Abstract
Background
Extrahepatic cholangiocarcinoma (EHC) is a rare malignancy with a relatively poor prognosis. There are no randomized, prospective data to help define the optimal method of radiation delivery for unresectable EHC. The purpose of this study was to evaluate the benefit of adding brachytherapy to external beam radiation therapy (EBRT) for unresectable EHC.
Methods
A retrospective review of 1,326 patients with unresectable EHC using the Surveillance, Epidemiology, and End Results (SEER) database was completed. Kaplan-Meier methods were used to analyze the primary endpoint, overall survival. Univariate and multivariate analysis was performed to identify and control for potential confounding variables, including age at diagnosis, sex, stage, grade, histology, race, year of diagnosis, and reason for no surgery.
Results
Of the 1,326 patients with unresectable EHC, 1,188 (92.9%) received EBRT only, while 91 (7.1%) received both EBRT and brachytherapy. Patients receiving combined modality radiation therapy were more likely to be treated prior to the year 2000. Median overall survival for patients receiving EBRT and EBRT plus brachytherapy was 9 and 11 months, respectively (P=0.04). Cause specific survival was 12 months for those receiving EBRT only, and 15 months for those who received EBRT + brachytherapy (P=0.10). Survival analysis performed on patients with locoregional disease only revealed a trend towards prolonged overall survival with those receiving EBRT + brachytherapy (P=0.08). Multivariate analysis revealed grade and stage of disease were correlated with both overall survival and cause specific survival (P≤0.05).
Conclusions
Among patients with unresectable EHC, the addition of brachytherapy to EBRT is associated with a prolonged median overall survival. However, the use of brachytherapy boost decreased in the last decade of the study.
Keywords: Brachytherapy, cholangiocarcinoma, extrahepatic, radiation
Introduction
Cholangiocarcinoma is a rare malignancy of the biliary tract, with only 0.6 to 1 of 100,000 persons in the United States being diagnosed per year (1-3). These tumors are subcategorized by location, either intrahepatic or extrahepatic, with the latter including both perihilar and distal bile duct tumors (2,4). Extrahepatic cholangiocarcinoma (EHC) portends a poor prognosis and surgery is generally thought to be the only curative modality (5,6). Unfortunately, surgery has to result in an R0 resection to provide benefit to the patient and this is not attainable most of the time (7-9).
Treatment options for unresectable extrahepatic cholangiocarcinoma (UEC) are limited to chemotherapy, radiation, or a combination of both (10). When utilized, external beam radiation therapy (EBRT) can be delivered with or without a brachytherapy boost (11,12). Combining EBRT with brachytherapy allows for the delivery of an elective dose to larger areas, including nodal basins when desired, while delivering a more definitive dose to the tumor that doesn’t tend to increase dose to organs at risk (13-18). The benefit of EBRT in combination with brachytherapy has been previously evaluated in multiple single-institution retrospective series with the majority either showing an overall survival or progression-free survival benefit (13-17,19).
There is also population-based evidence that the use of brachytherapy improves outcomes among unresectable cholangiocarcinoma patients (20). However, no large dataset analysis has investigated the benefit of adding a brachytherapy boost to EBRT. The purpose of this study was to evaluate if the addition of brachytherapy to EBRT provides a survival benefit among patients with UEC, using population-based data from the Surveillance, Epidemiology, and End Results (SEER) database.
Methods
The SEER database is an open access, population-based database that includes diagnostic, treatment, and survival data. It has been reported that SEER currently tracks 28% of the United States population. The data acquired for this study spanned from years 1974 to 2011 and all available registries were utilized for analysis. Internal review board approval was not required at our institution since the SEER database information is de-identified.
The International Classification of Diseases for Oncology (ICD-0-2 and ICD-0-3) codes were used to identify those with EHC according to anatomy and histology. The site code C24.0 for extrahepatic bile duct was included in our search with the following histology codes: 8010, 8020, 8040, 8070, 8140, 8144, 8160, 8161, 8162, 8260, 8310, 8480, 8490, and 8560. Only patients with unresectable disease who received radiation were included in the cohort. Patients who received brachytherapy only were excluded from our analysis. We included the following variables for the selected cohort: age at diagnosis, sex, race, and SEER registry location, year of diagnosis, grade, stage, type of radiation, and the reason for no-cancer-directed surgery. Survival months, vital status recode, and cause specific death classification were obtained for survival analysis.
