Abstract
Background: The spread of drug-resistant tuberculosis (TB) is one of the major public health problems through the world. Surveillance of anti-TB drug resistance is essential for monitoring of TB control strategies. The occurrence of drug resistance, particularly multi-drug resistance Mycobacterium tuberculosis (MDR), defined as resistance to at least rifampicin (RIF) and isoniazid (INH), has become a significant public health dilemma. The status of drug-resistance TB in Iran, one of the eastern Mediterranean countries locating between Azerbaijan and Armenia and high-TB burden countries (such as Afghanistan and Pakistan) has been reported inconsistently. Therefore, the aim of this study was to summarize reports of first-line anti-tubercular drug resistance in M. tuberculosis in Iran.
Material and Methods: We systematically reviewed published studies on drug-resistant M. tuberculosis in Iran. The search terms were “Mycobacterium tuberculosis susceptibility” or “Mycobacterium tuberculosis resistant” and Iran.
Results: Fifty-two eligible articles, published during 1998–2014, were included in this review. Most of the studies were conducted in Tehran. The most common used laboratory method for detecting M. tuberculosis drug resistant was Agar proportion. The highest resistance to first-line drugs was seen in Tehran, the capital city of Iran. The average prevalence of isoniazid (INH), rifampin (RIF), streptomycin (SM), and ethambotol (EMB) resistance via Agar proportion method in Tehran was 26, 23, 22.5, and 16%, respectively. In general, resistance to INH was more common than RIF, SM, and EMB in Tehran
Conclusions: In conclusion, this systematic review summarized the prevalence and distribution of first-line anti-tubercular drug resistance of M. tuberculosis in Iran. Our results suggested that effective strategies to minimize the acquired drug resistance, to control the transmission of resistance and improve the diagnosis measures for TB control in Iran.
Keywords: tuberculosis (TB), multidrug resistance tuberculosis (MDR), Iran
Introduction
Tuberculosis (TB) remains as one of the most common infectious disease in developing countries (Nasiri et al., 2014). In 2012, ~8.6 million people developed TB and 1.3 million died from the disease (Organization, 2013). TB is an important health problem, and this issue has become even more as a result of increasing number of drug resistant strains (Shamaei et al., 2009). There is not a complete data about first-line anti-tubercular drug resistance of Mycobacterium tuberculosis in Iran, one of the eastern Mediterranean countries locating between Azerbaijan and Armenia and high-TB burden countries (such as Afghanistan and Pakistan). Since 1996, when the national TB control programs established in Iran, TB incidence has been declining from 34 per 100,000 to 21 per 100,000 cases in 2011(Organization, 2011). Knowledge of geographic variations is essential for monitoring of antibiotic resistance within a defined population of patients infected with M. tuberculosis (Bahrmand et al., 2009). Isoniazid (INH), rifampin (RIF), streptomycin (SM), and ethambotol (EMB) are first-line chemotherapeutic drugs used in TB therapy (Mohammadi et al., 2002). Resistant to at least INH and RIF, is of great concern, because it requires the use of second-line drugs that are difficult to procure and are much more toxic and expensive than the first line regimen (Merza et al., 2011). Based on national wide survey conducted in 1999, among all M. tuberculosis isolates tested for drug susceptibility, 10.9% were resistant to = 1 anti-TB drug, and 6.7% were resistant to both INH and RIF (Organization, 2000). It has been proved that patients infected with strains resistant to RIF will experience a higher failure rate with short-course 6 months chemotherapy (Shamaei et al., 2009). Together with delayed diagnosis and lack or inadequacy of TB control programs, the emergence of MDR M. tuberculosis has complicated the epidemiology of TB (Yang et al., 2011). Although a number of original articles from different regions of Iran have been published in recent years, there has not been a systematic review of these data. Therefore, the aim of this study was to summarize reports on first-line anti-tubercular drug resistance of M. tuberculosis in Iran.
Materials and methods
Literature search
“Mycobacterium tuberculosis susceptibility,” “Mycobacterium tuberculosis resistant,” “M. tuberculosis susceptibility,” and “M. tuberculosis resistant” and Iran were searched with special strategies in PubMed and Google Scholar engines. Three Persian scientific search engines “Scientific Information Database,” “IranMedex,” and “MagIran” were searched as well. Reference articles were explored. Both studies published in English and Persian were included. Gray literature and Abstracts of articles which published in congress were not explored. Search strategies were followed until 30th November 2014.
