had been diagnosed with dementia, while 16.3% reported that their father had been diagnosed with dementia. Almost 18% of subjects reported depression in the last two years or lifetime depression, 10.2% of subjects were diagnosed with clinician-verified depression, and 10.6% reported a sleep disturbance. Percentages, means, and standard deviations (where applicable) are displayed in Tables 1 and 2. This study received approval from the Simmons College Institutional Review Board.
Figure 1.
Overall Eligible Sample and Sample Sizes by Predictor Variable
Table 1.
Demographic Overview of Sample and Predictor Variables
| Sample | – Overall Sample | Depressed - Last Two Years |
Depression – Lifetime |
Depression | Sleep Disturbance | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Present | Absent | Present | Absent | Present | Absent | Present | Absent | |||
| Sample with Normal Cognition |
11,453 | 2,049 (17.9%) |
9,357 (81.7%) |
2,017 (17.8%) |
9,300 (82.2%) |
1,164 (10.2%) |
10,289 (89.8%) |
1,120 (10.6%) |
9,456 (89.4%) |
|
|
| ||||||||||
| Female | 7,464 (65.2%) |
1,506 (73.5%) |
5,926 (63.3%) |
1,493 (74%) |
5,883 (63.3%) |
872 (74.9%) |
6,592 (64%) |
699 (62.4%) |
6,193 (65.5%) |
|
|
| ||||||||||
| Education | X = 16.01 years (SD: 6.72) |
X = 15.83 years (SD: 6.53) |
X = 16.23 years (SD: 7.13) |
X = 15.80 years (SD: 7.19) |
X = 15.88 years (SD: 7.25) |
|||||
|
| ||||||||||
| Age | X = 71.2 years (SD = 10.89) |
X = 68.7 years (SD:10.81) |
X = 68.1 years (SD:10.58) |
X = 68.7 years (SD: 10.88) |
X = 69.9 years (SD: 10.42) |
|||||
|
| ||||||||||
| Race | White | 9,188 (80.7%) |
1,765 (86.1%) |
7,388 (79.5%) |
1,726 (86.7%) |
7,359 (79.7%) |
955 (83.9%) |
8,233 (80.5%) |
941 (84.6%) |
7,552 (80.4%) |
|
| ||||||||||
| African- American |
1,504 (13.2%) |
161 (7.9%) |
1,337 (14.4%) |
169 (8.5%) |
1,313 (14.2%) |
108 (9.5%) |
1,396 (13.7%) |
100 (9%) |
1,284 (13.7%) |
|
|
| ||||||||||
| Other | 671 (5.9%) |
99 (4.9%) |
567 (6.1%) |
95 (4.8%) |
567 (6.1%) |
76 (6.7%) |
595 (5.8%) |
71 (6.4%) |
554 (5.9%) |
|
|
| ||||||||||
| Mother - Dementia |
Present 3,748 (34.6%) |
Absent 7,090 (65.4%) |
787 (40.3%) |
2,943 (33.3%) |
795 (41.2%) |
2,907 (33%) |
431 (39.3%) |
3,317 (34%) |
367 (34%) |
3,186 (35%) |
|
| ||||||||||
| Father - Dementia |
Present 1,722 (16.3%) |
Absent 8,850 (83.7%) |
344 (18%) |
1,373 (15.9%) |
365 (19.4%) |
1,343 (15.7%) |
191 (18%) |
1,531 (16.1%) |
196 (18.7%) |
1,446 (16.3%) |
Table 2.
Demographic Overview of Predictor Variables by APOE Genotype
| APOE Genotype (8,378 at visit one) |
ε3, ε3 | ε3, ε4 | ε4 ,ε4 | Contains ε2 | |||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| N | % | N | % | N | % | N | % | ||
| APOE Genotype – Distribution |
4,855 | 58% | 2,064 | 24.6% | 224 | 2.27% | 1,235 | 14.7% | |
|
| |||||||||
| 3,525 | 42% | 6,314 | 75.4% | 8,154 | 97.3% | 7,143 | 85.3% | ||
|
| |||||||||
| Depression – Last 2 Years |
Present | 834 | 57.8% | 383 | 26.5% | 41 | 2.8% | 186 | 12.9% |
|
| |||||||||
| Absent | 4,011 | 58% | 1,676 | 24.2% | 183 | 2.7% | 1,043 | 15% | |
|
| |||||||||
| Depression – Lifetime |
Present | 827 | 57% | 366 | 25.2% | 43 | 3% | 216 | 15% |
|
| |||||||||
| Absent | 3,975 | 58% | 1,676 | 24.5% | 178 | 2.6% | 1,006 | 14.7% | |
|
| |||||||||
| Depression – Clinician Verified |
Present | 427 | 55.3% | 211 | 27.3% | 24 | 3.1% | 110 | 14.3% |
|
| |||||||||
| Absent | 4,428 | 58.2% | 1,853 | 24.4% | 200 | 2.6% | 1,125 | 14.8% | |
|
| |||||||||
| Sleep Disturbance | Present | 488 | 60.7% | 190 | 23.6% | 22 | 2.7% | 104 | 12.9% |
|
| |||||||||
| Absent | 3,991 | 57.5% | 1,722 | 24.8% | 190 | 2.7% | 1,037 | 14.9% | |
|
| |||||||||
| Sex | Female | 3,135 | 57.5% | 1,352 | 24.8% | 146 | 2.7% | 819 | 15% |
|
| |||||||||
| Male | 1,720 | 58.8% | 712 | 24.3% | 78 | 2.7% | 416 | 14.2% | |
Measures
Assessment information in the UDS is gathered yearly from subjects during initial and follow-up visits. Data are collected by trained clinicians from subjects or provided by a chosen close friend, family member, neighbor, or caregiver. These interviews acquire demographic information; family history; medications used; health history; a physical; and imaging and labs. Dementia status of parents and siblings was obtained through self-or informant report. Participants respond to several rating scales encompassing cognitive, physical, psychological, and neuropsychological measures and a diagnosis regarding dementia status is determined. Diagnoses arising from these interviews are assigned either by a consensus team or the examining physician (NACC, 2010).
The variables utilized for this study include normal cognition, probable AD, self-report depression in the last two years, other episodes of depression, clinician-verified depression, sleep disturbance, and APOE genotype. Sporadic late-onset AD (probable Alzheimer’s disease) is the outcome of interest. Probable AD was diagnosed within the UDS using criteria set forth by the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (McKhann et al., 1984; McKhann et al., 2011).
Depression was measured in three different ways in this study. The first variable included depression self-reported by the participant to be active in the last two years. This includes depressive disorders for which a clinician was consulted, even if treatment or medication was not received. Depression includes major depressive disorder, situational depression, bipolar disorders, dysthymic disorders, and other mood disorders. The second variable, depression: other episodes, includes self-reported depression episodes prior to the last two years. For the purpose of this study, depression: other episodes is considered to be the measure of lifetime depression. The third depression variable is the clinician’s judgment of symptoms and measures the presence or absence of depression. The Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association, 1994) to inform the clinician’s diagnosis of depression.