Pearson chi-square analyses were used to compare treatment and tumor characteristics for categorical variables. Kaplan-Meier methods were then employed to analyze overall survival. Univariate and multivariate survival analyses were performed using Cox proportional hazards regression. To test whether overall survival becomes significantly different among groups, a log rank test was performed. Statistical analyses were performed using STATA 14.0. The level of significance was defined as P<0.05.
Results
A total of 1,326 patients were identified with UEC with available radiation data. Of these patients, 1,188 (89.6%) received EBRT only, 47 (3.5%) received brachytherapy only, and 91 (6.8%) received EBRT plus brachytherapy. Patients who received brachytherapy alone were excluded from the analysis. Fifty-nine percent of patients were diagnosed between the year 2000 and 2011. The cohort was predominantly male (56%), Caucasian (81%), and had locoregional disease (75%). The predominant reason for patients not undergoing surgery was due to surgery not being recommended (71%).
Patient demographic information was compared between those who received EBRT vs. those receiving combined modality treatment. Localized disease was more prevalent among those receiving both EBRT and brachytherapy (45% vs. 26%), while those receiving EBRT only were more likely to have distant disease (25% vs. 12%) (P=0.002). Patients receiving combined modality radiation therapy were also more likely to be treated prior to the year 2000 (P≤0.001). The percentages of those treated with EBRT and brachytherapy for each decade are as follows: 1973–1979, 1.5%; 1980–1989, 8.3%; 1990–1999, 15.9%; 2000–2011, 4.6%. There was no significant difference in gender, race, grade, or reason for being unresectable (see Table 1).
Table 1. Comparison of complete cohort patient demographics among different forms of radiation treatment.
| Variable | EBRT [%] | EBRT + brachytherapy [%] | P value |
|---|---|---|---|
| Total patients | 1,188 [93] | 91 [7] | |
| Sex | 0.769 | ||
| Female | 528 [44] | 39 [43] | |
| Male | 660 [56] | 52 [57] | |
| Race | 0.431 | ||
| White | 961 [81] | 72 [79] | |
| Black | 64 [5] | 3 [3] | |
| Other | 162 [14] | 16 [18] | |
| Year of diagnosis | <0.001 | ||
| 1973–1979 | 67 [6] | 1 [1] | |
| 1980–1989 | 200 [17] | 18 [20] | |
| 1990–1999 | 196 [16] | 37 [41] | |
| 2000–2011 | 725 [61] | 35 [38] | |
| Grade | 0.761 | ||
| Grade I | 99 [25] | 6 [19] | |
| Grade II | 153 [39] | 14 [45] | |
| Grade III | 134 [34] | 11 [35] | |
| Surgery recommendation | 0.988 | ||
| Not recommended | 848 [72] | 65 [71] | |
| Recommended | 338 [28] | 26 [29] | |
| SEER stage | 0.002 | ||
| Localized | 263 [26] | 30 [45] | |
| Regional | 487 [49] | 29 [43] | |
| Distant | 251 [25] | 8 [12] |
P values computed from Pearson’s χ2 test. EBRT, external beam radiation therapy; SEER, Surveillance, Epidemiology, and End Results.
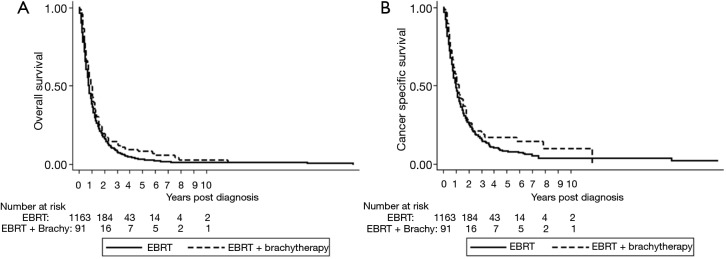
Median overall survival for patients receiving EBRT only was 9 months (95% CI, 9–10 months) vs. 11 months (95% CI, 9–14 months) for those receiving combined modality radiotherapy (P=0.04). Median cause specific survival was 12 months (95% CI, 11–13 months) for those receiving EBRT only, and 15 months (95% CI, 10–19 months) for those receiving EBRT plus brachytherapy (P=0.10) (see Figure 1). Survival analysis was also performed after excluding patients with metastatic disease. Among this cohort median overall survival was 10 months for patients receiving EBRT alone and 13 months for those receiving EBRT plus brachytherapy (P=0.08). Cause specific survival was 14 and 15 months for EBRT and EBRT plus brachytherapy, respectively (P=0.12).
