Abstract
Background
Primary human hepatocytes offer the best human in vitro model for studies on human liver cell metabolism. Investigators use a variety of different media supplements and matrix biocoatings and the type of culture system used may influence the outcome.
Objectives
To optimize in vitro conditions for primary human hepatocytes with regard to bile acid synthesis.
Methods
Human hepatocytes were isolated and cultured on collagen type I or EHS matrigel in cell media with or without dexamethasone. The glucocorticoid receptor (GR) antagonist RU486 was used to elucidate the involvement of GR.
Results
Hepatocytes cultured on EHS matrigel produced more bile acids and expressed higher levels of cholesterol 7α-hydroxylase (CYP7A1) than cells cultured on rat tail collagen. Supplementation with dexamethasone increased the formation of cholic acid (CA) and decreased chenodeoxycholic acid formation. In line with these results, the mRNA expression of sterol 12α-hydroxylase (CYP8B1) increased following dexamethasone treatment. Surprisingly, the mRNA expression of CYP7A1 and CYP27A1 was not increased to the same extent. By using the GR antagonist RU486, we concluded that CYP8B1 induction is mediated via a GR-independent pathway. An altered expression of retinoid-related orphan receptor (ROR) α and ROR α target gene Glucose-6-phosphatase (G6Pase) suggests that ROR α signaling may regulate CYP8B1 expression.
Conclusion
Primary human hepatocytes have an increased bile acid synthesis rate when cultured on matrigel as compared to collagen. Exposure to glucocorticoid hormones stimulates the expression of CYP8B1, leading to an increased formation of CA and alteration of the bile acid composition. The effect is most likely mediated through a GR-independent pathway, possibly through ROR α.
Abbreviations: BSEP, bile salt export pump; CA, cholic acid; CDCA, chenodeoxycholic acid; CYP7A1, cholesterol 7α-hydroxylase; CYP8B1, sterol 12α-hydroxylase; CYP27A1, sterol 27α-hydroxylase; FXR, farnesoid X receptor; G6Pase, glucose-6-phosphatase; GR, glucocorticoid receptor; NTCP, Na+-taurocholate cotransporting polypeptide; PXR, pregnane X receptor; ROR, retinoid-related orphan receptor
Keywords: cholic acid, chenodeoxycholic acid, dexamethasone, matrigel, primary hepatocytes
Production of bile is one of the many important functions of the liver. Disruption of this process, called cholestasis, causes considerable morbidity and need of medical attention. Good research tools to study bile acid metabolism are therefore important.
In humans, two primary bile acids are synthesized: cholic acid (CA) and chenodeoxycholic acid (CDCA). These bile acids are produced by two major pathways, the neutral pathway and the acidic pathway.1, 2 The initial step of the neutral pathway is a hydroxylation at the 7α-position of cholesterol, a reaction catalyzed by the rate-limiting enzyme cholesterol 7α-hydroxylase (CYP7A1). The acidic pathway begins with the oxidation of the cholesterol side chain, in which the first step is catalyzed by sterol 27-hydroxylase (CYP27A1). The enzyme responsible for determining the relative amounts of CA and CDCA in both pathways is sterol 12α-hydroxylase (CYP8B1). The ratio between the bile acids is important for feedback regulation of bile acid synthesis.3 It is also important for both cholesterol absorption and accumulation of cholesterol esters in the liver, as well as for gallstone formation.4, 5, 6
Many in vitro studies on human bile acid synthesis have been performed in HepG2 cells. However, HepG2 cells have limitations since these cells leak bile acid precursors and unconjugated bile acids that are not secreted by the liver under normal conditions.7, 8, 9, 10 The first reports on cholesterol conversion to bile acids in primary hepatocytes were from rat studies.11, 12 We have found that primary human and rat hepatocytes in culture exhibit significant species differences in both types of bile acids formed and, more importantly, in the regulation of bile acids homeostasis.13 We have also shown that primary human hepatocytes conjugate all secreted CA and CDCA by amidation to glycine or taurine13, 14 and thus, no unconjugated bile acids are found in the media.
Bile acid synthesis is affected by signaling from many different pathways including those from nuclear hormone receptors, such as FXR, pregnane X receptor (PXR), and signals derived from the extracellular matrix. There are numerous reports on the in vitro regulation of mRNA levels of enzymes important for bile acids synthesis, such as CYP7A1 and CYP8B1,15, 16, 17, 18, 19 but experimental data are not always consistent. This may be due to the different culture conditions used by investigators. Both the cell substrate and the composition of the culture media may affect the ability to produce bile acid. Thus there is a need for further investigation of how different commonly used culture conditions alter the production of bile acids. Even though culturing hepatocytes on matrigel or in a matrigel sandwich results in a more adequate cell morphology, the effect on P450 expression in human cells has been disputed. It has been reported that the expression of P450 enzymes is not changed and that the expression may be dependent on plating density rather than on the type of extracellular matrix used.20, 21 Since dexamethasone is a common additive to culture media, we have previously investigated their effect on bile acid transporters in primary hepatocytes and shown that dexamethasone addition in the cell culture media increased the expression of BSEP and NTCP.22 In this study, we investigated how different substrates, collagen and matrigel, and the addition of dexamethasone to the culture media influence bile acid synthesis and secretion.
Patients and Methods
Isolation of Primary Human Hepatocytes
Normal human liver tissue was obtained from patients (n = 19) undergoing surgical liver resection due to cancer or from donor livers that could not be used for transplantation. Approval to use parts of resected human liver specimens for research was given by the local Ethics Committee in Stockholm alternatively from the Institutional Review Board at University of Pittsburgh, see Table 1, for information on liver tissue donors. Hepatocytes were isolated by a three-step perfusion technique, utilizing EGTA and collagenase (collagenase XI from Sigma), as previously described by Strom et al.23 The hepatocytes were plated onto cell culture dishes precoated either with rat tail collagen type I or EHS matrigel. Hepatocytes were cultured under standard conditions in William's E medium supplemented with 12 nM insulin, amphotericin (250 μg/ml), and gentamicin (50 mg/ml). 1.5 million cells were plated onto 6-well plates and cultured in cell media with or without the addition of 100 nM dexamethasone. Untreated cells from the same liver always served as controls to the treated cells. In Figure 4, Figure 5B, cells were also treated with 1 μM RU486. The medium was changed one hour after plating and then daily until harvesting. On day 5, cells were harvested in Trizol for quantification of specific mRNAs, and cell culture medium was analyzed for bile acids.
