Abstract
Background/Objectives
Obesity among pregnant women may adversely affect both maternal iron status throughout pregnancy and placental transfer of iron. The objective of this study was to determine the association of maternal body mass index (BMI) with 1) maternal iron status and inflammation in mid and late pregnancy, 2) the change in maternal iron status throughout pregnancy, and 3) neonatal iron status.
Subjects/Methods
We examined longitudinal data from 1,613 participants in a pregnancy iron supplementation trial in rural China. Women with uncomplicated singleton pregnancies were enrolled in the early second trimester of pregnancy and followed through parturition. Maternal blood samples obtained at enrollment and in the third trimester, and cord blood samples were analyzed for a range of hematological and iron biomarkers.
Results
There was a negative association between maternal BMI and iron status at enrollment (transferrin receptor (sTfR): r=0.20, P<0.001; body iron (BI): r=−0.05; P=0.03). This association was markedly stronger among obese women. Maternal BMI was positively associated with maternal inflammation (C-reactive protein: r=0.33, P<0.001). In multiple linear regression models, maternal BMI was negatively associated with neonatal iron status (cord serum ferritin: −0.01, P=0.008; BI: −0.06, P=0.006) and associated with a lower decrease in iron status throughout pregnancy (sTfR: −4.6, P<0.001; BI: 1.1, P=0.004).
Conclusions
Maternal obesity during pregnancy may adversely affect both maternal and neonatal iron status, potentially through inflammatory pathways.
Keywords: Iron deficiency, obesity, pregnancy, double burden
Introduction
In many low- and middle-income countries, the rising prevalence of obesity amidst continuing conditions of poverty, and inadequate diets has led to a “double burden” of malnutrition (1). This co-occurrence of overweight and undernutrition has been observed at national levels, and even among different individuals within the same household (2). However, the individual-level burden of obesity and micronutrient malnutrition may be of equal public health concern, but is less commonly recognized.
Iron deficiency (ID) is a particular priority. It affects more than one billion individuals globally and is highly prevalent among women of childbearing age, who are also increasingly vulnerable to obesity (3). Several studies in non-pregnant women, adolescents, and children in high-income countries have shown that body mass index (BMI) is negatively correlated with iron status (4-6). Obesity may contribute to low iron status because obese individuals may have energy-dense, nutrient-poor diets that lead to low iron intakes. Alternatively, chronic inflammation linked to excessive adiposity may hinder iron absorption (7). However, other research has found that overweight and overfat are not associated with poor iron status (8). In fact, obesity has been shown to be positively associated with iron status in two studies (9, 10). Therefore, it is not clear the extent to which obesity may influence iron status in different populations or contexts. In particular, very little research to date has examined the relationship between obesity and iron status in pregnant women or the potential influence of maternal obesity on neonatal iron status. Only three studies, all recent, have examined associations between maternal obesity and the iron status of neonates (11-13). These studies examined distinct biomarkers of iron status in unique clinical contexts and reported contrasting findings.
Infants are highly dependent on iron stores accumulated in utero to meet their iron requirements in the first six months of life (14). Under conditions of restricted iron availability, the fetus may prioritize iron for production of hemoglobin (Hb) at the expense of other tissues, which could have deleterious impacts on brain development, and contribute to adverse developmental consequences throughout childhood (12, 15). Though infants born to obese women are more likely to be preterm and therefore have a lower iron endowment (16), it is not clear the extent to which maternal obesity during pregnancy may affect maternal and neonatal iron status independent of prematurity.
The objectives of this study were 1) to determine the association of maternal BMI with maternal iron status and inflammation in mid and late pregnancy, 2) to elucidate the association of maternal BMI with the change in maternal iron status throughout pregnancy, and 3) to determine the relation between maternal BMI and neonatal iron status. We also examined the associations of gestational weight gain with maternal inflammation in mid and late pregnancy and with neonatal iron status. We predicted that maternal BMI would be negatively associated with maternal and neonatal iron status, and positively associated with maternal inflammation.
Subjects and Methods
Study design and recruitment
The data used in this study are from a randomized controlled trial of prenatal iron supplementation in Hebei Province in northeastern China. Women with uncomplicated singleton pregnancies who attended their first prenatal visit at 20 weeks of gestation or earlier were recruited from prenatal clinics in three local hospitals between June 2009 and December 2011. Women with chronic health problems, including diabetes (for which screening occurred between 24 and 28 weeks of pregnancy), were excluded. A total of 2,367 women were enrolled. Women were randomized to receive daily supplements of 300 mg iron sulfate (60 mg elemental iron) or placebo and 0.4 mg folic acid from enrollment to delivery. Hematological data were collected for all women at the time of enrollment. Follow-up maternal blood collection during the third trimester was available for 1,613 women, and cord blood was available for 1,573 neonates. A comprehensive description of the trial design and the primary findings of the trial have been reported previously (17).
Data collection
At enrollment, women who consented to join the trial were administered a questionnaire on household demographics, reproductive history, and information about the current pregnancy. Maternal height and weight were also measured at this time, in addition to maternal pre-pregnancy weight based on maternal recall. Maternal BMI was calculated as pre-pregnancy weight in kilograms divided by the square of height in meters. In sensitivity analyses, we also examined associations using pregnancy BMI calculated from the weight measure taken at enrollment. The results were nearly identical to those using pre-pregnancy weight. We report analyses based on pre-pregnancy weight. Weight was measured again at or near term (i.e., within one week of birth), and gestational weight gain was determined by the difference between this weight and pre-pregnancy weight. Adequate, low, and excessive gestational weight gain were defined according to IOM recommendations (18). Gestational age was calculated based on the date of last menstrual period. Information on maternal diet during pregnancy was not available.
