Abstract
Background
Brain metastases from esophageal carcinoma have historically been rare and associated with poor prognosis. With improvements in systemic disease control, the incidence of brain metastases is expected to rise. To better inform management decisions, we sought to identify factors associated with survival in patients with brain metastasis from esophageal cancer.
Methods
We retrospectively identified 49 patients with brain metastasis from stage I–IV primary esophageal cancer treated with surgery, radiation, or a combination of modalities at our tertiary referral center between 1998 and 2015. Medical records were reviewed to collect demographic and clinical information.
Results
Median age at diagnosis of the primary esophageal cancer was 60 years. Forty-one (84%) patients were male and forty patients (82%) had adenocarcinoma. Median overall survival (MS) following esophageal cancer diagnosis was 24 months (range, 3–71 months), and median survival after the identification of brain metastases was 5 months (range, 1–52 months). On univariate analysis, only patients with poor Karnofsky performance status (KPS <70), recursive partitioning analysis (RPA) classification (III), or 3 or more brain metastases were found to have worsened survival after the diagnosis of brain metastases (all P<0.01). Factors not associated with survival were age, gender, histology (adenocarcinoma vs. other), palliative-intent treatment of the primary tumor, time to diagnosis of brain metastases from initial diagnosis, uncontrolled primary tumor at time of brain metastasis diagnosis, or extracranial metastases. On multivariate analysis (MVA, KPS excluded), patients with RPA class I (MS, 14.6 months) or II (MS, 5.0 months) disease had significantly improved overall survival compared to class III disease (MS, 1.6 months, P<0.01). Also on MVA, patients with 1 (MS, 10.7 months) or 2 (MS, 4.7 months) brain metastases had significantly improved overall survival compared to patients with 3 or more brain metastases (MS, 0.3 months, P<0.01). For the 36 patients with 1–2 brain metastases and KPS ≥70, MS was 11.1 months.
Conclusions
While the prognosis for esophageal cancer metastatic to brain remains poor overall, we found that patients with good performance status and limited number of brain lesions have superior survival. Aggressive management may further improve outcomes in these patients.
Keywords: Brain metastasis, esophageal cancer, recursive partitioning analysis (RPA) score
Introduction
Esophageal carcinoma has a high mortality rate, with the majority of patients ultimately succumbing to their disease. In the US, approximately 17,000 patients will be diagnosed and 15,000 are predicted to die from esophageal cancer in 2015, with the incidence expected to rise (1,2). Patients often present with advanced stage disease, with the lung, liver and bones as the most common sites of metastasis (3).
Brain metastases from esophageal carcinoma have been reported in small case series at a rate of less than 3% and have been associated with poorer prognosis than brain metastases from other solid tumors (4). The increased incidence over the last few years could be attributable to more sensitive imaging modalities, such as MRI, or from improved overall survival from the primary tumor (5,6).
It remains difficult to prospectively identify which esophageal cancer patients will develop brain metastases. Brain metastases have been associated with larger tumors and higher stage disease in retrospective reports (7,8), but have also been reported with early stage disease and even as the presenting symptom in other cases (9). Their infrequent incidence renders routine surveillance cost ineffective (10,11).
The recursive partitioning analysis (RPA) classification index has been established as an important prognostic tool for patients with brain metastasis, and could help determine optimal treatment courses for patients stratified by predicted survival (12,13). The index was developed by the Radiation Therapy Oncology Group (RTOG) using recursive-partitioning analysis for three consecutive brain metastasis trials consisting of 1,200 patients, and is based on four primary factors in determining survival after diagnosis of brain metastasis—Karnofsky performance status (KPS), control of the primary tumor, presence or absence of extracranial metastases, and age (13). Class I patients, with the best predicted survival, are defined as <65 years, having a KPS ≥70, and having no extracranial metastasis; class III patients, with the worst predicted survival, are defined as having KPS <70; and, class II patients are all others (12). Stratification by RPA can help evaluate the benefit of aggressive treatments for patients with better prognosis (RPA class I or II) (14).