Table 1.
List of Livers Used in the Experiments. F = female, M = male.
| Liver | Age | Gender | Diagnosis |
|---|---|---|---|
| HF79 | 39 | M | Hepatocellular carcinoma |
| HF82 | 47 | F | Pancreatic cancer |
| HF83 | 69 | F | Donor |
| HF107 | 64 | F | Colon cancer |
| HF108 | 45 | F | Crm |
| HF109 | 57 | F | Colon cancer |
| HF110 | 73 | M | Colon cancer |
| HF111 | 65 | F | Liver cancer |
| HF113 | 62 | F | Colon cancer |
| HH1436 | 49 | M | Colon cancer |
| HH1437 | 50 | F | Adeno cancer |
| HH1438 | 38 | F | Focal nodular hyperplasia |
| HH1439 | 68 | M | Focal nodular hyperplasia |
| HH1465 | 76 | M | Hepatocellular carcinoma |
| HH1467 | 52 | F | Colon cancer |
| HH1468 | 38 | M | Colon cancer |
| HH1469 | 46 | F | Donor |
| HH1571 | 62 | F | Colon cancer |
| HH1591 | 12 | F | Donor |
Figure 4.
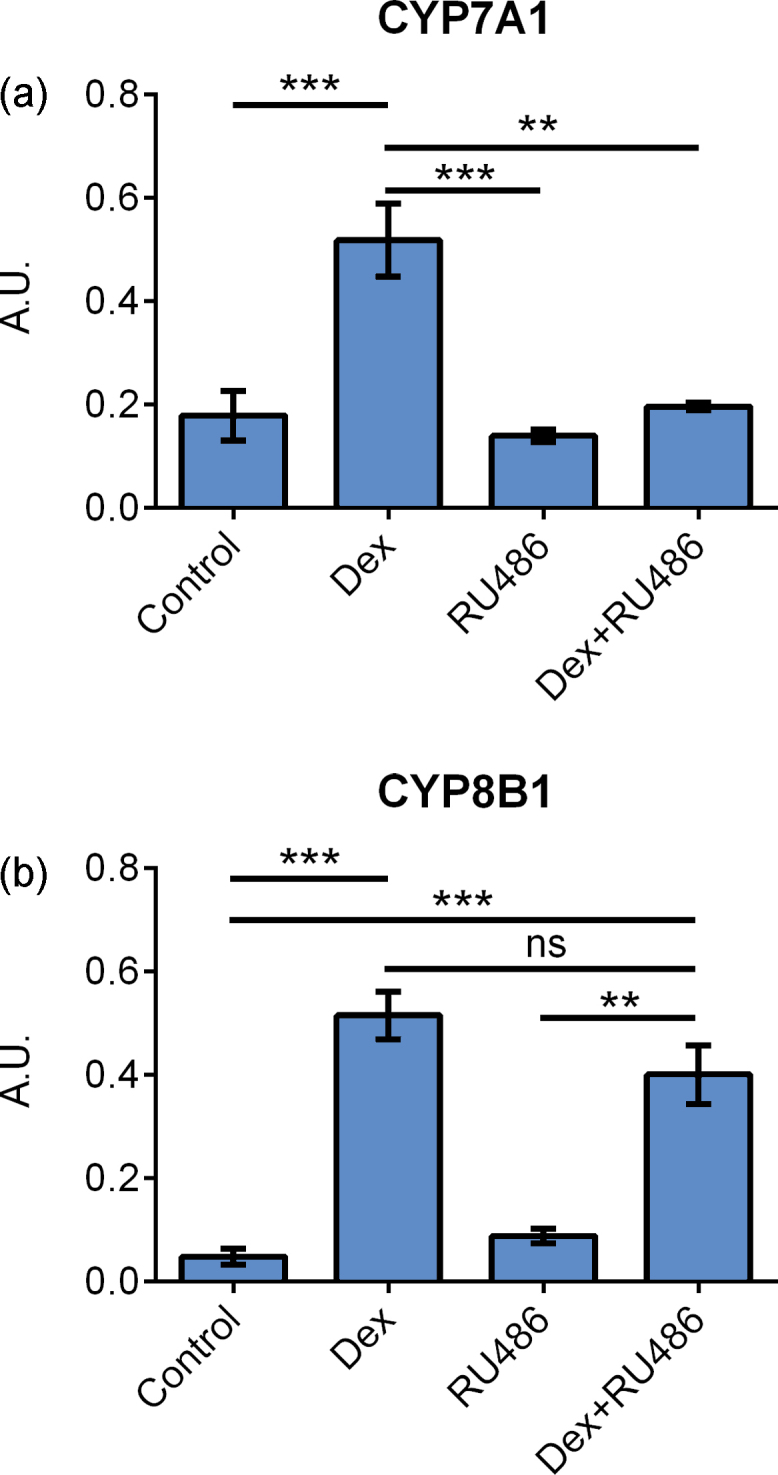
mRNA expression of (a) CYP7A1 and (b) CYP8B1 in primary hepatocytes cultured on collagen and treated with 100 nM dexamethasone, 1 μM RU486, or the combination of dexamethasone and RU486 for 24 h. Data represent means ± SEM, A. U., arbitrary unit, ** P ≤ 0.01, *** P ≤ 0.001, n = 4 livers.
Figure 5.