Sleep disturbance is measured by a variable recording the presence or absence of nighttime behaviors. These behaviors include awakening during the night, rising too early in the morning, or taking excessive naps during the day. Issues with sleep were measured utilizing the Neuropsychiatric Inventory Questionnaire (NPI-Q), which measures the presence or absence of psychiatric symptoms, including sleep disturbance (Cummings, 1977). The NPI-Q has demonstrated adequate test-retest reliability and convergent validity for the individual symptomology, with which the current study is concerned (Kaufer et al., 2000).
APOE genotypes were collected for 8,378 out of 11,453 subjects with normal cognition at visit one. The six possible genotypes (specified previously) were collapsed for this analysis into ε3, ε3; ε3, ε4; and ε4, ε4 and an additional category, contains ε2 (ε2, ε2; ε2, ε3; ε2, ε4). For the analysis, ε3, ε3 was used as the reference group, which is the standard reference group utilized in the literature (Multhammer et al., 2014; Luciano et al., 2015) given its neutral properties with relation to disease risk (de-Almada et al., 2012). APOE was collected from participants by either buccal swab or blood draw. APOE was also obtained at autopsy. The methods of APOE obtainment varied by ADC.
Survival analysis was used to estimate and interpret hazard functions (Kleinbaum & Klein, 2012). Survival analysis is often used in instances where researchers are concerned with time to event, time until the onset of a disease, relapse from a previous remission or recovery and return to work for those who have benefitted from short-and long-term disability, for example (Kleinbaum & Klein, 2012). Survival analysis is the optimal and most appropriate statistical analysis for time to event data. This is especially true in cases of staggered entry, such as is found in this dataset. While Ordinary Least Squares or logistic regression is used to estimate risk, survival analysis is touted for its efficiency in using all available information within a dataset (Kleinbaum & Klein, 2012). In addition, one of the strengths of the longitudinal design is that it allows each subject to serve as his/her own control (Portney & Watkins, 2009).The primary goal was to assess the relationship of predictor variables with the eventual diagnosis of AD dementia. The outcome variable was defined as a diagnosis of probable AD, which then constituted the failure point. Right censoring (Kleinbaum & Klein, 2012) was utilized to account for the fact that a subject was not necessarily diagnosed with AD prior to their last observation. True survival time is unobserved unless a subject’s diagnosis converts to AD by their last observation. Time zero was equal to the subject’s first observation (visit number 1). Subjects had differing observation intervals, with visits ranging from one to ten visits. Of the original 11,453 participants with normal cognition, 76% followed up to the second visit, 58.5% followed up to the third visit, 45% presented for a fourth visit, 34.5% followed up to the fifth visit, and 25.5% presented for the sixth visit. This number drops to 17.4% of the original group for the seventh visit, 8.5% for the eighth visit, and just over 2% for the ninth visit. Observation intervals were measured in days. Omitted responses were mapped to missing in order to exclude the value from the analysis but retain all other information from a participant’s visit.
A descriptive analysis of the baseline sample was conducted and included distributions across predictor variables, such as percentages, means, and standard deviations, where applicable. Baseline survival function was determined prior to the addition of predictors and covariates (age, race, maternal dementia, paternal dementia) in the model. Log-rank tests for equality were used to test for significant differences in survival curves between the various response categories within each predictor variable (such as: those reporting “yes” to depression in the last two years versus those reporting “no” to the same question).
The statistical software, STATA (StataCorp, Release 14, 2015), was utilized for the analyses, and a p value of < 0.05 was considered statistically significant for the analysis, though p values of < 0.001 were also reported.
Four models were developed to explore the main effects of the predictor variables. In the first model, unadjusted main effects of each individual predictor were examined. In the second model, covariates such as sex, age, race, and dementia status of parents were controlled. In the third model, the primary predictor of interest was examined in relation to the previous confounders with the addition of APOE genotypes. In the final model, all previous covariates were controlled as well as the presence of hypertension and hypercholesterolemia. A similar structure was applied to the exploration of interaction effects. The APOE genotype ε3, ε3 was used as the reference group for all analyses. The proportional hazards assumption was tested for all main effects, additive models, and interactions models. There was no evidence that the proportional hazards assumption was violated. The relationships of certain predictor variables were examined relative to the outcome variable using the Cox proportional hazards model (Cox, 1972). Regression modeling included simultaneous control of multiple predictors and covariates. The main effects were examined, and covariates such as sex, age, race, and dementia status of parents were controlled. The effect modification of primary predictors was tested, and the Efron approximation was used as a method for handling ties (Efron, 1977). Additive and multiplicative effects were adjusted for demographic variables, although parental dementia status was dropped as a confounder due to a diminished sample size. Both additive and multiplicative interactions were tested given their differing underlying assumptions about mortality and survival (Buckley, 1984). While multiplicative interactions are often solely reported, Buckley (1984) has noted that they may be “biologically suspect…. They postulate that the force of mortality attributable to the disease… is related to the mortality rates for all other diseases” (p. 53). Additive effects or interactions are utilized “when the effects of two or more identifiable and independently acting causes of death are under consideration” (p. 54). Though mortality is not the outcome of interest here, additive effects are desired as we examine two independently acting causes of neurodegeneration. The assumption of proportionality was examined in order to determine whether the proportional hazards assumption had been met.
Results
The minimum amount of time under observation was 286 days until the first occasion that AD diagnosis occurred, and the maximum was 3,229 days (M = 1469.37; Mdn: 1350.5 days). The mean number of visits for those with normal cognition was three (SD: 1.94), with a range of one to ten visits. There were 330 diagnoses of AD dementia (failures) by the end of the observation period among older adults who had at least an initial visit as well as a follow-up visit.
Preliminary log-rank tests for equality of survivor functions revealed that those who reported depression in the last two years, clinician verified depression, and sleep disturbance expressed statistically significant (p < 0.001) different survival curves than those who did not. The same was true for those reporting lifetime depression versus those who did not (p < 0.05). Visual inspection of Kaplan-Meier plots were consistent with log-rank tests. Chi-square statistical analyses and t-tests were performed to examine the relationships between the psychosocial predictor variables and important demographic covariates. The results of this analysis are displayed in Table 3.
Table 3.