Inclusion criteria
We sought any articles of antimicrobial susceptibility testing of M. tuberculosis isolates. In addition, the bibliography of each article were reviewed to identify additional relevant articles. Among English and Persian articles found with mentioned strategies, those with the following features were included in the study: (1) Full text was available. (2) An original article was performed. (3) Susceptibility data for at least one anti- tubercular drug was available. (4) The laboratory method was used.
Exclusion criteria
Studies with at least one of the following aspects were excluded: (1) Studies that were not relevant. (2) Articles with only available abstracts (without full text). (3) Studies that did not use laboratory methods (using patients records). (4) Articles that use of second line of antimicrobial drug resistance. (5) Articles that were review. (6) Articles which contain no eligible data. (7) Case series reports. (8) Articles that sample size is too small (N < 10).
Data collection
At this stage, articles with the following features were excluded as well: (1) Any articles were published both in English and Persian. (In these cases, the article published with more detailed results was chosen). (2) Duplicate publications. For all studies, we extracted the following data from the original publications. Literature identification and data extraction was performed by two researchers independently. Quality assessment of methodological sections and results of included articles was performed by use of STROBE checklist (http://www.equator-network.org).
Results
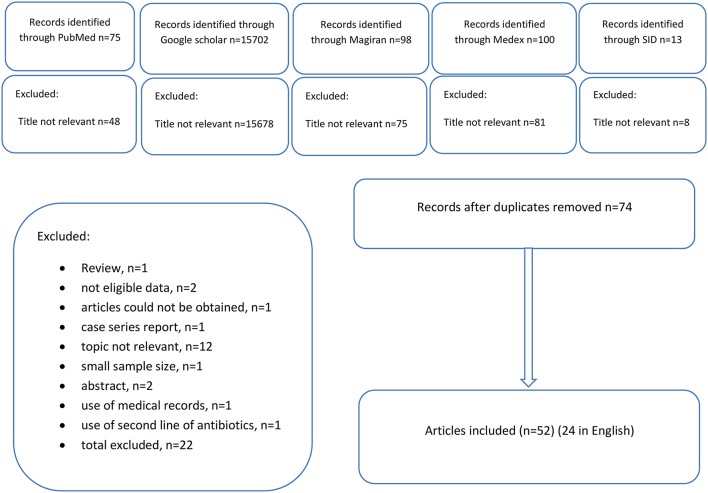
A total of 15,979 articles were achieved by literature search using different combination of key terms from the databases (Figure 1). After exclusion based on title not relevant and duplicates, 74 articles were retrieved for detailed full-text evaluation. Finally 52 studies, 24 in English, and 28 in Persian, addressing the prevalence of drug resistance TB were included (Tables 1, 2). The original articles were performed in different places of Iran. Most studies were conducted in Tehran (n = 25; Bahrmand et al., 2000; Mohammadi et al., 2002; Seyed-Davood Mansoori et al., 2003; Masjedi et al., 2006; Mirsaeidi et al., 2007; Mohammadzadeh et al., 2007; Farnia et al., 2008a,b; Shamaei et al., 2009; Dinmohammadi et al., 2010; Merza et al., 2011; Ostadzadeh et al., 2011; Sheikholslami et al., 2011; Taghavi et al., 2011; Tasbiti et al., 2011; Derakhshani Nezhad et al., 2012; Marjani et al., 2012; Mohammadi, 2012; Tahmasebi et al., 2012; Bahrami et al., 2013; Ali et al., 2014; Nasiri et al., 2014; Sheikh Ghomi et al., 2014; Varahram et al., 2014; Velayati et al., 2014) and Tabriz (n = 8; Hassan Heidarnejad and Nagili, 2001; Moadab and Rafi, 2006; Varshochi et al., 2006; Asgharzadeh et al., 2007, 2014; Rafi et al., 2009; Roshdi and Moadab, 2009; Zamanlou et al., 2009). Other studies were performed in Khorasan (n = 3; Namaei et al., 2006; Velayati et al., 2014; Sani et al., 2015), Ardebil (n = 1; Velayati et al., 2014), Isfahan (n = 3; Moniri, 2001; Nasiri et al., 2014; Velayati et al., 2014), Mazandaran (n = 3; Pourhajibagher et al., 2012; Babamahmoodi et al., 2014; Velayati et al., 2014), Gilan (n = 1; Velayati et al., 2014), Hamadan (n = 1; Velayati et al., 2014), Kerman(n = 1; Velayati et al., 2014), Kurdistan(n = 1; Velayati et al., 2014), Yazd (n = 1; Velayati et al., 2014), Qazvin (n = 1; Velayati et al., 2014), Kermanshah (n = 4; Izadi et al., 2011; Nasiri et al., 2014; Velayati et al., 2014; Mohajeri et al., 2014), Golestan (n = 3; Javid et al., 2009; Livani et al., 2011; Velayati et al., 2014), Markazi (n = 3; Farazi et al., 2013; Taherahmadi et al., 2013; Velayati et al., 2014), Lorestan (n = 1; Velayati et al., 2014), Khuzestan (n = 2; Khosravi et al., 2006; Velayati et al., 2014), Sistan va Baluchistan (n = 5; Bostanabad et al., 2007; Bahrmand et al., 2009; Haeili et al., 2013; Nasiri et al., 2014; Velayati et al., 2014), Qom (n = 1; Velayati et al., 2014), Fars (n = 1; Velayati et al., 2014), Hormozgan (n = 2; Nasiri et al., 2014; Velayati et al., 2014), and Semnan (n = 1; Velayati et al., 2014). A study which was conducted by Velayati et al. (2014), in years 2010–2011, has been investigated drug resistant in various places in Iran (Tehran, Sistan ba Balochestan, Khozestan, Khorasan, Ardebil, Qom, Golestan, Isfahan, Gilan, Fars, Hormozgan, Mazandaran, Semnan, Lorestan, Hamedan, Kerman, Kordestan, Kermanshah, Markazi, Yazd, and Qazvin (Velayati et al., 2014), but we identified it as 1 study in search flow diagram, it was considered for Nasiri et al. study too (Nasiri et al., 2014). In Isfahan, 4 surveys were performed but in one of them (Tavakoli et al.) only abstract was available, and it was excluded from total records. One study which was conducted by Moaddab et al. (2011) that did not note the location. One study has been done in Tehran and Zabol (Zakerbostanabad et al., 2008). One study has been conducted by Haeili et al. (2013) in Tehran, Alborz, Sistan va Blochestan, Hormozgan, and Kermanshah. Another study had been done in Tehran-Arak by Taheri et al. (2013). The reference method for determining drug resistance of M. tuberculosis was agar proportion. Using this method, the mean of resistance to INH, RIF, SM and EM in Iran was 20, 18, 18%, and to EM is 12%, respectively. Despite the reference method for susceptibility test is agar proportion (Rieder et al., 1998), the method that was used in most of the cities were PCR. For this reason, we determine the mean of resistance to INH and RIF in different geographical regions based on this method too. If Iran is divided into 8 geographical regions (Table 3), the mean of resistance to INH in Northern provinces of country was 5%, and maximum resistance was seen in Golestan and the minimum resistance was belonged to Gilan province. The mean of resistance to RIF was 4%, and the maximum and minimum resistance was seen in Gilan and Mazandaran, respectively. The mean of resistance to INH and RIF in Southern provinces of Iran was 6.45 and 10%, respectively. The mean of resistance to INH in Western provinces of country was 5%, and the maximum resistance belonged to Kordestan and minimum resistance was seen in Lorestan. The mean of resistance to RIF was 11%, and the highest and lowest resistance was seen in Lorestan and Kordestan. The mean of resistance to INH and RIF in Northwest provinces of Iran was 6.5 and 3%, respectively. The mean of resistance to INH in central provinces of country was 9%, and maximum resistance belonged to Markazi while minimum resistance belong to Yazd that no