Abstract
A variety of in vitro models has been developed to understand the mechanisms underlying the regenerative failure of central nervous system (CNS) axons, and to guide pre-clinical development of regeneration-promoting therapeutics. These range from single-cell based assays that typically focus on molecular mechanisms to organotypic assays aimed at recapitulating in vivo behavior. By utilizing a combination of models, researchers can balance the speed, convenience, and mechanistic resolution of simpler models with the biological relevance of more complex models. This review will discuss a number of the models that have been used to build our understanding of molecular mechanisms of CNS axon regeneration.
Keywords: axon regeneration, neurite outgrowth, in vitro models, cell based assays, organotypic assays, high content screening
II- Introduction
Axons regenerate poorly in the central nervous system (CNS) of adult mammals. This is in stark contrast to the robust regeneration observed in the mammalian peripheral nervous system (PNS), or in the CNS of many other organisms. The absence of a robust regenerative response after CNS injury contributes to permanent functional deficits and disability. Consequently, substantial efforts have been directed at understanding the biological basis for the disparity in regenerative behavior between axons in the CNS and the PNS, and towards identifying mechanisms for stimulating CNS regeneration.
In the late 1970s, it was observed that that PNS axons, while able to regenerate in PNS tissue, have difficulty regenerating through optic nerve grafts 1,2. A few years later, Aguayo et al demonstrated that some injured CNS axons are able to regrow long distances within sciatic nerve grafts but fail to reintegrate into CNS tissue 3,4. These observations led to the conclusion that the PNS microenvironment is permissive for axon regeneration, while factors in the CNS are non-permissive or even inhibit axon regrowth. In addition to these in vivo works, several groups demonstrated that certain diffusible (e.g. NGF), cell surface (e.g. L1), and matrix bound factors can positively regulate cell adhesion and axon growth 5–8. Later studies focused on characterizing the molecular mechanisms that mediate the CNS’s inhibitory activity 9–13. Multiple extracellular molecules with potent inhibitory properties, along with their cognate receptors and intracellular signaling pathways, were described. These include myelin associated proteins, tenascins, and chondroitin sulfate proteoglycans (CSPGs), 10,14–21.
Disrupting extracellular inhibitory signals, either individually or in combination, has thus far failed to produce robust axon regeneration 22–24. On the other hand, manipulating signaling pathways within CNS neurons could promote significant regeneration 25–28. These findings underscored another important aspect of regeneration failure, i.e. the genetically programmed decline of intrinsic regenerative capacity in CNS neurons. The complex interplay between neuron intrinsic and extrinsic factors suggests that strategies for affecting robust CNS axon regeneration must simultaneously target multiple mechanisms.
A large number of in vitro models have been developed to investigate axon regeneration. Cell-based assays are useful for dissecting subcellular and molecular mechanisms, but they do not reflect much of the complex cellular interactions that occur in vivo. Conversely, assays utilizing multiple cell types or tissue explants can have stronger biological relevance, but may be more difficult to dissect mechanistically. A broad range of experimental models will be reviewed here, with some discussion about the strengths and weaknesses of each. It should be noted that nerve regeneration is a complex process that may involve the genesis of new neurons, glia, axons, synapses, and connective tissue. This review specifically focuses on in vitro models of mammalian axon regeneration.
III. Cell-based models
A variety of in vitro assays have been developed to assess the intrinsic regenerative ability of CNS neurons and discover agents that can induce axon growth. Typically, cell preparations are obtained by mechanical dissection, enzymatic dissociation, and centrifugation. Filtration or affinity separation techniques may be utilized to produce relatively homogenous cultures from mixed cell preparations. It is important to note that the dissociation process itself is likely to cause extensive severing of preformed neuronal processes depending on the age and location of isolated cells, and therefore induce an injury response. Proper methodology and controls are necessary to isolate regenerative responses resulting from dissociation from those that are due to manipulations done to the animal, or to perturbagens applied to the cultured cells. Long standing research efforts and optimization of culture reagents has made it possible to grow dissociated neurons in chemically defined serum-free media. These media preparations can selectively favor the survival of certain cell types (e.g. neurons over glia) 29–32. This can help to maintain the purity of neuronal cultures and minimize variance in assay readout. The use of cells from mice in which apoptosis is impaired via knockout strategies, can assist in the dissociation of effects on regeneration and survival 33.
III. a. Low-density (sparse) culture models
The main advantage of low-density culture assays is their ability to extract detailed morphological information on individual cells. Another advantage is the relative ease with which they can be adapted to high content screening (HCS) approaches 34. HCS provides the ability to efficiently acquire morphological measurements for millions or billions of cells treated with different perturbagens. It permits detailed and unbiased investigation of the overall morphological response and population variance for any given treatment. This creates an efficient platform for identifying agents with high cellular efficacy and low toxicity, as reflected by the growing emphasis on phenotypic screening 34.
Most neuronal HCS assays use neurite outgrowth in vitro as a surrogate measure for process extension in vivo. This is due to multiple reasons including: 1- the time required for cultured neurons to reliably express axonal or dendritic protein markers (several days to weeks) 35,36 is beyond the optimal assay duration (1-5 days), and 2- axonal markers are typically restricted to the neurite at some distance away from the cell body, making it difficult to automatically connect traced processes to the correct cell body 34. Accordingly, assayed neurons are typically immunostained for β3-tubulin, and growth of putative axons and dendrites is often assessed simultaneously (FIGURE 1). Nevertheless, depending on the exact goals and desired throughput of a study, additional dendrite or axon-specific staining (e.g. Anti-tau or anti-MAP2) may be employed 37,38.
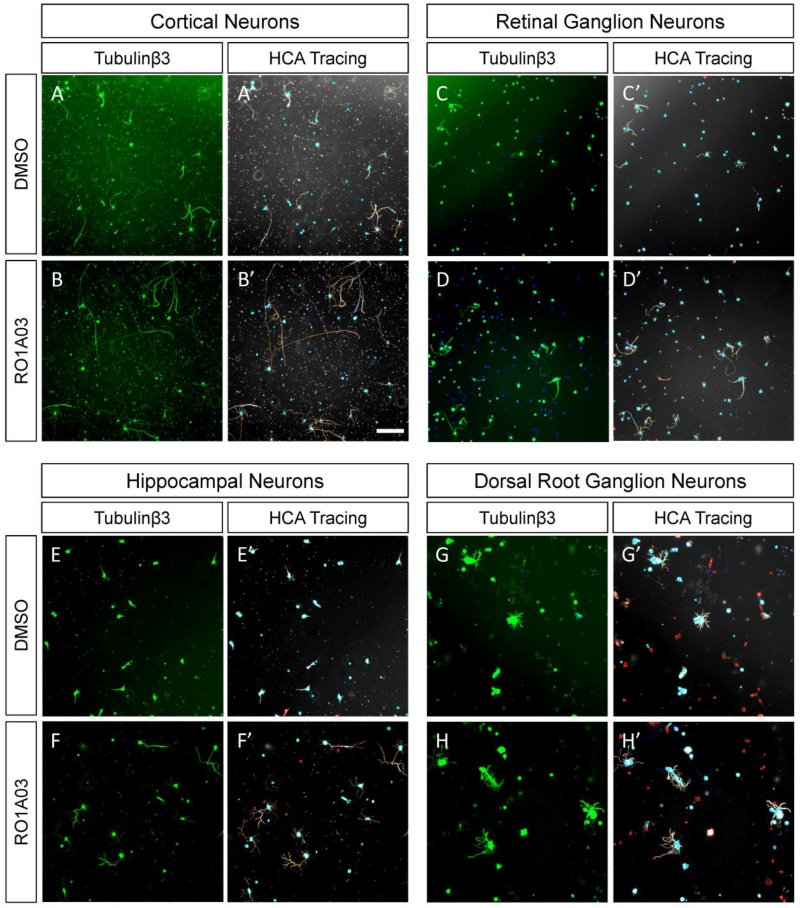
Figure 1. Examples of cell-based in vitro models of axon regeneration from neurite outgrowth experiments.
Micrographs show various types of primary neurons cultured in the absence (A-A’, C-C’, E-E’, G-G’) or presence (B-B’, D-D’, F-F’, H-H’) of a neurite growth promoting kinase inhibitor, RO1A03. A-H) Show anti-β3 Tubulin immunostaining used to visualize the neuronal cytoskeleton (green). A’-H’) Automated tracing of neuronal cytoskeleton (grey) obtained using the Cellomics Neuronal Profiler bioapplication shows cell bodies of valid neurons (blue), neurites (orange and purple), and cells rejected by the tracing algorithm (red) based on morphological parameters. A-B) Postnatal (P3) rat cortical neurons 2 DIV. C-D) Postnatal (P21) retinal ganglion cells 3 DIV. E-F) Embryonic (E18) rat hippocampal neurons 2DIV. G-H) Adult (10w) mouse dorsal root ganglion neurons 1 DIV. Images are single fields obtained with Cellomics VTI (5× objective) from cells cultured in 96-well plates. Scale bar is 200μm.
Neurite outgrowth assays
Neurite outgrowth assays are among the most commonly utilized phenotypic screens relevant to axon regeneration. They have been successfully used to identify genetic regulators of axon regeneration 25,39, perform mechanistic studies of the involved pathways 15,40–42, and identify small molecules that can promote regeneration in vivo 43. Neurons are typically grown on an adherent substrate that may be coated with one or more biological matrix proteins 44. The exact choice of substrate components depends on the phenomenon under investigation. For example, using a growth-inhibitory substrate (e.g. CSPGs or myelin) may be used to focus on mechanisms that mediate extrinsic inhibition from matrix elements. On the other hand, a more permissive substrate (e.g. laminin) may be used to more easily identify agents that inhibit growth. The substrate proteins are usually loaded on a coat of polylysine. Neurons plated directly on polylysine will adhere and extend neurites, although at a much slower rate than neurons grown on laminin. This may be beneficial in certain situations, particularly where a large effect window is desired for a given assay. Neurons grown on laminin will extend neurites at a high rate, making the window for detecting improvement on this growth relatively small. On the other hand, laminin can significantly improve neuronal survival and process extension, and has been extensively used in mechanistic studies of neurite outgrowth and axon regeneration.
Neurite outgrowth assays can be used to assess the effect of perturbagens on neurite extension. For chemical screens, cells are usually plated and allowed to adhere before compounds are added to the culture. For genetic screens, cells are often transfected prior to plating 25,39,41. Recent advances in transfection technologies, however, enable passive transfection after plating 43,45. Neurite outgrowth assays can also be used to assess latent effects of in vivo manipulations performed prior to extracting the cells from the animal. Examples may include pre-conditioning of dorsal root ganglia by damaging the peripheral nerve root, or virally transducing neurons in vivo. In vitro axotomy may also be performed where axons of cultured neurons are visually selected and transected 46. This allows the investigation of events that occur after an injury in individual cells.
Following treatment, cells are incubated for a pre-determined amount of time. The duration depends on a number of factors including: intended perturbation length, basal neurite extension rate, and the required amount of separation between readouts from positive controls and baseline 34. Larger separations (i.e. bigger effect windows) are desirable for screening 47.
For single endpoint assays, neurons are typically fixed and immunostained for a cytoskeletal marker, such as the neuron-specific βIII-tubulin protein 34. Staining for βIII-tubulin fills the cell body and neuronal extensions. This makes it possible to automatically image and analyze neuronal morphology, including parameters such as neurite count, length, and branching. Plasma membrane specific dyes can also be used for delineating cell bodies and neurites 48. Neurite outgrowth can also be measured dynamically in live low-density cultures in a label-free setting, using light microscopy 49. Alternatively, neurons can be fluorescently labeled using transfection or viral transduction. This permits measurement of neurite outgrowth in live neuron-only cultures as well as in neuron-astrocyte co-cultures 49.