Because of the paucity of esophageal brain metastasis cases, treatment for these patients has not been well defined and is generally based on individual clinician judgment. Treatment modalities include surgery, stereotactic radiation, whole brain radiation therapy (WBRT), or a combination. In order to better delineate the clinical characteristics of these patients and optimize future management options, we reviewed the clinical data from patients with brain metastases from primary esophageal cancer treated at our tertiary referral cancer center.
Methods
Institutional review board approval was obtained to conduct this study. We identified patients with a primary diagnosis of esophageal cancer who developed brain metastasis treated at our tertiary cancer center between 1998 and 2015 using our Total Cancer Care database (TCC™). This is a multi-institutional observational study of patients with cancer in which self-reported demographic and clinical data as well as medical record information is prospectively collected. Tissue is collected for research purposes. Every patient is eligible and there are no exclusion criteria. Informed consent was obtained through this protocol.
We obtained patient data from retrospective medical record review. Data collected included patient demographics, date of diagnosis of primary tumor and brain metastasis, tumor characteristics, and survival data.
Survival analysis was performed using the Kaplan-Meier method and compared by log-rank from the date of primary diagnosis as well as brain metastasis diagnosis to the date of death or last follow up. Univariate and multivariate analyses were performed by Cox Regression. Statistical analysis was performed using SPSS software (version 23).
Results
Patient characteristics
Forty-nine patients with esophageal cancer and brain metastases were identified for analysis. Table 1 summarizes the baseline characteristics of these patients. Forty-one patients were male. The median age at diagnosis was 60 years. The majority of patients (82%) had adenocarcinoma, four patients had squamous cell carcinoma, four had poorly differentiated carcinoma, and one patient had neuroendocrine carcinoma. There were 29 (59%) patients with supratentorial brain lesion(s), 9 (18%) patients with infratentorial lesion(s), and 11 (22%) patients with both supratentorial and infratentorial lesions. Patients generally presented with headaches (32.7%), dizziness or balance difficulties (28.6%), cognitive impairment (16.3%), nausea or vomiting (14.3%), weakness or numbness in the extremities (12.2%), vision changes (10.2%), or seizures (6.1%). Approximately 12.2% of patients were asymptomatic.
Table 1. Baseline characteristics.
| Characteristics | Number of patients [%] |
|---|---|
| Age at diagnosis [years] | |
| Median | 60 |
| Range | 29−77 |
| Gender | |
| Male | 41 [84] |
| Female | 8 [16] |
| Histology | |
| Adenocarcinoma | 40 [82] |
| Squamous cell carcinoma | 4 [8] |
| Carcinoma NOS | 4[8] |
| Neuroendocrine carcinoma | 1 [2] |
| Location of brain metastasis | |
| Supratentorial | 29 [59] |
| Infratentorial | 9 [18] |
| Both [supra and infra] | 11 [22] |
| Stage of primary tumor | |
| I | 3 [6] |
| II | 13 [27] |
| III | 11 [22] |
| IV | 19 [39] |
| Unknown | 3 [6] |
| Treatment of primary tumor | |
| Surgery | 2 [4] |
| Combined modality | 35 [71] |
| Chemotherapy | 12 [25] |
| Time to dx of brain metastasis from original dx [months] | |
| Median | 14 |
| Range | 0−70 |
| Number of brain metastasis | |
| 1 | 27 [55] |
| 2 | 12 [25] |
| 3 or more | 10 [20] |
| RPA class | |
| I | 13 [27] |
| II | 30 [61] |
| III | 6 [12] |
| Controlled primary | |
| Yes | 26 [53] |
| No | 23 [47] |
| Extracranial metastases at brain metastasis dx | |
| Yes | 20 [41] |
| No | 29 [59] |
| Age <65 at brain metastasis dx | |
| Yes | 37 [76] |
| No | 12 [24] |
| KPS <70 | |
| Yes | 6 [12] |
| No | 43 [88] |
Stage at diagnosis ranged from stage IA to stage IV. We were unable to collect complete staging data on three patients. Using the recursive partitioning score (RPA), 15 patients had class I disease and 28 had class II, and 6 had class III disease.
