Abstract
CD101 exerts negative-costimulatory effects in vitro, but its function in vivo remains poorly defined. CD101 is abundantly expressed on lymphoid and myeloid cells in intestinal tissues, but absent from naïve splenic T cells. Here, we assessed the impact of CD101 on the course of inflammatory bowel disease (IBD). Using a T cell transfer model of chronic colitis, we found that in recipients of naïve T cells from CD101+/+ donors up to 30% of the recovered lymphocytes expressed CD101, correlating with an increased IL-2-mediated FoxP3-expression. Transfer of CD101−/− T cells caused more severe colitis and was associated with an expansion of IL-17-producing T cells and an enhanced expression of IL-2Rα/β independently of FoxP3. The co-transfer of naïve and regulatory T cells (Treg) protected most effectively from colitis, when both donor and recipient mice expressed CD101. While the expression of CD101 on T cells was sufficient for Treg-function and the inhibition of T cell proliferation, sustained IL-10-production required additional CD101-expression by myeloid cells. Finally, in patients with IBD a reduced CD101-expression on peripheral and intestinal monocytes and CD4+ T cells correlated with enhanced IL-17-production and disease activity. Thus, CD101-deficiency is a novel marker for progressive colitis and potential target for therapeutic intervention.
Introduction
The inflammatory bowel diseases (IBD) ulcerative colitis (UC) and Crohn’s disease (CD) are driven by complex interactions of genetic susceptibility traits, environmental factors and enteric microbes1-2. Disturbances in T cell homeostasis contribute to the pathogenesis of both chronic intestinal disorders2. CD4+ Th17 cells expressing the lineage-determining transcription factor RORγt accumulate in intestinal tissues of IBD patients and perpetuate colitis in mouse models2-4. In contrast, regulatory T cells (Tregs) expressing the lineage-defining transcription factor FoxP3 protect from colitis5. Although Tregs and Th17 cells exhibit opposing functions, they both can develop from the same pool of naïve CD4+ T cell precursors. Once differentiated, they even display a certain degree of environment-dependent plasticity, with Tregs converting into IL-17-producers or RORγt-expressing cells becoming positive for FoxP3 and the anti-inflammatory cytokine IL-106.
IL-2Rα, IL-2Rβ and the common gamma chain form the IL-2 receptor, which is essential for T cell proliferation upon antigen encounter and the initiation of T cell responses7. IL-2Rα and its ligand IL-2 also act, together with the nuclear Foxo proteins and Smad-mediated signals, as pivotal regulators of Treg-function8-9. While IL-2 preferentially signals through STAT510 and promotes the generation of Tregs11-12, the Foxo transcription factors are tightly regulated by the PI3K and Akt pathways which induce the nuclear export of Foxo and thereby impede Treg activity13. Engagement of IL-2Rα minimizes the activation of Akt and PI3K-pathways9 and inhibits Th17-differentiation14. Although Tregs do not produce IL-2 themselves15-16, they are the only T cell population expressing constitutively IL-2Rα17.
Tregs express unique sets of costimulatory molecules at steady state18. One of these abundantly expressed molecules is CD10119,20. In vitro, CD101+ Tregs are more suppressive than their CD101− counterparts19. CD101 is also expressed on various myeloid cells19,21-22 which exhibit immunosuppressive functions23 upon engagement of CD101 by agonistic antibodies. The mechanisms underlying the regulation and abundant expression of CD101 in close proximity to the intestinal lumen24 and the interaction partner(s) of CD101 are unknown. The function of CD101 in vivo during intestinal inflammation has never been investigated.
Here, we analyzed the role of CD101 in mouse and human IBD. We observed that the transfer of naïve CD4+ T cells25 from CD101−/− donors accelerated the onset of intestinal inflammation in recipient mice which correlated with increased numbers of tissue-infiltrating T cells and enhanced IL-17-production. While the intrinsic CD101-expression on T cells was sufficient for the concomitant expression of FoxP3 with IL-2Rα and the inhibition of T cell proliferation, additional expression of CD101 by myeloid recipient cells was required for optimal Treg-function. In IBD patients a reduced CD101-expression on monocytes and CD4+ T cells correlated with enhanced IL-17-production and disease activity identifying CD101 as a novel marker for IBD disease activity.
Results
T cells become CD101+ upon transfer
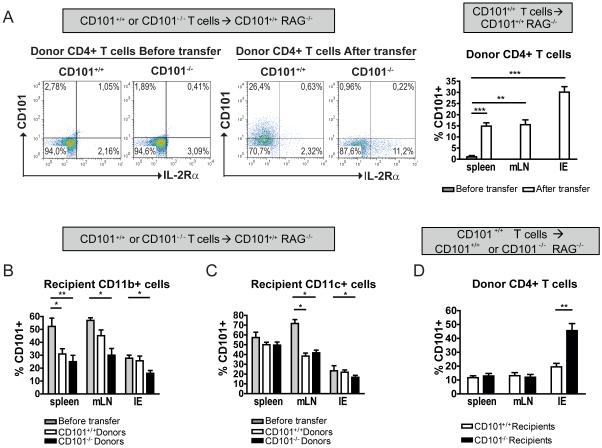
To study CD101 in vivo, we used the well-established T cell transfer-colitis model25 and first analyzed the expression of CD101 on donor and recipient cells. While purified naïve CD4+CD45RBhighIL-2Rα− T cells did not express CD101 before transfer, T cells became CD101-positive after transfer (Figure 1a), particularly in the intestinal epithelium (IE); 8.1±3.8%, 31.1±5.4%, 26.8±6.9% or 18.7±4.6% of all T cells in the IE were CD101+ at week 1, 3, 5 or 7 after transfer (mean + SEM of 2 experiments). Prior to T cell transfer, around 50% of CD11b+ and CD11c+ cells expressed CD101 in lymphopenic recipient mice (Figure 1b, c) while CD101 was undetectable on intestinal epithelial cells (data not shown). After T cell-transfer the numbers of CD101+ myeloid cells in the organs of recipient mice decreased (Figure 1b, c). While the number of CD101+ eosinophils remained unchanged (data not shown), transfer of CD101+/+ or CD101−/− T cells caused a significant reduction of CD101-expression by CD11b+Gr1+ neutrophils and CD11b+F4/80+ macrophages (Supplemental Table 1).
Figure 1. T cells become CD101+ upon transfer.
Naïve T-cells from CD101+/+ (A-D) and CD101−/− (A-C) donors were transferred into CD101+/+ (A-D) or CD101−/− RAG1−/−recipients (D). The expression of CD101 by donor T lymphocyte (TCRβ+, CD45+) and recipient myeloid cells (CD11b+, CD45+) was assessed by flow cytometry before (A, left panels for T cells obtained from mLNs; A-C, grey bars) and 3 weeks after the adoptive T cell transfer (A-D). The mean percentages (± SD) of CD101 positivity within the respective cell populations were derived from the analysis of 28 (A) or 18 (B-D) CD101+/+ mice and from 9 CD101−/− recipient mice (D) (one out of three experiments). Statistical significance was calculated using ANOVA with Bonferroni posthoc test (*, p<0.05; **, p<0.01; ***, p<0.001).
To evaluate whether the presence of CD101 in recipient mice influences CD101-expression by donor T cells, we performed comparative adoptive T cell transfers into CD101−/− and CD101+/+ recipients. Unlike to the regulation of CD101 on myeloid cells by transferred T lymphocytes, a lack of CD101-expression in recipient mice increased the frequency of CD101+ donor T cells in the intestine (Figure 1d) indicating a bidirectional impact of CD101 during the interaction of T cells and myeloid cells in vivo.