Neurite retraction assays
Neurite retraction assays 50 are a variation on the neurite outgrowth assay. Neurons are cultured for a given amount of time and allowed to extend neurites. Then, neurons are challenged with a substance (e.g. lysophatidic acid) that causes growth cone collapse and neurite retraction 51. The neurites are allowed time to respond (minutes to hours), after which the cultures are analyzed. The benefit of this approach is that it can investigate the mechanisms leading to collapse and retraction by inducing the process in an entire cell culture. As such, it is a valuable tool for identifying agents that can inhibit retraction in order to favor a pro-regenerative state 41,52–54,
Stripe assays
The stripe assay was originally developed to investigate axonal guidance mechanisms in vitro 55,56. It has been used to study mechanisms governing axon growth and guidance in the contexts of both development and regeneration 57–64. Assay setup consists of applying stripes of control and test substrates, with the aid of specially manufactured silicon matrices, to a glass or plastic surface in a culture dish 65,66. Control stripes typically contain a growth permissive substrate, while the alternating “guidance” stripes contain the substance of experimental interest. A fluorescent label can be added to the base solution of either condition for easy visualization of the stripes. Linear stripes are most common, but zigzag 67 and graded patterns have been used 68. Laminin can be applied homogenously to the entire substrate to provide a growth-promoting signal. Dissociated neurons are then applied to the dishes and incubated for a predetermined period, after which cultures are fixed and immunostained for analysis using fluorescence microscopy. Alternatively, cultures can be analyzed using time-lapse video with phase microscopy 67. Results are interpreted based on how frequently axons growing from the control stripes avoid or grow over the experimental stripes 65. One variation of the stripe assay, called the “step assay”, involves creating stripes with increasing concentration of the guide substance on each subsequent step of the gradient 69. This enables simultaneous testing of a range of conditions 8. The stripe assay can be combined with the turning assay to demonstrate the active repulsiveness of a given substance to axons. The turning assay was originally performed by applying a concentration gradient of a test substance to an individual growth cone via a micropipette 70,71. This was later combined with electrically gated pressure application systems to deliver repetitive pulsatile injections of picolitre volumes of solution containing the guidance cue 72. Ultimately, finely adjustable low pressure applicator systems eliminated the need for pulsatile application 72,73. Newer variations involve confronting growing axons with beads coated with a substance and observing growth cone behavior using time-lapse video microscopy 74. A turning of the growing axon or collapse of the growth cone upon contact with the beads confirms active repulsion by the coating substance.
Spot assay
Similar to the stripe assay, the spot assay involves creating a small boundary area containing a bioactive substance within a poly-lysine coated surface and then determining “boundary strength” by measuring the percentage of neurites from cells outside the spot perimeter that cross the spot’s rim 75. To achieve this, a small drop of solution containing the test substance is allowed to coat the culture surface after it has been coated with poly-lysine and allowed to dry. The solution is often mixed with a dye to facilitate visualization. The surface is then rinsed and dissociated cells are plated onto it. Cultures are maintained for enough time for neurites to grow, after which they are fixed, immunostained, and analyzed 76,77. A variant of this assay involves spotting a mixed solution of laminin with proteoglycans at different concentrations. The outcome is a circular area with laminin concentration decreasing from center to rim, while proteoglycan concentration decreases from rim to center. Thus, dissociated cells plated inside the spot extend neurites outwards against an increasing gradient of proteoglycan, which can be visualized by immunostaining with anti-proteoglycan antibodies 78.
Examples of low-density primary neuronal culture systems
Several major factors influence the choice of cell type for in vitro regeneration assays. The first consideration is, of course, the biological relevance of the cell model, which naturally predisposes the choice towards neurons that are directly involved in the disease phenotype in vivo. Another important consideration is the ability to culture the particular neuronal type in a setup that lends itself to the assay design. For example, cells that cannot survive at low density cannot be used to study neurite outgrowth in individual cells in a homogenous culture, and may require the presence of a supportive cell layer (see neuron-glia co-cultures below). Other considerations include the ease of acquisition, number of cells obtained per animal, and the requirements of the assay based on desired throughput. Low-density culture systems have been developed for a variety of neuronal cells, some of which are outlined below.
Retinal ganglion neuron cultures
Retinal ganglion cells (RGCs) can be purified from dissociated rodent retina using a series of immunopanning steps to remove macrophages and other cells 79. RGC survival in low-density cultures is enhanced by trophic factors that are normally produced along the visual pathway, such as BDNF and CNTF 80. RGCs have been used to study pathways that control axon growth 25,81,82. They have also been used to study growth cone dynamics and to investigate the effects of small molecules on CSPG-mediated axon growth inhibition 21,62,83. Finally, RGCs have been utilized in genetic screens to identify agents that mediate neuronal death 45.
Cortical neuron cultures
Cortical neuron cultures can be prepared from dissociated embryonic or postnatal rodent sensorimotor cortex 39. Glia conditioned media is usually required to support neuronal survival, particularly in low-density cultures grown on poly-lysine. Cortical neurons have been successfully used in genetic screens to identify genes that control intrinsic regeneration capacity 39. The KLF7 transcription factor, a member of a protein family implicated by a screen with cortical neurons, was later shown to promote axon regeneration of CST axons in vivo 27.
Hippocampal neuron cultures
Hippocampal neurons can be easily prepared from embryonic rodent brains to yield cultures that are relatively free of non-neuronal cells 84,85. An overexpression screen using embryonic hippocampal neurons was used to identify KLF4, a member of the KLF family of transcription factors, as a developmental regulator of axon growth 25. Small molecule screens with these neurons have also identified kinase inhibitors that were later shown to promote regeneration of axons in cortical slice cultures and of CST axons in vivo 43,86. Overexpression, biochemical, and computational studies have found that many of the kinases that regulate neurite outgrowth in other CNS neurons also regulate neurite outgrowth in hippocampal neurons 42,43. Thus, while there are clear variations in the biology and responses of different CNS neuronal types, there appears to be sufficient conservation in the major pathways that control neurite outgrowth and axon regeneration in CNS neurons to justify the use of these neurons for screening. Additionally, neurite outgrowth is strongly inducible in these cells with relatively consistent behavior in controls when grown in chemically defined media on polylysine 43. This allows for sensitive detection of neurite outgrowth promoters and accurate comparisons of the relative activities of various perturbagens. Such features are particularly useful for drug discovery and development campaigns, where structure activity relationship (SAR) studies following the initial step of hit discovery require reasonable effect sizes and relatively low variability.
Cerebral granule neuron cultures
Cerebral granule neurons (CGNs) can be purified from dissociated postnatal rodent cerebella by centrifugation on a Percoll gradient 87. They are a large population of relatively homogeneous neurons, and respond to inhibitory substrates such as myelin and CSPG 88. They can be grown in standard cell culture media without the need for trophic factors or glial conditioning. Low-density cultures are possible on growth-supportive substrates (laminin) but tend to have poorer survival on polylysine. CGN cultures have been used in chemical screens to identify small molecules that modulate neurite outgrowth on inhibitory substrates 15,89,90. They have also been used in genetic screens to identify genes that regulate neurite outgrowth 41,42.
Doral root ganglion neuron cultures
Enhancing sensory axon regeneration after PNS and CNS injury remains a goal of clinicians and scientists 91. Thus, being able to study sensory neuron cultures obtained from dissociated dorsal root ganglia (DRG) has obvious benefits. A feature that distinguishes DRG neurons from other CNS neurons is that their peripheral axons can regenerate long distances and often re-establish appropriate functional connections after PNS injuries 92,93. Furthermore, these neurons become “pre-conditioned” during such a process, such that their central axons acquire an enhanced regenerative/sprouting response 94,95. Using dissociated DRG cultures, many of the mechanisms by which these processes occur have been studied 96–99. Similarly, researchers have perturbed cells in vivo and then investigated how neurite outgrowth is affected on inhibitory substrates (e.g. myelin, CSPGs) in vitro 16,100. Additionally, mechanisms by which certain neurotrophic factors, matrix molecules, and cell surface receptors influence regeneration have been elucidated using DRGs 88,101,102. A salient benefit to using DRG neurons is that all neurites extended from these cells are technically axons, thereby eliminating the need for additional staining for axon-specific measurements. Drawbacks, however, include the fact that dissections can be time consuming owing to the ganglia’s location within the vertebral column. Furthermore, DRGs contain multiple classes of sensory neurons and these different populations may exhibit different phenotypes in vitro; this may affect assay performance 34,103,104. Additionally, multiple different enzymatic digestions and immunopanning steps may be needed to obtain enriched neuronal cultures 105,106. Finally, DRG neurons tend to extend neurites in culture at a much higher rate than other CNS neurons, which can shorten an assay’s duration and compress its dynamic range. This is particularly true for neurite outgrowth assays 34.
Non-primary neuron cultures
Short-term post-mitotic neuronal cultures, derived directly from primary tissue, have the benefit of phenotypic and gene expression profiles that mimic native neurons. Primary neurons are therefore considered more biologically relevant to neurons in vivo than immortalized cell lines 34. Nevertheless, non-primary neuronal cultures offer some advantages. Primary neurons are post mitotic, thus small amounts of cells cannot be amplified to yield sufficient quantities needed for molecular studies or drug discovery campaigns. Thus, expandable cell line models may be desirable in situations where large amounts of cells are required. Examples include the PC12, SH-SY5Y, and NTERA-2 cell lines. The PC12 cell line is derived from a rat adrenal pheochromocytoma 107. NGF-1 treatment ceases proliferation in PC12 cells and induces them to differentiate and extend neurites. This lead to their use as a model for neuronal differentiation, neuritogenesis, and neurite outgrowth, with some differences noted relative to neurite outgrowth in primary neurons 108. The human derived cell lines, NTERA-2 and SY5Y, were clonally isolated from a pluripotent human embryonal carcinoma and a human bone marrow neuroblastoma, respectively 109,110. Both neuron-like cell lines can be induced with retinoic acid to differentiate and extent neurites in culture. Neurite outgrowth in NTERA-2 differentiated neurons is potently inhibited by CSPGs, making them a useful model for studying mechanisms of CSPG inhibition 14.
Limited access to live primary neurons from human has made it challenging to study the molecular underpinnings of neurological disorders in a patient-specific manner. Pluripotent stem cells (PSC) derived from human embryonic tissues or directly reprogrammed from patient somatic cells (human-induced PSC, iPSC) can, in principle, be differentiated into any cell type, at a scale compatible with high-throughput technologies 111,112. Neurons differentiated from patient iPSCs may be able to model human neurological disorders in ways that animal model neurons cannot, owing to genetic and/or epigenetic hallmarks of a given disease. To date, human iPSCs have been differentiated into a variety of neuronal cells including glutamatergic, GABAergic, dopaminergic, retinal, sensory, and motor neurons 113–120. Phenotypic assays using iPSC-derived neurons have been developed and can be readily adapted for neurite outgrowth studies, including high content screening 121,122.
III. b. High-density culture models
High-density cultures can have several advantages over low-density neuronal cultures, especially if material is needed for follow-up molecular studies (e.g. proteomic or transcriptomic analyses). Additionally, high-density cultures may favor the generation of more biologically relevant neuronal phenotypes that are difficult to obtain with very low-density cultures (e.g. dendritic spines, synaptogenesis, neural networks, myelination). Unlike low-density cultures, high-density cultures often cannot produce readouts at the level of individual cells. Neurite outgrowth assays may still be performed using non-low density cultures, by normalizing overall neurite outgrowth to the total number of viable cells 121,123.
Scratch assay
The basic design of the scratch assay consists of plating cells at high-density to form a monolayer, followed by mechanical removal of cells from an area within the culture (scratch), and finally quantification of the occupancy in the scratch area over a given amount of time 124. A major goal for regeneration research is to identify agents that can stimulate pre-existing axons, destroyed by injury, to regrow and reestablish connectivity. The rate of neurite initiation from neuronal cell bodies, however, can strongly influence overall neurite outgrowth, making it difficult to distinguish between agents that promote initiation and those that promote elongation. The scratch assay has the benefit of more specifically quantifying growth from pre-existing neurites (or axons). Scratch assays were originally used to study ‘contact-inhibition’, cell proliferation, and cell migration 125,126. However, they have been adapted to study wound healing, polarization, and process extension from cells.