Blood sampling and hematologic assessment
Maternal blood samples (5-10 mL) were obtained by venipuncture for iron status measures at enrollment (i.e., mid pregnancy), and at or near term. Cord blood samples were obtained by sterile needle puncture immediately after cord clamping. Sampling procedures and protocols for the handling and transport of samples have been described previously (17). We assayed three biomarkers of iron status in both maternal and cord blood: 1) serum ferritin (SF), 2) transferrin receptor (sTfR), and 3) zinc protoporphyrin/heme (ZPP/H). SF concentrations reflect the amount of iron stored in tissues (19). Synthesis of sTfR is upregulated during conditions of tissue iron deficiency (20, 21). Similarly, serum concentrations of ZPP/H increase when insufficient iron is available for Hb production (22). ZPP/H was analyzed with a hematofluorometer (AVIV Biomedical, Lakewood, NJ, USA). SF and sTfR were determined by Beckman Coulter Access 2 Immunoassay System with chemiluminescent immunoassay method (Beckman Coulter Inc., Brea, CA, USA). We assessed hemoglobin (Hb) using a Sysmex KX-21N Auto Hematology Analyzer (SYSMEX Corporation, Kobe, Japan), and C-reactive protein (hsCRP) concentrations, a marker of inflammation, by rate nephelometry using a Hitachi 7600 modular chemistry analyzer (Hitachi Co., Tokyo, Japan). We defined maternal anemia as Hb concentration < 110 g/L according to WHO guidelines (23). Low storage iron, or iron depletion, among pregnant women was defined as SF <15 μg/L (22). Iron-deficient erythropoiesis was defined as ZPP/H >70 μmol/mol heme (24). We also calculated body iron (BI) using the equation: body iron (mg/kg) = − [log10(sTfR*1000/ferritin) – 2.8229]/0.1207 (17, 25). Positive values of this index reflect a surplus of iron in stores while negative values reflect an iron deficit in tissues. Anemia among neonates was defined as cord blood Hb <130 g/L (26, 27) and low SF among neonates was defined as cord SF <75 μg/L (28).
Statistical analysis
Statistical analyses were carried out in Stata v. 13.1 (College Station, TX, USA) and SAS 9.3 (SAS Institute, Cary, NC). Non-normally distributed variables were log transformed prior to analysis (i.e., SF, sTfR, ZPP/H). We calculated means and proportions of maternal characteristics by BMI categories and used ANOVA and Chi-square test statistics to assess differences in means and proportions, respectively, across these categories. We further used the Tukey–Kramer method to assess pair-wise differences among these categories adjusting for multiple comparisons. Pearson product-moment correlation coefficients (r) were calculated to assess the relation between maternal pre-pregnancy BMI with biomarkers of iron status as well as hsCRP concentrations. We further used Markov chain Monte Carlo (MCMC) methods for sampling from probability distributions based on constructing a Markov chain (29). We tested for and estimated a potential inflection point at which the association of maternal pre-pregnancy BMI with maternal iron status changed, and fitted different slopes for values of maternal BMI above or below the threshold (28). The PROC MCMC procedure in SAS was used to conduct these analyses.
We used unadjusted bivariate linear regression models and multiple linear regression models, adjusting for maternal age, parity, gestational weight gain, iron supplementation, iron status and inflammation in late pregnancy, cord hsCRP concentration, as well as the sex of the newborn to assess the association between maternal pre-pregnancy BMI and neonatal iron status. We initially included both birth weight and gestational age at birth in models. However, these variables were observed to be collinear. We therefore included them as covariates in separate models and observed the same results. The final models presented here include only birth weight because of differences observed at enrollment in this variable across women of differing BMIs. We further used multiple linear regression models, adjusting for a similar set of covariates, to examine the associations between maternal pre-pregnancy BMI and the change in maternal biomarkers of iron status from mid to late pregnancy.
Ethical approval
Informed consent was obtained for all subjects and the study was approved by the Institutional Review Boards of the School of Medicine at the University of Michigan and Peking University First Hospital.
Results
Prior to pregnancy, approximately one-fifth of women (19%) were overweight or obese (Table 1), while 14% were underweight. A larger proportion of overweight and obese women gained excessive weight during pregnancy as compared to normal weight women. Infants born to overweight and obese women weighed 117 and 73 g more, respectively, than children of normal weight women, though gestational age at birth did not differ by maternal BMI. Overall, 98% of infants were born at term.
Table 1.
Sample characteristics and iron status measures by body mass index.
| Body mass index (kg m−2) |
|||||
|---|---|---|---|---|---|
| Maternal characteristics at enrollment (mid-pregnancy) | Underweight (<18.5) |
Normal weight (≥18.5 and <25) |
Overweight (≥25 and <30) |
Obese (≥30) |
Test statistic |
| n | 219 | 1,084 | 233 | 77 | |
| Age (y) | 24 (3.0)a | 25 (3.5)b | 26 (4.3)c | 25 (3.6)b,c | 14*** |
| Height (cm) | 162 (4.6) | 161 (4.4) | 161 (4.5) | 161 (5.2) | 1.3 |
| Gave birth previously (%) | 14a | 21a | 29b,c | 24a,c | 15** |
| High school education or greater (%) | 40 | 33 | 28 | 32 | 17 |
| Week of gestation | 16 (2.0) | 16 (1.9) | 16 (2.1) | 16 (1.9) | 2.4 |
| Pregnancy and birth outcomes | |||||
|
| |||||
| Weight gain (kg) | 18.5 (5.2)a | 18.3 (5.8)a | 16.0 (6.0)b,d | 15.6 (6.1)c,d | 13*** |
| Excessive weight gain (%)§ | 47a | 65b | 79c,d | 92d | 78*** |
| Gestational age at birth (wk) | 39.8 (1.1) | 39.7 (1.1) | 39.6 (1.0) | 39.5 (1.2) | 1.1 |
| Birthweight (g) | 3 227 (362)a | 3 358 (375)b | 3 475 (398)c | 3 431 (433)b,c | 17*** |
| Macrosomic birth (birthweight > 4000 g) (%) | 1.8a | 3.6a | 8.2b,c | 6.5a,c | 14** |
| Maternal mid-pregnancy iron and anemia status | |||||
|
| |||||
| Hemoglobin (g/L) | 120 (9.8)a | 122 (9.2)b | 124 (8.5)c,d | 124 (8.7)b,d | 10*** |
| Anemia (Hb<110 g/L) (%) | 9.1 | 9.2 | 4.7 | 6.5 | 5.6 |
| Serum ferritin (SF) (μg/L) | 43 (36) | 41 (35) | 44 (36) | 37 (28) | 1.3 |
| Iron depletion (SF<15 μg/L) (%) | 18 | 20 | 17 | 22 | 1.9 |
| Transferrin receptor (sTfR) (nmol/L) | 14 (4.5)a | 15 (4.7)a | 16 (5.3)b,c | 17 (6.2)c | 9.1*** |
| Zinc protoporphyrin/heme (ZPP/H) (μmol/mol heme) | 53 (27) | 56 (25) | 57 (27) | 57 (27) | 1.2 |
| Body iron (BI) (mg/kg) | 5.7 (3.5) | 5.2 (3.5) | 5.3 (3.4) | 4.7 (3.3) | 1.8 |
| hsCRP (mg/L) | 2.6 (4.6)a | 3.4 (5.3)a | 5.6 (5.7)b | 5.7 (7.3)b | 18*** |
| High hsCRP (>5 mg/L) (%) | 12a | 18a | 45b | 40b | 104*** |
| Neonatal iron and anemia status (cord blood) | |||||
|
| |||||
| Hemoglobin (g/L) | 152 (15) | 152 (16) | 152 (15) | 151 (14) | 0.35 |
| Anemia (Hb<130 g/L) (%) | 6.4 | 5.7 | 5.2 | 5.2 | 0.36 |
| Serum ferritin (SF) (μg/L) | 139 (86) | 127 (86) | 118 (87) | 123 (84) | 2.5 |
| Low SF (SF<75 μg/L) | 21a | 26a,b | 33b | 31a,b | 9.2* |
Mean (SD) or proportion are shown; “Test statistic” refers to F-value for means and Chi-square statistic for proportions;
P<0.001
P<0.01
P<0.05.