Bivariate Analysis of Predictors and Covariates
| Depressed - Last Two Years |
t or χ2a | Depression – Lifetime |
t or χ2a | Depression Clinician- Verified |
t or χ2a | Sleep Disturbance |
t or χ2a | ||
|---|---|---|---|---|---|---|---|---|---|
| Normal Cognition at visit 1c |
2,049 (17.9%) |
2,017 (17.8%) |
1,164 (10.2%) |
1,120 (10.6%) |
|||||
|
| |||||||||
| Female | 1,506 (73.5%) |
21.9, df=1, p=0.00 |
1,493 (74%) |
24.2, df=1, p=0.00 |
872 (74.9%) |
17.01, df=1, p=0.00 |
699 (62.4%) |
1.3, df=1, p=0.041 |
|
|
| |||||||||
| Educationb | X = 15.83 years (SD: 6.53) |
2.76, df=42034, p= 0.0058 |
X = 16.23 years (SD: 7.13) |
−0.9513. df=41301, p= 0.3415 |
X = 15.80 years (SD: 7.19) |
3.4999, df=42206, p= 0.0005 |
X = 15.88 years (SD: 7.25) |
1.2555, df= 39291, p= 0.2093 |
|
|
| |||||||||
| Ageb | X = 68.7 yrs (SD:10.81) |
12.3505, df=42030, p=0.00 |
X = 68.1 yrs (SD:10.58) |
19.1535, df=41897, p=0.00 |
X = 68.7 yrs SD=10.88 |
1.5792, df=42202, p= 0.1143 |
X = 69.9 yrs SD=10.42 |
−2.2638, df=39287, p= 0.0236 |
|
|
| |||||||||
| Race | White | 1,765 (86.1%) |
9.9, df=2, p=0.00 |
1,726 (86.7%) |
8.4, df=2, p=0.00 |
955 (83.9%) |
1.3, df=2, p=0.00 |
941 (84.6%) |
1.9, df=2, p=0.00 |
|
| |||||||||
| African- American |
161 (7.9%) |
42.7, df=2, p=0.00 |
169 (8.5%) |
169, df=2, p=0.00 |
108 (9.5%) |
12.1, df=2, p=0.00 |
100 (9%) |
14.8, df=2, p=0.00 |
|
|
| |||||||||
| Other | 99 (4.9%) |
3.4, df=2, p=0.00 |
95 (4.8%) |
95, df=2, p=0.00 |
76 (6.7%) |
1.1, df=2, p=0.00 |
71 (6.4%) |
0.4, df=2, p=0.00 |
|
|
| |||||||||
| Mother - Dementia |
787 (40.3%) |
18.5, df=1, p=0.00 |
795 (41.2%) |
25, df=1, p=0.00 |
431 (39.3%) |
7.1, df=1, p=0.00 |
367 (34%) |
0.2, df=1, p=0.544 |
|
|
| |||||||||
| Father - Dementia | 344 (18%) |
3.6, df=1, p=0.021 |
365 (19.4%) |
11, df=1, p=0.00 |
191 (18%) |
1.8, df=1, p=0.121 |
196 (18.7%) |
3, df=1, p=0.045 |
|
χ2 test statistics are displayed for categorical variables, t test statistics for continuous variables.
Continuous variables are described with mean and standard deviation.
Categorical variables are described with sample size and percentage.
There was a significant association (p < 0.001) between individuals reporting depression in the last two years and the occurrence (diagnosis) of AD . Specifically, in model one, those who self-reported depression in the last two years experienced a significantly higher risk of AD dementia diagnosis (HR = 2.35 [95% CI, 1.88-2.94]) compared to those who had not. When adjusted for age, sex, race, and parental dementia in model two, the effect of those who had self-reported depression symptoms in the last two years was stronger compared to those who had not (HR = 5.66 [3.34-9.60]). Adjusting further for APOE genotype, the third model remained significant (HR = 5.75 [3.28-10.07]).
The main effect of lifetime depression symptoms, occurring more than two years earlier, presented a significant risk of AD diagnosis within the follow-up period compared to those who did not (HR = 1.35 [1.06-1.73]). When confounding covariates were added to the second model, the significance was much stronger (HR = 3.05 [1.75-5.23]). When the APOE genotype was controlled, the hazard for those who reported lifetime depression was similar (HR = 3.20 [1.78-5.73]).
Depression verified by a clinician was significantly associated with the diagnosis of AD dementia during the follow-up period as compared to those without depression symptoms in model one (HR = 2.82 [2.21-3.59]). When adjusted for sex, age, race, and parental dementia in the second model, the hazard increased two-fold and presented a stronger association (HR = 4.81 [2.79-8.30]). When adjusted further for the effect of APOE genotype as well as previous confounders, the hazard rose again and remained significant in the third model (HR = 5.50 [3.09-9.80]).
The presence of sleep disturbance was also significantly associated with the eventual diagnosis of AD dementia. In the first model, the hazard was similar to that of self-reported depression symptoms in the last two years, as well as clinician-verified depression (HR = 2.72 [2.11-3.50). When adjusted for demographic confounders, the hazard rose, and a stronger association between sleep disturbance and AD diagnosis was discovered (HR = 3.39 [1.88-6.12]). After adjusting for the APOE genotype, the hazard rose further and resulted in the strongest association between sleep disturbance and diagnosis of AD dementia in the period of follow-up evaluation (HR = 4.08 [2.19-7.61]).
When the effect of APOE genotype was considered as a main effect, those with two APOE ε4 alleles experienced the highest hazard (HR = 3.22 [1.91-5.40]) compared to those with ε3, ε3. APOE ε3, ε4 was also significant (p < .001), although the hazard was significantly decreased (HR = 1.78 [1.37-2.31]). The main effect genotypes containing ε2 did not produce a significant result (HR = .935 [.641-1.36]) as compared to the reference group. When the main effect of APOE genotype was adjusted for covariates, a considerably stronger hazard was demonstrated for ε4, ε4 carriers and with respect to eventual diagnosis of AD dementia (HR = 5.95 [1.68-21.07], p<.05). Main effects for all primary predictors are displayed in Table 4.
Table 4.
Main Effects (Alzheimer’s Disease Dementia as Outcome Variable)
| Predictor Variables | Model 1 Hazard Ratio (95% CI) |
Model 2 Hazard Ratio (95% CI) |
Model 3 Hazard Ratio (95% CI) |
Model 4 Hazard Ratio (95% CI) |
|
|---|---|---|---|---|---|
| Depression – Last 2 Years |
2.35 (1.88-2.94)** | 5.66 (3.34-9.60)** | 5.75 (3.28-10.07)** | 5.62 (3.22-9.81)** | |
|
| |||||
| Depression – Lifetime |
1.35 (1.06-1.73)* | 3.05 (1.75-5.29)** | 3.20 (1.78-5.73)** | 3.12 (1.75-5.59)** | |
|
| |||||
| Depression – Clinician Verified |
2.82 (2.21-3.59)** | 4.81 (2.79-8.30)** | 5.40 (3.03-9.64)** | 5.31 (2.99-9.49)** | |
|
| |||||
| Sleep Disturbance | 2.72 (2.11-3.50)** | 3.40 (1.88-6.11)** | 3.93 (2.10-7.36)** | 3.87 (2.07-7.24)** | |
|
| |||||
| ε3, ε3 | Reference group | Reference group | − | Reference group | |
| APOE Genotype |
|
||||
| ε3, ε4 | 1.78 (1.37-2.31)** | 2.28 (1.24-4.21)* | − | 1.90 (1.29-2.81)** | |
|
| |||||
| ε4, ε4 | 3.22 (1.91-5.40)** | 5.95 (1.68-21.07)* | − | 3.44 (1.90-6.23)** | |
|
|
|||||
| Contains ε2 |
.935 (.641-1.36) | .720 (.274-1.89) | − | 1.07 (.734-1.56) | |
Model 1: Main effect unadjusted
Model 2: Main effects adjusted for sex, age, race, maternal dementia, and paternal dementia.