CD101-deficiency of donor T cells accelerates the onset of intestinal inflammation
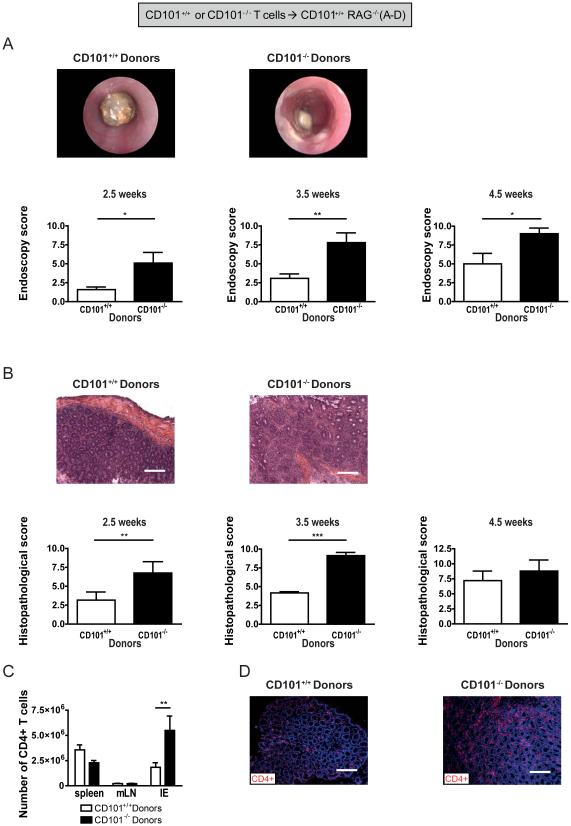
To study the impact of CD101 on disease, we assessed intestinal pathology in RAG1−/− mice that had received naïve CD101+/+ or CD101−/− T cells (Supplemental Figure 1a). Endoscopy and histopathology revealed a more severe inflammation and faster developing colitis in recipients of T lymphocytes from CD101−/− mice than recipients of T cells from CD101+/+ littermates (Figure 2a, b). This protective effect was CD101-intrinsic, as a co-transfer of naïve CD101+/+ and CD101−/− T cells in a 1:1 ratio into RAG1−/− recipients (Supplemental Figure 2a) ameliorated colitis compared to single transfers with CD101−/− T cells (Supplemental Figure 2b). The more severe colitis correlated with increased numbers of IE-infiltrating CD101−/− T cells (Figure 2c, d). The absence of CD101 in RAG1−/− recipient mice did not affect the severity of inflammation elicited by the transfer of naïve CD101+/+ T cells (Supplemental Figure 1b), neither at early nor at late time-points (Supplemental Figure 3a, b), although CD101−/− recipient mice contained significantly less T cells in spleen and intestine than their CD101+/+ counterparts (Supplemental Figure 3c). In contrast, when CD101−/− T cells were transferred into CD101−/− recipients (Supplemental Figure 1c), severe colitis was even more rapidly detected than after the transfer of CD101−/− T cells into CD101+/+ recipients (Supplemental Figure 3d). Thus, although both CD101+ myeloid and CD101+ lymphoid cells exhibit anti-colitogenic functions, the enhanced ability of CD101−/− T cells to induce colitis primarily results from the loss of a T cell-intrinsic negative-regulatory function of CD101.
Figure 2. CD101 on T cells is sufficient for ameliorating colitis.
(A+B) The induction of intestinal inflammation in CD101+/+ RAG1−/− recipient mice was monitored by high-resolution endoscopy (A) and histopathological analysis of H&E-stained tissue sections (B) 2.5, 3.5 and 4.5 weeks after T cell transfer from CD101+/+ and CD101−/− donor mice. Representative images from coloscopies (A) and H&E-stained tissue sections (B; bar, 100μm) as well as the means (± SD) of the endoscopic and histological scores from 6 individual recipient mice 4.5 weeks after T cells transfer are displayed (one out of two, five and three experiments, respectively). Statistical significance was calculated using a Mann-Whitney test (**, p<0.01; ***, p<0.001).
(C+D) The numbers of CD4+ T cells in the indicated tissues of 18 and 9 individual RAG1−/− recipient mice that had been injected with cells from CD101+/+ (n = 18) or CD101−/− donors (n = 9), respectively, 3.5 weeks before were assessed by flow cytometry (C) or immunofluorescence microscopy (CD4+ T lymphocytes are shown in red and intestinal epithelial cells in blue (D) (bar, 100μm) (mean ± SD of 3 experiments). Statistical significant differences within the respective organ sites were calculated using a student’s t-test (**, p<0.01).
CD101 supports the differentiation and function of Tregs
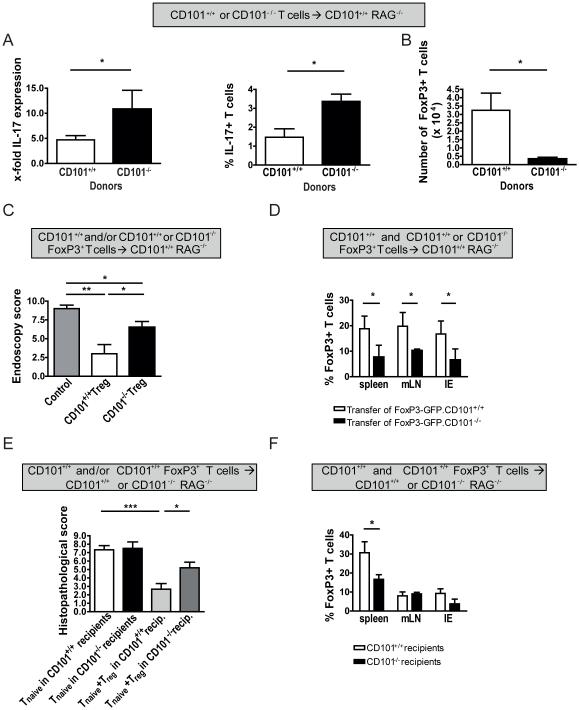
The induction of colitis in the T cell transfer model has been linked to an expansion of Th1 and Th17 cells3. Therefore, we tested the expression of cytokines and transcription factors associated with the generation of these Th cell populations in the organs of CD101+/+ or CD101−/− recipients after the transfer of naïve CD101+/+ or CD101−/− T cells. Although CD101 on either donor or recipient cells did not affect the generation of Th1 cells as indicated by similar levels of IFN-γ and T-bet (Supplemental Figure 4a, b; data not shown), the simultaneous deletion of CD101 on both, donor and recipient cells enhanced the production of IFN-γ (Supplemental Figure 4c). In contrast, the knockout of CD101 on donor T lymphocytes alone was sufficient to promote the expression of IL-17 (Figure 3a).
Figure 3. CD101 on T cells is sufficient for the inhibition of IL-17-production and the acquisition of FoxP3.
(A) Expression of mRNA copies for IL-17 (left panel) and the percentage of IL-17-positive cells (right panel) were evaluated by qPCR and flow cytometry in 1×106 T lymphocytes obtained from the IE of 8 individual recipient mice 4 weeks after T cell transfer from CD101+/+ or CD101−/− donors. All cells were pre-gated on CD45, CD4 and TCRβ before purification and intracellular cytokine staining (ICS) analysis. The ratio of the respective cytokine mRNA copies relative to the HPRT copies was calculated. The relative increase in IL-17 copy numbers of CD101−/− over CD101+/+ donor T cells is displayed (one out of two experiments).
(B) The absolute numbers of FoxP3+ cells (mean ± SD) isolated individually from the IE of 6 RAG1−/− mice 4 weeks after the transfer of T cells from either CD101+/+ or CD101−/− donors are displayed (one out of two experiments). Statistical significance was calculated using a student’s t-test (*, p<0.05). (C-F) nTregs from FoxP3-GFP×CD101+/+
(C-F) or FoxP3-GFP×CD101−/− (C+D) littermates were co-transferred together with naive T-cells from Ly5.1+ CD101+/+ mice into CD101+/+ (C+D) or CD101−/− (E+F) recipients. Intestinal inflammation was determined by endoscopy (C) and histopathology (E) and the relative number of FoxP3+ cells among all T cells in the various tissues was measured by flow cytometry (D+F) six weeks after transfer (one out of four experiments). All cells were pre-gated on CD45 before.Statistical significance for 8 individual recipient mice in each group was calculated using a Kruscal Wallis test with Dunn’s multiple comparison (*, p<0.05; **, p<0.01).