Figure 1.
Overall and cancer specific survival by treatment type for unresectable extrahepatic cholangiocarcinoma. (A) Kaplan-Meier curve of overall survival. Survival curves for EBRT and EBRT plus Brachytherapy shown; (B) Kaplan-Meier curve for cause specific survival. Survival curves for EBRT and EBRT plus Brachytherapy shown. EBRT, external beam radiation therapy; Brachy, brachytherapy.
On univariate analysis, a significant improvement in overall survival was noted in those who received combined modality radiation therapy [hazard ratio (HR) =0.78; 95% CI, 0.80–0.99] (Table 2). Increased grade (HR =1.18; 95% CI, 1.04–1.34) and greater extent of disease (HR =1.29; 95% CI, 1.18–1.42) were associated with a decrease in overall survival. On multivariate analysis, there was no association of combined modality radiation therapy with overall survival (HR =0.94; 95% CI, 0.72–1.23). However, grade (HR =1.15; 95% CI, 1.01–1.32) and extent of disease (HR =1.20; 95% CI, 1.02–1.41) continued to be negatively correlated with overall survival.
Table 2. Univariate and multivariate analysis for overall survival.
| Univariate covariate | Univariate | Multivariate* | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | ||
| Increasing age | 1.05 (1.00–1.10) | 0.051 | 1.01 (1.00–1.01) | |
| Treatment | ||||
| EBRT | 1 | – | 1 | |
| EBRT + brachy | 0.78 (0.64–0.99) | 0.049 | 0.94 (0.72-1.23) | |
| Sex | ||||
| Male | 1 | – | – | |
| Female | 1.05 (1.00–1.10) | 0.884 | – | |
| Race | ||||
| White | 1 | – | – | |
| Black | 0.88 (0.68–1.14) | 0.338 | – | |
| Other | 0.79 (0.59–1.06) | 0.122 | – | |
| Year of diagnosis | ||||
| 1973–1979 | 1 | – | 1 | |
| 1980–1989 | 0.78 (0.59–1.03) | 0.082 | 0.84 (0.62–1.14) | |
| 1990–1999 | 0.83 (0.63–1.09) | 0.172 | 0.92 (0.68–1.25) | |
| 2000–2011 | 0.80 (0.62–1.04) | 0.095 | 0.83 (0.63–1.09) | |
| Grade† | ||||
| Grade I | 1 | – | – | |
| Grade II | 1.38 (1.07–1.78) | 0.013 | – | |
| Grade III | 1.45 (1.11–1.89) | 0.006 | – | |
| Grade IV | 1.44 (0.58–3.53) | 0.431 | – | |
| Surgery recommendation | ||||
| Recommended | 1 | – | – | |
| Not recommended | 1.01 (0.89–1.15) | 0.861 | – | |
| SEER stage | ||||
| Localized | 1 | – | 1 | |
| Regional | 1.04 (0.89–1.20) | 0.639 | 1.06 (0.91–1.23) | |
| Distant | 1.70 (1.43–2.03) | <0.0001 | 1.77 (1.48–2.12) | |
*, Multivariate analysis included covariates significant (P<0.05) in univariate model and containing >30% of observations; †, missing >30% of data and not included in multivariate analysis. HR, hazard ratio; EBRT, external beam radiation therapy; SEER, Surveillance, Epidemiology, and End Results.