Excessive gestational weight gain was defined according to IOM recommendations based on the difference in weight pre-pregnancy and at or near term (28).
Means or proportions with: 1) different superscript letters correspond to groups wherein pair-wise differences are not consistent with random variation (i.e., P<0.05); 2) identical superscript letters correspond to groups wherein pair-wise differences are consistent with random variation (i.e., P>0.05); and 3) no superscripts correspond to groups wherein all pair-wise differences are consistent with random variation (i.e., P>0.05).
Less than 10% of women were anemic at enrollment across all BMI levels, though approximately one in five women was iron depleted (SF <15 μg/L). By late pregnancy, iron depletion was prevalent in two-thirds (67%) of women. Women who were both overweight and iron depleted at enrollment constituted 15% of the sample. Approximately one-quarter (26%) and one-third (33%) of neonates born to normal weight and overweight mothers, respectively, had low SF at birth.
Association of maternal BMI with iron status in mid and late pregnancy
We observed linear associations of maternal pre-pregnancy BMI with maternal sTfR concentrations and BI at enrollment (r=0.20; P<0.001 and r=−0.05; P=0.03, respectively), both indicating lower iron status with increasing BMI. However, maternal pre-pregnancy BMI was not linearly associated with maternal SF and ZPP/H concentrations at enrollment (r=0.01; P=0.83, and r=0.04; P=0.08, respectively). Biomarkers of maternal iron status in late pregnancy were not associated with maternal pre-pregnancy BMI (SF: r=0.01; P=0.60; sTfR: r=−0.04; P=0.11; ZPP/H: r=0.04; P=0.16; BI: r=0.03; P=0.31).
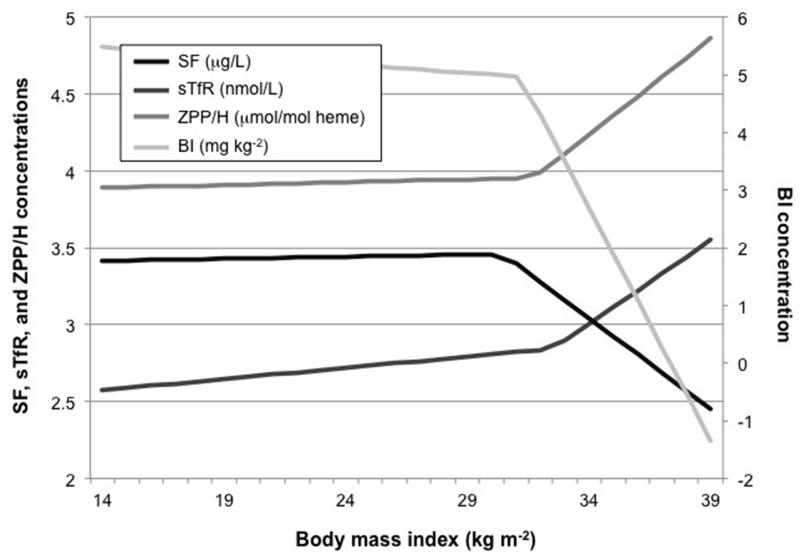
The associations between biomarkers of maternal iron status at enrollment (i.e., in mid pregnancy) showed inflection points such that the relation between maternal BMI and iron status was strongest among obese women (Figure 1). In fact, all four biomarkers of maternal iron status at enrollment demonstrated a similar inflection point near a BMI cut-off of 31 kg m−2 (i.e., SF: 30.5; sTfR: 32.5; ZPP/H: 31.8; BI: 31.2). The relation between maternal pre-pregnancy BMI and mid-pregnancy maternal iron status was stronger at BMI values above the inflection points.
Figure 1.
Markov chain Monte Carlo method inflection point analysis of the associations of maternal pre-pregnancy body mass index and biomarkers of iron status in mid pregnancy.
Inflection points for serum ferritin (SF), transferrin receptor (sTfR), zinc protoporphyrin/heme (ZPP/H), and body iron (BI) were observed at BMIs of 30.5, 32.5, 31.8, and 31.2, respectively.
Association of maternal BMI and gestational weight gain with inflammation in mid and late pregnancy
We observed a strong positive relation between maternal pre-pregnancy BMI and maternal inflammation in mid pregnancy as measured by hsCRP concentration (r=0.33; P<0.001). A similar but weaker relation was observed for hsCRP concentration in late pregnancy (r=0.12; P<0.001). Maternal gestational weight gain was also associated with maternal hsCRP concentrations in late pregnancy (r=0.1; P<0.001).