In high-density neuronal cultures, the scratch serves two important functions: 1- It clears out an area where regrowth of axonal projections can be measured and quantified, and 2- it provides an in vitro axonal injury paradigm. This model has been used to identify phosphatases that suppress axon regeneration after injury 127.
In relationship to glia, scratch assays can be used to model reactive gliosis and changes in astrocytes induced by trauma 128,129. In particular, the involvement of signaling molecules (e.g. Cdc42) that regulate reactivity in astrocytes and neurite outgrowth in neurons have been elucidated with this system 130,131.
Radial assay
The radial axon growth assay is a special application of high-density cultures where the cell bodies are sequestered into a dense cluster from which axons grow radially outward 132. This is achieved by applying a droplet of a highly concentrated suspension of dissociated neurons near the center of a well within a 1 mm diameter region followed by adding the appropriate amount of culture media. The resulting arrangement readily permits experimental axotomy and imaging of various axonal segments.
Fluidic Chambers
Fluidic technologies provide a platform for specifically isolating, treating, lesioning, and analyzing axons 133,134. This is achieved by culturing neurons in one compartment, and allowing the axons to grow into a second, fluidically isolated, compartment. The use of fluidically isolated chambers began with the ‘Campenot’ chambers, which are composed of a Teflon divider adhered with silicon grease to a petri dish 135,136. Campenot chambers require the use of a stimulating agent (e.g. NGF or BDNF) to induce the growth of neurites from cells within the Teflon divider into the outer chamber, which restricts the range of applications. Microfluidic devices were later developed to overcome this restriction 134. Microfluidic chambers take advantage of liquid behavior in very small volumes to create chambers that are fluidically isolated without being entirely physically separate 137. Typically, such devices consist of two or more small chambers connected by microgrooves of varying lengths (usually 0.5 mm or longer). A minute volume difference between the chambers creates a hydrostatic pressure differential, and the high fluidic resistance of the microgrooves allows a very small but sustained flow towards the chamber with less fluid 134. This unidirectional flow fluidically isolates the chambers. Consequently, neurons can be plated into the higher volume chamber and their axons will extend through the microgrooves towards the lower volume chamber. This enables selectively exposing axons to perturbagens in the axonal compartment without directly exposing the cell somas and dendrites. Conversely, the flow may be reversed after axons reach the “axonal compartment” by reversing the volume differential, therefor allowing selective treatment of cell bodies in isolation from the axons in the axonal compartment. The length of the microgrooves can be adjusted depending on the duration of the experiment and the rate of axon extension of the studied neurons. This can help to ensure that only axons and not dendrites make it to the axonal compartment 138. In related applications, microfluidic chambers can be used to create interconnected co-cultures with individual synapsis formed at defined locations. Such setups are useful for studying trafficking and other processes that occur along the length of axons connecting two cell populations 139.
Neuron-glia co-cultures
While neuron-only models are useful for dissecting neuron-intrinsic regenerative responses, they are not informative about extrinsic effects on axon regeneration from other cell types. Extrinsic factors from non-neuronal cells contribute to the limited capacity for axons to regrow 140,141. Astrocytes undergo morphological changes and upregulate expression of CSPGs in response to in vivo and in vitro injuries (reactive gliosis) 142–144. The astrocytic response is required for wound healing, but negatively influences axon regeneration in the chronically injured CNS 145,146. Accordingly, it is important that agents for promoting neurite outgrowth don’t contribute to the detrimental aspects of astrogliosis. Enriched astrocyte cultures have traditionally been obtained through passaging mixed glial cultures multiple times 147,148. Now, primary astrocytes can be efficiently purified from rodent cortex using a astrocyte-specific antibodies or genetic labels in concert with cell sorting 149,150. These cells have been utilized in phenotypic assays to examine the response of astrocytes to various perturbations 151–153.
Assays that utilize neurons cultured directly on astrocytes can provide a composite readout on the effect of perturbagens or astrocyte stressors on neurite outgrowth. While this setup may potentially be of higher relevance to what occurs in vivo, it provides less insight on individual cell-specific mechanisms. In the case of chemical perturbagens, these can be added to the co-cultures, or to either cell type prior to combining the cultures. In the case of genetic perturbagen, cells can be transfected prior to co-culturing, or neuron-selective transduction methods can be used (ex. AAV virus). Nevertheless, even in the case of cell-specific initial perturbation, the final outcome will be the result of complex neuron-astrocyte interactions, and will require follow up experiments to dissect mechanistically. Various protocols for culturing hippocampal or cortical neurons on astrocytes have been developed and can be used to study neurite outgrowth 75,154,155 or neurotoxicity 49,156. Three dimensional co-cultures have also been used, in attempt to better mimic the environment of axons growing in vivo 157,158. A stretch injury model has also been used to induce glial activation in co-cultures with DRG or cortical neurons 89,144,159. Single-cell axotomy can also be performed in co-cultures using a 2-photon laser to study morphological changes taking place after injury 160.
IV. Explant/Organotypic models
In organotypic cultures, most cell-cell and cell-matrix interactions are not disrupted by dissociation procedures. Thus, organotypic or explant cultures are, in principle, a middle ground between dissociated cells and in vivo studies. Explant studies have been instrumental in enhancing our understanding of axon guidance 161. While the initial preparation may require fewer steps than a typical dissociation protocol, downstream portions of the experimental protocol can be lengthier, owing to increased time requirement for immunostaining and imaging procedures. Additionally, analysis of neurite outgrowth can be complicated by tissue architecture.
DRG explants
Whole ganglia explants have been used extensively for identifying growth factors and matrix derived factors that influence axon growth and regeneration 70,162,163. A major advantage of this model stems from the fact that the ganglia can be rapidly isolated from organisms (typically, mouse, rat, or chicken) at various developmental stages and directly cultured 164. The cells are subjected to minimal trauma (i.e. axotomizing of peripheral and central branches) relative to the harsh enzymatic and/or mechanical stress of dissociated cell preparations. Once the ganglia adhere to the culture substrate, neurites extend radially from the explant 165. Because the explants are large, they can be handled with fine forceps or pipette tips and placed into specific regions within a culture dish. This makes the model particularly useful for carrying out variants of the stripe or spot assays experiments described above 166,167. DRGs can be obtained from specific spinal levels following particular in vivo manipulations (e.g. preconditioning lesion), and placed in culture to investigate downstream effects on neurite outgrowth within a chemically controlled environment 95,168.
Despite the tractability of this model, there are several drawbacks. For instance, given that neurons are retained within the DRG capsule, routine immunohistochemical studies of sensory neuron cell bodies and their proximal processes may not be possible. The extended neurites generally fasciculate and some may never directly contact a defined culture substrate. Additionally, the relatively large size of the explants makes them susceptible to fluid movement, which could shear neurites if the cultures aren’t handled with extreme care. Finally, it is important to note that DRG explants contain large numbers of satellite and supporting cells that migrate out from the capsule. Thus, distinguishing between direct and indirect effects on neurons may be challenging.
Retinal explants
The retina is an extension of the CNS and resembles other CNS tissues in terms of structure, function, and response to injury 169. Thus, the optic nerve, which contains retinal ganglion cell axons, is effectively CNS tissue 170). The retina is an attractive in vitro model system because the tissue can be easily obtained and cultured 171–173. Furthermore, retinal explants can yield tissue slices of relatively uniform thickness that survive well in culture and produce quantifiable neurite outgrowth 174. Early work with retinal tissue was focused on exploring the role of molecular gradients in modulating CNS axon growth 175–177. Culture systems using retinal explants with strips cut out of the optic tectum (hence, strip assay), and later stripes of defined matrix molecules (hence, stripe assay), were successfully used to elucidate receptor-ligand partners in axon guidance 65,173,178–180. Co-cultures of retinal explants and tectum have also been employed to study changes in neuron’s intrinsic regenerative capacity 181. Retina explants were also utilized to study the effect of Schwann cells on CNS axon growth and regeneration 182–184. Retinal explants have been useful for elucidating the dependence of axon growth on both electrical activity and neurotrophic factors 82,185. More recently, several studies using retinae demonstrated similarities between the growth of blood vessels and axons 186–188. Finally, retinal explants have been used to examine CNS axon regeneration in response to perturbation with small molecules or extracellular matrix elements 189–191.
Brain and Spinal Cord Slice cultures
Slice cultures (from brain, spinal cord or both) serve as contextual regeneration models because axons grow in a glial-rich environment. With tools like genetic fate mapping and viral vectors, it is possible to observe growing axons without classical antero- or retrograde tracers 86,192. In recent years, cortical slice cultures have been used to study axon regrowth following manipulations of regeneration associated transcription factors 193. They have also been used to study how kinase inhibitors affect axon growth 86. Recently, spinal cord slice cultures were used to study axon growth and remyelination, as well as astrocyte migratory and growth-supportive behavior 194–196. Several groups have reported co-culturing cortex and spinal cord tissue to investigate the regrowth of cortex derived axons into spinal cord tracts 197,198.
While some of the native context of neurons is preserved in organotypic cultures, the extraction and culture process is itself an injurious procedure that can induce widespread cell death and other alterations within the slice 199. For example, it has been demonstrated that some aspects of reactive gliosis are manifested in hippocampal slice cultures 200. This is important because it demonstrates that the neuronal environment within a slice culture is not equivalent to the intact in vivo setting. Additionally, analyzing axons or neurite outgrowth is complicated by non-uniform tissue thickness and tortuous growth paths, whereby confocal microscopy is required to visualize axons throughout the slice. Thus, appropriate sampling and analysis tools must be used in order to generate reliable data 86,193,201.
V. Special Considerations in High Content Screens
Information obtained from screens using “real” primary neurons is highly valuable and ultimately worthwhile. However, high content screens on primary neurons must be carefully designed and executed—the list of challenges is long. Major issues include:
-
1)
Variability in day-to-day and week-to-week performance of the assays, owing to subtle effects of cell density on neurite outgrowth, lot-to-lot variability of cell culture reagents purchased from vendors, and other less obvious variables.
-
2)
Selecting the “ideal” primary neuron for screening is fraught with challenges, not the least of which stem from grant/manuscript reviewers with little or no screening experience. In an ideal situation, researchers would obtain many millions of appropriately aged and homogeneous neurons that are highly relevant to the in vivo population being modeled. For most types of primary neurons, this is simply impossible. For example, obtaining large numbers of adult neurons from the brain, spinal cord, or retina is difficult or impossible. Dissociating nervous tissue when the neurons are more than 1-2 days post-generation typically leads to an injury response that may have substantial effects on their transcriptional and epigenetic state. As examples, while young cerebellar or hippocampal neurons are relatively homogenous, cortical or spinal cord neurons are clearly not. Dorsal root ganglion neurons and retinal ganglion cells, while relevant to specific important research questions, have multiple subpopulations and are difficult to obtain in large numbers.
-
3)
Even when working with a fairly homogenous population of neurons, there is large and non-Gaussian variability in the lengths of neurites produced by individual cells. The inherent problem of the heterogeneous response of primary neurons to neurite outgrowth promoting factors was illustrated by J. Raper and colleagues 202 and is observed today in all HCA assays of neurite outgrowth. Strong growth promoters like laminin and L1cam may increase mean neurite outgrowth by over 50% compared to non-biological substrates like poly-lysine. This leads to assays with small signal-to-noise ratios and Z’-factors that are considerably lower than biochemically based assays. Identifying and ranking robust hits is therefore challenging.
-
4)
Assay design can have significant impact on assay sensitivity. For instance, with immature cells like E18 hippocampal neurons or E18 DRG neurons growing on highly permissive substrate like laminin, neurites will grow rapidly and start contacting other cells or neurites in the culture. This has two consequences for the assay. The first is that analysis of neurite length at the cell level becomes impossible, since image analysis software cannot assign neurites to the soma of origin. The second consequence is less obvious. If basal neurite growth is rapid, it becomes difficult to detect an increase in growth rate. This “ceiling” effect is well known in biological assays 203. We have overcome these issues in several cases by using culture conditions that are less permissive for neurite outgrowth, such as using polylysine without laminin as a substrate.