A trend towards improved cause specific survival was noted for those receiving EBRT plus brachytherapy (HR =0.90; 95% CI, 0.79–1.02) (Table 3). A later decade of diagnosis was associated with a significant improvement in cause specific survival (HR =0.92; 95% CI, 0.86–0.99), while increasing grade (HR =1.22; 95% CI, 1.06–1.40) and extent of disease (HR =1.36; 95% CI, 1.22–1.51) were also associated with a decrease in cause specific survival. On multivariate analysis, there was no association between the addition of brachytherapy and cause specific survival (HR =1.09; 95% CI, 0.86–1.40). Grade (HR =1.19; 95% CI, 1.02–1.39) and extent of disease (HR =1.28; 95% CI, 1.07–1.54) were associated with worsening of cause specific survival as well. Recent decades of diagnoses were associated with an improved cause specific survival (HR 0.85; 95% CI, 0.75–0.96).
Table 3. Univariate and multivariate analysis for cause-specific survival.
| Univariate covariate | Univariate | Multivariate* | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | ||
| Increasing age | 1.05 (1.00–1.010) | 0.051 | – | |
| Treatment | ||||
| EBRT | 1 | – | 1 | |
| EBRT + Brachy | 0.80 (0.64–0.99) | 0.049 | 0.95 (0.70–1.29) | |
| Sex | ||||
| Male | 1 | – | – | |
| Female | 1.01 (0.90–1.13) | 0.884 | – | |
| Race | ||||
| White | 1 | – | – | |
| Black | 0.88 (0.68–1.14) | 0.338 | – | |
| Other | 0.79 (0.59–1.06) | 0.122 | – | |
| Year of diagnosis | ||||
| 1973–1979 | 1 | – | 1 | |
| 1980–1989 | 0.78 (0.59–1.03) | 0.082 | 0.82 (0.59–1.13) | |
| 1990–1999 | 0.83 (0.63–1.09) | 0.172 | 0.89 (0.64–1.24) | |
| 2000–2011 | 0.80 (0.62–1.04) | 0.095 | 0.72 (0.53–0.97) | |
| Grade† | ||||
| Grade I | 1 | – | – | |
| Grade II | 1.38 (1.07–1.78) | 0.013 | – | |
| Grade III | 1.45 (1.11–1.89) | 0.006 | – | |
| Grade IV | 1.44 (0.58–3.53) | 0.431 | – | |
| Surgery recommendation | ||||
| Recommended | 1 | – | – | |
| Not recommended | 1.01 (0.89–1.15) | 0.861 | – | |
| SEER stage | ||||
| Localized | 1 | – | 1 | |
| Regional | 1.04 (0.89–1.20) | 0.639 | 1.15 (0.96–1.37) | |
| Distant | 1.70 (1.43–2.03) | <0.0001 | 1.90 (1.55–2.33) | |
*, Multivariate analysis included covariates significant (P<0.05) in univariate model and containing >30% of observations; †, missing >30% of data and not included in multivariate analysis. EBRT, external beam radiation therapy; Brachy, brachytherapy
After excluding metastatic patients, univariate analysis revealed a trend towards improved overall survival among those receiving EBRT plus brachytherapy (HR =0.82; 95% CI, 0.65–1.03). This trend was also seen on cause specific survival (HR =0.81; 95% CI, 0.61–1.06). Multivariate analysis on this cohort again revealed no correlation between combined modality radiation and overall survival (HR =0.97; 95% CI, 0.84–1.12) or cause specific survival (HR =0.97; 95% CI, 0.82–1.14).
Discussion
This is the first large, population-based study evaluating the survival benefit of brachytherapy when added to EBRT for UEC patients. We selected for patients that were not surgical candidates and specifically evaluated the benefit of combined modality radiotherapy by comparing those treated with EBRT alone and those receiving EBRT plus brachytherapy. Due to the size of our cohort, we were able to elucidate demographic and clinical factors predictive of overall survival. This is the first study of its kind which shows a potential overall survival benefit when brachytherapy is added to EBRT in unresectable UEC. Our study reveals a two month overall survival benefit of adding brachytherapy to EBRT (P=0.04), which was also seen on univariate analysis (HR =0.89; 95% CI, 0.80–0.99). In spite of its apparent efficacy, brachytherapy boost saw declining use from the 1990’s to the 2000’s.