To define the CD101-intrinsic effects on T cell differentiation, we performed competitive index experiments and co-transferred naive T cells from CD101+/+ mice or CD101−/− mice allelically marked by CD45.1 or CD45.2 in a 1:1 mixture into RAG1−/− mice (Supplemental Figure 2a). T cells from CD101+/+ mice expressed also significantly more FoxP3 and less IL-17 than their CD101−/− counterparts after co-transfer implying CD101-intrinsic effects on T cell differentiation (Supplemental Figure 2c, d).
The balance between Th17 and Tregs maintains intestinal immune homeostasis6. Whereas only few RORγt+ T cells were CD101+ (6.9±3.7% and 8.1±4.3% CD101+ for mLN and IE, respectively; mean + SEM of 5 mice), Tregs abundantly expressed CD101 (Supplemental Figure 5a)19. Therefore, we analyzed the role of CD101 in the differentiation of Tregs. Although the CD4+CD45RBhigh T cells were devoid of IL-2Rα− expression prior to transfer (Figure 1a), they still contained few FoxP3+ cells (0.87±0.24% versus 0.99±0.21% from CD101+/+ versus CD101−/− donors; mean + SEM of 6 mice in 2 experiments). In accordance with previous studies reporting an upregulation of FoxP3 in transferred T cells26-27, significant numbers of FoxP3+ T cells were detected in CD101+/+ RAG1−/− recipients (Figure 3b). Most notably, recipients of CD101+/+ donor T cells contained significantly higher numbers of FoxP3+ T cells in the IE than recipients of CD101−/− T cells (Figure 3b, Supplemental Figure 5b). In contrast, comparing the T cell transfer into CD101+/+ and CD101−/− recipient mice, the numbers of FoxP3+ T cells recovered from CD101+/+ or CD101−/− recipient mice were comparable (Supplemental Figure 5c). These observations indicate that the expression of CD101 by naïve T cells intrinsically inhibits IL-17-production and promotes their differentiation into Tregs.
To evaluate CD101-expression on FoxP3+ T cells, we transferred purified natural Tregs (nTregs) from mice expressing FoxP3 under a GFP promoter28 into recipient mice. Following transfer the number of recovered CD101+ FoxP3+ T cells was significantly increased (Supplemental Figure 5a). To test whether CD101-expression correlated with the suppressive capacity of Tregs as previously proposed19, we co-transferred nTregs from CD101+/+ or CD101−/− FoxP3-GFP mice (CD45.2+) along with naive T cells from CD101+/+ mice (CD45.1+). Although T cells from CD101−/− FoxP3-GFP donors accumulated in greater absolute numbers in the IE than from their CD101-expressing counterparts (Supplemental Figure 5d), nTregs from CD101−/− mice were significantly less efficient in suppressing colitis than nTregs from CD101+/+ mice (Figure 3c), likely due to the enhanced loss of FoxP3-expression in the absence of CD101 (Supplemental Figure 5e) resulting in a reduced representation of FoxP3+ cells in relation to all T cells (Figure 3d). While CD101+ cells of the recipient were dispensable for the induction of FoxP3 in transferred naïve T cells (Supplemental Figure 5c), they were required for the optimal function of Tregs and the maintenance of FoxP3 expression, because colitis in CD101−/− recipients was significantly more severe than in their CD101+/+ counterparts and associated with a decreased relative accumulation of Foxp3+ cells and enhanced FoxP3 loss (Figure 3e-f, Supplemental Figure 5f). Thus, CD101-expression by both donor T cells and myeloid recipient cells is critical for the protective function of Tregs.
The T cell-intrinsic expression of CD101 inhibits the expansion of T lymphocytes
To test whether intrinsic effects of CD101 inhibit the accumulation of CD101+/+ donor T cells in recipient mice, we performed competitive index experiments and co-transferred naive T cells from CD101+/+ mice or CD101−/− mice allelically marked by CD45.1 or CD45.2 (Supplemental Figure 2a) in a 1:1 mixture into RAG1−/− mice. The numbers of T cells from CD101+/+ donors accumulating in recipient mice were significantly lower compared to CD101−/− donors, independent of whether T cells from CD101−/− donors were transferred separately (Figure 2c) or together with CD101+/+ T cells (Figure 4a). Still, the absolute number of CD101−/− T cells accumulating in the single transfer studies was larger than in the co-transfer set-up, which might be due to variabilities in the numbers of intraepithelial lymphocytes and/or CD101-dependent, but cell-extrinsic mechanisms that affect, for example, the production and/or consumption of cytokines.
Figure 4. CD101 inhibits the expansion of T cells and the FoxP3-independent expression of IL-2Rα.
(A+B) Naïve T cells from CD45.1+ CD101+/+ and CD45.2+ CD101−/− mice were transferred together in a 1:1 mixture into RAG1−/− mice. 4 weeks after the transfer, the absolute numbers of CD45.1+ and CD45.2+ T cells (A) and the percentage of IL-2Rα+ cells within the TCRβ+ T cell population (B) was determined in the indicated tissues of 7 individual recipient mice (one out of 5 experiments). The results shown are based on the analysis of CD45.1+ and CD45.2+ T cells from 11 individual recipient mice. (*, p<0.05; **, p<0.01).
(C) CFSE-labeled naïve T cells from CD101+/+ and CD101−/− mice were transferred into RAG1−/− recipient mice. 4 weeks later the proliferation of T cells was determined by CFSE dilution analysis (see Supplemental Fig. 3E). The number of T cells from 5 individual CD101+/+ and CD101−/− mice within the respective division cycles are shown (mean±SD) (one out of two experiments).
(D) FoxP3+ T cells from CD101−/− or CD101+/+ mice were transferred into 10 individual RAG1−/− recipient mice each. The percentage of IL-2Rα+ cells on FoxP3− is displayed (one out of three experiments).
(E) Naïve T cells from CD101+/+ or CD101−/− donors were transferred into 8 individual RAG1−/− mice. The relative number of FoxP3+ among IL-2Rα+ T cells is displayed (one out of five experiments).
(F) Purified Tregs from FoxP3-GFP×CD101+/+ mice were transferred into 6 individual RAG1−/− recipient mice each. The percentage of IL-2Rα+ CD101+ double positive cells among the T cells which maintained (FoxP3+) or lost (FoxP3−) their FoxP3 expression is displayed (one out of two experiments).
Statistical significance was calculated using a student’s t-test for each organ separately (*, p<0.05; **, p < 0.01).
Engagement of CD101 inhibited IL-2-production and proliferation of human T cells29-30. To evaluate whether CD101 intrinsically inhibits the T cell response to IL-2, which is essential for T cell proliferation7, we evaluated IL-2Rα/β− and IL-2-expression of CD101+/+ and CD101−/− T cells after co-transfer. Confirming this in vitro inhibitory effect of CD10129-30, we observed that T cells from CD101+/+ mice expressed significantly less IL-2Rα and IL-2Rβ than T cells from CD101−/− mice (Figure 4b, Supplemental Figure 2e). The IL-2 mRNA levels in purified CD101+/+ (i.e. CD45.1+) T cells were significantly lower than in CD101−/− (i.e. CD45.2+) T cells (Supplemental Figure 2f), suggesting that CD101 intrinsically inhibited IL-2Rα/β− expression and IL-2-production by T cells.
Vice versa, in the absence of CD101 on myeloid recipient cells IL-2Rα− and IL-2Rβ− expression on CD101+/+ donor T cells were reduced compared to CD101+/+ T cells transferred into CD101+/+ recipients (Supplemental Figure 3e, f), which is in accordance with their enhanced CD101-expression (Figure 1d) and reduced recovery from the organs of CD101-deficient recipient mice (Supplemental Figure 3c).