Association of maternal BMI with the change in maternal iron status throughout pregnancy
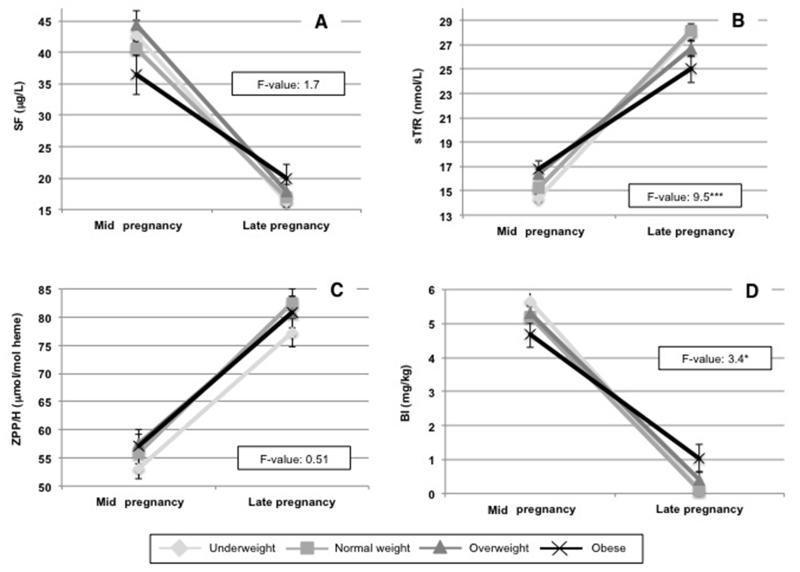
As expected, maternal iron status declined from mid to late pregnancy among women in the entire sample (mean (SD) change in biomarker of iron status): SF: −24 μg/L (36); sTfR: 12 nmol/L (10); ZPP/H: 26 μmol/mol heme (39); BI: −5.1 mg/kg (4.2)). However, the deterioration in maternal sTfR and BI were less severe among obese women than among non-obese women (Figure 2). These differences by BMI in the change in iron status throughout pregnancy were consistent even after adjusting for the iron status of women at enrollment (Table 2).
Figure 2.
Change in biomarkers of maternal iron status from mid to late pregnancy.
F-values are from ANOVA comparing the change in maternal iron status from mid to late pregnancy across BMI categories. Mean values with standard errors of each biomarker at each time point are shown.
***P<0.001, **P<0.01, *P<0.05.
Table 2.
Maternal pre-pregnancy body mass index and change in maternal iron status from mid to late pregnancy.
| ΔSF (μg/L) |
ΔsTfR (nmol/L) |
ΔZPP/H (μmol/mol heme) |
ΔBI (mg/kg) |
|||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Coeff. | P-value | Coeff. | P-value | Coeff. | P-value | Coeff. | P-value | |
|
|
||||||||
| Unadjusted analysis | ||||||||
|
| ||||||||
| Maternal pre-pregnancy BMI (kg m−2) | ||||||||
| Normal weight (reference) | - | - | - | - | - | - | - | - |
| Underweight | −3.2 | 0.24 | 0.61 | 0.43 | −2.1 | 0.48 | −0.43 | 0.18 |
| Overweight | −2.6 | 0.32 | −2.7 | <0.001 | −3.0 | 0.30 | 0.22 | 0.47 |
| Obese | 6.5 | 0.13 | −4.8 | <0.001 | −2.8 | 0.56 | 1.4 | 0.01 |
| Adjusted analysis | ||||||||
|
| ||||||||
| Maternal pre-pregnancy BMI (kg m−2) | ||||||||
| Normal weight (reference) | - | - | - | - | - | - | - | - |
| Underweight | −0.92 | 0.42 | 0.68 | 0.37 | −2.2 | 0.42 | −0.11 | 0.63 |
| Overweight | 0.69 | 0.54 | −2.5 | 0.001 | −3.1 | 0.25 | 0.31 | 0.19 |
| Obese | 3.5 | 0.06 | −4.6 | <0.001 | −3.8 | 0.39 | 1.1 | 0.004 |
| Maternal age (y) | 0.11 | 0.30 | −0.09 | 0.20 | −0.11 | 0.66 | 0.05 | 0.04 |
| Iron supplementation | ||||||||
| No (reference) | - | - | - | - | - | - | - | - |
| Yes | 6.3 | <0.001 | −4.7 | <0.001 | −18 | <0.001 | 1.8 | <0.001 |
| Gestational weight gain | ||||||||
| Adequate (reference) | - | - | - | - | - | - | - | - |
| Low | 0.80 | 0.59 | 0.17 | 0.87 | −3.3 | 0.37 | 0.24 | 0.43 |
| Excessive | 2.2 | 0.02 | −0.18 | 0.77 | −1.4 | 0.54 | 0.33 | 0.09 |
| Birth weight (kg) | −0.003 | 0.003 | 0.004 | <0.001 | 0.009 | <0.001 | −0.001 | <0.001 |
Maternal iron status in mid pregnancy and maternal hsCRP concentration in mid and late pregnancy were included as additional covariates in models.
Association of maternal BMI with neonatal iron status
In bivariate linear regression models, maternal pre-pregnancy BMI was negatively associated with neonatal iron status. Cord SF concentrations were lower (P<0.001) and sTfR concentrations were higher (P=0.04) for infants born to women with higher BMIs (Table 3). Cord body iron, though not a well-established biomarker of iron status among neonates, was also lower (P<0.001) among infants born to women with higher BMIs (Table 3). In multiple linear regression models adjusting for maternal iron supplementation, gestational age at birth, gestational weight gain, and several other covariates, maternal pre-pregnancy BMI remained negatively associated with neonatal iron status using both SF and BI as biomarkers of iron status (P=0.002) (Table 3). No inflection points were observed for the relation between maternal pre-pregnancy BMI and neonatal iron status.
Table 3.