Of the patients who received neoadjuvant chemoradiation, 18 out of 26 showed downstaging of their tumor, including 6 patients who had pathological complete response (pCR). HER-2 status was available for seven patients, and two had HER-2 amplified tumors.
The time between primary diagnosis of esophageal cancer and development of brain metastases ranged between zero and 70 months, with a median of 14 months. Twenty-seven patients had only one brain lesion, 12 patients had two brain lesions and 10 patients had more than two lesions.
Brain metastasis management
Of the patients with a solitary CNS lesion, 7 were treated with a combination of radiation [which included stereotactic radiosurgery (SRS) and WBRT], 2 were treated with surgery alone (both passed away prior to further therapy), and 16 were treated with surgery followed by radiation (which included SRS and WBRT). One patient was treated with WBRT alone and one patient was not treated due to declining performance status. Among the patients with two brain metastases, a combination of surgery and radiation was performed in four and SRS and/or WBRT was done in six. Two patients had surgery but passed away prior to further therapy. The ten patients with multiple brain lesions were treated as follows: two with surgery alone (passed away prior to further therapy), six with radiation, and one patient had resection of one large lesion followed by WBRT.
In our analysis, using the recursive partitioning score (RPA), 15 patients had class I disease and 28 had class II, and 6 had class III disease. For the RPA class I patients, two received surgery and SRS, two received SRS alone, four received surgery alone, three received surgery and WBRT, and four received SRS and WBRT. Among the patients who were RPA class II, one received surgery and SRS, six received SRS alone, five received surgery alone, seven received surgery and WBRT, one received SRS and WBRT, and eight received WBRT alone. For the patients who were RPA class III, one received surgery and WBRT, three received surgery alone, and two did not receive treatment.
Survival data
Figure 1 displays median survival after the diagnosis of brain metastasis, with a median of 5.3 months, and a range from 1 to 52 months. Median overall survival from time of diagnosis was 24 months, with a range from 3 to 71 months. The median survival from time of brain metastasis diagnosis for patients with a single brain metastasis, two brain metastases, and more than two brain metastases were 10.7, 4.7, and 0.3 months, respectively. The median survival based on RPA were 14.6 months for class I, 5.0 months for class II, and 1.6 months for class III, respectively.
Figure 1.
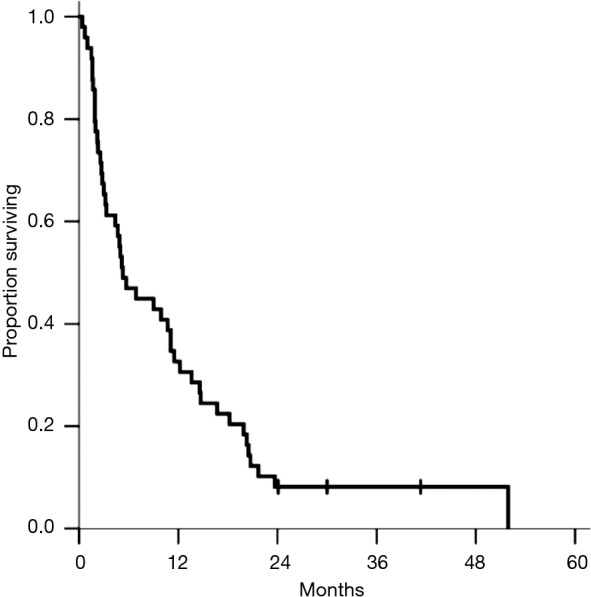
Overall survival from brain metastasis diagnosis. Median survival for all patients from the time of brain metastasis diagnosis is 5.3 months.