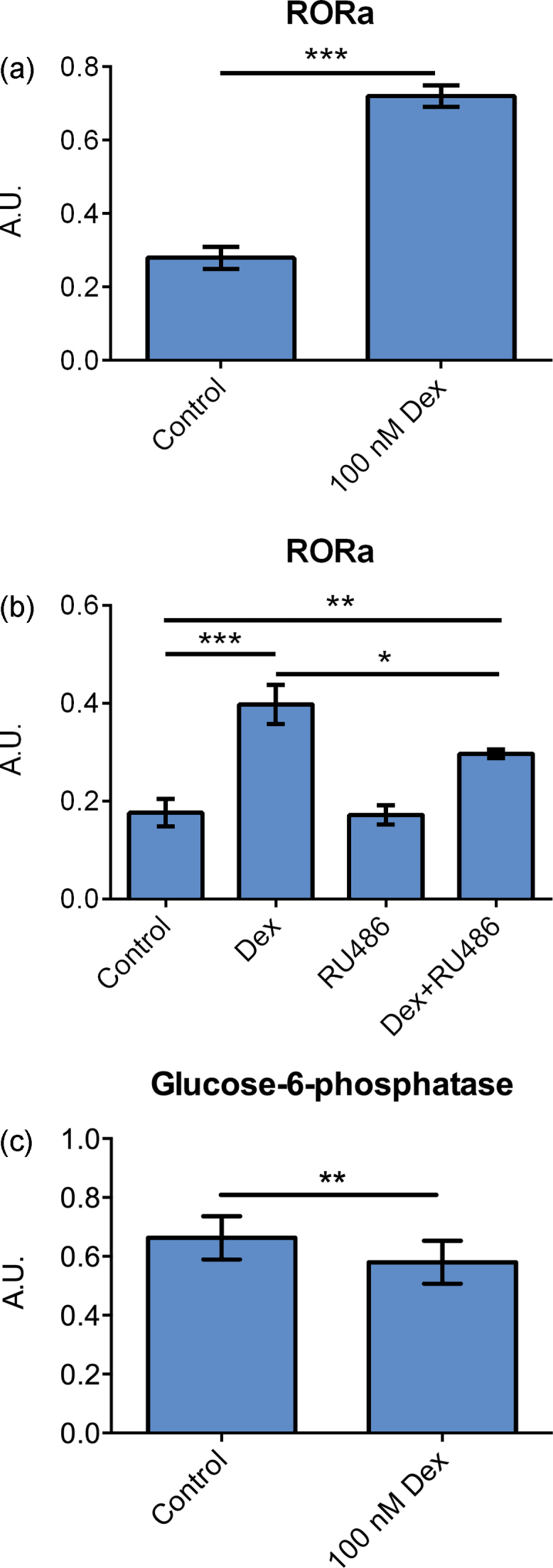
(a) mRNA expression of ROR α in primary hepatocytes cultured on matrigel and treated with 100 nM dexamethasone for 96 h. Data represent means ± SEM, A. U., arbitrary unit, *** P ≤ 0.01, n = 8 livers. (b) mRNA expression of ROR α in primary hepatocytes cultured on collagen and treated with 100 nM dexamethasone, 1 μM RU486, or the combination of dexamethasone and RU486 for 24 h. Data represent means ± SEM, A. U., arbitrary unit, * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001, n = 4 livers. (c) mRNA expression of glucose-6-phosphatase in primary hepatocytes cultured on matrigel and treated with 100 nM dexamethasone for 96 h. Data represent means ± SEM, A. U., arbitrary unit, ** P ≤ 0.01, n = 8 livers.
Real-Time PCR
RNA was isolated using Trizol reagent (Invitrogen, Stockholm, Sweden) and cDNA synthesis was performed using MultiScribe Reverse Transcriptase (Applied Biosystems, Stockholm, Sweden). mRNA expression was quantified with Quantitative real-time PCR using Taqman probes from ABI, and analysis was performed on an ABI Prism 7000 instrument (Applied Biosystems, Stockholm, Sweden). As endogenous control cyclophilin was used.
Analysis of Bile Acids
Bile acids in cell culture media were analyzed as described previously.13 Briefly, 500 ng of deuterium-labeled CA and CDCA was added to 1 ml of cell culture medium. The mixture was hydrolyzed in 1 M KOH at 120 °C overnight. The samples were extracted with ethyl ether, and following acidification with hydrochloric acid to pH 1, the samples were extracted again with ethyl ether. The samples were washed until neutral, evaporated and methylated with trimethylsilyldiazomethane and derivatized using hexamethyldisilazane and trimethylchlorosilane in pyridine. Samples were analyzed by gas chromatography/mass spectrometry (Column HP-1, 6890N GC System, 5973 Mass Selective Detector, Agilent Technologies, Stockholm, Sweden).
Statistics
Data are presented as means ± SEM. The significance of differences between treatments was tested by T-test (Figure 1, Figure 2, Figure 3, Figure 5A) and by ANOVA followed by Tukey's HSD (Figure 4, Figure 5B) (IBM SPSS Statistics).