To further prove that alterations in the production of and the responsiveness to IL-2 underlie the divergent recovery of T cells from CD101−/− and CD101+/+ donors, we transferred CFSE-labeled T cells into RAG1−/− mice and defined the divisions of T cells by CFSE dilution assays (Supplemental Figure 6a). We observed that CFSE dilution (i.e. T cell proliferation) was much faster in recipients of CD101−/− T cells than in mice that had received CD101+/+ T cells (Figure 4c). The percentage of CD101+ cells also gradually decreased with higher cell division cycles (Supplemental Figure 6b), while the expression of IL-2Rα/β increased (Supplemental Figure 6c), further supporting that CD101 intrinsically inhibits T cell proliferation due to the restriction of IL-2-production and IL-2Rα/β− expression. Accordingly, the addition of an IL-2 blocking antibody reduced the IL-2-induced expression of IL-2Rα on T cells and subsequently T cell proliferation (Supplemental Figure 6d). These data support the concept that CD101 intrinsically inhibits T cell proliferation by restricting the production of IL-2 and, via downregulation of IL-2R-expression, also the responsiveness to IL-2.
CD101 inhibits the FoxP3-independent expression of IL-2Rα on T cells
CD101 promoted the development of naïve T cells into Tregs (Figure 3b, Supplemental Figure 5b), which are characterized by an IL-2-dependent differentiation and high IL-2Rα− and FoxP3-expression9. At the same time CD101+/+ T cells expressed less IL-2Rα than their CD101−/− counterparts (Figure 1a, 4b). To further unravel the link between CD101, FoxP3 and IL-2Rα, we compared the expression of IL-2Rα and CD101 on FoxP3+ and FoxP3− T cells after the transfer of naïve or purified Tregs from CD101+/+ and CD101−/− FoxP3-GFP littermates28 as Tregs loose their FoxP3-expression when adoptively transferred into lymphopenic hosts (Supplemental Figure 3e). Within the FoxP3− subset significantly less T cells expressed IL-2Rα when originating from CD101+/+ FoxP3-GFP as compared to CD101−/− FoxP3-GFP donors (Figure 4d). While the numbers of IL-2Rα+ cells among the FoxP3+ subset were comparable between CD101+/+ and CD101−/− donors after the transfer of purified Tregs or naïve T cells (Supplemental Figures 7a, 7b), the recipients of T cells from CD101+/+ mice showed an increase in FoxP3-expression within the IL-2Rα+ T cell population when compared to naïve T cells (Figure 4e, Supplemental Figure 7c) or purified Tregs (not shown) donated from CD101−/− mice. We again detected significantly more FoxP3-/IL-2Rα− double-positive cells among T cells from CD101+/+ compared to CD101−/− mice upon stimulation with rIL-2 in vitro (Supplemental Figure 7d). Furthermore, while hardly any IL-2Rα/CD101-double positive cells were detected within the FoxP3− T cell compartment (Figure 1a, 4f), FoxP3+ cells contained a large population of IL-2Rα/CD101-coexpressing cells before19 and after transfer (Figure 4f). Thus, CD101 does not only promote the induction, but also the maintenance of FoxP3-expression and coordinates the co-expression of FoxP3 and IL-2Rα in response to IL-2. In contrast, FoxP3− cells express IL-2Rα without upregulating CD101 simultaneously.
CD101 modulates signaling pathways to promote Treg and to suppress Th17 differentiation
To corroborate the role of CD101 for the simultaneous stabilization of FoxP3 and IL-2Rα on a molecular basis, we assessed the mRNA-expression of the IL-2 target genes SOCS 2 and TGFβ132 and evaluated the activation of STAT5, a major component of the IL-2 signaling pathway11-12, in rIL-2-stimulated T cell cultures obtained from the mLNs of recipient mice after transfer of purified CD101+/+ and CD101−/− Tregs. While SOCS2-transcription (Figure 5a) and STAT5-phosphorylation (Figure 5b, c) were increased in CD101+/+ compared to CD101−/− T cells, TGFβ1-expression remained unaltered (Supplemental Figure 7d). SOCS2-transcription was also enhanced in the mLNs of naïve CD101+/+ compared to CD101−/− mice upon IL-2-stimulation (data not shown) suggesting an enhanced sensitivity of T cells to IL-2 signals in the presence of CD101. Thus, CD101 promotes the differentiation and function of Tregs by fostering distinct IL-2-mediated signaling pathways.
Figure 5. CD101 modulates signaling pathways to promote Treg and to suppress Th17 differentiation.
(A-C) 2.5×105 T cells purified from the mLNs of six recipients of purified CD101+/+ and CD101−/− FoxP3-GFP+ T cells 3 weeks after transfer were stimulated with 50ng/mL rIL-2 (A+B) or 100ng/mL anti-CD3 (B). mRNA (A) and protein lysates (B) from purified T cells were extracted 30 minutes later. SOCS2 mRNA copies were determined by RT-qPCR (A). The ratio of the Socs2 mRNA copies relative to the HPRT copies was calculated. The ratios Socs2/ HPRT in each panel were set to 1 for the tissues of one control sample. STAT5-expression and -phosphorylation was analyzed by Western blotting (B). Western Blot signals were quantitated using Image J software (C) (one out of three (A+B) experiments).
(D-G) The expression of mRNA copies for COX2 (D) as well as the expression and phosphorylation of ERK (E, F) and Akt (G) were evaluated in the mLNs of 9-12 recipient mice 4 weeks after the transfer of naïve T cells (D+E) or purified Tregs (F+G) from CD101+/+ and CD101−/− mice. mRNA was extracted and COX2 mRNA copies were determined by RT-qPCR (D). The ratio of the COX2 mRNA copies relative to the HPRT copies was calculated. The relative increase in COX2 copy numbers of CD101−/− over CD101+/+ donor T cells is displayed. Representative Western blots for ERK1 and Akt are displayed (E-G) (one out of three experiments). Statistical significance was calculated using a student’s t-test for each experimental condition separately (**, p<0.01).
The absence of CD101 did not only largely abolish FoxP3-expression (Figure 3b), but also enhanced T cell proliferation (Figure 4c) and IL-17-production (Figure 3a). We therefore assessed the regulation of signaling pathways and target genes that are critical for the differentiation of Tregs and Th17 cells8,33, in tissues of recipient mice after the transfer of naïve T cells or purified Tregs. The expression of COX2, which has been associated with Th17 differentiation34-35, was strikingly reduced in recipients of naïve CD101+/+ compared to CD101−/− T cells (Figure 5d). The phosphorylation of ERK1, which restrains the generation of Tregs36 and is upstream of COX2, but not of the Akt pathways37, was similarly decreased in the organs of recipients of naïve T cells (Figure 5e) and purified Tregs (Figure 5f) from CD101+/+ donors. The phosphorylation of the phosphatidylinositide-3 kinase (PI3K)-dependent serine/threonine kinase Akt, which induces the nuclear export of the Foxo transcription factors8,13, was also decreased in transferred purified Tregs from CD101+/+ donors (Figure 5g). As PI3K-phosphorylation itself was only marginally altered between CD101+ and CD101− T cells (data not shown), CD101-engagement likely affects the differentiation of Th17 cells through an alternative pathway of Akt-activation independent of PI3K.
CD101 expression by T cells or myeloid cells mutually regulates the production of IL-10
The expression of CD101 on T cells ameliorated the colitis (Figure 2a, b) and was sufficient for the generation of Tregs (Figure 3b) and the inhibition of T cell proliferation (Figure 4c). In contrast, CD101 on myeloid cells had no impact on the severity of the colitis induced by CD101+/+ T cells (supplemental Figure 3a, b), which raises questions on the exact role of myeloid cells for the regulation of intestinal homeostasis.