Table 2 reports univariate and multivariable analysis from the time of brain metastasis. Three or more brain metastases as well as RPA class III were significantly associated with worse survival on multivariate analysis. Presence of one or two brain metastasis did not reflect a difference in survival, but patients with three or more had worse outcomes (Figure 2). While there was no significant association between decreased survival and number of brain metastases for 1 vs. 2 metastases, the hazard ratio for 1 vs. 3 brain metastases was statistically significant for both univariate analysis (5.70; 95% CI, 2.51−13.0; P<0.01) and multivariate analysis (4.77; 95% CI, 1.99−11.4; P<0.01). There was no significant association found for RPA class I vs. class II but RPA class III had much worse survival (Figure 3). Patients with RPA class I or class II disease had significantly improved overall survival compared to those who were RPA class III upon both univariate analysis (8.22; 95% CI, 2.76−24.4; P<0.01) and multivariate analysis (4. 77; 95% CI, 1.99−11.4; P<0.01).
Table 2. Univariate and multivariate analysis from time of brain metastasis.
| Characteristic (comparison group) | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |||
| Age (continuous) | 1.00 (0.96−1.03) | 0.80 | − | − | ||
| Gender | 0.54 (0.23−1.28) | 0.16 | − | − | ||
| Histology (other vs. adenocarcinoma) | 1.62 (0.78−3.37) | 0.20 | − | − | ||
| Location (vs. supratentorial) | ||||||
| Infratentorial | 1.70 (0.82−3.53) | 0.16 | − | − | ||
| Both | 0.96 (0.27−2.12) | 0.92 | − | − | ||
| Initial primary tumor stage (vs. IV) | ||||||
| I | 0.93 (0.27−3.21) | 0.91 | − | − | ||
| II | 0.85 (0.40−1.82) | 0.67 | − | − | ||
| III | 1.39 (0.64−3.03) | 0.41 | − | − | ||
| Unknown | 0.58 (0.17−1.99) | 0.39 | − | − | ||
| Definitive primary therapy (vs. chemotherapy) | 0.89 (0.46−1.73) | 0.37 | − | − | ||
| Time to diagnosis of brain metastasis from original diagnosis (continuous) | 1.00 (0.98−1.03) | 0.79 | − | − | ||
| Number of brain metastases (vs. 1) | ||||||
| 2 | 0.97 (0.46−2.02) | 0.93 | 0.90 (0.43−1.89) | 0.77 | ||
| 3 or more | 5.70 (2.51−13.0) | <0.01 | 4.77 (1.99−11.4) | <0.01 | ||
| RPA classification (vs. I) | ||||||
| II | 1.37 (0.69−2.73) | 0.36 | 1.42 (0.71−2.84) | 0.32 | ||
| III | 8.22 (2.76−24.4) | <0.01 | 4.77 (1.99−11.4) | <0.01 | ||
| KPS <70 (vs. ≥70) | 6.60 (2.50−17.4) | <0.01 | − | − | ||
| Controlled primary (vs. uncontrolled) | 0.79 (0.44−1.42) | 0.43 | − | − | ||
| No extracranial metastasis (vs. yes) | 1.54 (0.85−2.78) | 0.16 | − | − | ||
| Age <65 (vs. ≥65) | 0.97 (0.49−1.92) | 0.93 | − | − | ||
Figure 2.

Survival stratified by number of brain metastases. Median survivals for 1, 2, and 3+ metastases are 10.7, 4.7, and 0.3 months, respectively. Log-rank for 1 vs. 3+ yields, P<0.001; 2 vs. 3+, P=0.002; and 1 vs. 2, P=0.953.
Figure 3.