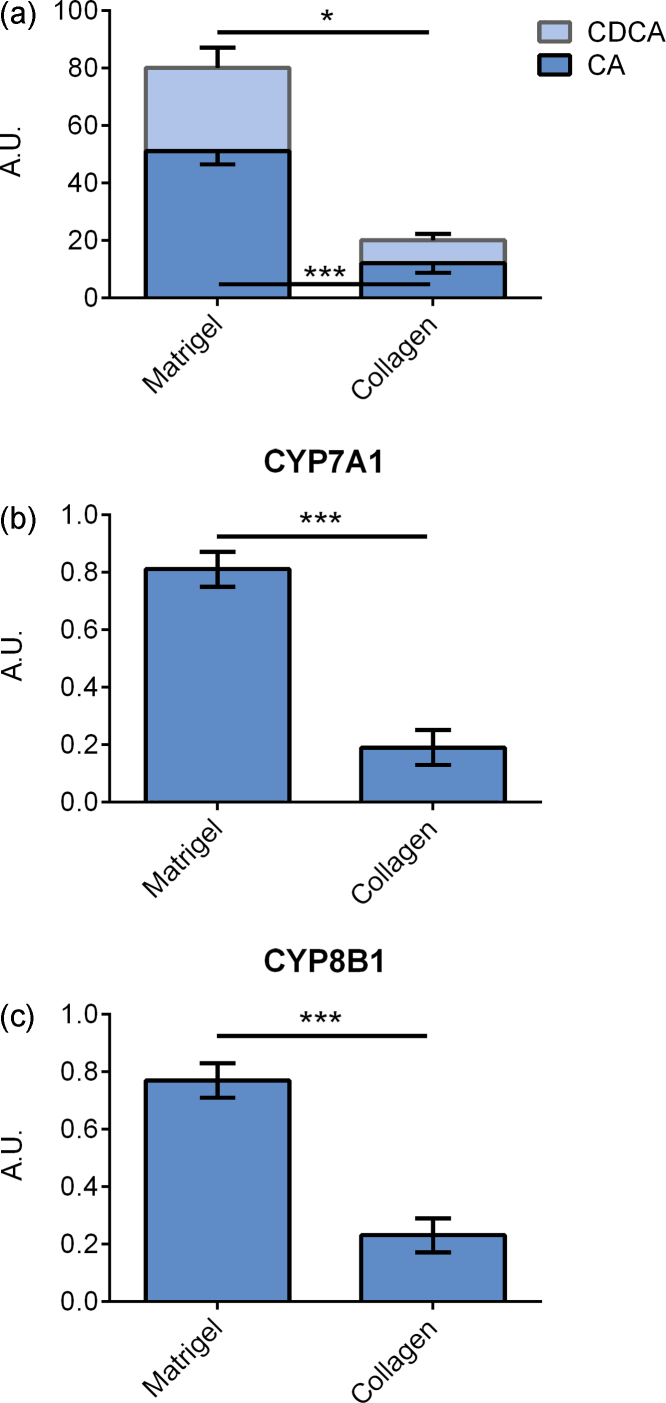
Figure 1.
Primary hepatocytes cultured on matrigel and collagen type I. (a) Bile acid levels in cell culture medium. (b) CYP7A1 mRNA expression. (c) CYP8B1 mRNA expression. Data represent means ± SEM, A. U., arbitrary unit, * P ≤ 0.05, *** P ≤ 0.01, n = 4 livers.
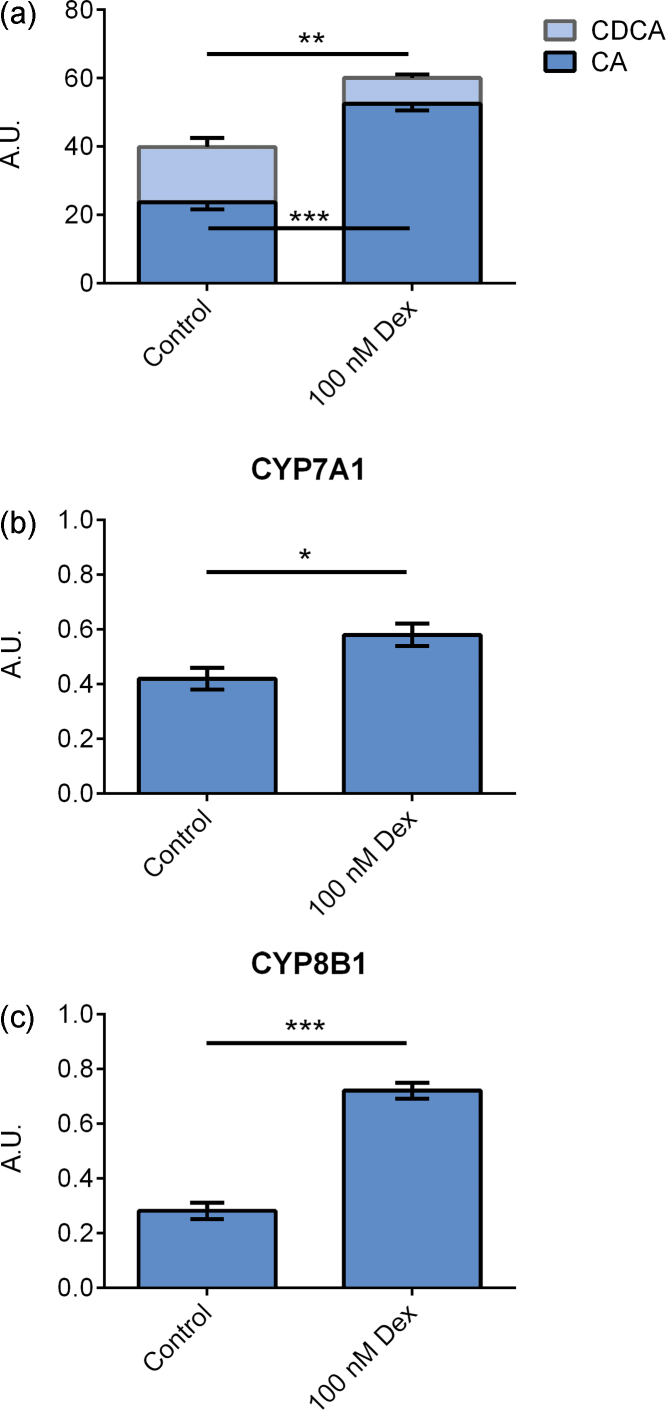
Figure 2.
Primary hepatocytes cultured on matrigel and treated with 100 nM dexamethasone for 96 h. (a) Bile acid levels in cell culture medium. (b) CYP7A1 mRNA expression. (c) CYP8B1 mRNA expression. Data represent means ± SEM, A. U., arbitrary unit, * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.01, n = 9 livers.