Maternal pre-pregnancy body mass index and neonatal iron status.
| Neonatal iron status biomarkers |
||||||||
|---|---|---|---|---|---|---|---|---|
| Log SF (μg/L) | Log sTfR (nmol/L) | Log ZPP/H (μmol/mol heme) | BI (mg/kg) | |||||
|
|
||||||||
| Coeff. | P-value | Coeff. | P-value | Coeff. | P-value | Coeff. | P-value | |
| Unadjusted analysis | ||||||||
|
| ||||||||
| Maternal pre-pregnancy BMI (kg m−2) | −0.02 | <0.001 | 0.005 | 0.04 | −0.0002 | 0.95 | −0.09 | <0.001 |
| Adjusted analysis | ||||||||
|
| ||||||||
| Maternal pre-pregnancy BMI (kg m−2) | −0.01 | 0.008 | 0.003 | 0.17 | −0.001 | 0.62 | −0.06 | 0.006 |
| Maternal age (y) | −0.01 | 0.01 | 0.003 | 0.34 | 0.004 | 0.17 | −0.06 | 0.01 |
| Previous birth | ||||||||
| No (reference) | - | - | - | - | - | - | - | - |
| Yes | 0.07 | 0.17 | 0.01 | 0.56 | −0.02 | 0.46 | 0.21 | 0.33 |
| Iron supplementation | ||||||||
| No (reference) | - | - | - | - | - | - | - | - |
| Yes | −0.02 | 0.50 | −0.001 | 0.96 | −0.02 | 0.17 | 0.12 | 0.45 |
| Gestational weight gain§ | ||||||||
| Adequate (reference) | - | - | - | - | - | - | - | - |
| Low | −0.03 | 0.63 | −0.01 | 0.73 | −0.03 | 0.39 | −0.07 | 0.81 |
| Excessive | −0.17 | <0.001 | 0.02 | 0.22 | 0.04 | 0.06 | −0.69 | <0.001 |
| Child sex | ||||||||
| Male (reference) | - | - | - | - | - | - | - | - |
| Female | 0.09 | 0.01 | −0.04 | 0.01 | −0.04 | 0.01 | 0.47 | 0.002 |
| Birth weight (kg) | −0.00 | 0.03 | −0.00 | 0.94 | −0.00 | 0.03 | −0.00 | 0.09 |
Gestational weight gain and maternal pre-pregnancy BMI were not found to be collinear, and so were simultaneously included in adjusted regression analyses.
Maternal iron status in late pregnancy, maternal hsCRP concentration in late pregnancy, and cord hsCRP concentration were included as additional covariates in models.
Discussion
We examined the association of maternal BMI with both maternal iron status during pregnancy and neonatal iron status in a cohort of non-diabetic Chinese women among whom there was a high prevalence of iron depletion, and very few macrosomic or preterm births. Maternal pre-pregnancy BMI was negatively associated with maternal markers of iron status in mid, but not late pregnancy. This trend was most pronounced among obese women. The inflection points for these associations (i.e., between BMIs of 31-32) were consistent across all four markers of iron status that we examined. These findings suggest that obesity, rather than overweight, has a potential negative influence on the iron status of pregnant women.
One previous study in non-pregnant adult women that examined absorption of iron from meals fortified with isotopically labeled iron observed a linear decrease in iron absorption with increasing BMI Z-score (4). However, no obese women were included in this sample (BMI range: 15.8 – 26.7). One small study in pregnant women that may have lacked statistical power found that serum iron and transferrin saturation did not differ at 24-28 weeks of gestation between normal weight (n=15) and obese (n=15) pregnant women (11). A more recent study (n=240) by Garcia-Valdes et al. found no differences in iron status across normal weight, overweight, and obese pregnant women in mid pregnancy; however, at term, SF and sTfR concentrations among obese women were lower and higher, respectively, as compared to normal weight women (13). The authors hypothesized that this may have been due to elevated hepcidin concentrations among obese women.
It is plausible that hepcidin, a hormone that regulates cellular iron export, influenced the differences in iron status observed across normal weight as compared to obese women in our sample. Expression of hepcidin is feedback regulated by iron concentrations, erythropoietic requirements for iron, and inflammation (30). The low-grade, chronic inflammation associated with obesity may upregulate hepcidin, and inhibit intestinal iron absorption or the release of iron from hepatic stores and splenic macrophages (31), thereby decreasing circulating iron. Though we did not measure serum hepcidin concentrations in this study, we did find that maternal BMI was strongly positively associated with maternal inflammation as measured by hsCRP concentrations in mid and late pregnancy. If circulating maternal hepcidin concentrations, which normally decline throughout pregnancy to maximize the availability of iron to the fetus (32), do not decline to the same extent among obese women due to chronic inflammation, maternal iron may be sequestered later in pregnancy. This sequestration may inhibit placental iron transfer and potentiate greater maternal iron retention. Though we observed no differences in late pregnancy in any of the biomarkers of iron status across BMI levels, we did observe that the decline in maternal iron status throughout pregnancy was less severe among obese as compared to normal weight women even after adjusting for the lower iron status of obese women in mid pregnancy and for infant birth weight. Taken together, these findings suggest that among obese women, maternal iron is less available to both mother and placenta. While obese women are likely to begin pregnancy with poorer iron status, they may also transfer less iron to the placenta (as evidenced by a lower decline in iron status throughout pregnancy among obese women), thus minimizing differences in iron status in late pregnancy by BMI. The substantial decline in iron status across all women in the sample with no apparent rebound near term may explain the contrasting findings in our study as compared to those of Garcia-Valdes et al., who observed a less pronounced absolute decline in iron status among women of all BMI levels (13).
Maternal pre-pregnancy BMI was also strongly positively associated with maternal hsCRP concentrations in mid and late pregnancy and, in analyses adjusting for covariates, negatively associated with neonatal iron status. These observations align with the hypothesis that the placental transfer of iron may be inhibited among obese women. Maternal pre-pregnancy BMI was associated with lower cord SF and BI independent of birth weight, maternal iron status in mid pregnancy, iron supplementation, and several other covariates. Excessive weight gain during pregnancy was similarly associated with poorer neonatal iron status, and showed a greater magnitude of effect as compared to maternal pre-pregnancy BMI. Maternal gestational weight gain was also associated with maternal hsCRP concentrations in late pregnancy (P<0.001), which may have contributed to the diminished association between maternal pre-pregnancy BMI and hsCRP concentration in late pregnancy as compared to mid pregnancy.