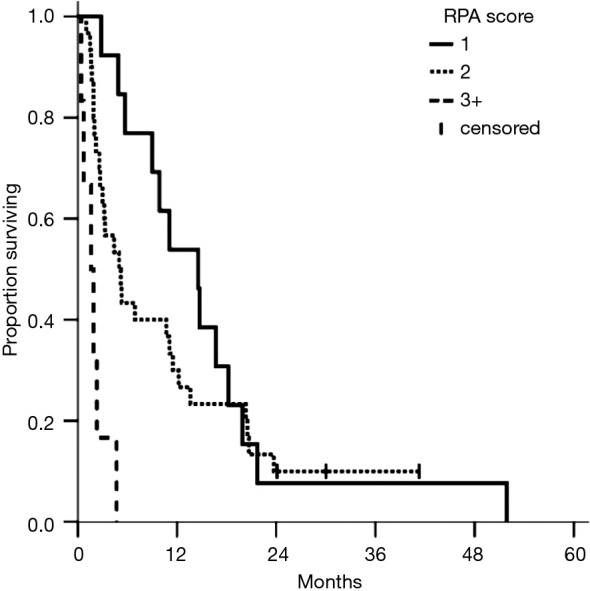
Survival based on recursive partitioning assessment (RPA). Median survivals for RPA class I, II, and III are 14.6, 5.0, and 1.6 months, respectively. Log-rank for I vs. III yields, P<0.001; II vs. III, P=0.001; and I vs. II, P=0.363.
Upon further analysis, this survival difference was found to be largely driven by KPS. KPS <70 was associated with RPA class III and significantly decreased survival. The hazard ratio for KPS <70 vs. ≥70 was 6.60 (95% CI, 2.50−17.4; P<0.01). For patients with 1−2 brain metastases who had a KPS score ≥70 (n=36, 73%), median survival (11.1 months) was found to be significantly better than RPA estimates from the RTOG (13). There was no significant difference in any of the other factors determining RPA, including control of the primary tumor, presence of extracranial metastases, or age.
Discussion
Metastasis of esophageal carcinoma to the brain is rare and associated with overall poor prognosis. Because of a combination of improved treatment modalities for the primary tumor and improved imaging modalities, the frequency of brain metastasis diagnosis is increasing. Historically, brain metastases in this disease have been treated with WBRT alone. Though WBRT has been associated with long-term side effects of neurocognitive decline, it has been utilized extensively because of ease of delivery and cost effectiveness in a patient population that has a perceived shortened survival. In our series, 73% of patients survived for greater than 11 months. Contrary to previously held beliefs, these patients do have sufficient time to develop the neurocognitive side effects associated with WBRT and should be considered for more focal therapies like surgery and SRS in select cases.
Though WBRT may improve the short term neurological symptoms associated with brain metastasis and prevent the development of further metastases in the short term, it has not been shown to significantly improve survival as an independent treatment modality (14,15). In patients with a single, resectable lesion, there is a survival benefit associated with surgery followed by WBRT compared to WBRT alone (16-18). Increased survival has been reported in patients with multiple brain lesions who underwent surgical removal of all metastases, and there is similar prognosis between such patients and those who underwent resection of a single lesion (19).
The value of SRS in the treatment of brain metastases, particularly in unresectable lesions, has also been established (14). Specifically, WBRT with SRS boost compared to WBRT alone is associated with improved local control in patients with small lesions (generally ≤3 cm) or a small number of metastases (20,21). Overall survival from SRS alone is comparable to that from combined SRS and WBRT (22,23). Although the numbers were limited in our study, those who had definitive local therapy appeared to do as well as those patients who had local therapy followed by WBRT. As the former treatment modality could be associated with fewer cognitive symptoms, treating with local therapy without WBRT could be considered in the future.
Careful patient selection for treatment could be the basis for determining which patients are likely to benefit from aggressive local therapies. The RPA classification has been established as a valid prognostic index and can help determine treatment options for patients stratified by predicted response (24,25). RPA class I or II patients have improved survival compared to class III, and may benefit from aggressive treatment (26). Rades et al. found that patients in RPA classes I and II with 1−3 brain metastases had improved local control and brain control when treated with SRS alone (18 to 25 Gy) compared to WBRT alone (30 to 40 grays) (27). The value of aggressive treatment—SRS, resection, or both—in patients with multiple brain metastases stratified by RPA class has also been evaluated. RPA class has been significantly correlated with survival suggesting aggressive treatment may benefit patients with RPA class I and II with a limited number of brain lesions and controlled primary disease (28).