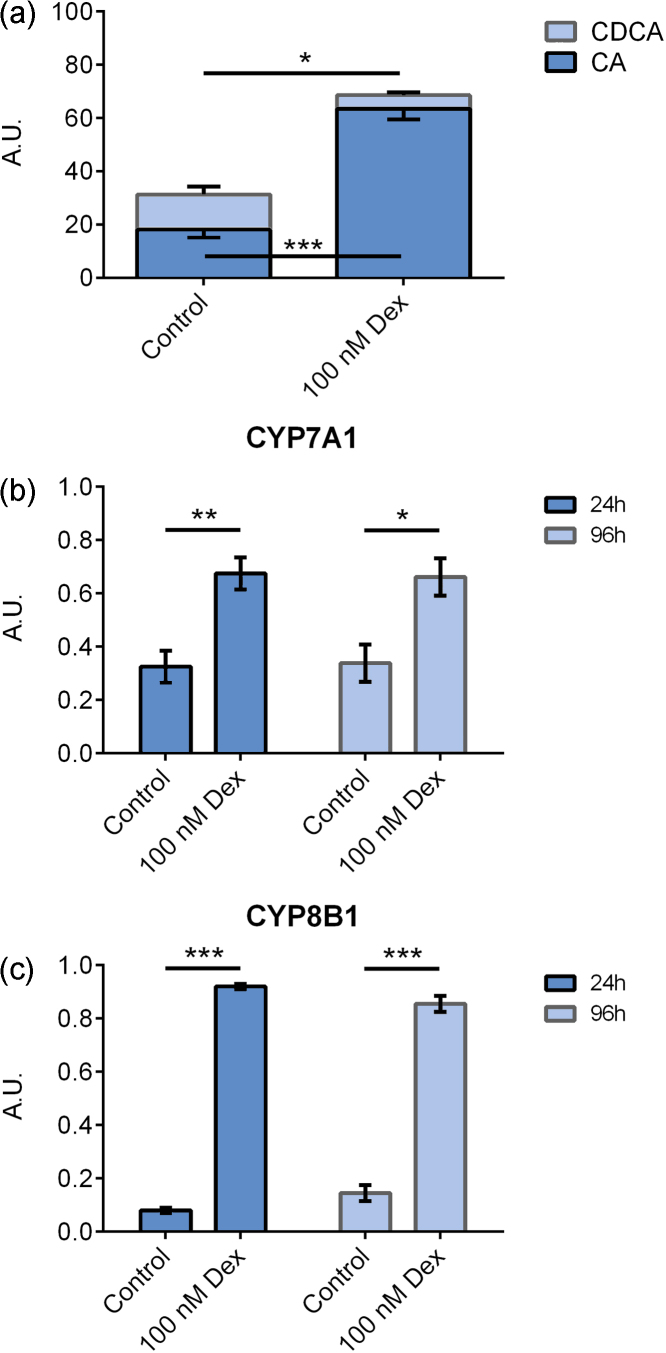
Figure 3.
(a) Bile acid levels in cell culture medium from primary hepatocytes cultured on collagen and treated with 100 nM dexamethasone for 96 h. mRNA expression of (b) CYP7A1 and (c) CYP8B1 in primary hepatocytes cultured on collagen and treated with 100 nM dexamethasone for 24 h (n = 6) or 96 h (n = 4). Data represent means ± SEM, A. U., arbitrary unit, * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.01.
Results
Matrix Regulation of Bile Acid Synthesis
Primary hepatocyte cultures are usually maintained on some form of extracellular matrix in order to maintain the hepatocyte phenotype. We tested the two commonly used matrixes collagen type I and EHS matrigel. As described earlier, hepatocytes cultured on matrigel displayed morphology completely different from that on collagen type I. Cells cultured on matrigel migrate and moved together and formed large cell aggregate, whereas cells cultured on collagen spread out and achieved a flattened polygonal shape.
Cells produced more bile acids, both CA (P ≤ 0.001) and CDCA (P ≤ 0.05), when cultured on matrigel compared to cells cultured on collagen, Figure 1A. The mRNA expression of the key enzymes involved in bile acid production, CYP7A1 (P ≤ 0.001), CYP8B1 (P ≤ 0.001), and CYP27A1, (P ≤ 0.001), was 3-fold higher in cells cultured on matrigel compared to cells cultured on collagen, Figure 1B and C.
Influence of Dexamethasone on Bile Acid Synthesis
Using matrigel only, we studied the effect of supplementation of the cell media with dexamethasone (n = 9). Addition of dexamethasone increased the levels of CA (P ≤ 0.001) and decreased the levels of CDCA (P ≤ 0.01) simultaneously, Figure 2A. This altered bile acid synthesis caused the ratio between CA and CDCA to change from 2.0 to 9.3 (P ≤ 0.01).
Following dexamethasone treatment, there was a slight increase in CYP7A1 expression (P ≤ 0.05), Figure 2B, and a three-fold increase in CYP8B1 expression (P ≤ 0.001), Figure 2C. The expression of CYP27A1 was not changed; data not shown.
In the above experiments (Figure 2), the cells were cultured with dexamethasone from plating on day one until harvest on day 5, thus for 96 hours. We also investigated the short-term effects of dexamethasone. Cells were plated in media without dexamethasone (n = 6), and on day 4, dexamethasone was added to the media and the cells were harvested after 24 h. We also studied the effect of dexamethasone in cells cultured on collagen.
Dexamethasone caused an increased CA synthesis (P ≤ 0.001) and a decreased CDCA synthesis (P ≤ 0.05) also when cells were cultured on collagen, Figure 3A. The CA/CDCA ratio changed from 1.6 in controls to 14.5 in dexamethasone-treated cells (P ≤ 0.01).
Short-term treatment of dexamethasone caused induction of CYP7A1 (P ≤ 0.01) that were in the same range as the induction observed following 96 h of treatment. However, short-term treatment caused a higher induction of CYP8B1 (11-fold) (P ≤ 0.001) compared to a 6-fold induction observed after 96 h treatment (P ≤ 0.001), Figure 3C. The expression of CYP27A1 was increased after 24 h (P ≤ 0.001) but not after 96 h; data not shown.
Stimulation of CYP8B1 mRNA Expression is not Mediated by glucocorticoid Receptor (GR) Signaling
The effects of dexamethasone in hepatocytes can be mediated by either GR or PXR.24 To determine if the effect was mediated through GR, primary hepatocytes from 4 donor cases were treated with dexamethasone in combination with RU486, a GR antagonist.25
When cells were treated with dexamethasone only, the expression of CYP7A1 increased, but addition of RU486 inhibited the dexamethasone CYP7A1 induction, Figure 4A. The difference between dexamethasone and dexamethasone + RU486 (P ≤ 0.01) observed suggests that CYP7A1 is regulated by GR.