A limited number of recent studies, in much smaller samples and in unique clinical contexts, have reported contrasting findings on the association of maternal obesity with neonatal iron status. Dao et al. (2013) (n=30) found that infants of obese women had lower serum iron and transferrin saturation in cord blood as compared to normal weight women (11). Phillips et al. (2014) also found that cord blood iron status (i.e., measured using ZPP/H and SF) was lower in neonates of mothers who were obese at delivery (n=176) compared to non-obese (n=140) (12). They also observed that high gestational weight gain (i.e., ≥18 kg) was associated with poorer newborn iron status. However, nearly one third of these mothers were diabetic, and many had multiple risk factors, independent of obesity status, for their newborn to develop iron-deficiency anemia. Garcia-Valdes et al. found no correlations between maternal BMI and hematological parameters of cord blood with the exception of transferrin saturation (ρ=−0.2). In fact, they observed higher serum iron and transferrin saturation in the cord blood of pregnant women with excessive gestational weight gain (13). Placental transferrin receptor protein expression and mRNA levels were upregulated in iron-deficient women, but were not associated with maternal BMI.
Maternal iron status in late pregnancy and maternal pre-pregnancy BMI demonstrated independent associations with neonatal iron status among the mother-infant dyads in our sample. Based on our findings and the limited evidence available to date, it seems likely that although maternal iron deficiency may negatively influence neonatal iron status, maternal obesity also plays an important role. However, the mechanisms underlying this association remain unclear.
It is plausible that the larger size of fetuses of obese mothers may negatively influence cord blood iron markers (33). However, similar to a previous study (12), we observed that maternal obesity and excessive gestational weight gain were independently negatively associated with neonatal iron status even after adjusting for infant size at birth. Alternatively, it is possible that the upregulation of hepcidin under proinflammatory conditions in obese individuals may lead to iron sequestration and impaired iron transfer to the placenta (34). The linear increase in maternal hsCRP concentrations that we observed with increasing BMI supports this hypothesis. However, further research is needed to understand the mechanisms that govern the regulation of placental iron transfer and the extent to which maternal overweight may influence these (35). Some evidence suggests that fetal, and not maternal hepcidin, regulates placental iron transfer (36). Yet, other studies have found that maternal hepcidin may explain much of the variation in placental transfer of dietary iron (37). Furthermore, maternal and cord hepcidin concentrations have been shown to be associated in several (13, 38, 39), though not all studies (36), suggesting that the expression of maternal and fetal hepcidin may share a common regulatory mechanism (13). If this is the case, proinflammatory stimuli among obese mothers could influence both maternal and fetal regulation of placental iron transfer.
This study is the largest study to date examining the associations of maternal overweight during pregnancy with maternal and neonatal iron status. We examined these associations among a well-characterized cohort of non-diabetic pregnant mothers for whom iron status was measured using several distinct, complementary blood biomarkers. Our study was limited, however, in that we did not measure hepcidin in maternal or cord blood. It was therefore not possible to examine one of the hypothesized mechanisms by which maternal overweight may affect placental iron transfer. Though we measured hsCRP to assess maternal inflammation, future work would benefit from assessing other markers of immune activation, including the pro-inflammatory cytokine interleukin-6, which signals activation of hepcidin gene transcription (40). Assessment of α1-acid glycoprotein in particular would have allowed for more in-depth analysis of the stage of the acute phase inflammatory response within individuals (i.e., incubation, early or late convalescence) that would have permitted alternative ferritin corrections (41, 42). However, our adjustment for hsCRP in multiple regression models likely accounts for much of the differences in iron biomarker concentrations due to inflammation. We were further limited in that we were unable to examine maternal body fat percentage or distinguish between central or peripheral fat given that measures of body fat were not assessed as part of this trial. BMI, an imperfect proxy indicator of body fat, was the only indicator available for examining the relationships between obesity and iron status that we assessed (43). Finally, data on the diets of women during pregnancy were not collected, thus limiting our ability to adjust for the potential confounding influence of diet on observed relationships. Subsequent data collected on the complementary foods fed to the infants born to women in the study indicated low intakes of bioavailable, iron-rich animal-source foods, possibly reflective of household access to these foods (e.g., meat purée, animal liver purée, animal blood curd: consumed by 17%, 12% and 2% of infants, respectively). These data align with anecdotal evidence of the largely rice- and root crop-based diets in the study region.
As the prevalence of obesity climbs in countries that continue to face substantial burdens of ID among women of childbearing age, the public health consequences of these two synergistic forms of malnutrition could be profound. Our findings indicate that maternal obesity during pregnancy is negatively associated with both the iron status of women in mid pregnancy, and neonatal iron status, suggesting that the placental transfer of iron may be inhibited among obese individuals. Further research is needed to understand the mechanisms by which obesity may affect maternal and neonatal iron status, as well as the extent to which heterogeneity in pre-pregnancy iron stores, and adiposity affect these relations. This evidence could help to inform intervention strategies to mitigate the potential adverse consequences of this nutritional double burden.
Acknowledgements
We are grateful to Blair Richards for his assistance with data management and data cleaning.
Funding: This study was financially supported by a grant from Vifor Pharma Ltd., Gengli Zhao, Principal Investigator. The laboratory measures of iron status were supported by a grant from the US National Institutes of Health (NIH), R01 HD052069, Betsy Lozoff, Principal Investigator, which included funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the Office of Dietary Supplements. The content is solely the responsibility of the authors and does not necessarily represent the official views of Vifor or NIH. Authors had full control of primary data and did not have an agreement with the funders that limited their ability to complete the research as planned.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
Author contributions: A.J. developed the research question, designed the primary analyses, and drafted the manuscript. G.Z., Z.Z., and B.L. contributed to the study conception and design. G.Z., Y.P., M.Z, and G.X. contributed to data collection. N.K. contributed to statistical analyses. A.J. and B.L. had primary responsibility for the final content of the manuscript. All authors critically revised the manuscript and read and approved the final manuscript.