In an analysis of 27 patients with brain metastasis specifically from esophageal carcinoma, treated at MD Anderson Cancer Center, it was shown that RPA class I was associated with improved survival compared to class II–III (29). It was also found that that the longest survival following diagnosis of brain metastasis occurred in patients treated with surgery and WBRT for a single brain metastasis, suggesting that patients with controlled disease can benefit from aggressive treatment of the CNS lesions. Similar results were found in another retrospective review of 27 patients with brain metastasis originating from esophageal primary disease (30). Low RPA class, single lesion, KPS score ≥70, lack of systemic disease, and aggressive treatment (resection, SRS & WBRT, SRS & resection & WBRT, resection & WBRT) were associated with a significant increase in survival upon univariate analysis. Moreover, multivariate analysis showed improved survival in patients with a higher KPS score who received aggressive treatment, indicating the benefit of aggressive treatment for patients with better prognosis. The role of KPS in predicting prognosis in patients with brain metastasis from esophageal carcinoma has been confirmed by several studies (31-34). In our analysis, we found a statistically significant improvement in survival based on lower RPA class. Subset analysis showed that the primary driver of this survival advantage was the patient’s KPS. Rather than relying on RPA, KPS alone represents a more simplified selection criterion to identify patients who would benefit from aggressive intervention in the future.
Disease biology might also help to identify patients who are susceptible to brain metastasis in the future. For example, amplification of the HER2 oncogene in esophageal adenocarcinoma ranges from 19−43%, and overexpression of HER2 may result in increased risk of developing brain metastasis in esophageal cancer (35-37). Although we were able to collect HER2 data for only a limited number of patients, this variable is being increasingly evaluated in patients presenting with brain metastasis and could impact future management of disease, especially with regards to targeted agents.
We found that those patients with a limited number of CNS lesions who had definitive therapy (surgery or SRS) had improved outcome even without WBRT and that performance status was a strong predictor of outcome. Although our study findings represent the largest series to date on brain metastasis from esophageal carcinoma, there are still several limitations to our results. The patients included in our cohort were retrospectively identified. It is also possible that asymptomatic patients with brain metastases were not identified, resulting in an observed rate of brain metastasis that was lower than the actual rate. Finally, our study consisted of a heterogeneous patient population, as patients who were treated from 1998 to 2015 were included for analysis and treatment approaches to this disease have changed over time.
Conclusions
The incidence of brain metastases arising from esophageal carcinoma is increasing. Although these metastases are still rare with overall poor prognosis, aggressive management allows for prolonged survival. In the largest series to date, we found that those patients with a limited number of CNS lesions who had definitive therapy (surgery or SRS) had improved outcome from aggressive management. Though prospective studies are not possible given the rarity of the tumor, reporting of large series such as ours can help define the most effective management for these patients.
Acknowledgements
None.