Model 3: Main effects adjusted for adjusted for sex, age, race, maternal dementia, paternal dementia, and APOE genotype.
Model 4: Main effects adjusted for adjusted for sex, age, race, maternal dementia, paternal dementia, APOE genotype, the presence of hypertension and hypercholesterolemia
indicates statistical significance at p < 0.05,
indicates p < 0.001
Additive Interactions
In the additive effects models, the hazard of developing AD was nine times higher for ε4, ε4 carriers with recent depression (HR = 8.15 [3.70-17.95])]) than for those without depression and with two ε3 alleles (reference group). Most remarkably, when adjusted for demographic factors, the ε4, ε4 group with depression in the last two years experienced a hazard almost 18 times that of the reference group (HR = 17.71 [7.93-39.53]).]). Interestingly, carriers with genotypes containing ε2 who also reported depression symptoms in the last two years experienced a statistically significant hazard of eventual AD development (HR = 2.28 [1.43-3.63]), even though the main effects model for ε2 did not demonstrate significance.
When exploring the additive effect of lifetime depression and APOE genotype, only the ε3, ε4 (HR = 2.07 [1.19-3.57])]) carriers exhibited a significant association (p < .05) with AD diagnosis during follow-up compared to the reference group.
When the additive effect of clinician-verified depression and APOE genotype was analyzed, the hazard for ε3, ε4 and ε3, ε4 was similar to previous models (HR = 3.58 [2.02-6.32)]], while the hazard for ε4, ε4 carriers was ten times that of the reference group (HR = 10.11 [4.43-23.09]. Remarkably, the hazard of those with ε4, ε4 almost doubled when adjusted for covariates, with hazards 20 times that of the reference group (HR = 20.26 [8.76-46.69]).
For participants reporting sleep disturbance hazards for all allele combinations were significant as compared to the reference group. Additive effects for predictors are displayed in Table 5.
Table 5.
Cox Proportional Hazards Model - Additive Effects among Predictor Variables and APOE Genotype
| Predictor | (Unadjusted) Hazard Ratio (95% CI) |
p- value |
Omnibus Test for Model Fit* Log- likelihood; LR chi2 |
(Adjusted) Hazard Ratio (95% CI) |
p-value | Omnibus Test for Model Fit * Log-likelihood; LR chi2 |
||
|---|---|---|---|---|---|---|---|---|
| Depressed 2 Years × APOE Genotype |
ε3, ε3 | Reference group |
− | −2144.1685; (7)78.09 |
ε3, ε3 | Reference group |
− | −2043.2575; (11)261.60 |
|
| ||||||||
| ε3, ε4 | 3.13 (1.89- 5.19)** |
p=0.00 | ε3, ε4 | 4.26 (2.57- 7.10)** |
p=0.00 | |||
|
| ||||||||
| ε4, ε4 | 8.15 (3.70- 17.95)** |
p=0.00 | ε4, ε4 | 17.71 (7.93- 39.53)** |
p=0.00 | |||
|
|
||||||||
| Contains ε2 |
2.28 (1.43- 3.63)** |
p=0.00 | Contains ε2 |
2.48 (.1.58- 3.95)** |
p=0.00 | |||
|
| ||||||||
| Depression Lifetime × APOE Genotype |
ε3, ε3 | Reference group |
− | −2161.8633; (7)43.84 |
ε3, ε3 | Reference group |
− | −2062.5857; (11)224.06 |
|
| ||||||||
| ε3, ε4 | 2.07 (1.19- 3.57)* |
p=.009 | ε3, ε4 | 3.00 (1.73- 5.19)** |
p=0.00 | |||
|
|
||||||||
| ε4, ε4 | 1.74 (.414-7.30) |
ns | ε4, ε4 | 3.76 (.890-15.92) |
ns | |||
|
|
||||||||
| Contains ε2 |
1.59 (.990- 2.58) |
ns | Contains ε2 | 1.87 (1.16- 3.02)* |
p=0.011 | |||
|
| ||||||||
| Clinician-Verified Depression × APOE Genotype |
ε3, ε3 | Reference group |
- | −2168.886; (7)98.55 |
ε3, ε3 | Reference group |
- | −2072.522; (11)272.92 |
|
| ||||||||
| ε3, ε4 | 3.58 (2.02- 6.32)** |
p=0.00 | ε3, ε4 | 4.34 (2.45- 7.68)** |
p=0.00 | |||
|
| ||||||||
| ε4, ε4 | 10.11 (4.43- 23.09)** |
p=0.00 | ε4, ε4 | 20.26 (8.76- 46.69)** |
p=0.00 | |||
|
| ||||||||
| Contains ε2 |
3.51 (2.21 – 5.58) |
p=0.00 | Contains ε2 | 3.42 (2.15- 5.44)** |
p=0.00 | |||
|
| ||||||||
| Sleep Disturbance × APOE Genotype |
ε3, ε3 | Reference group |
- | −2014.0897; (7)87.36 |
ε3, ε3 | Reference group |
- | 1927.1757; (11)243.27 |
|
| ||||||||
| ε3, ε4 | 3.93 (2.23- 6.92)** |
p=0.00 | ε3, ε4 | 4.67 (2.66- 8.27)** |
p=0.00 | |||
|
|
||||||||
| ε4, ε4 | 6.79 (2.38- 19.37)** |
p=0.00 | ε4, ε4 | 12.05 (4.18- 34.71)** |
p=0.00 | |||
|
|
||||||||
| Contains ε2 |
3.01 (.188- 4.84)** |
p=0.00 | Contains ε2 |
2.72 (1.69- 4.38)** |
p=0.00 | |||
Model 2: Adjusted for age, sex, and race.
indicates p < 0.05;
indicates p < 0.001.
ns = not significant
Multiplicative Interactions
In the multiplicative effects models, unexpected trends emerged. The hazard of developing AD was statistically significant for ε2 carriers with recent depression (HR =2.77 [1.07-7.17]) as compared to the reference group. Interactions with other alleles were not significant. None of the interactions between lifetime depression and the APOE isoforms were significant when compared to the reference group.
When the interaction between clinician-verified depression and APOE genotype was analyzed, a statistically significant hazard was demonstrated for ε2 carriers with reporting sleep disturbance. The interaction effects are displayed in Table 6.
Table 6.