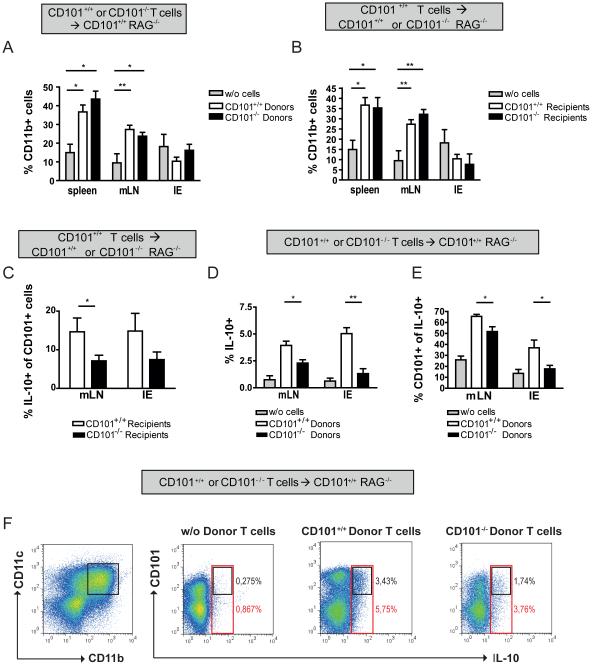
We first evaluated the distribution of myeloid cells in recipient mice before and after T cell transfer. Compared to naïve RAG1−/− mice, recipients of CD101+/+ or CD101−/− T cells had an increased frequency of CD11b+ cells in the spleen and mLNs (Figure 6a). While Gr1+ and F4/80+ cells were enriched within the CD11b+ population, there were no significant changes detected for Siglec-F+ or CD11c+ cells (data not shown). Also, upon transfer of CD101+/+ T cells the frequency of CD11b+ cells was augmented in the spleens and mLNs of CD101+/+ and CD101−/− recipient mice (Figure 6b).
Figure 6. CD101 promotes IL-10 production by myeloid cells.
(A+B) 1×106 naïve T-cells from CD101+/+ (A+B) and CD101−/− (A) mice were transferred into 18 CD101+/+ RAG1−/− (A+B) or 9 CD101−/− × RAG1−/− (B) mice. A group of 4 individual RAG1−/− mice did not receive any T cells (grey bars). The numbers of CD11b+ cells in the spleens, mLNs and the IE were determined by flow cytometry. Statistical significance was calculated using ANOVA with Bonferroni posthoc test (One out of two experiments). (*, p<0.05; **, p< 0.01).
(C-F) 1×106 naïve T cells from CD101+/+ VERT-X (C-F) and CD101−/− VERT-X mice (D-F) were transferred into 8 B6 RAG1−/− CD101+/+ VERT-X (C-F) and B6 RAG1−/− CD101−/− VERT-X mice (C) each. The numbers of IL-10-GFP+ T cells (C) and of IL-10+ CD11b+ myeloid cells (D) as well as the percentages of IL-10+ CD11b+ myeloid cells expressing CD101 simultaneously (E) were determined by flow cytometry. Representative FACS dot plots for the co-expression of CD101 and IL-10 on CD11b+ CD11c+ myeloid cells from the intestinal epithelium are displayed (F). All cells were pre-gated on CD45 before (one out of two experiments). Statistical significance for 8 individual recipients mice of T cells from CD101+/+ and CD101−/− donors was calculated using a student’s test for each organ separately (C) or using ANOVA with Bonferroni posthoc test (D) (*, p<0.05).
Next, we investigated whether CD101 is involved in the regulation of IL-10, which exhibits potent anti-inflammatory effects38, regulates the growth, function and/or differentiation of multiple cell types39-40 and protects against IBD or severe enterocolitis in humans and mice, respectively41-42. To assess the effect of CD101 on the expression of IL10, we transferred naïve T cells from CD101+/+ or CD101−/− VERT-X mice, an IL-10-GFP transcriptional reporter strain43, into CD101+/+ or CD101−/− recipient mice. Furthermore, we crossed CD101+/+ or CD101−/− VERT-X mice with RAG1−/− mice and transferred naïve T cells as well as Tregs from CD101+/+ or CD101−/− mice into CD101+/+ or CD101−/− RAG1−/− VERT-X recipients. While the deletion of CD101 on T cells did not affect their expression of IL-10 (data not shown), a significantly reduced percentage of CD101+/+ T cells produced IL-10 when transferred into CD101−/− recipients (Figure 6c). Conversely, significantly more CD11b+ myeloid cells expressed IL-10 when CD101 was also expressed on donor T cells (Figure 6d) with the majority of IL-10-expressing cells being positive for CD101 (Figure 6e-f). While the IL-10+ cell populations in the spleen and mLNs mainly consisted of F4/80+, CD11b+ and Siglec-F+ cells (data not shown), the majority of IL-10+ cells within the IE co-expressed CD11c and CD11b (Figure 6f). From these data we conclude that CD101-expression by T cells or myeloid cells supports IL-10 production by myeloid cells or T cells, respectively.
CD101-expression correlates inversely with the severity of IBD in humans
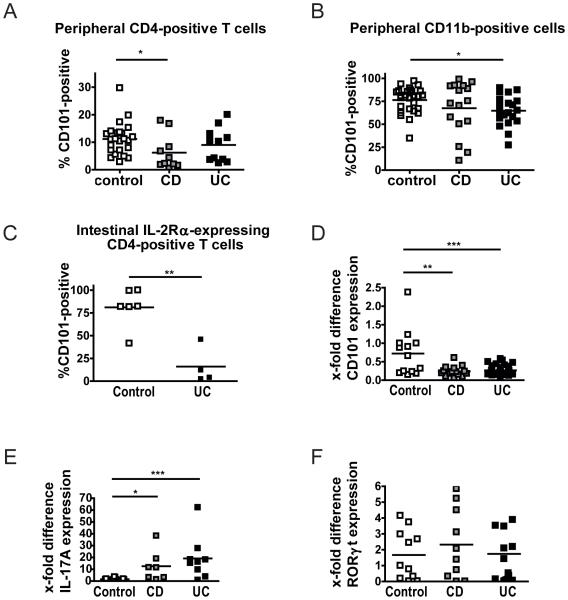
Having seen that CD101 has a strong impact on the severity of colitis in mice, we evaluated CD101-expression on lymphoid and myeloid cell subsets of IBD patients. Compared to peripheral blood T lymphocytes from control individuals, CD and UC patients, particularly those with active disease, exhibited a markedly reduced frequency of CD4+ T cells that co-expressed CD101 (Figure 7a; Supplemental Figure 8a). Furthermore, significantly fewer CD101-expressing CD11b+ cells were recovered from the peripheral blood of UC and CD patients as compared to controls (Figure 7b). Within that CD11b+ population the number of CD14+CD15− monocytes was significantly reduced (7.8±10.9 vs. 2.9±2.2; mean + SEM of 28 controls and 36 IBD patients; p < 0.05).
Figure 7. Human IBD is associated with a reduced expression of CD101 and enhanced IL-17-production.
(A+B) PBMCs from healthy individuals as well as CD and UC patients were evaluated for the expression of CD101. The number of CD101+ CD4+ T cells (A) and CD11b+ myeloid cells (B) for 10 CD patients with a score > 6 on the Harvey-Bradshaw index (HBI) and 20 UC patients with a score > 2 on the Mayo scale compared to 24 (A) and 27 (B) control individuals are displayed. Statistical significance was calculated using using ANOVA with Bonferroni posthoc test (*, p<0.05).