Ethical Statement: The study was approved by institutional ethics board (No. Pro000049780 USF) and written informed consent was obtained from all patients.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. 10.3322/caac.21254 [DOI] [PubMed] [Google Scholar]
- 2.Simard EP, Ward EM, Siegel R, et al. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin 2012;62:118-28. 10.3322/caac.20141 [DOI] [PubMed] [Google Scholar]
- 3.Quint LE, Hepburn LM, Francis IR, et al. Incidence and distribution of distant metastases from newly diagnosed esophageal carcinoma. Cancer 1995;76:1120-5. [DOI] [PubMed] [Google Scholar]
- 4.Go PH, Klaassen Z, Meadows MC, et al. Gastrointestinal cancer and brain metastasis: a rare and ominous sign. Cancer 2011;117:3630-40. 10.1002/cncr.25940 [DOI] [PubMed] [Google Scholar]
- 5.Feng W, Zhang P, Zheng X, et al. Incidence and treatment of brain metastasis in patients with esophageal carcinoma. World J Gastroenterol 2015;21:5805-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith RS, Miller RC. Incidence of brain metastasis in patients with esophageal carcinoma. World J Gastroenterol 2011;17:2407-10. 10.3748/wjg.v17.i19.2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogawa K, Toita T, Sueyama H, et al. Brain metastases from esophageal carcinoma: natural history, prognostic factors, and outcome. Cancer 2002;94:759-64. 10.1002/cncr.10271 [DOI] [PubMed] [Google Scholar]
- 8.Yoshida S. Brain metastasis in patients with esophageal carcinoma. Surg Neurol 2007;67:288-90. 10.1016/j.surneu.2006.05.065 [DOI] [PubMed] [Google Scholar]
- 9.Spallone A, Izzo C. Esophageal cancer presenting as a brain metastasis: A case report. Oncol Lett 2013;6:722-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gabrielsen TO, Eldevik OP, Orringer MB, et al. Esophageal carcinoma metastatic to the brain: clinical value and cost-effectiveness of routine enhanced head CT before esophagectomy. AJNR Am J Neuroradiol 1995;16:1915-21. [PMC free article] [PubMed] [Google Scholar]
- 11.Wadhwa R, Taketa T, Correa AM, et al. Incidence of brain metastases after trimodality therapy in patients with esophageal or gastroesophageal cancer: implications for screening and surveillance. Oncology 2013;85:204-7. 10.1159/000354736 [DOI] [PubMed] [Google Scholar]
- 12.Gaspar LE, Scott C, Murray K, et al. Validation of the RTOG recursive partitioning analysis (RPA) classification for brain metastases. Int J Radiat Oncol Biol Phys 2000;47:1001-6. 10.1016/S0360-3016(00)00547-2 [DOI] [PubMed] [Google Scholar]
- 13.Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 1997;37:745-51. 10.1016/S0360-3016(96)00619-0 [DOI] [PubMed] [Google Scholar]
- 14.Goetz P, Ebinu JO, Roberge D, et al. Current standards in the management of cerebral metastases. Int J Surg Oncol 2012;2012:493426. [DOI] [PMC free article] [PubMed]
- 15.Biswas G, Bhagwat R, Khurana R, et al. Brain metastasis--evidence based management. J Cancer Res Ther 2006;2:5-13. 10.4103/0973-1482.19768 [DOI] [PubMed] [Google Scholar]
- 16.Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 1990;322:494-500. 10.1056/NEJM199002223220802 [DOI] [PubMed] [Google Scholar]
- 17.Noordijk EM, Vecht CJ, Haaxma-Reiche H, et al. The choice of treatment of single brain metastasis should be based on extracranial tumor activity and age. Int J Radiat Oncol Biol Phys 1994;29:711-7. 10.1016/0360-3016(94)90558-4 [DOI] [PubMed] [Google Scholar]
- 18.Vecht CJ, Haaxma-Reiche H, Noordijk EM, et al. Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann Neurol 1993;33:583-90. 10.1002/ana.410330605 [DOI] [PubMed] [Google Scholar]
- 19.Bindal RK, Sawaya R, Leavens ME, et al. Surgical treatment of multiple brain metastases. J Neurosurg 1993;79:210-6. 10.3171/jns.1993.79.2.0210 [DOI] [PubMed] [Google Scholar]
- 20.Linskey ME, Andrews DW, Asher AL, et al. The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol 2010;96:45-68. 10.1007/s11060-009-0073-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kondziolka D, Patel A, Lunsford LD, et al. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys 1999;45:427-34. 10.1016/S0360-3016(99)00198-4 [DOI] [PubMed] [Google Scholar]