resistance has been seen. The mean of resistance to RIF was 10%, and the highest and lowest level of resistance was seen in Markazi and Qom. One of the provinces of central regions is Isfahan. In Isfahan the mean of resistance to INH based on agar proportion was 12.6% which was similar to PCR method (12%), but mean of resistance to RIF based on agar proportion was 26%, that was higher than PCR method (7%). In Markazi provinces, the mean of resistance to INH, based on reference method was 2.6% that was lower than PCR; there was the same result about RIF too. In Southwest of Iran, resistance to INH and RIF was 6 and 5%, respectively. The mean of resistance to INH and RIF in Northeast provinces of Iran was 4%. In Southeast provinces of Iran such as Sistan- Blochestan and Kerman, the mean of resistance to INH and RIF was 4.4 and 9% based on PCR method. Because the most of method that use in Sistan- Blochestan was agar proportion, we calculate the mean of resistance to INH and RIF based on the mentioned method, (INH 20% and RIF 12%). Due to the large number of studies in Tehran and Tabriz, these provinces were examined separately. The most common laboratory method that used was agar proportion in Tehran. The average prevalence of resistant against INH in Tehran was 26%, RIF 23%, SM 22.5%, and EMB 16% by agar proportion method. In general, resistance to INH was more common than RIF, SM and EMB in Tehran. The average prevalence of resistant against INH in Tabriz was 15%, RIF 5%, SM 19%, and EMB 2.43% by agar proportion. The highest resistance to first-line drugs was seen in Tehran. Most studies about two drug resistances were conducted in Tehran by proportional method on INH and RIF. The mean of resistance to INH and RIF in Tehran was 22% by proportional method. The mean of resistance to INH and RIF in IRAN was 15% by this method. Due to the limited number of studies on other two drug resistance, the results are given only in Table 2. Most studies on three drug resistance were conducted on INH, RIF and SM. The mean of resistance to these three drugs was 4% using proportional method. The mean of resistance to all first line drugs in IRAN was 3.57% by this method.
Figure 1.
Flow diagram of study identification.
Table 1.
Summary of studies on resistance to a single drug among Mycobacterium tuberculosis isolates in Iran.
| Study | Resistance to a single drug | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Location | Author | Years | Method | No. of isolates tested | INH | RIF | SM | EMB | ||||
| N | % | N | % | N | % | N | % | |||||
| Ardebil | Velayati et al., 2014 | 2010–2011 | PCR(a) | 65 | 2 | 3 | 4 | 6 | … | … | … | … |
| Fars | Velayati et al., 2014 | 2010–2011 | PCR(a) | 40 | 2 | 5 | 5 | 12.5 | … | … | … | … |
| Gilan | Velayati et al., 2014 | 2010–2011 | PCR(a) | 39 | 1 | 2.5 | 2 | 5 | … | … | … | … |
| Golestan | Javid et al., 2009 | 2008 | PCR(b) | 87 | 6 | 7 | 4 | 5 | … | … | … | … |
| Agar proportion | 45 | 4 | 9 | 6 | 13 | … | … | … | … | |||
| Livani et al., 2011 | … | MGIT | 148 | 26 | 18 | 5 | 3 | … | … | … | … | |
| Velayati et al., 2014 | 2010–2011 | PCR(a) | 47 | 3 | 6 | 2 | 4 | … | … | … | … | |
| Qom | Velayati et al., 2014 | 2010–2011 | PCR(a) | 61 | 3 | 5 | 3 | 5 | … | … | … | … |
| Hormozgan | Velayati et al., 2014 | 2010–2011 | PCR(a) | 38 | 3 | 8 | 3 | 8 | … | … | … | … |