(C-F) Intestinal tissue specimens obtained from colectomies of 4 UC patients with severe disease (with a score of 3) and six non-IBD control patients (with a score of 0) were analyzed for the expression of CD101 on IL-2Rα+ CD4+ T cells by flow cytometry (C). 105 T cells from intestinal biopsies of control individuals, CD and UC patients were evaluated for the mRNA copy numbers of CD101 (D), Rorγt (E) and IL-17 (F) by RT-qPCR. Each square in the panels depicts the numbers of CD101+ T cells (C) or the relative mRNA levels for CD101 (D), RORγt (E) and IL-17 (F) of one individual control, CD or UC patient. β− actin (D) and PPIA (E+F) mRNA copies were used to normalize expression levels, and the ratio of the respective cytokine over the β− actin or PPIA copies was calculated. The ratios CD101/β− actin, Rorγt/PPIA or IL-17/PPIA in each panel were set to 1 for the tissues of one control patient. Statistical significance was calculated using Mann-Whitney test (C) or ANOVA with Bonferroni posthoc test (D-E) (*, p<0.05; **, p<0.01; ***, p<0.001).
To investigate CD101-expression within the intestine, we obtained tissue samples from UC patients undergoing selective colectomy due to a failure of conservative treatment (n = 4) and non-IBD control patients (n = 6). CD101 was almost completely absent from CD4+ T lymphocytes (1.53±1.24 vs. 11.48±9.67; mean + SEM of 6 controls and 4 UC patients; p=0.08) and CD11b+ myeloid cells in colon specimens of UC patients as compared to controls (8.34±7.71 vs. 35.27±11.21; mean + SEM of 6 controls and 4 UC patients; p<0.01). Furthermore, whereas more than 90% of IL-2Rα− expressing T cells were positive for CD101 in the control group, less than 20% of the IL-2Rα+ cells co-expressed CD101 in UC patients (Figure 7c; Supplemental Figure 8b) suggesting that the expression of IL-2Rα is usually linked to the expression of CD101 under physiologic conditions. The drastic reduction in the expression of CD101 protein was associated with similar changes of the expression of CD101 mRNA in both CD and UC patients (Figure 7d). Underlining a dominant role of Th17 cells in the pathogenesis of IBD2-3, we detected significantly increased levels of IL-17A-mRNA in T cells of CD or UC patients compared to non-IBD controls (Figure 7e) and an inverse expression of CD101 and IL-17A (Supplemental Figure 8c), although the Th17-related transcription factor RORγt was comparably expressed in all three groups (Figure 7f). Collectively, these data document a reduced intestinal and peripheral CD101-expression and an enhanced IL-17-production in IBD patients.
Discussion
The data presented here support the concept that loss of CD101 expression is a novel marker for progressive IBD. The association of a reduced CD101-expression on CD4+ T cells with an increased disease activity in UC and CD patients was mirrored by the results obtained in the mouse model of T cell-transfer-induced colitis, which allowed detailed analyses of the mechanism of action of CD101. Whereas a lack of CD101 on transferred T cells clearly correlated with more severe colitis, the clinical phenotypes of CD101−/− and CD101+/+ recipient mice after the transfer of CD101+/+ T cells were comparable. This latter observation is best explained by the enhanced recovery of CD101+ and FoxP3+ T cells from CD101−/− recipients which compensate the reduced Treg-function mediated by CD101− myeloid cells. In IBD patients the expression of CD101 was reduced on myeloid as well as lymphoid cells, suggesting that both cell populations are unable to properly exhibit their compensatory anti-inflammatory functions.
CD101, IL-2 and Tregs
Our mechanistic analyses point to an unexpected dichotomic activity of CD101 on IL-2-production and -signaling. On the one hand CD101 restricted IL-2R-expression and IL-2-release. The resulting lack of IL-2-protein likely limits T lymphocyte expansion and/or survival, although the T cell-intrinsic CD101-expression was already sufficient for the inhibition of T cell proliferation. Thus, it is conceivable that CD101 acts as a critical modulator of the recently suggested IL-2-dependent quorum sensing-like mechanisms between IL-2-positive T cells and Tregs44 due to its negative-regulatory functions on IL-2-production and IL-2R-expression. On the other hand, CD101 amplified the IL-2-mediated SOCS2-expression and STAT5-phosphorylation, both of which reflect an improved IL-2 signaling10,45 and thus, might reflect an enhanced consumption of IL-2, for example, due to a larger number of CD101+/+ T cells differentiating into Tregs. Consequently, the question arises how these seemingly contradictory molecular events associated with the engagement of CD101 nevertheless lead to a uniform phenotypic effect on a defined T cell subset. While IL-2 and IL-2R are pivotal for T cell proliferation and expansion in general, they are in particular critical for the stability of FoxP3-expression and Treg-function46. Based on our observation that CD101 coordinated the expression of IL-2Rα and FoxP3 on a single cell level, we postulate that CD101 sensitizes Tregs for IL-2 and stabilizes IL-2-signaling, despite the overall reduced expression of IL-2 and IL-2Rα within the FoxP3− T cell compartment in CD101+/+ compared to CD101−/− mice. Furthermore, the engagement of CD101 might contribute to the FoxP3-dependent repression of the IL-2 gene15-16.
CD101 and the Treg/Th17 balance
In the presence of CD101 the transformation of naïve T cells into colitogenic, IL-17+ T cells was attenuated and the phosphorylation of ERK and Akt was blocked. Akt is a phosphatidylinositide-3 kinase (PI3K)-dependent serine/threonine kinase which induces the nuclear export of Foxo transcription factors and thereby antagonizes the function of Tregs8,13,47. Similar to Akt, ERK restrains the generation of Treg36 and promotes the proliferation of naïve T cells and their differentiation into Th17 cells48-49. Thus, CD101 modulates signaling cascades downstream of the TCR in such a way that the development of Tregs is facilitated, whereas the generation of Th17 cells is inhibited. Importantly, a deficiency of CD101 in T cells promoted the production of IL-17, which fosters T cell-mediated pathology in lymphopenic recipients4, but this was not sufficient for an enhanced production of IFN-γ, which requires an additional deletion of CD101 on myeloid cells (supplemental Figure 4c) and/or another modulation of myeloid cells in addition to CD101-suppression as observed during bacterial infection22. While this enhanced IFN-γ production by CD101−/− T cells stipulated the more severe enterocolitis in CD101−/− recipients, an augmented IL-2 and IL-17 production, an increased IL-2Rα/β expression and an enhanced T cell accumulation contributed to the more severe intestinal inflammation in CD101+/+ recipients after the transfer of T cells from CD101−/− compared to CD101+/+ donors.
CD101 and myeloid cells
CD101 is not only expressed on lymphoid, but also myeloid cell populations19,21-22. We previously observed that the expression of CD101 was lost on granulocytes, macrophages and dendritic cells following infection22. As the expression of MHC class II antigens was at the same enhanced, we believe that the downregulation of CD101 is a critical event in the initiation of effective anti-bacterial immune responses22. In the present study we show that CD101 was critical for the sustained production of the anti-inflammatory cytokine IL-10. Furthermore, the function of and the differentiation into regulatory T cells was most potent, when both donor and recipient cells expressed CD101 (see Figures 3b-f and Supplemental Figure 2c). Thus, the enhanced IL-10-production in CD101-competent recipients and/or protective interactions between CD101+ myelojd and CD101+ lymphoid cells most likely reduces the pathogenicity of transferred T cells and helps to explain the comparable clinical outcome in CD101+/+ vs. CD101−/− recipients, despite a reduced T cell accumulation in the latter ones (see Supplemental Figures 3a-c). Whether certain bacterial species promote the expression of CD101 and IL-10 is part of our ongoing analyses.
In conclusion, we describe a novel anti-inflammatory function of CD101 in mice and identify the loss of CD101 as a potential marker for progressive IBD in humans. CD101 serves as a dual regulator of lymphoid and myeloid cell subsets: First, CD101 acts as a stabilizer of FoxP3-expression via distinct IL-2-dependent mechanisms. It promotes the generation of Tregs and suppresses the conversion of naïve into IL-17-producing T cells by interfering with various signaling pathways and inhibiting FoxP3-independent IL-2Rα− expression and T cell expansion. Second, CD101+ myeloid cells support the function of Tregs (Supplemental Figure 9). Future studies need to delineate the exact mechanisms and signaling pathways that connect CD101 with the promotion of SOCS2 and STAT5 expression and the suppression of Akt/ERK-signaling, to define the extracellular ligand(s) of CD101, and to identify the molecular pathways regulating the expression of CD101.