In contrary, CYP8B1 expression was not altered by the addition of RU486 indicating that GR does not mediate induction of CYP8B1 by dexamethasone, Figure 4B.
The expression of retinoid-related orphan receptor (ROR) was investigated. Dexamethasone treatment increased the expression of ROR α 2-fold (P ≤ 0.001), Figure 5A. Combining dexamethasone with RU486, ROR α expression was increased compared to non-treated cells (P ≤ 0.01) but lower compared to cells treated with dexamethasone alone (P ≤ 0.05), Figure 5B. Expression of the ROR α target gene Glucose-6-phosphatase (G6Pase) was also investigated. Dexamethasone caused a doubling of G6Pase mRNA expression (P ≤ 0.01), Figure 5C.
Discussion
De novo bile acid synthesis is a liver-specific function that is difficult to maintain in cultured cells. Moreover, there are significant species differences in the types of bile acids formed and in the regulation of bile acid homeostasis. Thus, animal experimental models do not necessarily reflect the human situation. Isolated primary human hepatocytes have the ability to synthesize, conjugate, and secrete bile acids and thus provide a good human in vitro model. In this study, we illustrate the importance of different culture conditions, such as choice of culture substrate and media supplements on the total bile acid production, as well as the bile acid composition. Our experiments show that primary human hepatocytes synthesize more bile acids when cultured on matrigel compared to collagen. This finding can be explained by increased mRNA expression of the main enzymes in bile acid production, CYP7A1, CYP8B1, and CYP27A1.
Dexamethasone is a synthetic glucocorticoid frequently included as a supportive or protective factor in hepatocyte culture media and even in flushing and transport solutions used to cold preserve livers for transplantation. Supplementation of culture media with dexamethasone is reported to improve attachment, survival, morphology, and expression of CYP enzymes in primary hepatocytes,26, 27 and is therefore a very common media additive. In this study, we demonstrate that dexamethasone increased the formation of CA and decreased the CDCA formation; similar results have been reported in dogs where CA concentration in bile increased 9 times following hydrocortisone treatment, whereas CDCA only increased 3 times.28 In our study, the changed bile acid ratio was achieved by an increased expression of CYP8B1. The shift in bile composition toward more CA and less CDCA can be of clinical importance as different bile acids exert different feedback signals. CDCA is the most potent regulator in humans followed by DCA and CA,3 and thus the regulation of bile acid production would be affected by a changed composition. This is in line with previous data, where transplanted patients that undergo rejection treatment, with methylprednisolone, have a decrease in total bile acids.29 Furthermore, previous studies have shown that CA facilitates intestinal absorption of lipids, especially cholesterol, and may have a role in gallstone formation.5, 6 Bile composition may also be of importance for development of atherosclerosis as studies on double knockout animals of Cyp8b1 and ApoE have a 50% reduction of atherosclerotic lesions compared to ApoE knockout mice.4
Our results revealed that dexamethasone treatment increased the expression of CYP8B1 more than the expression of CYP7A1. Previous studies in primary rat hepatocytes and HepG2 cells have shown that CYP7A1 is stimulated by dexamethasone treatment.30, 31, 32
However, in our experiments, only a small increase in CYP7A1 mRNA expression was detected, whereas we observed a much more pronounced increase in CYP8B1 expression. The amount of bile acid produced varies substantially between different individuals and thus also between different cultures of hepatocytes. In this study, we saw a trend toward higher induction of CYP7A1 levels following dexamethasone treatment in livers with a low basal bile acid production, which is the case when cells are cultured on collagen. It is also reported that CYP27A1 is induced by dexamethasone in HepG2 cells.33 We could only detect a difference in CYP27A1 expression with short-term stimulation with dexamethasone when cells were cultured on collagen. Thus, it is conceivable that increased CYP27A1 and CYP7A1 expression in response to dexamethasone in HepG2 cells is due to the very low basal levels of the enzymes in these cells as compared to primary hepatocytes cultured in conditions that maintain the adult liver phenotype. Another possibility is that the insulin present in the cell media prevents the induction of CYP7A1; this is shown to be the case in rat hepatocytes.31, 32, 34 However, when we cultured hepatocytes in two different insulin concentrations (12 nM and 120 nM), no difference was detected (data not shown).
Furthermore, in a previous study using human hepatocytes, CYP7A1 and bile acid synthesis was not inhibited by higher insulin concentrations (3 nM and 30 nM) compared to a lower concentration (0.3 nM).35
By using the GR antagonist RU486, we concluded that CYP8B1 induction is mediated via a GR-independent pathway. We found no evidence that this would be via PXR signaling in that the PXR agonist rifampicin did not change the expression of neither CYP7A1 nor CYP8B1; data not shown.
One possible regulation pathway is that CDCA regulates its own synthesis through a negative feedback system.3 However, the concentration of bile acids produced by the hepatocytes and released into the cell media ranges between 0.2 and 1.5 μmol/L, which is too low to affect the bile acid synthesis.
Another nuclear receptor family suggested to have a role in bile acid regulation is the ROR family.36 This receptor family consists of three members: ROR α, ROR β, and ROR γ, where ROR α and ROR γ are expressed in the liver.37 ROR α and ROR γ double knockout mice have reduced hepatic levels of CYP8B1, whereas CYP7A1 expression is not affected.36 ROR α has previously been described to induce the expression of CYP8B1 during fasting; in a study by Pathak et al. a functional ROR α response element was found in the CYP8B1 promotor.38 With this knowledge, we decided to investigate ROR α expression following dexamethasone treatment and indeed the expression of ROR α was increased. Blocking GR signaling with RU486 resulted in a reduced but not blocked expression of ROR α. This indicates that dexamethasone stimulation of ROR α is not only partly regulated via the GR but also that another mechanism is involved, possibly through competition of ROR responsive elements with REV-ERBs, as described by Solt et al.39 One target gene of ROR α is glucose 6-phosphatase (G6Pase).40 Thus, the increased expression of G6Pase following dexamethasone treatment further strengthens the hypothesis that ROR α is a likely candidate for the observed effects. Furthermore, it has previously been shown in mouse hepatocytes that RNAi depletion of ROR α diminishes the effect of dexamethasone on G6Pase.40
In conclusion, out of all different conditions tested, primary human hepatocytes cultured on matrigel produce the highest amounts of bile acids. Addition of dexamethasone increased the mRNA expression and activity of CYP8B1, altering the ratio of CA to CDCA. ROR α signaling provides one possible pathway for CYP8B1 regulation.