-
5)
Lack of effective controls is also a major hindrance to assay development. Without robust positive and negative controls, most screening centers will not accept a project, since it is impossible to calculate assay performance metrics and normalize data across plates and experiments. In the early 2000’s, there were almost no compounds or genes that could robustly enhance neurite outgrowth. For some cell types (DRG neurons, sympathetic neurons, retinal ganglion cells), soluble growth factors could serve this purpose. But for other cells, such as cerebellar, cortical, or hippocampal neurons, no positive controls existed, especially for overexpression and knockdown screens. Our lab currently uses several treatments as positive controls (e.g. doublecortin for overexpression screens and a kinase inhibitor, ML7, for compound library screens) for several different neuronal types.
The advent of RNAseq has provided a means for investigators to compare the transcriptional landscapes of neurons in vivo and in vitro. The field is still relatively immature, with only about 200 papers mentioning RNAseq and neuron in PubMed in January, 2016. However, in the next two to three years there will likely be massive amounts of information that researchers can mine to evaluate the similarity of neurons used in screening assays to those in various developmental or disease states. Most importantly, it will allow investigators to assess whether neurons suitable for screening (e.g. E18 hippocampal neurons or iPSC derived human neurons) express genes and pathways relevant to neurons that are less suitable for HCS (e.g. RGCs or layer 5 corticospinal tract neurons). With this knowledge, massive libraries could first be screened with the HCS-suitable neurons to identify hits, and then these hits can be validated in small-scale secondary assays with the non-HCS-suitable neurons, in vitro or even in vivo.
VI. Summary and Discussion
It has been known for thousands of years that the CNS does not experience sufficient regeneration in most cases to restore function following injury 204. Experiments into why regeneration is so poor in the CNS were carried out by Cajal and colleagues at the turn of the 20th century 93,205. In the almost 100 years that have passed since then, scientists have been extracting individual components of the nervous system and studying their behavior in attempts to deconstruct the phenomena that control regeneration. Such approaches, and the in vitro models that resulted from them, have produced an extensive body of knowledge with molecular-level resolution.
These studies began with whole tissue explants, where CNS components were extracted from a live organism and maintained in culture. Examples include retinal, DRG, cortical, and spinal cord tissue explants. By mixing and matching tissues from various CNS and PNS origins, strip assays were born. These led to the discovery of numerous matrix, secreted, or membrane-bound factors that influence axon growth, pathfinding, and regeneration. Strip assays were later refined into stripe and spot assays. These permitted more detailed investigation of the effects of purified or mixed bioactive molecules, over homogenously or gradient coated surfaces. Relatively recent advances in cell culture technologies have made it possible to maintain purified primary neuron-only cultures in vitro. Improved media formulations can support the survival of low-density neuronal cultures for days or weeks. Such setups permit detailed molecular and morphological investigation of neuron-specific responses, even at the level of individual neurons.
From whole tissue explants to single cells, every model comes with its own set of strengths and weaknesses. With the possibility of culturing almost every neuronal tissue or cell type, the choice of in vitro model can be prioritized by biological relevance at every level. For example, one may choose cortical neurons to study CST regeneration, DRG neurons to study sensory axon regeneration, RGC neurons to study optic nerve regeneration, etc. Sufficient conservation in the mechanisms that control neurite outgrowth across different cell types may permit some flexibility with cell choice, particularly where strong technical considerations factor into assay design (e.g. using hippocampal neurons instead of cortical neurons for large scale screening campaigns focused on spinal cord injury). It is possible that iPSC derived neurons will become major tools for such studies in the foreseeable future, especially for HTS screening. It may take years, however, for the field to agree on robust and comprehensive markers to define “signatures” for different kinds of neurons (e.g., cortical and spinal motor neurons) and the culture conditions required to produce them from iPSC cells. In the end, a carefully selected battery of in vitro regeneration models, with varying levels of complexity, will likely constitute the best approach for understanding CNS regeneration biology. It is also of particular value for prioritizing therapeutic strategies before embarking on the expensive and time consuming in vivo studies.
Acknowledgments
This work was supported by the National Institute of Child Health and Human Development [grant number HD057632] and the National Institute of Neurological Disorders and Stroke [grant number NS080145], as well as the Miami Project to Cure Paralysis and the Walter G. Ross Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- (1).Aguayo AJ, Dickson R, Trecarten J, Attiwell M, Bray GM, Richardson P. Ensheathment and myelination of regenerating PNS fibres by transplanted optic nerve glia. Neurosci. Lett. 1978;9:97–104. doi: 10.1016/0304-3940(78)90055-1. [DOI] [PubMed] [Google Scholar]
- (2).Weinberg EL, Spencer PS. Studies on the control of myelinogenesis. 3. Signalling of oligodendrocyte myelination by regenerating peripheral axons. Brain Res. 1979;162:273–9. doi: 10.1016/0006-8993(79)90289-0. [DOI] [PubMed] [Google Scholar]
- (3).Aguayo AJ, David S, Bray GM. Influences of the glial environment on the elongation of axons after injury: transplantation studies in adult rodents. J. Exp. Biol. 1981;95:231–40. doi: 10.1242/jeb.95.1.231. [DOI] [PubMed] [Google Scholar]
- (4).Richardson PM, Issa VM, Aguayo AJ. Regeneration of long spinal axons in the rat. J. Neurocytol. 1984;13:165–82. doi: 10.1007/BF01148324. [DOI] [PubMed] [Google Scholar]
- (5).Edelman GM. Surface modulation in cell recognition and cell growth. Science. 1976;192:218–26. doi: 10.1126/science.769162. [DOI] [PubMed] [Google Scholar]
- (6).Levi-Montalcini R, Hamburger V. Selective growth stimulating effects of mouse sarcoma on the sensory and sympathetic nervous system of the chick embryo. J. Exp. Zool. 1951;116:321–61. doi: 10.1002/jez.1401160206. [DOI] [PubMed] [Google Scholar]
- (7).Takeichi M. Functional correlation between cell adhesive properties and some cell surface proteins. J. Cell Biol. 1977;75:464–74. doi: 10.1083/jcb.75.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Lemmon V, Burden SM, Payne HR, Elmslie GJ, Hlavin ML. Neurite growth on different substrates: permissive versus instructive influences and the role of adhesive strength. J. Neurosci. 1992;12:818–826. doi: 10.1523/JNEUROSCI.12-03-00818.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Case LC, Tessier-Lavigne M. Regeneration of the adult central nervous system. Curr. Biol. 2005;15:R749–53. doi: 10.1016/j.cub.2005.09.008. [DOI] [PubMed] [Google Scholar]
- (10).Filbin MT. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat. Rev. Neurosci. 2003;4:703–13. doi: 10.1038/nrn1195. [DOI] [PubMed] [Google Scholar]
- (11).Filbin MT. Recapitulate development to promote axonal regeneration: good or bad approach? Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2006;361:1565–74. doi: 10.1098/rstb.2006.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Pernet V, Joly S, Jordi N, Dalkara D, Guzik-Kornacka A, Flannery JG, Schwab ME. Misguidance and modulation of axonal regeneration by Stat3 and Rho/ROCK signaling in the transparent optic nerve. Cell Death Dis. 2013;4:e734. doi: 10.1038/cddis.2013.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Schwab ME, Bartholdi D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol. Rev. 1996;76:319–70. doi: 10.1152/physrev.1996.76.2.319. [DOI] [PubMed] [Google Scholar]
- (14).Lingor P, Teusch N, Schwarz K, Mueller R, Mack H, Bähr M, Mueller BK. Inhibition of Rho kinase (ROCK) increases neurite outgrowth on chondroitin sulphate proteoglycan in vitro and axonal regeneration in the adult optic nerve in vivo. J. Neurochem. 2007;103:181–9. doi: 10.1111/j.1471-4159.2007.04756.x. [DOI] [PubMed] [Google Scholar]
- (15).Sivasankaran R, Pei J, Wang KC, Zhang YP, Shields CB, Xu X-M, He Z. PKC mediates inhibitory effects of myelin and chondroitin sulfate proteoglycans on axonal regeneration. Nat. Neurosci. 2004;7:261–8. doi: 10.1038/nn1193. [DOI] [PubMed] [Google Scholar]
- (16).Shen Y, Tenney AP, Busch SA, Horn KP, Cuascut FX, Liu K, He Z, Silver J, Flanagan JG. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science. 2009;326:592–6. doi: 10.1126/science.1178310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Yamashita T, Higuchi H, Tohyama M. The p75 receptor transduces the signal from myelin-associated glycoprotein to Rho. J. Cell Biol. 2002;157:565–70. doi: 10.1083/jcb.200202010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Nakamura F, Vartanian T. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature. 2000;7199:439–444. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- (19).Fournier a E., GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409:341–6. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- (20).Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat. Rev. Neurosci. 2006;7:617–27. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).McKeon RJ, Schreiber RC, Rudge JS, Silver J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J. Neurosci. 1991;11:3398–411. doi: 10.1523/JNEUROSCI.11-11-03398.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Lee JK, Geoffroy CG, Chan AF, Tolentino KE, Crawford MJ, Leal MA, Kang B, Zheng B. Assessing spinal axon regeneration and sprouting in Nogo-, MAG-, and OMgp-deficient mice. Neuron. 2010;66:663–70. doi: 10.1016/j.neuron.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Lee JK, Chow R, Xie F, Chow SY, Tolentino KE, Zheng B. Combined genetic attenuation of myelin and semaphorin-mediated growth inhibition is insufficient to promote serotonergic axon regeneration. J. Neurosci. 2010;30:10899–904. doi: 10.1523/JNEUROSCI.2269-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Shields LBE, Zhang YP, Burke DA, Gray R, Shields CB. Benefit of chondroitinase ABC on sensory axon regeneration in a laceration model of spinal cord injury in the rat. Surg. Neurol. 2008;69:568–77. doi: 10.1016/j.surneu.2008.02.009. discussion 577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Moore DL, Blackmore MG, Hu Y, Kaestner KH, Bixby JL, Lemmon VP, Goldberg JL. KLF family members regulate intrinsic axon regeneration ability. Science. 2009;326:298–301. doi: 10.1126/science.1175737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Park KK, Liu K, Hu Y, Kanter JL, He Z. PTEN/mTOR and axon regeneration. Exp. Neurol. 2010;223:45–50. doi: 10.1016/j.expneurol.2009.12.032. [DOI] [PubMed] [Google Scholar]