Methods
Mice
CD45.1+/+, FoxP3-GFP28, IL-2Rα−/−, (all on a C57BL/6 background), B6 RAG1−/− mice and B6 scid mice were obtained from the Jackson Laboratory (Bar Harbor, USA). The IL-10-GFP transcriptional reporter mouse strain VERT-X43 was kindly provided by Dr. Christopher Karp (University of Cincinnati). All mouse strains including CD101−/−20 mice were kept and bred in the Franz-Penzoldt-Zentrum at the University of Erlangen. The B6 CD101−/− mice were crossed with B6 RAG1−/− mice, B6 scid mice, IL-2Rα+/− or with B6 VERT-X mice; the resulting F1 mice were crossed to obtain the respective double k.o. mice and heterozygous control mice. B6 scid CD101−/− and B6 RAG1−/− CD101−/− mice were also paired with B6 VERT-X mice to generate B6 scid CD101−/− VERT-X and B6 RAG1−/− CD101−/− VERT-X mice. For experiments utilizing CD101−/− mice on a B6, B6 scid or B6 RAG1−/− background, heterozygous CD101+/−, as well as homozygous CD101+/+ littermate controls were used.
All mice were raised and kept in a specific pathogen-free environment and used at the 5-9 weeks of age. The experiments were conducted according to the Institutional Animal Care and Use Committee guidelines of the Cincinnati Children’s Hospital and approved by the Animal Welfare Committee of the local government (Regierung von Mittelfranken, Ansbach, Germany).
Adoptive T cell transfer studies
1×106 splenic CD4+CD45RBhighIL-2Rα− (i.e. naïve) T-cells from CD101+/+ and CD101−/− mice were transferred intraperitoneally into RAG1−/− mice. For cell division studies donor T cells were labeled with 5μM CFSE prior to transfer according to the manufacturer's instructions (Molecular Probes and BD Pharmingen). For co-transfer experiments with Tregs recipient mice were injected with 3×105 CD4+CD45RBhighIL-2Rα− T cells together with 3×105 CD4+FoxP3-GFP+ Tregs (nTregs). For competitive index experiments, RAG1−/− recipient mice were injected with a 1:1 mixture of 5×105 CD4+CD45RBhighIL-2Rα− T-cells from CD45.1+CD101+/+ and CD45.2+CD101−/− donor mice. Recipient mice were monitored for the development of colitis 3-6 weeks after the adoptive T cell transfer and the numbers of the T cell populations in the spleens, IE and mLNs of the recipient mice were determined.
Colonoscopy
The inflammation of the gut was analyzed by high resolution mini-endoscopy of the colon (Karl Storz GmbH, Tuttlingen, Germany) utilizing the MEICS system as previously described50. Briefly, the score was based on the following five parameters: thickening of the colon wall, changes in the normal vascular pattern, presence of fibrin, mucosal granularity and stool consistency. An endoscopic grading was performed for each parameter (score between 0 and 3), leading to a cumulative score between 0 (no signs of inflammation) and 15 (very severe inflammation).
Histology and H&E staining
Intestinal tissue was fixed in 10% buffered formalin, embedded in paraffin, and cut into 2 μm thick sections. Colonic and ileal sections were deparaffinized, stained with H&E by the Department of Pathology and the Medical Department I of the FAU Erlangen-Nürnberg, and evaluated microscopically in a double-blinded manner. Briefly, the intestinal damage shown in the presented figures was evaluated using the following parameters: (a) Inflammatory infiltrate of the lamina propia, (b) thickness of the subepithelial collagenous layer, (c) presence of intraepithelial lymphocytes and (d) epithelial damage. A histologic grading of the severity of the tissue damage was performed for each parameter (score between 0 and 3), leading to a cumulative score between 0 (no signs of inflammation) and 12 (very severe inflammation and epithelial damage).
Immunofluorescence microscopy
Cryosections obtained from intestinal samples of mice were incubated with primary rat-antibodies against mouse CD4 (Pharmingen) overnight. Subsequently, the sections were incubated for one hour with a secondary antibody against rat IgG (Dianova). For the detection of double-positive cells, anti-mouse CD101 antibody (R&D Systems) was biotinylated and labeled with Alexa555-conjugated streptavidin. The nuclei were counterstained with Hoechst 3342 (Invitrogen). Images were taken with a fluorescence microscope (IX70; Olympus).
Human tissue samples
The collection of human samples was approved by the local Ethics Committee and the Reviewing Board of the University of Erlangen-Nürnberg (number 4032). Each patient gave written informed consent. Human PBMCs were obtained after Ficoll centrifugation of heparinized blood of IBD patients and healthy controls. Colonic biopsies of sigmoid colon and rectum from UC patients and biopsies of ileum and colon from CD patients with a score of 3 were investigated in this study along with samples from non-inflammatory controls (diverticulosis, cancer screening, functional bowel disorders) with a score of 0.
Flow cytometry and intracellular cytokine staining
Antibodies against mouse TCRβ, CD4, CD101, CD25, Cd122, CD45.1, CD45.2, FoxP3, CD11b and CD11c were purchased from eBioscience. Antibodies against human CD3, CD4, CD25 and CD11b were purchased from eBioscience; anti-human CD101 was obtained from Abcam. Intracellular staining for Foxp3 and intracellular cytokines was performed with a staining kit (eBioscience) following the manufacturer’s instructions. Cells were analyzed on a BD FACS Canto II (BD Biosciences, San Diego, CA) with FlowJo software (Tree Star).
Statistical analysis
Samples were analyzed for normal distribution by a Kolmogorov-Smirnov test. According to the results, statistical significance in normal distributed samples were analyzed by one-way ANOVA with posthoc test (Bonferroni) and student’s t test, and samples failing the normal distribution test by Kruscal-Wallis Test with posthoc (Dunn’s multiple comparison) or Mann-Whitney U test as indicated in the respective experiments. A sample size of at least three (n = 3) was used for each sample group in a given experiment, and a p value of 5% (*; p 0.05), 1% (**; p 0.01) or 0.1% (***; p 0.001) was considered significant to accept the alternate hypothesis. GraphPad Prism software was used for statistical analysis.
Supplementary Material
Acknowledgements
We are grateful to Prof. Markus Neurath for critical reading of the manuscript, discussion and advice, to Harald Arnold, Claudia Giessler, Stephanie Schmidt, and Alexandra Wandersee for technical assistance, and to Dr. Jonas Mudter as well as all medical personnel of the Department of Medicine I at the University Hospital Erlangen for collecting human blood and tissue samples for our studies. We also thank Simon Schey for his help with the graphical illustrations. This study was supported by the Interdisciplinary Center for Clinical Research of the Universitätsklinikum Erlangen (IZKF project grant A48 to JM; IZKF project grant A62 to CB), the Staedtler Stiftung (to JM), the German Research Foundation DFG (grant MA 2621/2-1 and MA2621/3-1 to JM; SFB643 project A5 grant to CB) and by a grant (award number R01DK084054 to JM) from the National Institute of Diabetes and Digestive and Kidney Diseases.