Brachytherapy has the advantage of delivering higher doses of radiation therapy with less dose to the surrounding normal tissues, thus minimizing toxicity in patients with UEC (14,21). Early studies revealed survival was proportional to the dose of radiation delivered. Patients receiving greater than 55 Gray (Gy) have an improved 2-year survival when compared to those receiving less than 55 Gy (48% vs. 0%) (22). More recently, ablative doses of EBRT of up to 100 Gy in 25 fractions prescribed as a boost to the internal portion of intrahepatic cholangiocarcinomas were shown to outperform more conventional lower doses in overall survival (23). Better outcomes with higher doses have led many to the practice of using brachytherapy as a boost to EBRT. Several retrospective series have shown a progression-free and overall survival benefit with the addition of brachytherapy to EBRT (13-17). One of the proposed mechanisms in these small studies was felt to be secondary to increasing longevity of stent patency (17,24). Stent patency can also enhance patients’ tolerability to chemotherapy by decreasing bilirubin levels (25).
For malignancies with a low-incidence such as EHC, the SEER database is a useful way to aggregate larger numbers of patients to analyze outcomes (26). Shinohara et al. utilized the SEER database to evaluate the benefit of radiation therapy of all patients with EHC. In patients with unresectable disease, radiotherapy improved overall survival with a HR of 0.61 (95% CI, 0.54–0.70) (27). However, no analysis was performed on the type of radiotherapy delivered. In a separate analysis, Shinohara et al. utilized the SEER database to evaluate the impact of brachytherapy (without regard to the use of EBRT) among patients with both intra- and extrahepatic cholangiocarcinoma (20). The cohort also included patients who received surgery as a part of their definitive treatment. When compared to patients who did not receive any type of radiation, the use of brachytherapy (with or without EBRT) was associated with a significant overall survival benefit of 11 vs. 4 months (P<0.05). Race, stage of disease, and an earlier year of diagnosis were associated with a poorer prognosis. A comparison of patients receiving EBRT plus or minus brachytherapy was not completed.
In contrast to the studies performed by Shinohara et al. we selected for patients with EHC that were not surgical candidates. Our results also differ, as stage and grade were found to be a significant prognostic indicator in this cohort, while race was not. The potential benefit of including brachytherapy appears to be among those patients surviving greater than 2 years following diagnosis. This could be explained by an existing subpopulation within the cohort with more aggressive tumor biology more likely to die of distant rather than local disease. Those with local tumors would be the most likely to benefit from brachytherapy. Our subset analysis excluding patients with metastatic disease showed a trend toward improved survival with combined modality radiation, though this was not found to be statistically significant. Given that patients with metastatic disease had decreased survival rates, regardless of the type of radiation delivered, may be due to decreased statistical power, or may hint at the importance of local control in EHC.
Our study has some unavoidable limitations. The SEER database itself is known to be associated with several pitfalls including coding reliability and underreporting of radiation therapy (28). There is no information about dose, fractionation, field size, prescription point/volume, or other RT parameters. The lack of important variables such as performance status and use of chemotherapy could confound the correlations we found. In our particular cohort, there were significant differences between the two treatment groups. Patients receiving combined modality treatment were more likely to have local disease and to be treated before 2000. The benefit of combined modality RT was shown on univariate and log-rank analysis; however, the correlation did not hold on multivariate analysis. This could be due to confounding of variables that are unequal between the two arms. For example, patients treated with brachytherapy were more likely to be diagnosed before 2000, when suboptimal or antiquated chemotherapy regimens would have been used. The benefit of newer agents such as gemcitabine, or concurrent chemotherapy with 5-fluorouracil was not established until the early 2000s (29,30). Brachytherapy could also have been implemented to compensate for the lack of chemotherapy use due to either contraindication, intolerance, or both.
It is important to note that these results do not negate other potential benefits of combined modality radiotherapy. The SEER database lacks information concerning local recurrence or patient reported outcomes. Such data could inform the discussion of brachytherapy in this setting in terms of providing palliation of symptoms or prolonging the disease-free interval. Our results also suggest that the addition of brachytherapy to EBRT in patients with UEC for these reasons would not be associated with a survival decrement.
Conclusions
UEC is rare malignancy associated with a poor prognosis. Among UEC patients treated with radiotherapy, grade and extent of disease are negatively associated with overall and cause specific survival. When compared to those receiving EBRT alone, those receiving EBRT plus brachytherapy have a prolonged median survival. In spite of this apparent benefit, brachytherapy boost utilization has dropped significantly over the last decade of this study.