References
- 1.Prentice AM. The emerging epidemic of obesity in developing countries. International Journal of Epidemiology. 2006;35(1):93–9. doi: 10.1093/ije/dyi272. [DOI] [PubMed] [Google Scholar]
- 2.Doak CM, Adair LS, Bentley M, Monteiro C, Popkin BM. The dual burden household and the nutrition transition paradox. International Journal of Obesity. 2005;29(1):129–36. doi: 10.1038/sj.ijo.0802824. [DOI] [PubMed] [Google Scholar]
- 3.Rozowski J, Parodi CG. Implications of the Nutrition Transition in the Nutritional Status on Pregant Women. In: Lammi-Keefe C, Couch SC, Philipson E, editors. Handbook of Nutrition and Pregnancy. Humana Press; Totowa, NJ: 2008. pp. 307–318. [Google Scholar]
- 4.Zimmermann MB, Zeder C, Muthayya S, Winichagoon P, Chaouki N, Aeberli I, et al. Adiposity in women and children from transition countries predicts decreased iron absorption, iron deficiency and a reduced response to iron fortification. International Journal of Obesity. 2008;32(7):1098–104. doi: 10.1038/ijo.2008.43. [DOI] [PubMed] [Google Scholar]
- 5.Pinhas-Hamiel O, Newfield RS, Koren I, Agmon A, Lilos P, Phillip M. Greater prevalence of iron deficiency in overweight and obese children and adolescents. International Journal of Obesity and Related Metabolic Disorders. 2003;27(3):416–8. doi: 10.1038/sj.ijo.0802224. [DOI] [PubMed] [Google Scholar]
- 6.Tussing-Humphreys LM, Liang H, Nemeth E, Freels S, Braunschweig CA. Excess adiposity, inflammation, and iron-deficiency in female adolescents. J Am Diet Assoc. 2009;109(2):297–302. doi: 10.1016/j.jada.2008.10.044. [DOI] [PubMed] [Google Scholar]
- 7.Tussing-Humphreys L, Pusatcioglu C, Nemeth E, Braunschweig C. Rethinking iron regulation and assessment in iron deficiency, anemia of chronic disease, and obesity: introducing hepcidin. Journal of the Academy of Nutrition and Dietetics. 2012;112(3):391–400. doi: 10.1016/j.jada.2011.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karl JP, Lieberman HR, Cable SJ, Williams KW, Glickman EL, Young AJ, et al. Poor iron status is not associated with overweight or overfat in non-obese pre-menopausal women. Journal of the American College of Nutrition. 2009;28(1):37–42. doi: 10.1080/07315724.2009.10719759. [DOI] [PubMed] [Google Scholar]
- 9.Wendt AS, Jefferds ME, Perrine CG, Halleslevens P, Sullivan KM. Obese women less likely to have low serum ferritin, Nicaragua. Public Health Nutrition. 2015;18(4):736–741. doi: 10.1017/S1368980014000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kordas K, Fonseca Centeno ZY, Pachon H, Jimenez Soto AZ. Being overweight or obese is associated with lower prevalence of anemia among Colombian women of reproductive age. Journal of Nutrition. 2013;143(2):175–81. doi: 10.3945/jn.112.167767. [DOI] [PubMed] [Google Scholar]
- 11.Dao MC, Sen S, Iyer C, Klebenov D, Meydani SN. Obesity during pregnancy and fetal iron status: Is hepcidin the link? Journal of Perinatology. 2013;33(3):177–81. doi: 10.1038/jp.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phillips A, Roy S, Lundberg R, Guilbert T, Auger A, Blohowiak S, et al. Neonatal iron status is impaired by maternal obesity and excessive weight gain during pregnancy. Journal of Perinatology. 2014;34(7):513–518. doi: 10.1038/jp.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Valdes L, Campoy C, Hayes H, Florido J, Rusanova I, Miranda MT, et al. The impact of maternal obesity on iron status, placental transferrin receptor expression and hepcidin expression in human pregnancy. International Journal of Obesity. 2015;39:571–8. doi: 10.1038/ijo.2015.3. [DOI] [PubMed] [Google Scholar]
- 14.Domellof M. Iron requirements in infancy. Annals of Nutrition & Metabolism. 2011;59(1):59–63. doi: 10.1159/000332138. [DOI] [PubMed] [Google Scholar]
- 15.Lozoff B, Georgieff MK. Iron deficiency and brain development. Seminars in Pediatric Neurology. 2006;13(3):158–65. doi: 10.1016/j.spen.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 16.McDonald SD, Han Z, Mulla S, Beyene J. Overweight and obesity in mothers and risk of preterm birth and low birth weight infants: systematic review and meta-analyses. BMJ. 2010;341:c3428. doi: 10.1136/bmj.c3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao G, Xu G, Zhou M, Jiang Y, Richards B, Clark KM, et al. Prenatal iron supplementation reduces maternal anemia, iron deficiency, and iron deficiency anemia in a randomized clinical trial in rural China, but iron deficiency remains widespread in mothers and neonates. Journal of Nutrition. 2015;145(8):1916–23. doi: 10.3945/jn.114.208678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Institute of Medicine. National Research Council . Weight Gain During Pregnancy: Reexamining the Guidelines. National Academies Press; Washington, DC: 2009. [PubMed] [Google Scholar]
- 19.Worwood M. Ferritin in human tissues and serum. Clinics in Haematology. 1982;11(2):275–307. [PubMed] [Google Scholar]
- 20.Carriaga MT, Skikne BS, Finley B, Cutler B, Cook JD. Serum transferrin receptor for the detection of iron deficiency in pregnancy. American Journal of Clinical Nutrition. 1991;54(6):1077–81. doi: 10.1093/ajcn/54.6.1077. [DOI] [PubMed] [Google Scholar]