Abbreviations used
- FoxP3
forkhead box P3
- IBD
inflammatory bowel disease
- i.p.
intraperitoneally
- i.v.
intravenously
- IE
intestinal epithelium
- IELs
intraepithelial lymphocytes
- mLN
mesenteric lymph node
- PBMCs
peripheral blood mononuclear cells
- r(m)IL-2
recombinant (murine) interleukin-2
- TCR
T cell receptor
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011;365:1713–1725. doi: 10.1056/NEJMra1102942. [DOI] [PubMed] [Google Scholar]
- 2.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ostanin DV, et al. T cell transfer model of chronic colitis: concepts, considerations, and tricks of the trade. Am J Physiol Gastrointest Liver Physiol. 2009;296:G135–146. doi: 10.1152/ajpgi.90462.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leppkes M, et al. RORgamma-expressing Th17 cells induce murine chronic intestinal inflammation via redundant effects of IL-17A and IL-17F. Gastroenterology. 2009;136:257–267. doi: 10.1053/j.gastro.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 5.Izcue A, Coombes JL, Powrie F. Regulatory lymphocytes and intestinal inflammation. Annu Rev Immunol. 2009;27:313–338. doi: 10.1146/annurev.immunol.021908.132657. [DOI] [PubMed] [Google Scholar]
- 6.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 7.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 8.Ouyang W, Li MO. Foxo: in command of T lymphocyte homeostasis and tolerance. Trends Immunol. 2011;32:26–33. doi: 10.1016/j.it.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng G, Yu A, Malek TR. T-cell tolerance and the multi-functional role of IL-2R signaling in T-regulatory cells. Immunol Rev. 2011;241:63–76. doi: 10.1111/j.1600-065X.2011.01004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burchill MA, Yang J, Vang KB, Farrar MA. Interleukin-2 receptor signaling in regulatory T cell development and homeostasis. Immunol Lett. 2011;114:1–8. doi: 10.1016/j.imlet.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang XP, et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol. 2011;12:247–254. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quintana FJ, et al. Aiolos promotes T(H)17 differentiation by directly silencing Il2 expression. Nat Immunol. 2012;13:770–777. doi: 10.1038/ni.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunet A, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 14.Nagai S, Kurebayashi Y, Koyasu S. Role of PI3K/Akt and mTOR complexes in Th17 cell differentiation. Ann N Y Acad Sci. 2013;1280:30–34. doi: 10.1111/nyas.12059. [DOI] [PubMed] [Google Scholar]
- 15.Wu Y, et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375–387. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 16.Ono M, et al. Foxp3 controls regulatory T-cell function by interacting with AML1/Runx1. Nature. 2007;446:685–689. doi: 10.1038/nature05673. [DOI] [PubMed] [Google Scholar]
- 17.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 18.Bour-Jordan H, Bluestone JA. Regulating the regulators: costimulatory signals control the homeostasis and function of regulatory T cells. Immunol Rev. 2009;229:41–66. doi: 10.1111/j.1600-065X.2009.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandez I, et al. CD101 surface expression discriminates potency among murine FoxP3+ regulatory T cells. J Immunol. 2007;179:2808–2814. doi: 10.4049/jimmunol.179.5.2808. [DOI] [PubMed] [Google Scholar]
- 20.Rainbow DB, et al. Evidence that Cd101 is an autoimmune diabetes gene in nonobese diabetic mice. J Immunol. 2011;187:325–336. doi: 10.4049/jimmunol.1003523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruegg CL, et al. V7, a novel leukocyte surface protein that participates in T cell activation. II. Molecular cloning and characterization of the V7 gene. J Immunol. 1995;154:4434–4443. [PubMed] [Google Scholar]
- 22.Mohammed JP, et al. Identification of Cd101 as a susceptibility gene for Novosphingobium aromaticivorans-induced liver autoimmunity. J Immunol. 2011;187:337–349. doi: 10.4049/jimmunol.1003525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bouloc A, Bagot M, Delaire S, Bensussan A, Boumsell L. Triggering CD101 molecule on human cutaneous dendritic cells inhibits T cell proliferation via IL-10 production. Eur J Immunol. 2000;30:3132–3139. doi: 10.1002/1521-4141(200011)30:11<3132::AID-IMMU3132>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 24.Russell GJ, et al. p126 (CDw101), a costimulatory molecule preferentially expressed on mucosal T lymphocytes. J Immunol. 1996;157:3366–3374. [PubMed] [Google Scholar]
- 25.Powrie F, et al. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 26.Huber S, et al. Th17 cells express interleukin-10 receptor and are controlled by Foxp3(-) and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity. 2011;34:554–565. doi: 10.1016/j.immuni.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valatas V, et al. Host-dependent control of early regulatory and effector T-cell differentiation underlies the genetic susceptibility of RAG2-deficient mouse strains to transfer colitis. Mucosal Immunol. 2013;6:601–611. doi: 10.1038/mi.2012.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin W, et al. Regulatory T cell development in the absence of functional Foxp3. Nat Immunol. 2007;8:359–368. doi: 10.1038/ni1445. [DOI] [PubMed] [Google Scholar]
- 29.Soares LR, Tsavaler L, Rivas A, Engleman EG. V7 (CD101) ligation inhibits TCR/CD3-induced IL-2 production by blocking Ca2+ flux and nuclear factor of activated T cell nuclear translocation. J Immunol. 1998;161:209–217. [PubMed] [Google Scholar]
- 30.Rivas A, et al. V7, a novel leukocyte surface protein that participates in T cell activation. I. Tissue distribution and functional studies. J Immunol. 1995;154:4423–4433. [PubMed] [Google Scholar]
- 31.Willerford DM, et al. Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 32.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 33.Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–479. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- 34.Boniface K, et al. Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling. J Exp Med. 2009;206:535–548. doi: 10.1084/jem.20082293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khayrullina T, Yen JH, Jing H, Ganea D. In vitro differentiation of dendritic cells in the presence of prostaglandin E2 alters the IL-12/IL-23 balance and promotes differentiation of Th17 cells. J Immunol. 2008;181:721–735. doi: 10.4049/jimmunol.181.1.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Powell JD, Pollizzi KN, Heikamp EB, Horton MR. Regulation of immune responses by mTOR. Annu Rev Immunol. 2012;30:39–68. doi: 10.1146/annurev-immunol-020711-075024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steelman LS, et al. Roles of the Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity to therapy-implications for cancer and aging. Aging (Albany NY) 2011;3:192–222. doi: 10.18632/aging.100296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bogdan C, Vodovotz Y, Nathan C. Macrophage deactivation by interleukin 10. J Exp Med. 1991;174:1549–1555. doi: 10.1084/jem.174.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 40.Murai M, et al. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol. 2009;10:1178–1184. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 42.Glocker EO, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009;361:2033–2045. doi: 10.1056/NEJMoa0907206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Madan R, et al. Nonredundant Roles for B Cell-Derived IL-10 in Immune Counter-Regulation. J Immunol. 2009;183:2312–2320. doi: 10.4049/jimmunol.0900185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amado IF, et al. IL-2 coordinates IL-2-producing and regulatory T cell interplay. J Exp Med. 2013;210:2707–2720. doi: 10.1084/jem.20122759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tannahill GM, et al. SOCS2 can enhance interleukin-2 (IL-2) and IL-3 signaling by accelerating SOCS3 degradation. Mol Cell Biol. 2005;25:9115–9126. doi: 10.1128/MCB.25.20.9115-9126.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gavin MA, Clarke SR, Negrou E, Gallegos A, Rudensky A. Homeostasis and anergy of CD4(+)CD25(+) suppressor T cells in vivo. Nat Immunol. 2002;3:33–41. doi: 10.1038/ni743. [DOI] [PubMed] [Google Scholar]
- 47.Ouyang W, et al. Novel Foxo1-dependent transcriptional programs control T(reg) cell function. Nature. 2012;491:554–559. doi: 10.1038/nature11581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu H, et al. ERK differentially regulates Th17- and Treg-cell development and contributes to the pathogenesis of colitis. Eur J Immunol. 2013;43:1716–1726. doi: 10.1002/eji.201242889. [DOI] [PubMed] [Google Scholar]
- 49.Tybulewicz VL, Henderson RB. Rho family GTPases and their regulators in lymphocytes. Nat Rev Immunol. 2009;9:630–644. doi: 10.1038/nri2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Becker C, Fantini MC, Neurath MF. High resolution colonoscopy in live mice. Nat Protoc. 2006;1:2900–2904. doi: 10.1038/nprot.2006.446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.