Cox Proportional Hazards Model - Interaction Effects among Predictor Variables and APOE Genotype
| Predictor | (Unadjusted) Hazard Ratio (95% CI) |
p-value | Omnibus Test for Model Fita Log-likelihood; LR chi2 |
(Adjusted) Hazard Ratio (95% CI) |
p-value | Omnibus Test for Model Fit Log-likelihood; LR chi2 |
||
|---|---|---|---|---|---|---|---|---|
| Depressed 2 Years × APOE Genotype |
ε3, ε3 | Reference Group |
−2144.1685; (7)78.09 |
ε3, ε3 | Reference Group | −2043.2575; (12)261.60 |
||
|
| ||||||||
| ε3, ε4 | 1.90 (.714-5.06) | ns | ε3, ε4 | 2.04 (1.29-3.21)* | p=0.002 | |||
|
| ||||||||
| ε4, ε4 | 3.49 (.932- 13.09) |
ns | ε4, ε4 | 4.31 (1.94- 9.55)** |
p=0.000 | |||
|
| ||||||||
| Contains ε2 |
2.77 (1.07- 7.17)* |
p=0.036 | Contains ε2 | .824 (.531- 1.28) |
ns | |||
|
| ||||||||
| Depression Lifetime × APOE Genotype |
ε3, ε3 | Reference Group |
−2161.8633; (7)43.84 |
ε3, ε3 | Reference Group |
−2062.5857 (11)224.06 |
||
|
| ||||||||
| ε3, ε4 | 1.39 (.515- 3.77) |
ns | ε3, ε4 | 1.32 (.486- 3.57) |
ns | |||
|
|
||||||||
| ε4, ε4 | .535 (.095- 3.00) |
ns | ε4, ε4 | .506 (.090- 2.84) |
ns | |||
|
|
||||||||
| Contains ε2 |
2.19 (.842-5.69) | ns | Contains ε2 |
2.08 (.800- 5.41) |
ns | |||
|
| ||||||||
| Clinician-Verified Depression × APOE Genotype |
ε3, ε3 | Reference Group |
− | −2168.886; (7)98.55 |
ε3, ε3 | Reference Group |
− | −2072.522; (12)272.92 |
|
| ||||||||
| ε3, ε4 | 1.37 (.470- 3.99) |
ns | ε3, ε4 | 1.24 (.427- 3.63) |
ns | |||
|
| ||||||||
| ε4, ε4 | 2.88 (.731- 11.33) |
ns | ε4, ε4 | 2.95 (.749- 11.62) |
ns | |||
|
|
||||||||
| Contains ε2 |
2.94 (1.07- 8.09)** |
p=0.037 | Contains ε2 | 2.64 (.958- 7.28) |
ns | |||
|
| ||||||||
| Sleep Disturbance × APOE Genotype |
ε3, ε3 | Reference Group |
− | −2014.0897; (7)87.36 |
ε3, ε3 | Reference Group |
− | −1927.1757; (11)243.27 |
|
| ||||||||
| ε3, ε4 | 1.68 (1.08- 2.60)* |
p=0.020 | ε3, ε4 | 2.10 (1.35- 3.26)** |
p=0.001 | |||
|
|
||||||||
| ε4, ε4 | 2.87 (1.43- 5.76)* |
p=0.003 | ε4, ε4 | 2.38 (.503- 11.26) |
ns | |||
|
|
||||||||
| Contains ε2 |
.764 (.496- 1.18) |
ns | Contains ε2 |
3.77 (1.24- 11.43)* |
p=0.019 | |||
Model 2: Adjusted for age, sex, and race.
indicates p < 0.05;
indicates p < 0.001,
ns = not significant
Discussion
This study described the presence of sleep disturbance, depression, and APOE genotypes among cognitively asymptomatic subjects and the association with subsequent diagnosis of AD dementia. The findings support the hypothesis that the presence of sleep disturbance, depression, and APOE genotype increase the likelihood of meeting the criteria for AD dementia diagnosis during follow-up visits among a group of cognitively intact participants at baseline.
The results relative to the main effects of APOE were consistent with previous research (Schipper, 2011). This study reinforced the association between APOE and AD, especially for ε4 carriers. A surprising result was the interaction between some of the psychosocial factors and genotypes containing ε2. The literature has previously shown that participants with genotypes containing ε2 may be at a higher risk for depression symptoms (Ballard et al., 1997), while other researchers have speculated that ε2 may be a susceptibility gene for depression (López-León et al., 2008). Additionally, ε2 is commonly thought to provide neuroprotective benefits with regard to AD and may even delay its onset (Corder et al., 1994).A novel finding in this study was the additive effect of lifetime depression and APOE genotype. Previous research in this area produced heterogeneous results. Based on the literature, one would expect the genotypes containing ε4 to produce significant hazards. However, ε4 did not produce any significant effects in unadjusted models except among those with ε3, ε4 (p < .05) when additive effects with lifetime depression were tested. Significance was not achieved in any of the interaction models targeting life depression and APOE. The ε3 genotype has often been thought to have no consequence and buffer patients with certain comorbid disorders from the development of AD (Aboud, Mrak, Boop, & Griffin, 2012). By contrast, the results of this study indicate that ε3 may present a greater hazard among those with the psychosocial risk factors described.
For ε4, ε4 carriers with self-reported recent depression or clinician-verified depression, the additive effects produced a stronger hazard than the main effects of depression or the ε4 isoform alone. This result is contrary to previously reported results in the literature, which suggested that ε4 may serve as a protective mechanism with regard to those with depression (Ballard et al., 1997) or other studies that simply reported no association between depression and ε4 in relation to AD (Cantillon et al., 1997; Harwood, Barke, Ownby, Mullan, & Duara, 2004; Hollingworth et al., 2006). The findings in this study provide support for the association between depression, APOE ε3 and ε4, and AD.
The effect of sleep disturbance combined with specific APOE genotypes is often overlooked in the literature, except when sleep disturbance is defined as obstructive sleep apnea (Nikodemova, Finn, Mignot, Salzieder, & Peppard, 2013; Osorio et al., 2014).
Three recent studies (Lim et al., 2013; Spira et al., 2013; Xie et al., 2013) explored the link between sleep disorders and AD and also included an analysis of the role of APOE. The results of this study provide evidence for the increased hazard experienced by those with combinations of APOE ε3 and ε4 as well those with sleep disturbance as compared to those without sleep disturbance and genotypes containing ε2. Contrary to the results presented by Lim et al. (2013), an increased hazard was associated with ε3, ε4 and ε4, ε4 in both the additive and multiplicative models. An expansion of the types of ‘reversible sleep disorders’ under investigation in relation to AD may be one avenue for addressing earlier phases of AD (Segal, 2013).