Acknowledgements
The authors would like to thank Michelle Denney for her assistance in editing and manuscript formatting.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Altekruse SF, Kosary CL, Krapcho M, et al. editors. SEER cancer statistics review, 1975–2007, National Cancer Institute. Bethesda, MD. Available online: http://seer.cancer.gov/csr/1975_2007/, based on November 2009 SEER data submission, posted to the SEER web site, 2010.
- 2.Vauthey JN, Blumgart LH. Recent advances in the management of cholangiocarcinomas. Semin Liver Dis 1994;14:109-14. 10.1055/s-2007-1007302 [DOI] [PubMed] [Google Scholar]
- 3.Welzel TM, McGlynn KA, Hsing AW, et al. Impact of classification of hilar cholangiocarcinomas (Klatskin tumors) on the incidence of intra- and extrahepatic cholangiocarcinoma in the United States. J Natl Cancer Inst 2006;98:873-5. 10.1093/jnci/djj234 [DOI] [PubMed] [Google Scholar]
- 4.Curley SA. Diagnosis and management of intrahepatic and extrahepatic cholangiocarcinoma. Cancer Treat Res 2001;109:117-44. 10.1007/978-1-4757-3371-6_7 [DOI] [PubMed] [Google Scholar]
- 5.Fuller CD, Wang SJ, Choi M, et al. Multimodality therapy for locoregional extrahepatic cholangiocarcinoma: a population-based analysis. Cancer 2009;115:5175-83. 10.1002/cncr.24572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet 2014;383:2168-79. 10.1016/S0140-6736(13)61903-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burke EC, Jarnagin WR, Hochwald SN, et al. Hilar Cholangiocarcinoma: patterns of spread, the importance of hepatic resection for curative operation, and a presurgical clinical staging system. Ann Surg 1998;228:385-94. 10.1097/00000658-199809000-00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ben-David MA, Griffith KA, Abu-Isa E, et al. External-beam radiotherapy for localized extrahepatic cholangiocarcinoma. Int J Radiat Oncol Biol Phys 2006;66:772-9. 10.1016/j.ijrobp.2006.05.061 [DOI] [PubMed] [Google Scholar]
- 9.Sakamoto E, Nimura Y, Hayakawa N, et al. The pattern of infiltration at the proximal border of hilar bile duct carcinoma: a histologic analysis of 62 resected cases. Ann Surg 1998;227:405-11. 10.1097/00000658-199803000-00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malhi H, Gores GJ. Review article: the modern diagnosis and therapy of cholangiocarcinoma. Aliment Pharmacol Ther 2006;23:1287-96. 10.1111/j.1365-2036.2006.02900.x [DOI] [PubMed] [Google Scholar]
- 11.Skipworth JR, Keane MG, Pereira SP. Update on the management of cholangiocarcinoma. Dig Dis 2014;32:570-8. 10.1159/000360507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayes JK, Jr, Sapozink MD, Miller FJ. Definitive radiation therapy in bile duct carcinoma. Int J Radiat Oncol Biol Phys 1988;15:735-44. 10.1016/0360-3016(88)90319-7 [DOI] [PubMed] [Google Scholar]
- 13.Kamada T, Saitou H, Takamura A, et al. The role of radiotherapy in the management of extrahepatic bile duct cancer: an analysis of 145 consecutive patients treated with intraluminal and/or external beam radiotherapy. Int J Radiat Oncol Biol Phys 1996;34:767-74. 10.1016/0360-3016(95)02132-9 [DOI] [PubMed] [Google Scholar]
- 14.Shin HS, Seong J, Kim WC, et al. Combination of external beam irradiation and high-dose-rate intraluminal brachytherapy for inoperable carcinoma of the extrahepatic bile ducts. Int J Radiat Oncol Biol Phys 2003;57:105-12. 10.1016/S0360-3016(03)00410-3 [DOI] [PubMed] [Google Scholar]
- 15.Golfieri R, Giampalma E, Renzulli M, et al. Unresectable hilar cholangiocarcinoma: multimodality approach with percutaneous treatment associated with radiotherapy and chemotherapy. In Vivo 2006;20:757-60. [PubMed] [Google Scholar]