Conflicts of Interest
The authors have none to declare.
References
- 1.Chiang J.Y. Regulation of bile acid synthesis: pathways, nuclear receptors, and mechanisms. J Hepatol. 2004;40:539–551. doi: 10.1016/j.jhep.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Russell D.W. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137–174. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- 3.Ellis E., Axelson M., Abrahamsson A. Feedback regulation of bile acid synthesis in primary human hepatocytes: evidence that CDCA is the strongest inhibitor. Hepatology. 2003;38:930–938. doi: 10.1053/jhep.2003.50394. [DOI] [PubMed] [Google Scholar]
- 4.Slatis K., Gafvels M., Kannisto K. Abolished synthesis of cholic acid reduces atherosclerotic development in apolipoprotein E knockout mice. J Lipid Res. 2010;51:3289–3298. doi: 10.1194/jlr.M009308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy C., Parini P., Wang J., Bjorkhem I., Eggertsen G., Gafvels M. Cholic acid as key regulator of cholesterol synthesis, intestinal absorption and hepatic storage in mice. Biochim Biophys Acta. 2005;1735:167–175. doi: 10.1016/j.bbalip.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Wang J., Gafvels M., Rudling M. Critical role of cholic acid for development of hypercholesterolemia and gallstones in diabetic mice. Biochem Biophys Res Commun. 2006;342:1382–1388. doi: 10.1016/j.bbrc.2006.02.108. [DOI] [PubMed] [Google Scholar]
- 7.Everson G.T., Polokoff M.A. HepG2. A human hepatoblastoma cell line exhibiting defects in bile acid synthesis and conjugation. J Biol Chem. 1986;261:2197–2201. [PubMed] [Google Scholar]
- 8.Axelson M., Mork B., Everson G.T. Bile acid synthesis in cultured human hepatoblastoma cells. J Biol Chem. 1991;266:17770–17777. [PubMed] [Google Scholar]
- 9.Cooper A.D., Craig W.Y., Taniguchi T., Everson G.T. Characteristics and regulation of bile salt synthesis and secretion by human hepatoma HepG2 cells. Hepatology. 1994;20:1522–1531. doi: 10.1002/hep.1840200623. [DOI] [PubMed] [Google Scholar]
- 10.Ellis E., Roeb E., Marschall H. Primary cultures of human hepatocytes but not HepG2 hepatoblastoma cells are suitable for the study of glycosidic conjugation of bile acids. Biochim Biophys Acta. 2001;1530:155–161. doi: 10.1016/s1388-1981(00)00179-7. [DOI] [PubMed] [Google Scholar]
- 11.Davis R.A., Highsmith W.E., McNeal M.M., Schexnayder J.A., Kuan J.C. Bile acid synthesis by cultured hepatocytes. Inhibition by mevinolin, but not by bile acids. J Biol Chem. 1983;258:4079–4082. [PubMed] [Google Scholar]
- 12.Davis R.A., Hyde P.M., Kuan J.C., Malone-McNeal M., Archambault-Schexnayder J. Bile acid secretion by cultured rat hepatocytes. Regulation by cholesterol availability. J Biol Chem. 1983;258:3661–3667. [PubMed] [Google Scholar]
- 13.Ellis E., Goodwin B., Abrahamsson A. Bile acid synthesis in primary cultures of rat and human hepatocytes. Hepatology. 1998;27:615–620. doi: 10.1002/hep.510270241. [DOI] [PubMed] [Google Scholar]
- 14.Axelson M., Ellis E., Mork B. Bile acid synthesis in cultured human hepatocytes: support for an alternative biosynthetic pathway to cholic acid. Hepatology. 2000;31:1305–1312. doi: 10.1053/jhep.2000.7877. [DOI] [PubMed] [Google Scholar]
- 15.Goodwin B., Jones S.A., Price R.R. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6:517–526. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 16.Holt J.A., Luo G., Billin A.N. Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev. 2003;17:1581–1591. doi: 10.1101/gad.1083503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jahan A., Chiang J.Y. Cytokine regulation of human sterol 12alpha-hydroxylase (CYP8B1) gene. Am J Physiol Gastrointest Liver Physiol. 2005;288:G685–G695. doi: 10.1152/ajpgi.00207.2004. [DOI] [PubMed] [Google Scholar]
- 18.Li T., Jahan A., Chiang J.Y. Bile acids and cytokines inhibit the human cholesterol 7 alpha-hydroxylase gene via the JNK/c-jun pathway in human liver cells. Hepatology. 2006;43:1202–1210. doi: 10.1002/hep.21183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodwin B., Watson M.A., Kim H., Miao J., Kemper J.K., Kliewer S.A. Differential regulation of rat and human CYP7A1 by the nuclear oxysterol receptor liver X receptor-alpha. Mol Endocrinol. 2003;17:386–394. doi: 10.1210/me.2002-0246. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton G.A., Jolley S.L., Gilbert D., Coon D.J., Barros S., LeCluyse E.L. Regulation of cell morphology and cytochrome P450 expression in human hepatocytes by extracellular matrix and cell-cell interactions. Cell Tissue Res. 2001;306:85–99. doi: 10.1007/s004410100429. [DOI] [PubMed] [Google Scholar]
- 21.Silva J.M., Morin P.E., Day S.H. Refinement of an in vitro cell model for cytochrome P450 induction. Drug Metab Dispos. 1998;26:490–496. [PubMed] [Google Scholar]