Previous research has attempted to explain the effect of psychosocial factors such as depression and sleep disturbance in relation to AD. Some studies have suggested that depression and sleep disturbance may be prodromal symptoms of AD (Caraci, Copani, Nicoletti, & Drago, 2010; Lyketsos & Olin, 2002). Other studies have speculated that depression, especially symptoms occurring later in life, may be causally related to AD as a result of hippocampal insult or atrophy (Arnone et al., 2013; Sheline, Gado, & Kraemer, 2003). Associations between the hypothalamic-pituitary-adrenal axis, APOE ε4, and damaging cortisol levels have also been suggested as mechanisms responsible for increased AD risk (Peavy et al., 2007). The exact causal pattern remains under debate (Gil-Bea et al., 2010).
This study has several strengths. Multiple measures of depression allow for higher confidence in the reliability of the concept. Deviations in the participant’s conceptualization of a psychological variable or its importance may affect responses for a self-report measure (Lee & Dugan, 2015). The addition of clinician-verified depression was provided for additional certainty beyond self-report alone. Given that depression may be an early symptom of AD, a lifetime depression measure was added to include and account for those cases where depression may have occurred more than two years prior. The longitudinal nature of this dataset allowed for multiple measures for each subject over time and allowed for each participant to act as his or her own control. Several limitations to this study also exist. Forward causal inferences cannot be made in the NACC UDS. Additionally, reverse causation is a possibility, making it difficult to determine whether a predictor is an early symptom of AD or a risk factor. The UDS is not a nationally representative sample of the U.S. population with regard to dementia, ethnicity, and race (Morris et al., 2006). Care should be taken not to derive associations between the role nor the impact of race or ethnicity from this analysis. The sample selected for analysis included participants deemed to be cognitively asymptomatic using standard guidelines. Nevertheless, it is accepted theory that the pathophysiological process leading to observable signs of AD dementia may begin up to 25 years before observable clinical signs (Bateman et al., 2012). Small sample sizes occurred when variables were combined in the analysis. This was the case, specifically for ε4, ε4 carriers with clinician-verified depression, as well as ε4, ε4 carriers reporting sleep disturbance. In some cases, confidence intervals for statistically significant hazard ratios were extremely wide, calling into question the exact precision of the estimate, though the hazard was within a favorable margin of error.
This study sought to identify modifiable psychosocial variables that, on their own or in concert with APOE, may present an increased risk of developing AD. Identification of variables that may respond to treatments already available is a potential avenue for delaying the onset or decreasing the risk of AD. While the APOE gene continues to present a risk, research continues to explore how such a hazard might be mitigated based on behavioral and lifestyle changes. Barnes and Yaffe (2011) reviewed risk factors for AD, including diabetes mellitus, hypertension, obesity, smoking, cognitive inactivity, and depression, through Cochrane Reviews and PubMed. It was estimated that these risk factors contribute to half of AD cases globally and in the United States. Projections estimate that a reduction of these factors at their current levels found in the population by 10% may result in a 1.1 million case decrease globally, while a decline by 25% would further result in a reduction of three million cases globally (Barnes & Yaffe, 2011).
Social workers are uniquely positioned to assist clients in modifying behavioral patterns that are associated with a neurodegenerative brain disease with no current cure and few efficacious treatments. Although modification of the APOE genotype is not yet possible, behavioral changes such as the alleviation of depression and sleep disturbance may greatly decrease a person’s risk profile, despite the presence of APOE ε4. Given that the pathophysiological process may begin more than two decades earlier than any observable clinical signs of AD (Bateman et al., 2012), it is crucial that behavioral risk factors are identified early and addressed. The role of frontline social workers in this intervention process is similar to the work they are often charged with at present, including accurately identifying behavioral and psychological conditions. Population interventions geared toward education about the increase in long-term physiological risks derived from psychological processes, especially in concert with one’s specific genetic profile, are increasingly important and needed. Continued research is necessary to determine the exact mechanisms facilitating AD risk, as well as public health campaigns and individual-level interventions which may decrease mental health symptomology and risk of AD.
Footnotes
Conflict of Interests: None
Description of authors’ roles:
S.B. was involved in all aspects of the study, including conceptualization, data analysis, writing the manuscript, and revisions.
P.M. assisted in study conceptualization, supervised data analysis, edited multiple drafts, and supervised entire study process.
T.C. assisted in study conceptualization, supervised data analysis, edited multiple drafts, and supervised entire study process.
W.K. assisted in study conceptualization, provided data set and guidance on use thereof, supervised data analysis, edited multiple drafts, and supervised entire study process.
Contributor Information
Shanna L. Burke, Florida International University, Robert Stempel College of Public Health and Social Work, School of Social Work, Modesto A. Maidique Campus, 11200 S.W. 8th Street, AHC5 564, Miami, Florida 33199, 305-348-7462.
Peter Maramaldi, Hartford Faculty Scholar & National Mentor, Director of the PhD Program, Simmons College School of Social Work, Boston, MA 02115-5820; HSDM-Oral Health Policy and Epidemiology, Harvard School of Dental Medicine; Department of Social and Behavioral Sciences, Harvard School of Public Health.
Tamara Cadet, Simmons College School of Social Work; Lecturer on Oral Health Policy and Epidemiology, HSDM-Oral Health Policy and Epidemiology, Harvard School of Dental Medicine.
Walter Kukull, National Alzheimer's Coordinating Center (NACC), University of Washington, Department of Epidemiology, Box 357236, Seattle WA 98195-7236, Phone: 206-543-4560.
References
- Aboud O, Mrak RE, Boop F, Griffin ST. Apolipoprotein epsilon 3 alleles are associated with indicators of neuronal resilience. BMC Medicine. 2012;10(1):35–44. doi: 10.1186/1741-7015-10-35. doi:10.1186/1741-7015-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’s Association 2015 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia. 2015;11(3):332–384. doi: 10.1016/j.jalz.2015.02.003. doi: http://dx.doi.org/10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th American Psychiatric Publishing; Washington, DC: 1994. [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5th American Psychiatric Publishing; Arlington, VA: 2013. [Google Scholar]
- Anderson KN, Bradley AJ. Sleep disturbance in mental health problems and neurodegenerative disease. Nature & Science of Sleep. 2013:561–75. doi: 10.2147/NSS.S34842. doi: 10.2147/NSS. S34842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone D, et al. State dependent changes in hippocampal grey matter. Molecular Psychiatry. 2013;18(12):1265–1272. doi: 10.1038/mp.2012.150. [DOI] [PubMed] [Google Scholar]
- Ballard C, Massey H, Lamb H, Morris C. Apolipoprotein E: non-cognitive symptoms and cognitive decline in late onset Alzheimer’s disease. Journal of Neurology, Neurosurgery & Psychiatry. 1997;63(2):273–273. doi: 10.1136/jnnp.63.2.273b. doi:10.1136/jnnp.63.2.273b. [DOI] [PubMed] [Google Scholar]
- Barnes D, Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurology. 2011;10(9):819–828. doi: 10.1016/S1474-4422(11)70072-2. doi:10.1016/S1474-4422(11)70145-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman R, et al. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. New England Journal of Medicine. 2012;367(9):795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky DW, Moffitt TE, Caspi A. Genetics in population health science: Strategies and opportunities. American Journal of Public Health. 2013;103(S1):S73–S83. doi: 10.2105/AJPH.2012.301139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainerd CJ, Reyna VF, Petersen RC, Smith GE, Taub ES. Is the apolipoprotein e genotype a biomarker for mild cognitive impairment? Findings from a nationally representative study. Neuropsychology. 2011;25(6):679–689. doi: 10.1037/a0024483. doi: 10.1037/a0024483. [DOI] [PubMed] [Google Scholar]
- Brainerd CJ, et al. The apolipoprotein E genotype predicts longitudinal transitions to mild cognitive impairment but not to Alzheimer's dementia: Findings from a nationally representative study. Neuropsychology. 2013;27(1):86–94. doi: 10.1037/a0030855. doi: 10.1037/a0030855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley JD. Additive and multiplicative models for relative survival rates. Biometrics. 1984;40(1):51–62. [PubMed] [Google Scholar]
- Cantillon M, Barker W, Harwood D, Mullan M, Duara R. Phenotype of depression in Alzheimer's disease (AD) with APOE genotype. Biological Psychiatry. 1996;39(7):661. [Google Scholar]
- Caraci F, Copani A, Nicoletti F, rago F. Depression and Alzheimer’s disease: Neurobiological links and common pharmacological targets. European Journal of Pharmacology. 2010;626:64–71. doi: 10.1016/j.ejphar.2009.10.022. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Underlying mortality data provided by National Center for Health Statistics. 2013 Mar; Retrieved October 27, 2013, from http://www.cdc.gov/nchs/data/databriefs/db116.pdf.