- 16.Flickinger JC, Epstein AH, Iwatsuki S, et al. Radiation therapy for primary carcinoma of the extrahepatic biliary system. An analysis of 63 cases. Cancer 1991;68:289-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qian XJ, Zhai RY, Dai DK, et al. Treatment of malignant biliary obstruction by combined percutaneous transhepatic biliary drainage with local tumor treatment. World J Gastroenterol 2006;12:331-5. 10.3748/wjg.v12.i2.331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foo ML, Gunderson LL, Bender CE, et al. External radiation therapy and transcatheter iridium in the treatment of extrahepatic bile duct carcinoma. Int J Radiat Oncol Biol Phys 1997;39:929-35. 10.1016/S0360-3016(97)00299-X [DOI] [PubMed] [Google Scholar]
- 19.Buskirk SJ, Gunderson LL, Schild SE, et al. Analysis of failure after curative irradiation of extrahepatic bile duct carcinoma. Ann Surg 1992;215:125-31. 10.1097/00000658-199202000-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shinohara ET, Guo M, Mitra N, et al. Brachytherapy in the treatment of cholangiocarcinoma. Int J Radiat Oncol Biol Phys 2010;78:722-8. 10.1016/j.ijrobp.2009.08.070 [DOI] [PubMed] [Google Scholar]
- 21.Kocak Z, Ozkan H, Adli M, et al. Intraluminal brachytherapy with metallic stenting in the palliative treatment of malignant obstruction of the bile duct. Radiat Med 2005;23:200-7. [PubMed] [Google Scholar]
- 22.Alden ME, Mohiuddin M. The impact of radiation dose in combined external beam and intraluminal Ir-192 brachytherapy for bile duct cancer. Int J Radiat Oncol Biol Phys 1994;28:945-51. 10.1016/0360-3016(94)90115-5 [DOI] [PubMed] [Google Scholar]
- 23.Tao R, Krishnan S, Bhosale PR, et al. Ablative radiotherapy doses lead to a substantial prolongation of survival in patients with inoperable intrahepatic cholangiocarcinoma: a retrospective dose response analysis. J Clin Oncol 2016;34:219-26. 10.1200/JCO.2015.61.3778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y, Wang XL, Yan ZP, et al. HDR-192Ir intraluminal brachytherapy in treatment of malignant obstructive jaundice. World J Gastroenterol 2004;10:3506-10. 10.3748/wjg.v10.i23.3506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rizvi S, Gores GJ. Current diagnostic and management options in perihilar cholangiocarcinoma. Digestion 2014;89:216-24. 10.1159/000360791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lloyd S, Park HS, Decker RH, et al. Using the Surveillance, Epidemiology, and End Results database to investigate rare cancers, second malignancies, and trends in epidemiology, treatment, and outcomes. Curr Probl Cancer 2012;36:191-9. 10.1016/j.currproblcancer.2012.03.008 [DOI] [PubMed] [Google Scholar]
- 27.Shinohara ET, Mitra N, Guo M, et al. Radiotherapy is associated with improved survival in adjuvant and palliative treatment of extrahepatic cholangiocarcinomas. Int J Radiat Oncol Biol Phys 2009;74:1191-8. 10.1016/j.ijrobp.2008.09.017 [DOI] [PubMed] [Google Scholar]
- 28.Park HS, Lloyd S, Decker RH, et al. Limitations and biases of the Surveillance, Epidemiology, and End Results database. Curr Probl Cancer 2012;36:216-24. 10.1016/j.currproblcancer.2012.03.011 [DOI] [PubMed] [Google Scholar]
- 29.Malik IA, Aziz Z, Zaidi SH, et al. Gemcitabine and Cisplatin is a highly effective combination chemotherapy in patients with advanced cancer of the gallbladder. Am J Clin Oncol 2003;26:174-7. 10.1097/00000421-200304000-00015 [DOI] [PubMed] [Google Scholar]
- 30.Phelip JM, Vendrely V, Rostain F, et al. Gemcitabine plus cisplatin versus chemoradiotherapy in locally advanced biliary tract cancer: Fédération Francophone de Cancérologie Digestive 9902 phase II randomised study. Eur J Cancer 2014;50:2975-82. 10.1016/j.ejca.2014.08.013 [DOI] [PubMed] [Google Scholar]