- (27).Blackmore MG, Wang Z, Lerch JK, Motti D, Zhang YP, Shields CB, Lee JK, Goldberg JL, Lemmon VP, Bixby JL. Krüppel-like Factor 7 engineered for transcriptional activation promotes axon regeneration in the adult corticosal tract. Proc. Natl. Acad. Sci. U. S. A. 2012;109:7517–22. doi: 10.1073/pnas.1120684109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–6. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Chen Y, Stevens B, Chang J, Milbrandt J, Barres BA, Hell JW. NS21: re-defined and modified supplement B27 for neuronal cultures. J. Neurosci. Methods. 2008;171:239–47. doi: 10.1016/j.jneumeth.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J. Neurosci. Res. 1993;35:567–76. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- (31).Brewer GJ. Isolation and culture of adult rat hippocampal neurons. J. Neurosci. Methods. 1997;71:143–55. doi: 10.1016/s0165-0270(96)00136-7. [DOI] [PubMed] [Google Scholar]
- (32).Brewer GJ. Serum-free B27/neurobasal medium supports differentiated growth of neurons from the striatum, substantia nigra, septum, cerebral cortex, cerebellum, and dentate gyrus. J. Neurosci. Res. 1995;42:674–683. doi: 10.1002/jnr.490420510. [DOI] [PubMed] [Google Scholar]
- (33).Patel TD, Kramer I, Kucera J, Niederkofler V, Jessell TM, Arber S, Snider WD. Peripheral NT3 Signaling Is Required for ETS Protein Expression and Central Patterning of Proprioceptive Sensory Afferents. Neuron. 2003;38:403–416. doi: 10.1016/s0896-6273(03)00261-7. [DOI] [PubMed] [Google Scholar]
- (34).Al-Ali H, Blackmore M, Bixby JL, Lemmon VP. High Content Screening with Primary Neurons. Assay Guid. Man. 2013 [Google Scholar]
- (35).Dotti CG, Sullivan CA, Banker GA. The establishment of polarity by hippocampal neurons in culture. J. Neurosci. 1988;8:1454–68. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Bartlett WP, Banker GA. An electron microscopic study of the development of axons and dendrites by hippocampal neurons in culture. I. Cells which develop without intercellular contacts. J. Neurosci. 1984;4:1944–53. doi: 10.1523/JNEUROSCI.04-08-01944.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Ohara R, Hata K, Yasuhara N, Mehmood R, Yoneda Y, Nakagawa M, Yamashita T. Axotomy induces axonogenesis in hippocampal neurons by a mechanism dependent on importin β. Biochem. Biophys. Res. Commun. 2011;405:697–702. doi: 10.1016/j.bbrc.2011.01.108. [DOI] [PubMed] [Google Scholar]
- (38).Beaudoin GMJ, Lee S-H, Singh D, Yuan Y, Ng Y-G, Reichardt LF, Arikkath J. Culturing pyramidal neurons from the early postnatal mouse hippocampus and cortex. Nat. Protoc. 2012;7:1741–54. doi: 10.1038/nprot.2012.099. [DOI] [PubMed] [Google Scholar]
- (39).Blackmore MG, Moore DL, Smith RP, Goldberg JL, Bixby JL, Lemmon VP. High content screening of cortical neurons identifies novel regulators of axon growth. Mol. Cell. Neurosci. 2010;44:43–54. doi: 10.1016/j.mcn.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Gopalakrishnan SM, Teusch N, Imhof C, Bakker MHM, Schurdak M, Burns DJ, Warrior U. Role of Rho kinase pathway in chondroitin sulfate proteoglycan-mediated inhibition of neurite outgrowth in PC12 cells. J. Neurosci. Res. 2008;86:2214–26. doi: 10.1002/jnr.21671. [DOI] [PubMed] [Google Scholar]
- (41).Loh SHY, Francescut L, Lingor P, Bähr M, Nicotera P. Identification of new kinase clusters required for neurite outgrowth and retraction by a loss-of-function RNA interference screen. Cell Death Differ. 2008;15:283–98. doi: 10.1038/sj.cdd.4402258. [DOI] [PubMed] [Google Scholar]
- (42).Buchser WJ, Slepak TI, Gutierrez-Arenas O, Bixby JL, Lemmon VP. Kinase/phosphatase overexpression reveals pathways regulating hippocampal neuron morphology. Mol. Syst. Biol. 2010;6:391. doi: 10.1038/msb.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Al-Ali H, Lee D-H, Danzi MC, Nassif H, Gautam P, Wennerberg K, Zuercher B, Drewry DH, Lee JK, Lemmon VP, Bixby JL. Rational Polypharmacology: Systematically Identifying and Engaging Multiple Drug Targets To Promote Axon Growth. ACS Chem. Biol. 2015;10:1939–51. doi: 10.1021/acschembio.5b00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Carbonetto ST, Gruver MM, Turner DC. Nerve fiber growth on defined hydrogel substrates. Science. 1982;216:897–9. doi: 10.1126/science.7079743. [DOI] [PubMed] [Google Scholar]
- (45).Welsbie DS, Yang Z, Ge Y, Mitchell KL, Zhou X, Martin SE, Berlinicke CA, Hackler L, Fuller J, Fu J, Cao L-H, Han B, Auld D, Xue T, Hirai S-I, Germain L, Simard-Bisson C, Blouin R, Nguyen JV, Davis C-HO, Enke RA, Boye SL, Merbs SL, Marsh-Armstrong N, Hauswirth WW, Diantonio A, Nickells RW, Inglese J, Hanes J, Yau K-W, Quigley HA, Zack DJ. Functional genomic screening identifies dual leucine zipper kinase as a key mediator of retinal ganglion cell death. Proc. Natl. Acad. Sci. U. S. A. 2013;110:4045–50. doi: 10.1073/pnas.1211284110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Chuckowree JA, Vickers JC. Cytoskeletal and morphological alterations underlying axonal sprouting after localized transection of cortical neuron axons in vitro. J. Neurosci. 2003;23:3715–25. doi: 10.1523/JNEUROSCI.23-09-03715.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Zhang JJ-H, Chung T, Oldenburg K. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J. Biomol. Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- (48).Yeyeodu ST, Witherspoon SM, Gilyazova N, Ibeanu GC. A rapid, inexpensive high throughput screen method for neurite outgrowth. Curr. Chem. Genomics. 2010;4:74–83. doi: 10.2174/1875397301004010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).B. McManus O, McEwen D. Measuring Neurite Dynamics In Vitro. Genet. Eng. Biotechnol. News. 2015;35:14–15. [Google Scholar]
- (50).Kapfhammer JP, Raper JA. Collapse of growth cone structure on contact with specific neurites in culture. J. Neurosci. 1987;7:201–12. doi: 10.1523/JNEUROSCI.07-01-00201.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Fukushima N. LPA in neural cell development. J. Cell. Biochem. 2004;92:993–1003. doi: 10.1002/jcb.20093. [DOI] [PubMed] [Google Scholar]
- (52).Roloff F, Scheiblich H, Dewitz C, Dempewolf S, Stern M, Bicker G. Enhanced neurite outgrowth of human model (NT2) neurons by small-molecule inhibitors of Rho/ROCK signaling. PLoS One. 2015;10:e0118536. doi: 10.1371/journal.pone.0118536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Jin Z, Strittmatter SM. Rac1 Mediates Collapsin-1-Induced Growth Cone Collapse. J. Neurosci. 1997;17:6256–6263. doi: 10.1523/JNEUROSCI.17-16-06256.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Sakisaka T, Baba T, Tanaka S, Izumi G, Yasumi M, Takai Y. Regulation of SNAREs by tomosyn and ROCK: implication in extension and retraction of neurites. J. Cell Biol. 2004;166:17–25. doi: 10.1083/jcb.200405002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Walter J, Kern-Veits B, Huf J, Stolze B, Bonhoeffer F. Recognition of position-specific properties of tectal cell membranes by retinal axons in vitro. Development. 1987;101:685–96. doi: 10.1242/dev.101.4.685. [DOI] [PubMed] [Google Scholar]
- (56).Walter J, Henke-Fahle S, Bonhoeffer F. Avoidance of posterior tectal membranes by temporal retinal axons. Development. 1987;101:909–13. doi: 10.1242/dev.101.4.909. [DOI] [PubMed] [Google Scholar]
- (57).Alabed YZ, Pool M, Tone SO, Fournier AE, Ong Tone S. Identification of CRMP4 as a convergent regulator of axon outgrowth inhibition. J. Neurosci. 2007;27:1702–11. doi: 10.1523/JNEUROSCI.5055-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Wang HU, Anderson DJ. Eph family transmembrane ligands can mediate repulsive guidance of trunk neural crest migration and motor axon outgrowth. Neuron. 1997;18:383–96. doi: 10.1016/s0896-6273(00)81240-4. [DOI] [PubMed] [Google Scholar]
- (59).Vielmetter J, Stolze B, Bonhoeffer F, Stuermer CA. In vitro assay to test differential substrate affinities of growing axons and migratory cells. Exp. brain Res. 1990;81:283–7. doi: 10.1007/BF00228117. [DOI] [PubMed] [Google Scholar]
- (60).Nguyen-Ba-Charvet KT, Brose K, Marillat V, Sotelo C, Tessier-Lavigne M, Chédotal A. Sensory axon response to substrate-bound Slit2 is modulated by laminin and cyclic GMP. Mol. Cell. Neurosci. 2001;17:1048–58. doi: 10.1006/mcne.2001.0994. [DOI] [PubMed] [Google Scholar]
- (61).Pozas E, Pascual M, Nguyen Ba-Charvet KT, Guijarro P, Sotelo C, Chédotal A, Del Río JA, Soriano E. Age-dependent effects of secreted Semaphorins 3A, 3F, and 3E on developing hippocampal axons: in vitro effects and phenotype of Semaphorin 3A (−/−) mice. Mol. Cell. Neurosci. 2001;18:26–43. doi: 10.1006/mcne.2001.0999. [DOI] [PubMed] [Google Scholar]
- (62).Monnier PP, Sierra A, Schwab JM, Henke-Fahle S, Mueller BK. The Rho/ROCK pathway mediates neurite growth-inhibitory activity associated with the chondroitin sulfate proteoglycans of the CNS glial scar. Mol. Cell. Neurosci. 2003;22:319–330. doi: 10.1016/s1044-7431(02)00035-0. [DOI] [PubMed] [Google Scholar]
- (63).Oertle T, van der Haar ME, Bandtlow CE, Robeva A, Burfeind P, Buss A, Huber AB, Simonen M, Schnell L, Brösamle C, Kaupmann K, Vallon R, Schwab ME. Nogo-A inhibits neurite outgrowth and cell spreading with three discrete regions. J. Neurosci. 2003;23:5393–406. doi: 10.1523/JNEUROSCI.23-13-05393.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Snow DM, Lemmon V, Carrino DA, Caplan AI, Silver J. Sulfated proteoglycans in astroglial barriers inhibit neurite outgrowth in vitro. Exp. Neurol. 1990;109:111–130. doi: 10.1016/s0014-4886(05)80013-5. [DOI] [PubMed] [Google Scholar]
- (65).Knöll B, Weinl C, Nordheim A, Bonhoeffer F. Stripe assay to examine axonal guidance and cell migration. Nat. Protoc. 2007;2:1216–24. doi: 10.1038/nprot.2007.157. [DOI] [PubMed] [Google Scholar]
- (66).Jain A, Brady-Kalnay SM, Bellamkonda RV. Modulation of Rho GTPase activity alleviates chondroitin sulfate proteoglycan-dependent inhibition of neurite extension. J. Neurosci. Res. 2004;77:299–307. doi: 10.1002/jnr.20161. [DOI] [PubMed] [Google Scholar]
- (67).Hahn CM, Kleinholz H, Koester MP, Grieser S, Thelen K, Pollerberg GE. Role of cyclin-dependent kinase 5 and its activator P35 in local axon and growth cone stabilization. Neuroscience. 2005;134:449–465. doi: 10.1016/j.neuroscience.2005.04.020. [DOI] [PubMed] [Google Scholar]
- (68).von Philipsborn AC, Lang S, Bernard A, Loeschinger J, David C, Lehnert D, Bastmeyer M, Bonhoeffer F. Microcontact printing of axon guidance molecules for generation of graded patterns. Nat. Protoc. 2006;1:1322–8. doi: 10.1038/nprot.2006.251. [DOI] [PubMed] [Google Scholar]