- Chui HC, Zheng L, Reed BR, Vinters HV, Mack WJ. Vascular risk factors and Alzheimer’s disease: Are these risk factors for plaques and tangles or for concomitant vascular pathology that increases the likelihood of dementia? An evidence-based review. Alzheimer’s Research and Therapy. 2012;4(1):1–13. doi: 10.1186/alzrt98. doi:10.1186/alzrt98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder EH, et al. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nature Genetics. 1994;7:181–184. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- Corder EH, et al. Gene dose of apolipoprotein E type allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Cox DR. Regression models and life-tables. Journal of the Royal Statistical Society, Series B. 1972;34(2):187–220. [Google Scholar]
- Cummings JL. The Neuropsychiatric Inventory: Assessing psychopathology in dementia patients. Neurology. 1997;48:S10–S16. doi: 10.1212/wnl.48.5_suppl_6.10s. [DOI] [PubMed] [Google Scholar]
- de-Almada BVP, et al. Protective effect of the APOE-e3 allele in Alzheimer’s disease. Brazilian Journal of Medical and Biological Research. 2012;45(1):8–12. doi: 10.1590/S0100-879X2011007500151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira FF, Ferreira Bertolucci PH, Chen ES, Smith MC. Assessment of risk factors for earlier onset of sporadic Alzheimer's disease dementia. Neurology India. 2014;62(6):625–630. doi: 10.4103/0028-3886.149384. doi:10.4103/0028-3886.149384. [DOI] [PubMed] [Google Scholar]
- Donix M, Small GW, Bookheimer SY. Family history and APOE-4 genetic risk in Alzheimer’s disease. Neuropsychological Review. 2012;22:298–309. doi: 10.1007/s11065-012-9193-2. doi: 10.1007/s11065-012-9193-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B. The efficiency of Cox’s likelihood function for censored data. Journal of the American Statistical Association. 1977;72:557–565. [Google Scholar]
- Farrer LA, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. JAMA, The Journal of the American Medical Association. 1997;278(16):1349–1356. [PubMed] [Google Scholar]
- Fortune DA, Lang R, Cook S, Byrd GS. African Americans and Alzheimer’s disease: Role of health educators in addressing this silent epidemic. American Journal of Health Studies. 2013;28(3):92–100. [Google Scholar]
- Ghebranious N, et al. A pilot study of gene/gene and gene/environment interactions in Alzheimer Disease. Clinical Medicine & Research. 2011;9(1):17–25. doi: 10.3121/cmr.2010.894. doi:10.3I2l/cmr.20I0.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Bea FJ, et al. HPA axis dysregulation associated to apolipoprotein e4 genotype in Alzheimer’s disease. Journal of Alzheimer’s Disease. 2010;22:829–838. doi: 10.3233/JAD-2010-100663. doi: 10.3233/JAD-2010-100663. [DOI] [PubMed] [Google Scholar]
- Harwood DG, Barker WW, Ownby RL, Mullan M, Duara R. No association between subjective memory complaints and apolipoprotein E genotype in cognitively intact elderly. International Journal of Geriatric Psychiatry. 2004;19(12):1131–1139. doi: 10.1002/gps.1193. doi:10.1002/gps.1193. [DOI] [PubMed] [Google Scholar]
- Hollingworth P, et al. Four components describe behavioral symptoms in 1,120 individuals with late-onset Alzheimer's disease. Journal of the American Geriatrics Society. 2006;54(9):1348–1354. doi: 10.1111/j.1532-5415.2006.00854.x. [DOI] [PubMed] [Google Scholar]
- Hollingworth P, Harold D, Jones L, Owen MJ, Williams J. Alzheimer’s disease genetics: Current knowledge and future challenges. International Journal of Geriatric Psychiatry. 2011;26:793–802. doi: 10.1002/gps.2628. doi: 10.1002/gps.2628. [DOI] [PubMed] [Google Scholar]
- Kaufer DI, et al. Validation of the NPI-Q: A brief clinical form of the Neuropsychiatric Inventory. The Journal of Neuropsychiatry and Clinical Neurosciences. 2000;12(2):233–239. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- Kleinbaum DG, Klein M. Survival analysis: A self-learning text. 3rd Springer; New York: 2012. [Google Scholar]
- Kumar A, Chanana P. Sleep reduction: A link to other neurobiological diseases. Sleep and Biological Rhythms. 2014;12:150–161. doi:10.1111/sbr.12066. [Google Scholar]
- Lee HJ, Dugan E. How Large Is the Gap Between Self-Report and Assessed Mental Health and Does It Impact Older Adult Mental Health Service Utilization? Journal of Gerontological Social Work. 2015;58(1):3–19. doi: 10.1080/01634372.2014.919978. doi:10.1080/01634372.2014.919978. [DOI] [PubMed] [Google Scholar]
- Lim A, Yu L, Kowgier M, Schneider J, Buchman A, Bennett D. Modification of the relationship of the apolipoprotein E ε4 allele to the risk of Alzheimer disease and neurofibrillary tangle density by sleep. JAMA Neurology. 2013;70(12):1544–1551. doi: 10.1001/jamaneurol.2013.4215. doi:10.1001/jamaneurol.2013.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-León SS, et al. Meta-analyses of genetic studies on major depressive disorder. Molecular Psychiatry. 2008;13(8):772–785. doi: 10.1038/sj.mp.4002088. doi:10.1038/sj.mp.4002088. [DOI] [PubMed] [Google Scholar]
- Luciano M, et al. Current versus lifetime depression, APOE variation, and their interaction on cognitive performance in younger and older adults. Psychosomatic Medicine. 2015;77(5):480–492. doi: 10.1097/PSY.0000000000000190. doi: 10.1097/PSY.0000000000000190. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Olin J. Depression in Alzheimer’s disease: Overview and treatment. Biological Psychiatry. 2002;52:243–252. doi: 10.1016/s0006-3223(02)01348-3. [DOI] [PubMed] [Google Scholar]