- 22.Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 2006;295:2483-91. 10.1001/jama.295.21.2483 [DOI] [PubMed] [Google Scholar]
- 23.Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 2004;363:1665-72. 10.1016/S0140-6736(04)16250-8 [DOI] [PubMed] [Google Scholar]
- 24.Chidel MA, Suh JH, Reddy CA, et al. Application of recursive partitioning analysis and evaluation of the use of whole brain radiation among patients treated with stereotactic radiosurgery for newly diagnosed brain metastases. Int J Radiat Oncol Biol Phys 2000;47:993-9. 10.1016/S0360-3016(00)00527-7 [DOI] [PubMed] [Google Scholar]
- 25.Kepka L, Cieslak E, Bujko K, et al. Results of the whole-brain radiotherapy for patients with brain metastases from lung cancer: the RTOG RPA intra-classes analysis. Acta Oncol 2005;44:389-98. 10.1080/02841860510029699 [DOI] [PubMed] [Google Scholar]
- 26.Nieder C, Nestle U, Motaref B, et al. Prognostic factors in brain metastases: should patients be selected for aggressive treatment according to recursive partitioning analysis (RPA) classes? Int J Radiat Oncol Biol Phys 2000;46:297-302. 10.1016/S0360-3016(99)00416-2 [DOI] [PubMed] [Google Scholar]
- 27.Rades D, Pluemer A, Veninga T, et al. Whole-brain radiotherapy versus stereotactic radiosurgery for patients in recursive partitioning analysis classes 1 and 2 with 1 to 3 brain metastases. Cancer 2007;110:2285-92. 10.1002/cncr.23037 [DOI] [PubMed] [Google Scholar]
- 28.Pollock BE, Brown PD, Foote RL, et al. Properly selected patients with multiple brain metastases may benefit from aggressive treatment of their intracranial disease. J Neurooncol 2003;61:73-80. 10.1023/A:1021262218151 [DOI] [PubMed] [Google Scholar]
- 29.Weinberg JS, Suki D, Hanbali F, et al. Metastasis of esophageal carcinoma to the brain. Cancer 2003;98:1925-33. 10.1002/cncr.11737 [DOI] [PubMed] [Google Scholar]
- 30.Khuntia D, Sajja R, Chidel MA, et al. Factors associated with improved survival in patients with brain metastases from esophageal cancer: a retrospective review. Technol Cancer Res Treat 2003;2:267-72. 10.1177/153303460300200309 [DOI] [PubMed] [Google Scholar]
- 31.Song Z, Lin B, Shao L, et al. Brain metastases from esophageal cancer: clinical review of 26 cases. World Neurosurg 2014;81:131-5. 10.1016/j.wneu.2013.02.058 [DOI] [PubMed] [Google Scholar]
- 32.Bowden G, Kano H, Tempel ZJ, et al. Gamma knife radiosurgery for management of cerebral metastases from esophageal carcinoma. J Neurooncol 2014;118:141-6. 10.1007/s11060-014-1408-3 [DOI] [PubMed] [Google Scholar]
- 33.Bartelt S, Momm F, Weissenberger C, et al. Patients with brain metastases from gastrointestinal tract cancer treated with whole brain radiation therapy: prognostic factors and survival. World J Gastroenterol 2004;10:3345-8. 10.3748/wjg.v10.i22.3345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rades D, Dziggel L, Bartscht T, et al. Predicting overall survival in patients with brain metastases from esophageal cancer. Anticancer Res 2014;34:6763-5. [PubMed] [Google Scholar]
- 35.Safran H, Dipetrillo T, Akerman P, et al. Phase I/II study of trastuzumab, paclitaxel, cisplatin and radiation for locally advanced, HER2 overexpressing, esophageal adenocarcinoma. Int J Radiat Oncol Biol Phys 2007;67:405-9. 10.1016/j.ijrobp.2006.08.076 [DOI] [PubMed] [Google Scholar]
- 36.Abu Hejleh T, Deyoung BR, Engelman E, et al. Relationship between HER-2 overexpression and brain metastasis in esophageal cancer patients. World J Gastrointest Oncol 2012;4:103-8. 10.4251/wjgo.v4.i5.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Preusser M, Berghoff AS, Ilhan-Mutlu A, et al. Brain metastases of gastro-oesophageal cancer: evaluation of molecules with relevance for targeted therapies. Anticancer Res 2013;33:1065-71. [PubMed] [Google Scholar]