- 21.Zimmermann MB, Molinari L, Staubli-Asobayire F, Hess SY, Chaouki N, Adou P, et al. Serum transferrin receptor and zinc protoporphyrin as indicators of iron status in African children. American Journal of Clinical Nutrition. 2005;81(3):615–23. doi: 10.1093/ajcn/81.3.615. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention Recommendations to Prevent and Control Iron Deficiency in the United States. Morbidity and Mortality Weekly Report. 1998;47:RR-3. [PubMed] [Google Scholar]
- 23.Stevens GA, Finucane MM, De-Regil LM, Paciorek CJ, Flaxman SR, Branca F, et al. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: a systematic analysis of population-representative data. Lancet Global Health. 2013;1(1):e16–e25. doi: 10.1016/S2214-109X(13)70001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Metzgeroth G, Adelberger V, Dorn-Beineke A, Kuhn C, Schatz M, Maywald O, et al. Soluble transferrin receptor and zinc protoporphyrin--competitors or efficient partners? European Journal of Haematology. 2005;75(4):309–17. doi: 10.1111/j.1600-0609.2005.00515.x. [DOI] [PubMed] [Google Scholar]
- 25.Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood. 2003;101(9):3359–64. doi: 10.1182/blood-2002-10-3071. [DOI] [PubMed] [Google Scholar]
- 26.Diagne I, Archambeaud MP, Diallo D, d’Oiron R, Yvart J, Tchernia G. [Erythrocyte indices and iron stores in cord blood] Archives de Pediatrie. 1995;2(3):208–14. doi: 10.1016/0929-693x(96)81129-8. [DOI] [PubMed] [Google Scholar]
- 27.Paterakis GS, Lykopoulou L, Papassotiriou J, Stamulakatou A, Kattamis C, Loukopoulos D. Flow-cytometric analysis of reticulocytes in normal cord blood. Acta Haematologica. 1993;90(4):182–5. doi: 10.1159/000204454. [DOI] [PubMed] [Google Scholar]
- 28.Shao J, Lou J, Rao R, Georgieff MK, Kaciroti N, Felt BT, et al. Maternal serum ferritin concentration is positively associated with newborn iron stores in women with low ferritin status in late pregnancy. Journal of Nutrition. 2012;142(11):2004–9. doi: 10.3945/jn.112.162362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gelman A, Carlin JB, Stern HS, Rubin DB. Bayesian Data Analysis. Chapman and Hall; London: 1995. [Google Scholar]
- 30.Ganz T. Hepcidin and iron regulation, 10 years later. Blood. 2011;117(17):4425–33. doi: 10.1182/blood-2011-01-258467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dao MC, Meydani SN. Iron Biology, Immunology, Aging, and Obesity: Four Fields Connected by the Small Peptide Hormone Hepcidin. Advances in Nutrition. 2013;4(6):602–17. doi: 10.3945/an.113.004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rao R, Georgieff MK. Perinatal aspects of iron metabolism. Acta Paediatrica. 2002;91(438):124–9. doi: 10.1111/j.1651-2227.2002.tb02917.x. [DOI] [PubMed] [Google Scholar]
- 33.Kleven KJ, Blohowiak SE, Kling PJ. Zinc protoporphyrin/heme in large-for-gestation newborns. Neonatology. 2007;92(2):91–5. doi: 10.1159/000100807. [DOI] [PubMed] [Google Scholar]
- 34.Schmatz M, Madan J, Marino T, Davis J. Maternal obesity: the interplay between inflammation, mother and fetus. Journal of Perinatology. 2010;30(7):441–6. doi: 10.1038/jp.2009.182. [DOI] [PubMed] [Google Scholar]
- 35.McClung JP, Karl JP. Iron deficiency and obesity: the contribution of inflammation and diminished iron absorption. Nutrition Reviews. 2009;67(2):100–4. doi: 10.1111/j.1753-4887.2008.00145.x. [DOI] [PubMed] [Google Scholar]
- 36.Rehu M, Punnonen K, Ostland V, Heinonen S, Westerman M, Pulkki K, et al. Maternal serum hepcidin is low at term and independent of cord blood iron status. European Journal of Haematology. 2010;85(4):345–52. doi: 10.1111/j.1600-0609.2010.01479.x. [DOI] [PubMed] [Google Scholar]
- 37.Young MF, Griffin I, Pressman E, McIntyre AW, Cooper E, McNanley T, et al. Maternal hepcidin is associated with placental transfer of iron derived from dietary heme and nonheme sources. Journal of Nutrition. 2012;142(1):33–9. doi: 10.3945/jn.111.145961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gambling L, Czopek A, Andersen HS, Holtrop G, Srai SK, Krejpcio Z, et al. Fetal iron status regulates maternal iron metabolism during pregnancy in the rat. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2009;296(4):R1063–70. doi: 10.1152/ajpregu.90793.2008. [DOI] [PubMed] [Google Scholar]
- 39.Ervasti M, Sankilampi U, Luukkonen S, Heinonen S, Punnonen K. Maternal pro-hepcidin at term correlates with cord blood pro-hepcidin at birth. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 2009;147(2):161–5. doi: 10.1016/j.ejogrb.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 40.Wrighting DM, Andrews NC. Interleukin-6 induces hepcidin expression through STAT3. Blood. 2006;108(9):3204–9. doi: 10.1182/blood-2006-06-027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thurnham DI, McCabe GP. Priorities in the assessment of vitamin A and iron status in populations, Panama City, Panama, 15-17, September 2010. World Health Organization; Geneva, Switzerland: 2012. Influence of infection and inflammation on biomarkers of nutritional status with an emphasis on vitamin A and iron; pp. 63–80. [Google Scholar]
- 42.Thurnham DI, McCabe LD, Haldar S, Wieringa FT, Northrop-Clewes CA, McCabe GP. Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: a meta-analysis. American Journal of Clinical Nutrition. 2010;92(3):546–55. doi: 10.3945/ajcn.2010.29284. [DOI] [PubMed] [Google Scholar]
- 43.Ahima RS, Lazar MA. The Health Risk of Obesity—Better Metrics Imperative. Science. 2013;341(6148):856–858. doi: 10.1126/science.1241244. [DOI] [PubMed] [Google Scholar]