- Marques SF, Oliveira CR, Outeiro TF, Pereira CF. Alzheimer's disease: The quest to understand complexity. Journal of Alzheimer's Disease. 2010;21(2):373–383. doi: 10.3233/JAD-2010-100303. doi:10.3233/JAD2010100303. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- McKhann G, et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. doi:10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn AR, Larrick JW. Sleep facilitates clearance of metabolites from the brain: Glymphatic function in aging and neurodegenerative diseases. Rejuvenation Research. 2013;18(6):518–523. doi: 10.1089/rej.2013.1530. doi: 10.1089/rej.2013.1530. [DOI] [PubMed] [Google Scholar]
- Meng X, D’Arcy C. Apolipoprotein E gene, environmental risk factors, and their interactions in dementia among seniors. International Journal of Geriatric Psychiatry. 2013;28:1005–1014. doi: 10.1002/gps.3918. doi: 10.1002/gps.3918. [DOI] [PubMed] [Google Scholar]
- Morris JC, et al. The uniform data set (UDS): Clinical and cognitive variables and descriptive data from Alzheimer’s Disease Centers. Alzheimer Disease and Associated Disorders. 2006;20(4):210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- Multhammer M, Michels A, Zintl M, Mendoza MC, Klünemann HH. A large ApoE ε4/ε4 homozygous cohort reveals no association with Parkinson’s disease. Acta Neurologica Belgica. 2014;114(1):25–31. doi: 10.1007/s13760-013-0223-5. [DOI] [PubMed] [Google Scholar]
- National Alzheimer’s Coordinating Center The UDS Study Population. 2010 Retrieved from: https://www.alz.washington.edu/WEB/study_pop.html.
- Nikodemova M, Finn L, Mignot E, Salzieder N, Peppard PE. Association of sleep disordered breathing and cognitive deficit in APOE ε4 carriers. Sleep. 2013;36(6):873–880. doi: 10.5665/sleep.2714. doi:10.5665/sleep.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio RS, et al. The interaction between sleep-disordered breathing and apolipoprotein E genotype on cerebrospinal fluid biomarkers for Alzheimer’s disease in cognitively normal elderly individuals. Neurobiology of Aging. 2014;35:1318–1324. doi: 10.1016/j.neurobiolaging.2013.12.030. doi:10.1016/j.neurobiolaging.2013.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peavy GM, et al. The effects of prolonged stress and APOE genotype on memory and cortisol in older adults. Biological Psychiatry. 2007;62(5):472–478. doi: 10.1016/j.biopsych.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistacchi M, Gioulis M, Contin F, Sanson F, Marsala SZ. Sleep disturbance and cognitive disorder: Epidemiological analysis in a cohort of 263 patients. Journal of the Neurological Sciences. 2014 doi: 10.1007/s10072-014-1870-x. doi: 10.1007/s10072-014-1870-x. [DOI] [PubMed] [Google Scholar]
- Portney LG, Watkins MP. Foundations of clinical research: Applications to practice. 3rd Pearson Education, Inc; Upper Saddle River, NJ: 2009. [Google Scholar]
- Qiu C, Xu W, Winblad B, Fratiglioni L. Vascular risk profiles for dementia and Alzheimer’s disease in very old people: A population-based longitudinal study. Journal of Alzheimer’s Disease. 2010;20(1):293–300. doi: 10.3233/JAD-2010-1361. doi: 10.3233/JAD-2010-1361. [DOI] [PubMed] [Google Scholar]
- Reitz C, et al. A summary risk score for the prediction of Alzheimer disease in elderly persons. Archives of Neurolology. 2010;67(7):835–841. doi: 10.1001/archneurol.2010.136. doi: 10.1001/archneurol.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]; Saunders A, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- Schipper HM. Apolipoprotein E: Implications for AD neurobiology, epidemiology and risk assessment. Neurobiology of Aging. 2011;32:778–790. doi: 10.1016/j.neurobiolaging.2009.04.021. doi:10.1016/j.neurobiolaging.2009.04.021. [DOI] [PubMed] [Google Scholar]
- Segal AZ. Poor sleep quality may predict preclinical Alzheimer's disease. Neurology Alert. 2013;31(11):86–88. [Google Scholar]
- Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. American Journal of Psychiatry. 2003;160:1516–1519. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- Sperling RA, et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. doi:10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira AP, et al. Self-reported sleep and β-amyloid deposition in community-dwelling older adults. JAMA Neurology. 2013;70(12):1537–1543. doi: 10.1001/jamaneurol.2013.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software: Release 14. StataCorp LP; College Station, TX: 2015. [Google Scholar]
- Talbot C, Lendon C, Craddock N, Shears S, Morris JC, Goate A. Protection against Alzheimer’s disease with APOE epsilon 2. Lancet. 1994;343(8910):1432–1433. doi: 10.1016/s0140-6736(94)92557-7. [DOI] [PubMed] [Google Scholar]
- Thies W, Bleiler L. 2013 Alzheimer's disease facts and figures. Alzheimer's & Dementia: The Journal of the Alzheimer's Association. 2013;9(2):208–245. doi: 10.1016/j.jalz.2013.02.003. doi:10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Virta J, et al. Midlife sleep characteristics associated with late life cognitive function. Sleep. 2013;36(10):1533–1541. doi: 10.5665/sleep.3052. doi:10.5665/sleep.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S, et al. The Alzheimer’s disease centers’ uniform data set (UDS): The neuropsychological test battery. Alzheimer Disease and Associated Disorders. 2009;23(2):91. doi: 10.1097/WAD.0b013e318191c7dd. doi: 10.1097/WAD.0b013e31819c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weldemichael DA, Grossberg GT. Circadian rhythm disturbances in patients with Alzheimer's disease: A review. International Journal of Alzheimer's Disease. 2010;2010:1–9. doi: 10.4061/2010/716453. doi:10.4061/2010/716453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373–377. doi: 10.1126/science.1241224. doi:10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage JA, et al. Sleep/Wake disruption in Alzheimer's disease: APOE status and longitudinal course. Journal of Geriatric Psychiatry & Neurology. 2004;17(1):20–24. doi: 10.1177/0891988703261994. doi:10.1177/0891988703261994. [DOI] [PubMed] [Google Scholar]