- (69).Snow DM, Letourneau PC. Neurite outgrowth on a step gradient of chondroitin sulfate proteoglycan (CS-PG) J. Neurobiol. 1992;23:322–36. doi: 10.1002/neu.480230311. [DOI] [PubMed] [Google Scholar]
- (70).Gundersen RW, Barrett JN. Neuronal chemotaxis: chick dorsal-root axons turn toward high concentrations of nerve growth factor. Science. 1979;206:1079–80. doi: 10.1126/science.493992. [DOI] [PubMed] [Google Scholar]
- (71).Lohof AM, Quillan M, Dan Y, Poo MM. Asymmetric modulation of cytosolic cAMP activity induces growth cone turning. J. Neurosci. 1992;12:1253–61. doi: 10.1523/JNEUROSCI.12-04-01253.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Hong K, Nishiyama M, Henley J, Tessier-Lavigne M, Poo M. Calcium signalling in the guidance of nerve growth by netrin-1. Nature. 2000;403:93–98. doi: 10.1038/47507. [DOI] [PubMed] [Google Scholar]
- (73).Sun QL, Wang J, Bookman RJ, Bixby JL. Growth cone steering by receptor tyrosine phosphatase delta defines a distinct class of guidance cue. Mol. Cell. Neurosci. 2000;16:686–95. doi: 10.1006/mcne.2000.0893. [DOI] [PubMed] [Google Scholar]
- (74).Weinl C, Drescher U, Lang S, Bonhoeffer F, Löschinger J. On the turning of Xenopus retinal axons induced by ephrin-A5. Development. 2003;130:1635–43. doi: 10.1242/dev.00386. [DOI] [PubMed] [Google Scholar]
- (75).Yu P, Wang H, Katagiri Y, Geller HM. An in vitro model of reactive astrogliosis and its effect on neuronal growth. Methods Mol. Biol. 2012;814:327–40. doi: 10.1007/978-1-61779-452-0_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Karumbaiah L, Anand S, Thazhath R, Zhong Y, Mckeon RJ, Bellamkonda RV. Targeted downregulation of N-acetylgalactosamine 4-sulfate 6-O-sulfotransferase significantly mitigates chondroitin sulfate proteoglycan-mediated inhibition. Glia. 2011;59:981–996. doi: 10.1002/glia.21170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Laabs TL, Wang H, Katagiri Y, McCann T, Fawcett JW, Geller HM. Inhibiting glycosaminoglycan chain polymerization decreases the inhibitory activity of astrocyte-derived chondroitin sulfate proteoglycans. J. Neurosci. 2007;27:14494–14501. doi: 10.1523/JNEUROSCI.2807-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Tom VJ, Steinmetz MP, Miller JH, Doller CM, Silver J. Studies on the development and behavior of the dystrophic growth cone, the hallmark of regeneration failure, in an in vitro model of the glial scar and after spinal cord injury. J. Neurosci. 2004;24:6531–9. doi: 10.1523/JNEUROSCI.0994-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Barres BA, Silverstein BE, Corey DP, Chun LL. Immunological, morphological, and electrophysiological variation among retinal ganglion cells purified by panning. Neuron. 1988;1:791–803. doi: 10.1016/0896-6273(88)90127-4. [DOI] [PubMed] [Google Scholar]
- (80).Meyer-Franke A, Kaplan MR, Pfieger FW, Barres BA. Characterization of the signaling interactions that promote the survival and growth of developing retinal ganglion cells in culture. Neuron. 1995;15:805–819. doi: 10.1016/0896-6273(95)90172-8. [DOI] [PubMed] [Google Scholar]
- (81).Bischof J, Müller A, Fänder M, Knippschild U, Fischer D. Neurite Outgrowth of Mature Retinal Ganglion Cells and PC12 Cells Requires Activity of CK1δ and CK1ε. PLoS One. 2011;6:e20857. doi: 10.1371/journal.pone.0020857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Goldberg JL, Espinosa JS, Xu Y, Davidson N, Kovacs GTA, Barres BA. Retinal ganglion cells do not extend axons by default: promotion by neurotrophic signaling and electrical activity. Neuron. 2002;33:689–702. doi: 10.1016/s0896-6273(02)00602-5. [DOI] [PubMed] [Google Scholar]
- (83).Trakhtenberg EF, Goldberg JL. Epigenetic regulation of axon and dendrite growth. Front. Mol. Neurosci. 2012;5:24. doi: 10.3389/fnmol.2012.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Banker GA, Cowan WM. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977;126:397–42. doi: 10.1016/0006-8993(77)90594-7. [DOI] [PubMed] [Google Scholar]
- (85).Dotti CG, Banker GA. Experimentally induced alteration in the polarity of developing neurons. Nature. 330:254–6. doi: 10.1038/330254a0. [DOI] [PubMed] [Google Scholar]
- (86).Al-Ali H, Schürer SC, Lemmon VP, Bixby JL. Chemical interrogation of the neuronal kinome using a primary cell-based screening assay. ACS Chem. Biol. 2013;8:1027–36. doi: 10.1021/cb300584e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Beattie CE, Siegel RE. Developmental cues modulate GABAA receptor subunit mRNA expression in cultured cerebellar granule neurons. J. Neurosci. 1993;13:1784–92. doi: 10.1523/JNEUROSCI.13-04-01784.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Mukhopadhyay G, Doherty P, Walsh FS, Crocker PR, Filbin MT. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994;13:757–67. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- (89).Usher LC, Johnstone A, Erturk A, Hu Y, Strikis D, Wanner IB, Moorman S, Lee JW, Min J, Ha H-HH, Duan Y, Hoffman S, Goldberg JL, Bradke F, Chang YT, Lemmon VP, Bixby JL, Lemmon P, Ertürk A. A chemical screen identifies novel compounds that overcome glial-mediated inhibition of neuronal regeneration. J. Neurosci. 2010;30:4693–4706. doi: 10.1523/JNEUROSCI.0302-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Koprivica V, Cho KS, Park JB, Yiu G, Atwal J, Gore B, Kim JA, Lin E, Tessier-Lavigne M, Chen DF, He Z. EGFR activation mediates inhibition of axon regeneration by myelin and chondroitin sulfate proteoglycans. Science (80-.) 2005;310:106–110. doi: 10.1126/science.1115462. [DOI] [PubMed] [Google Scholar]
- (91).Chen Z-L, Yu W-M, Strickland S. Peripheral regeneration. Annu. Rev. Neurosci. 2007;30:209–33. doi: 10.1146/annurev.neuro.30.051606.094337. [DOI] [PubMed] [Google Scholar]
- (92).Liuzzi FJ, Tedeschi B. Peripheral nerve regeneration. Neurosurg. Clin. N. Am. 1991;2:31–42. [PubMed] [Google Scholar]
- (93).y Cajal SR. Degeneration & regeneration of the nervous system. Oxford University Press; Humphrey Milford: 1928. [Google Scholar]
- (94).Neumann S, Bradke F, Tessier-Lavigne M, Basbaum AI. Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron. 2002;34:885–93. doi: 10.1016/s0896-6273(02)00702-x. [DOI] [PubMed] [Google Scholar]
- (95).Neumann S, Woolf CJ. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron. 1999;23:83–91. doi: 10.1016/s0896-6273(00)80755-2. [DOI] [PubMed] [Google Scholar]
- (96).Cai D, Qiu J, Cao Z, McAtee M, Bregman BS, Filbin MT. Neuronal cyclic AMP controls the developmental loss in ability of axons to regenerate. J. Neurosci. 2001;21:4731–9. doi: 10.1523/JNEUROSCI.21-13-04731.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).Song H, Ming G, He Z, Lehmann M, McKerracher L, Tessier-Lavigne M, Poo M. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science. 1998;281:1515–8. doi: 10.1126/science.281.5382.1515. [DOI] [PubMed] [Google Scholar]
- (98).Lindner R, Puttagunta R, Nguyen T, Di Giovanni S. DNA methylation temporal profiling following peripheral versus central nervous system axotomy. Sci. data. 2014;1:140038. doi: 10.1038/sdata.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).Cho Y, Sloutsky R, Naegle KM, Cavalli V. Injury-Induced HDAC5 Nuclear Export Is Essential for Axon Regeneration. Cell. 2013;155:894–908. doi: 10.1016/j.cell.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (100).Fisher D, Xing B, Dill J, Li H, Hoang HH, Zhao Z, Yang X-L, Bachoo R, Cannon S, Longo FM, Sheng M, Silver J, Li S. Leukocyte common antigen-related phosphatase is a functional receptor for chondroitin sulfate proteoglycan axon growth inhibitors. J. Neurosci. 2011;31:14051–66. doi: 10.1523/JNEUROSCI.1737-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (101).Lindsay RM. Nerve growth factors (NGF, BDNF) enhance axonal regeneration but are not required for survival of adult sensory neurons. J. Neurosci. 1988;8:2394–405. doi: 10.1523/JNEUROSCI.08-07-02394.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).Gilbert RJ, McKeon RJ, Darr A, Calabro A, Hascall VC, Bellamkonda RV. CS-4,6 is differentially upregulated in glial scar and is a potent inhibitor of neurite extension. Mol. Cell. Neurosci. 2005;29:545–58. doi: 10.1016/j.mcn.2005.04.006. [DOI] [PubMed] [Google Scholar]
- (103).Usoskin D, Furlan A, Islam S, Abdo H, Lönnerberg P, Lou D, Hjerling-Leffler J, Haeggström J, Kharchenko O, Kharchenko PV, Linnarsson S, Ernfors P. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat. Neurosci. 2014;18:145–153. doi: 10.1038/nn.3881. [DOI] [PubMed] [Google Scholar]
- (104).Chen C-L, Broom DC, Liu Y, de Nooij JC, Li Z, Cen C, Samad OA, Jessell TM, Woolf CJ, Ma Q. Runx1 determines nociceptive sensory neuron phenotype and is required for thermal and neuropathic pain. Neuron. 2006;49:365–77. doi: 10.1016/j.neuron.2005.10.036. [DOI] [PubMed] [Google Scholar]
- (105).Zuchero JB. Purification of dorsal root ganglion neurons from rat by immunopanning. Cold Spring Harb. Protoc. 2014;2014:826–38. doi: 10.1101/pdb.prot074948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (106).Malin SA, Davis BM, Molliver DC. Production of dissociated sensory neuron cultures and considerations for their use in studying neuronal function and plasticity. Nat. Protoc. 2007;2:152–60. doi: 10.1038/nprot.2006.461. [DOI] [PubMed] [Google Scholar]
- (107).Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc. Natl. Acad. Sci. U. S. A. 1976;73:2424–8. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (108).Das KP, Freudenrich TM, Mundy WR. Assessment of PC12 cell differentiation and neurite growth: a comparison of morphological and neurochemical measures. Neurotoxicol. Teratol. 2004;26:397–406. doi: 10.1016/j.ntt.2004.02.006. [DOI] [PubMed] [Google Scholar]
- (109).Biedler JL, Roffler-Tarlov S, Schachner M, Freedman LS. Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer Res. 1978;38:3751–7. [PubMed] [Google Scholar]
- (110).Lee VM, Andrews PW. Differentiation of NTERA-2 clonal human embryonal carcinoma cells into neurons involves the induction of all three neurofilament proteins. J. Neurosci. 1986;6:514–21. doi: 10.1523/JNEUROSCI.06-02-00514.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (111).Marchetto MCN, Winner B, Gage FH. Pluripotent stem cells in neurodegenerative and neurodevelopmental diseases. Hum. Mol. Genet. 2010;19:R71–R76. doi: 10.1093/hmg/ddq159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (112).Phillips BW, Crook JM. Pluripotent Human Stem Cells. BioDrugs. 2010;24:99–108. doi: 10.2165/11532270-000000000-00000. [DOI] [PubMed] [Google Scholar]
- (113).Kitazawa A, Shimizu N. Differentiation of mouse induced pluripotent stem cells into neurons using conditioned medium of dorsal root ganglia. N. Biotechnol. 2011;28:326–33. doi: 10.1016/j.nbt.2011.03.011. [DOI] [PubMed] [Google Scholar]