- 22.Mork L.M., Isaksson B., Boran N. Comparison of culture media for bile acid transport studies in primary human hepatocytes. J Clin Exp Hepatol. 2012;2:315–322. doi: 10.1016/j.jceh.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strom S.C., Pisarov L.A., Dorko K., Thompson M.T., Schuetz J.D., Schuetz E.G. Use of human hepatocytes to study P450 gene induction. Methods Enzymol. 1996;272:388–401. doi: 10.1016/s0076-6879(96)72044-x. [DOI] [PubMed] [Google Scholar]
- 24.Pascussi J.M., Drocourt L., Fabre J.M., Maurel P., Vilarem M.J. Dexamethasone induces pregnane X receptor and retinoid X receptor-alpha expression in human hepatocytes: synergistic increase of CYP3A4 induction by pregnane X receptor activators. Mol Pharmacol. 2000;58:361–372. doi: 10.1124/mol.58.2.361. [DOI] [PubMed] [Google Scholar]
- 25.Chobert M.N., Barouki R., Finidori J. Antiglucocorticoid properties of RU 38486 in a differentiated hepatoma cell line. Biochem Pharmacol. 1983;32:3481–3483. doi: 10.1016/0006-2952(83)90380-5. [DOI] [PubMed] [Google Scholar]
- 26.Laishes B.A., Williams G.M. Conditions affecting primary cell cultures of functional adult rat hepatocytes. II. Dexamethasone enhanced longevity and maintenance of morphology. In Vitro. 1976;12:821–832. doi: 10.1007/BF02796367. [DOI] [PubMed] [Google Scholar]
- 27.Sidhu J.S., Omiecinski C.J. Modulation of xenobiotic-inducible cytochrome P450 gene expression by dexamethasone in primary rat hepatocytes. Pharmacogenetics. 1995;5:24–36. doi: 10.1097/00008571-199502000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Kook P.H., Schellenberg S., Rentsch K.M., Reusch C.E., Glaus T.M. Effect of twice-daily oral administration of hydrocortisone on the bile acids composition of gallbladder bile in dogs. Am J Vet Res. 2011;72:1607–1612. doi: 10.2460/ajvr.72.12.1607. [DOI] [PubMed] [Google Scholar]
- 29.Ericzon B.G., Eusufzai S., Kubota K., Einarsson K., Angelin B. Characteristics of biliary lipid metabolism after liver transplantation. Hepatology. 1990;12:1222–1228. doi: 10.1002/hep.1840120524. [DOI] [PubMed] [Google Scholar]
- 30.Princen H.M., Meijer P., Hofstee B. Dexamethasone regulates bile acid synthesis in monolayer cultures of rat hepatocytes by induction of cholesterol 7 alpha-hydroxylase. Biochem J. 1989;262:341–348. doi: 10.1042/bj2620341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crestani M., Stroup D., Chiang J.Y. Hormonal regulation of the cholesterol 7 alpha-hydroxylase gene (CYP7) J Lipid Res. 1995;36:2419–2432. [PubMed] [Google Scholar]
- 32.Sporstol M., Mousavi S.A., Eskild W., Roos N., Berg T. ABCA1, ABCG1 and SR-BI: hormonal regulation in primary rat hepatocytes and human cell lines. BMC Mol Biol. 2007;8:5. doi: 10.1186/1471-2199-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang W., Norlin M., Wikvall K. Glucocorticoid receptor-mediated upregulation of human CYP27A1, a potential anti-atherogenic enzyme. Biochim Biophys Acta. 2008;1781:718–723. doi: 10.1016/j.bbalip.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 34.Twisk J., Hoekman M.F., Lehmann E.M., Meijer P., Mager W.H., Princen H.M. Insulin suppresses bile acid synthesis in cultured rat hepatocytes by down-regulation of cholesterol 7 alpha-hydroxylase and sterol 27-hydroxylase gene transcription. Hepatology. 1995;21:501–510. [PubMed] [Google Scholar]
- 35.Kotokorpi P., Ellis E., Parini P. Physiological differences between human and rat primary hepatocytes in response to liver X receptor activation by 3-[3-[N-(2-chloro-3-trifluoromethylbenzyl)-(2,2-diphenylethyl)amino]propyloxy]phe nylacetic acid hydrochloride (GW3965) Mol Pharmacol. 2007;72:947–955. doi: 10.1124/mol.107.037358. [DOI] [PubMed] [Google Scholar]
- 36.Kang H.S., Angers M., Beak J.Y. Gene expression profiling reveals a regulatory role for ROR alpha and ROR gamma in phase I and phase II metabolism. Physiol Genomics. 2007;31:281–294. doi: 10.1152/physiolgenomics.00098.2007. [DOI] [PubMed] [Google Scholar]
- 37.Jetten A.M., Kurebayashi S., Ueda E. The ROR nuclear orphan receptor subfamily: critical regulators of multiple biological processes. Prog Nucleic Acid Res Mol Biol. 2001;69:205–247. doi: 10.1016/s0079-6603(01)69048-2. [DOI] [PubMed] [Google Scholar]
- 38.Pathak P., Li T., Chiang J.Y. Retinoic acid-related orphan receptor alpha regulates diurnal rhythm and fasting induction of sterol 12alpha-hydroxylase in bile acid synthesis. J Biol Chem. 2013;288:37154–37165. doi: 10.1074/jbc.M113.485987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Solt L.A., Griffin P.R., Burris T.P. Ligand regulation of retinoic acid receptor-related orphan receptors: implications for development of novel therapeutics. Curr Opin Lipidol. 2010;21:204–211. doi: 10.1097/MOL.0b013e328338ca18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chopra A.R., Louet J.F., Saha P. Absence of the SRC-2 coactivator results in a glycogenopathy resembling Von Gierke's disease. Science. 2008;322:1395–1399. doi: 10.1126/science.1164847. [DOI] [PMC free article] [PubMed] [Google Scholar]