- (114).Kim J-E, O’Sullivan ML, Sanchez CA, Hwang M, Israel MA, Brennand K, Deerinck TJ, Goldstein LSB, Gage FH, Ellisman MH, Ghosh A. Investigating synapse formation and function using human pluripotent stem cell-derived neurons. Proc. Natl. Acad. Sci. U. S. A. 2011;108:3005–10. doi: 10.1073/pnas.1007753108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (115).Marchetto MCN, Carromeu C, Acab A, Yu D, Yeo GW, Mu Y, Chen G, Gage FH, Muotri AR. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell. 2010;143:527–39. doi: 10.1016/j.cell.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (116).Shi Y, Kirwan P, Livesey FJ. Directed differentiation of human pluripotent stem cells to cerebral cortex neurons and neural networks. Nat. Protoc. 2012;7:1836–46. doi: 10.1038/nprot.2012.116. [DOI] [PubMed] [Google Scholar]
- (117).Pedrosa E, Sandler V, Shah A, Carroll R, Chang C, Rockowitz S, Guo X, Zheng D, Lachman HM. Development of patient-specific neurons in schizophrenia using induced pluripotent stem cells. J. Neurogenet. 2011;25:88–103. doi: 10.3109/01677063.2011.597908. [DOI] [PubMed] [Google Scholar]
- (118).Zeng H, Guo M, Martins-Taylor K, Wang X, Zhang Z, Park JW, Zhan S, Kronenberg MS, Lichtler A, Liu H-X, Chen F-P, Yue L, Li X-J, Xu R-H. Specification of Region-Specific Neurons Including Forebrain Glutamatergic Neurons from Human Induced Pluripotent Stem Cells. In: Reh TA, editor. PLoS One. Vol. 5. 2010. p. e11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (119).Brennand KJ, Simone A, Jou J, Gelboin-Burkhart C, Tran N, Sangar S, Li Y, Mu Y, Chen G, Yu D, McCarthy S, Sebat J, Gage FH. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011;473:221–225. doi: 10.1038/nature09915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (120).Kramer AS, Harvey AR, Plant GW, Hodgetts SI. Systematic Review of Induced Pluripotent Stem Cell Technology as a Potential Clinical Therapy for Spinal Cord Injury. Cell Transplant. 2013;22:571–617. doi: 10.3727/096368912X655208. [DOI] [PubMed] [Google Scholar]
- (121).Sirenko O, Hesley J, Rusyn I, Cromwell EF. High-content high-throughput assays for characterizing the viability and morphology of human iPSC-derived neuronal cultures. Assay Drug Dev. Technol. 2014;12:536–47. doi: 10.1089/adt.2014.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (122).Hancock MK, Kopp L, Kaur N, Hanson BJ. A Facile Method for Simultaneously Measuring Neuronal Cell Viability and Neurite Outgrowth. Curr. Chem. Genomics Transl. Med. 2015;9:6–16. doi: 10.2174/2213988501509010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (123).Wu C, Schulte J, Sepp KJ, Littleton JT, Hong P. Automatic robust neurite detection and morphological analysis of neuronal cell cultures in high-content screening. Neuroinformatics. 2010;8:83–100. doi: 10.1007/s12021-010-9067-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (124).Liang C-C, Park AY, Guan J-L. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007;2:329–33. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- (125).Todaro GJ, Lazar GK, Green H. The initiation of cell division in a contact-inhibited mammalian cell line. J. Cell. Physiol. 1965;66:325–33. doi: 10.1002/jcp.1030660310. [DOI] [PubMed] [Google Scholar]
- (126).Cory G. Scratch-wound assay. Methods Mol. Biol. 2011;769:25–30. doi: 10.1007/978-1-61779-207-6_2. [DOI] [PubMed] [Google Scholar]
- (127).Zou Y, Stagi M, Wang X, Yigitkanli K, Siegel CS, Nakatsu F, Cafferty WBJ, Strittmatter SM. Gene-Silencing Screen for Mammalian Axon Regeneration Identifies Inpp5f (Sac2) as an Endogenous Suppressor of Repair after Spinal Cord Injury. J. Neurosci. 2015;35:10429–39. doi: 10.1523/JNEUROSCI.1718-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (128).Nishio T, Kawaguchi S, Yamamoto M, Iseda T, Kawasaki T, Hase T. Tenascin-C regulates proliferation and migration of cultured astrocytes in a scratch wound assay. Neuroscience. 2005;132:87–102. doi: 10.1016/j.neuroscience.2004.12.028. [DOI] [PubMed] [Google Scholar]
- (129).Faber-Elman A, Solomon A, Abraham J. a, Marikovsky M, Schwartz M. Involvement of wound-associated factors in rat brain astrocyte migratory response to axonal injury: in vitro simulation. J. Clin. Invest. 1996;97:162–71. doi: 10.1172/JCI118385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (130).Robel S, Bardehle S, Lepier A, Brakebusch C, Götz M. Genetic deletion of cdc42 reveals a crucial role for astrocyte recruitment to the injury site in vitro and in vivo. J. Neurosci. 2011;31:12471–82. doi: 10.1523/JNEUROSCI.2696-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (131).Etienne-Manneville S. In vitro assay of primary astrocyte migration as a tool to study Rho GTPase function in cell polarization. Methods Enzymol. 2006;406:565–78. doi: 10.1016/S0076-6879(06)06044-7. [DOI] [PubMed] [Google Scholar]
- (132).Gerdts J, Sasaki Y, Vohra B, Marasa J, Milbrandt J. Image-based screening identifies novel roles for IKK and GSK3 in axonal degeneration. J. Biol. Chem. 2011;286:28011–8. doi: 10.1074/jbc.M111.250472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (133).Campenot RB. NGF and the local control of nerve terminal growth. J. Neurobiol. 1994;25:599–611. doi: 10.1002/neu.480250603. [DOI] [PubMed] [Google Scholar]
- (134).Taylor AM, Blurton-Jones M, Rhee SW, Cribbs DH, Cotman CW, Jeon NL. A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nat. Methods. 2005;2:599–605. doi: 10.1038/nmeth777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (135).Campenot RB. Local control of neurite development by nerve growth factor. Proc. Natl. Acad. Sci. U. S. A. 1977;74:4516–9. doi: 10.1073/pnas.74.10.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (136).MacInnis BL. Retrograde Support of Neuronal Survival Without Retrograde Transport of Nerve Growth Factor. Science (80-.) 2002;295:1536–1539. doi: 10.1126/science.1064913. [DOI] [PubMed] [Google Scholar]
- (137).Sackmann EK, Fulton AL, Beebe DJ. The present and future role of microfluidics in biomedical research. Nature. 2014;507:181–9. doi: 10.1038/nature13118. [DOI] [PubMed] [Google Scholar]
- (138).Taylor AM, Rhee SW, Tu CH, Cribbs DH, Cotman CW, Jeon NL. Microfluidic Multicompartment Device for Neuroscience Research. Langmuir. 2003;19:1551–1556. doi: 10.1021/la026417v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (139).Dinh N-D, Chiang Y-Y, Hardelauf H, Waide S, Janasek D, West J. Preparation of neuronal co-cultures with single cell precision. J. Vis. Exp. 2014 doi: 10.3791/51389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (140).Cregg JM, DePaul MA, Filous AR, Lang BT, Tran A, Silver J. Functional regeneration beyond the glial scar. Exp. Neurol. 2014;253:197–207. doi: 10.1016/j.expneurol.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (141).David S, Aguayo AJ. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science. 1981;214:931–3. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- (142).Bardehle S, er M, Buggenthin F, Schwausch J, Ninkovic J, Clevers H, Snippert HJ, Theis FJ, Meyer-Luehmann M, Bechmann I, Dimou L, Götz M. Live imaging of astrocyte responses to acute injury reveals selective juxtavascular proliferation. Nat. Neurosci. 2013;16:580–6. doi: 10.1038/nn.3371. [DOI] [PubMed] [Google Scholar]
- (143).McKeon RJ, Jurynec MJ, Buck CR. The chondroitin sulfate proteoglycans neurocan and phosphacan are expressed by reactive astrocytes in the chronic CNS glial scar. J. Neurosci. 1999;19:10778–88. doi: 10.1523/JNEUROSCI.19-24-10778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (144).Wanner IB, Deik A, Torres M, Rosendahl A, Neary JT, Lemmon VP, Bixby JL. A new in vitro model of the glial scar inhibits axon growth. Glia. 2008;56:1691–709. doi: 10.1002/glia.20721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (145).Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN, Mucke L, Johnson MH, Sofroniew MV. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23:297–308. doi: 10.1016/s0896-6273(00)80781-3. [DOI] [PubMed] [Google Scholar]
- (146).Rolls A, Shechter R, Schwartz M. The bright side of the glial scar in CNS repair. Nat. Rev. Neurosci. 2009;10:235–41. doi: 10.1038/nrn2591. [DOI] [PubMed] [Google Scholar]
- (147).Noble M, Mayer-Pröschel M. Culture of Astrocytes, Oligodendrocytes, and O-2A Progenitor Cells. In: Banker G, Goslin K, editors. Culturing Nerve Cells. 2nd 1998. p. 678. [Google Scholar]
- (148).McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J. Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (149).Jungblut M, Tiveron MC, Barral S, Abrahamsen B, Knöbel S, Pennartz S, Schmitz J, Perraut M, Pfrieger FW, Stoffel W, Cremer H, Bosio A. Isolation and characterization of living primary astroglial cells using the new GLAST-specific monoclonal antibody ACSA-1. Glia. 2012;60:894–907. doi: 10.1002/glia.22322. [DOI] [PubMed] [Google Scholar]
- (150).Foo LC. Purification and culture of astrocytes. Cold Spring Harb. Protoc. 2013;8:485–487. doi: 10.1101/pdb.top070912. [DOI] [PubMed] [Google Scholar]
- (151).Beckerman SR, Jimenez JE, Shi Y, Al-Ali H, Bixby JL, Lemmon VP. Phenotypic Assays to Identify Agents That Induce Reactive Gliosis: A Counter-Screen to Prioritize Compounds for Preclinical Animal Studies. Assay Drug Dev. Technol. 2015;13:377–388. doi: 10.1089/adt.2015.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (152).Wang R, Zhang X, Zhang J, Fan Y, Shen Y, Hu W, Chen Z. Oxygen-Glucose Deprivation Induced Glial Scar-Like Change in Astrocytes. In: Deli MA, editor. PLoS One. Vol. 7. 2012. p. e37574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (153).Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA. Genomic Analysis of Reactive Astrogliosis. J. Neurosci. 2012 doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (154).Lee JO, Yang H, Georgescu MM, Di Cristofano a, Maehama T, Shi Y, Dixon JE, Pandolfi P, Pavletich NP. Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell. 1999;99:323–34. doi: 10.1016/s0092-8674(00)81663-3. [DOI] [PubMed] [Google Scholar]
- (155).Doering LC, editor. Protocols for Neural Cell Culture. Humana Press; Totowa, NJ: 2010. [Google Scholar]
- (156).Anderl JL, Redpath S, Ball AJ. A neuronal and astrocyte co-culture assay for high content analysis of neurotoxicity. J. Vis. Exp. 2009:2–7. doi: 10.3791/1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (157).Fawcett JW, Housden E, Smith-Thomas L, Meyer RL. The growth of axons in three-dimensional astrocyte cultures. Dev. Biol. 1989;135:449–458. doi: 10.1016/0012-1606(89)90193-0. [DOI] [PubMed] [Google Scholar]
- (158).East E, de Oliveira DB, Golding JP, Phillips JB. Alignment of astrocytes increases neuronal growth in three-dimensional collagen gels and is maintained following plastic compression to form a spinal cord repair conduit. Tissue Eng. Part A. 2010;16:3173–84. doi: 10.1089/ten.tea.2010.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (159).Ellis EF, McKinney JS, Willoughby KA, Liang S, Povlishock JT. A new model for rapid stretch-induced injury of cells in culture: characterization of the model using astrocytes. J. Neurotrauma. 1995;12:325–39. doi: 10.1089/neu.1995.12.325. [DOI] [PubMed] [Google Scholar]
- (160).Gomis-Rüth S, Stiess M, Wierenga CJ, Meyn L, Bradke F. Single-cell axotomy of cultured hippocampal neurons integrated in neuronal circuits. Nat. Protoc. 2014;9:1028–37. doi: 10.1038/nprot.2014.069. [DOI] [PubMed] [Google Scholar]
- (161).Serafini T, Kennedy TE, Galko MJ, Mirzayan C, Jessell TM, Tessier-Lavigne M. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell. 1994;78:409–24. doi: 10.1016/0092-8674(94)90420-0. [DOI] [PubMed] [Google Scholar]
- (162).Enomoto M, Bunge MB, Tsoulfas P. A multifunctional neurotrophin with reduced affinity to p75NTR enhances transplanted Schwann cell survival and axon growth after spinal cord injury. Exp. Neurol. 2013;248:170–82. doi: 10.1016/j.expneurol.2013.06.013. [DOI] [PubMed] [Google Scholar]
- (163).Cohen S, Levi-Montalcini R, Hamburger V. A NERVE GROWTH-STIMULATING FACTOR ISOLATED FROM SARCOM AS 37 AND 180. Proc. Natl. Acad. Sci. U. S. A. 1954;40:1014–8. doi: 10.1073/pnas.40.10.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (164).Guan W, Puthenveedu MA, Condic ML. Sensory Neuron Subtypes Have Unique Substratum Preference and Receptor Expression before Target Innervation. J. Neurosci. 2003;23:1781–1791. doi: 10.1523/JNEUROSCI.23-05-01781.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (165).Melli G, Höke A. Dorsal Root Ganglia Sensory Neuronal Cultures: a tool for drug discovery for peripheral neuropathies. Expert Opin. Drug Discov. 2009;4:1035–1045. doi: 10.1517/17460440903266829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (166).Chan CCM, Roberts CR, Steeves JD, Tetzlaff W. Aggrecan components differentially modulate nerve growth factor-responsive and neurotrophin-3-responsive dorsal root ganglion neurite growth. J. Neurosci. Res. 2008;86:581–92. doi: 10.1002/jnr.21522. [DOI] [PubMed] [Google Scholar]
- (167).Tuttle R, Matthew WD. Neurotrophins affect the pattern of DRG neurite growth in a bioassay that presents a choice of CNS and PNS substrates. Development. 1995;121:1301–9. doi: 10.1242/dev.121.5.1301. [DOI] [PubMed] [Google Scholar]
- (168).Zhang H, Verkman AS. Aquaporin-1 water permeability as a novel determinant of axonal regeneration in dorsal root ganglion neurons. Exp. Neurol. 2015;265:152–9. doi: 10.1016/j.expneurol.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (169).London A, Benhar I, Schwartz M. The retina as a window to the brain-from eye research to CNS disorders. Nat. Rev. Neurol. 2013;9:44–53. doi: 10.1038/nrneurol.2012.227. [DOI] [PubMed] [Google Scholar]
- (170).Berson DM. Retinal Ganglion Cell Types and Their Central Projections. In: Basbaum AI, Kaneko A, Shepard GM, Westheimer G, editors. The Senses: A Comprehensive Reference. Elsevier; 2008. pp. 491–519. [Google Scholar]
- (171).Bähr M, Vanselow J, Thanos S. In vitro regeneration of adult rat ganglion cell axons from retinal explants. Exp. brain Res. 1988;73:393–401. doi: 10.1007/BF00248232. [DOI] [PubMed] [Google Scholar]
- (172).Landreth GE, Agranoff BW. Explant culture of adult goldfish retina: effect of prior optic nerve crush. Brain Res. 1976;118:299–303. doi: 10.1016/0006-8993(76)90714-9. [DOI] [PubMed] [Google Scholar]
- (173).Halfter W, Newgreen DF, Sauter J, Schwarz U. Oriented axon outgrowth from avian embryonic retinae in culture. Dev. Biol. 1983;95:56–64. doi: 10.1016/0012-1606(83)90006-4. [DOI] [PubMed] [Google Scholar]
- (174).Landreth GE, Agranoff BW. Explant culture of adult goldfish retina: a model for the study of CNS regeneration. Brain Res. 1979;161:39–55. doi: 10.1016/0006-8993(79)90194-x. [DOI] [PubMed] [Google Scholar]
- (175).Turner JE, Schwab ME, Thoenen H. Nerve growth factor stimulates neurite outgrowth from goldfish retinal explants: the influence of a prior lesion. Brain Res. 1982;256:59–66. doi: 10.1016/0165-3806(82)90096-7. [DOI] [PubMed] [Google Scholar]
- (176).Snow DM, Watanabe M, Letourneau PC, Silver J. A chondroitin sulfate proteoglycan may influence the direction of retinal ganglion cell outgrowth. Development. 1991;113:1473–85. doi: 10.1242/dev.113.4.1473. [DOI] [PubMed] [Google Scholar]
- (177).Gottlieb DI, Rock K, Glaser L. A gradient of adhesive specificity in developing avian retina. Proc. Natl. Acad. Sci. U. S. A. 1976;73:410–4. doi: 10.1073/pnas.73.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (178).Bonhoeffer F, Huf J. In vitro experiments on axon guidance demonstrating an anterior-posterior gradient on the tectum. EMBO J. 1982;1:427–31. doi: 10.1002/j.1460-2075.1982.tb01186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (179).Deiner MS, Kennedy TE, Fazeli A, Serafini T, Tessier-Lavigne M, Sretavan DW. Netrin-1 and DCC mediate axon guidance locally at the optic disc: loss of function leads to optic nerve hypoplasia. Neuron. 1997;19:575–89. doi: 10.1016/s0896-6273(00)80373-6. [DOI] [PubMed] [Google Scholar]
- (180).Drescher U, Kremoser C, Handwerker C, Löschinger J, Noda M, Bonhoeffer F. In vitro guidance of retinal ganglion cell axons by RAGS, a 25 kDa tectal protein related to ligands for Eph receptor tyrosine kinases. Cell. 1995;82:359–370. doi: 10.1016/0092-8674(95)90425-5. [DOI] [PubMed] [Google Scholar]
- (181).Chen DF, Jhaveri S, Schneider GE. Intrinsic changes in developing retinal neurons result in regenerative failure of their axons. Proc. Natl. Acad. Sci. U. S. A. 1995;92:7287–91. doi: 10.1073/pnas.92.16.7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (182).Kleitman N, Wood P, Johnson MI, Bunge RP. Schwann cell surfaces but not extracellular matrix organized by Schwann cells support neurite outgrowth from embryonic rat retina. J. Neurosci. 1988;8:653–63. doi: 10.1523/JNEUROSCI.08-02-00653.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (183).Hopkins JM, Bunge RP. Regeneration of axons from adult human retina in vitro. Exp. Neurol. 1991;112:243–51. doi: 10.1016/0014-4886(91)90124-u. [DOI] [PubMed] [Google Scholar]
- (184).Hopkins JM, Bunge RP. Regeneration of axons from adult rat retinal ganglion cells on cultured Schwann cells is not dependent on basal lamina. Glia. 1991;4:46–55. doi: 10.1002/glia.440040106. [DOI] [PubMed] [Google Scholar]
- (185).Feller MB, Wellis DP, Stellwagen D, Werblin FS, Shatz CJ. Requirement for cholinergic synaptic transmission in the propagation of spontaneous retinal waves. Science. 1996;272:1182–7. doi: 10.1126/science.272.5265.1182. [DOI] [PubMed] [Google Scholar]
- (186).Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- (187).Böcker-Meffert S, Rosenstiel P, Röhl C, Warneke N, Held-Feindt J, Sievers J, Lucius R. Erythropoietin and VEGF promote neural outgrowth from retinal explants in postnatal rats. Invest. Ophthalmol. Vis. Sci. 2002;43:2021–6. [PubMed] [Google Scholar]
- (188).Sawamiphak S, Ritter M, Acker-Palmer A. Preparation of retinal explant cultures to study ex vivo tip endothelial cell responses. Nat. Protoc. 2010;5:1659–65. doi: 10.1038/nprot.2010.130. [DOI] [PubMed] [Google Scholar]
- (189).Gaublomme D, Buyens T, De Groef L, Stakenborg M, Janssens E, Ingvarsen S, Porse A, Behrendt N, Moons L. Matrix metalloproteinase 2 and membrane type 1 matrix metalloproteinase co-regulate axonal outgrowth of mouse retinal ganglion cells. J. Neurochem. 2014;129:966–79. doi: 10.1111/jnc.12703. [DOI] [PubMed] [Google Scholar]
- (190).Douglas MR, Morrison KC, Jacques SJ, Leadbeater WE, Gonzalez AM, Berry M, Logan A, Ahmed Z. Off-target effects of epidermal growth factor receptor antagonists mediate retinal ganglion cell disinhibited axon growth. Brain. 2009;132:3102–21. doi: 10.1093/brain/awp240. [DOI] [PubMed] [Google Scholar]
- (191).Biermann J, Grieshaber P, Goebel U, Martin G, Thanos S, Di Giovanni S, Lagrèze WA. Valproic acid-mediated neuroprotection and regeneration in injured retinal ganglion cells. Invest. Ophthalmol. Vis. Sci. 2010;51:526–34. doi: 10.1167/iovs.09-3903. [DOI] [PubMed] [Google Scholar]
- (192).Tuszynski MH, Steward O. Concepts and methods for the study of axonal regeneration in the CNS. Neuron. 2012;74:777–91. doi: 10.1016/j.neuron.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (193).Lerch JK, Martínez-Ondaro YR, Bixby JL, Lemmon VP. CJun promotes CNS axon growth. Mol. Cell. Neurosci. 2014;59:97–105. doi: 10.1016/j.mcn.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (194).Tsai H-H, Li H, Fuentealba LC, Molofsky AV, Taveira-Marques R, Zhuang H, Tenney A, Murnen AT, Fancy SPJ, Merkle F, Kessaris N, Alvarez-Buylla A, Richardson WD, Rowitch DH. Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science. 2012;337:358–62. doi: 10.1126/science.1222381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (195).Molofsky AV, Kelley KW, Tsai H-H, Redmond SA, Chang SM, Madireddy L, Chan JR, Baranzini SE, Ullian EM, Rowitch DH. Astrocyte-encoded positional cues maintain sensorimotor circuit integrity. Nature. 2014;509:189–94. doi: 10.1038/nature13161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (196).Thomson CE, Hunter AM, Griffiths IR, Edgar JM, McCulloch MC. Murine spinal cord explants: a model for evaluating axonal growth and myelination in vitro. J. Neurosci. Res. 2006;84:1703–15. doi: 10.1002/jnr.21084. [DOI] [PubMed] [Google Scholar]
- (197).Pohland M, Glumm R, Stoenica L, Höltje M, Kiwit J, Ahnert-Hilger G, Strauss U, Bräuer AU, Paul F, Glumm J. Studying Axonal Outgrowth and Regeneration of the Corticospinal Tract in Organotypic Slice Cultures. J. Neurotrauma. 2015;32:1465–1477. doi: 10.1089/neu.2014.3467. [DOI] [PubMed] [Google Scholar]
- (198).Stavridis SI, Dehghani F, Korf H-W, Hailer NP. Cocultures of rat sensorimotor cortex and spinal cord slices to investigate corticospinal tract sprouting. Spine (Phila. Pa. 1976) 2009;34:2494–9. doi: 10.1097/BRS.0b013e3181b4abd8. [DOI] [PubMed] [Google Scholar]
- (199).Krassioukov AV, Ackery A, Schwartz G, Adamchik Y, Liu Y, Fehlings MG. An in vitro model of neurotrauma in organotypic spinal cord cultures from adult mice. Brain Res. Protoc. 2002;10:60–68. doi: 10.1016/s1385-299x(02)00180-0. [DOI] [PubMed] [Google Scholar]
- (200).Takano T, He W, Han X, Wang F, Xu Q, Wang X, Oberheim Bush NA, Cruz N, Dienel GA, Nedergaard M. Rapid manifestation of reactive astrogliosis in acute hippocampal brain slices. Glia. 2014;62:78–95. doi: 10.1002/glia.22588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (201).Torres-Espín A, Santos D, González-Pérez F, del Valle J, Navarro X. Neurite-J: an image-J plug-in for axonal growth analysis in organotypic cultures. J. Neurosci. Methods. 2014;236:26–39. doi: 10.1016/j.jneumeth.2014.08.005. [DOI] [PubMed] [Google Scholar]
- (202).Kapfhammer J, Raper J. Interactions between growth cones and neurites growing from different neural tissues in culture. J. Neurosci. 1987;7:1595–1600. doi: 10.1523/JNEUROSCI.07-05-01595.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (203).Gutierrez OA, Danielson UH. Sensitivity analysis and error structure of progress curves. Anal. Biochem. 2006;358:1–10. doi: 10.1016/j.ab.2006.07.007. [DOI] [PubMed] [Google Scholar]
- (204).Breasted JH. The Edwin Smith Surgical Papayrus. 1st The University of Chicago Press; Chicago: 1930. [Google Scholar]
- (205).del Rio Hortega P, Penfield W. Cerebral Cicatrix: The Reaction of Neuroglia and Microglia to Brain Wounds. Johns Hopkins; Baltimore: 1927. [Google Scholar]