| Nasiri et al., 2014 | 2010–2012 | Agar proportion | 48 | 3 | 6 | 2 | 4 | 4 | 8 | 2 | 4 | |
| Hamedan | Velayati et al., 2014 | 2010–2011 | PCR(a) | 21 | 1 | 5 | 2 | 10 | … | … | … | … |
| Isfahan | Velayati et al., 2014 | 2010–2011 | PCR(a) | 42 | 5 | 12 | 3 | 7 | … | … | … | … |
| Nasiri et al., 2014 | 2010–2012 | Agar proportion | 45 | 2 | 7 | 2 | 9 | 1 | 2 | 0 | 0 | |
| Moniri, 2001 | 1998–2009 | Agar proportion | 94 | 17 | 18 | 41 | 44 | 14 | 15 | 3 | 3 | |
| Khorasan | Namaei et al., 2006 | 2001–2002 | indirect proportion | 105 | 1 | 1 | … | … | 27 | 26 | … | … |
| Velayati et al., 2014 | 2010–2011 | PCR(a) | 117 | 10 | 8.5 | 9 | 8 | … | … | … | … | |
| Sani et al., 2015 | 2012–2013 | Agar proportion | 100 | 7 | 7 | 7 | 7 | 9 | 9 | 3 | 3 | |
| Kermanshah | Izadi et al., 2011 | 2006–2008 | Agar proportion | 14 | 8 | 57 | 6 | 43 | … | … | … | … |
| Velayati et al., 2014 | 2010–2011 | PCR(a) | 16 | 1 | 6 | 2 | 12.5 | … | … | … | … | |
| Nasiri et al., 2014 | 2010–2012 | Agar proportion | 15 | 4 | 26.6 | 3 | 20 | 3 | 20 | 3 | 20 | |
| Mohajeri et al., 2014 | 2011–2012 | Agar proportion | 112 | 18 | 16 | 16 | 14 | 25 | 22 | 15 | 13 | |
| Kermanshah | Mohajeri et al., 2015 | 2011–2013 | Agar proportion | 125 | … | … | 35 | 28 | … | … | … | … |
| Khozestan | Khosravi et al., 2006 | 2001 | PCR(c) | 80 | 5 | 6 | 6 | 7.5 | … | … | … | … |
| Velayati et al., 2014 | 2010–2011 | PCR(a) | 119 | 7 | 6 | 3 | 2.5 | … | … | … | … | |
| Kerman | Velayati et al., 2014 | 2010–2011 | PCR(a) | 24 | 1 | 4 | 3 | 12.5 | … | … | … | … |
| Kordestan | Velayati et al., 2014 | 2010–2011 | PCR(a) | 16 | 2 | 12.5 | 0 | 0 | … | … | … | … |
| Lorestan | Velayati et al., 2014 | 2010–2011 | PCR(a) | 24 | 0 | 0 | 5 | 21 | … | … | … | … |
| Mazandaran | Pourhajibagher et al., 2012 | 2010–2011 | PCR(d) | 59 | 4(use of katG gene) 3(use of inhA gene) | 7 5 | 1 | 2 | … | … | … | … |
| Velayati et al., 2014 | 2010–2011 | PCR(a) | 26 | 1 | 4 | 1 | 4 | … | … | … | … | |
| Babamahmoodi et al., 2014 | … | LPA(e) | 54 | 2 | 4 | 3 | 5.5 | 4 | 7 | … | … | |
| Markazi | Taherahmadi et al., 2013 | … | Agar proportion PCR-RFLP (f) | 60 | … | … | … | … | … | … | 43 19 | 72 32 |
| Farazi et al., 2013 | 2011–2012 | Agar proportion | 115 | 3 | 3 | 2 | 2 | 3 | 3 | 8 | 7 | |
| Velayati et al., 2014 | 2010–2011 | PCR(a) | 15 | 3 | 20 | 3 | 20 | … | … | … | … | |
| Qazvin | Velayati et al., 2014 | 2010–2011 | PCR(a) | 10 | 1 | 10 | 0 | 0 | … | … | … | … |
| Semnan | Velayati et al., 2014 | 2010–2011 | PCR(a) | 21 | 0 | 0 | 0 | 0 | … | … | … | … |
| Zakerbostanabad et al., 2008 | 2005–2006 | Agar proportion | 91 | 28 | 31 | 4 | 4 | 23 | 25 | 8 | 9 | |
| Sistan va Balochestan | Bahrmand et al., 2009 | 2005–2006 | Agar proportion | 286 | … | … | 78 | 27 | … | … | … | … |
| Velayati et al., 2014 | 2010–2011 | PCR(a) | 165 | 8 | 5 | 10 | 6 | … | … | … | … | |
| Nasiri et al., 2014 | 2010–2012 | Agar proportion | 59 | 5 | 8 | 3 | 5 | 8 | 13.5 | 3 | 5 | |
| Tehran | Ostadzadeh et al., 2011 | … | Agar proportion | 50 | … | … | 25 | 50 | … | … | … | … |
| Farnia et al., 2008a | … | Agar proportion MGMT Both of them | 60 | 0 0 30 | 0 0 50 | 0 0 30 | 0 0 50 | 4 3 29 | 7 5 48 | 3 5 28 | 5 8 47 | |
| Sheikholslami et al., 2011 | … | Agar proportion PCR-SSCP(g) | 74 | 17 10 | 23 13.5 | 7 4 | 9 5 | … | … | … | … | |
| Seyed-Davood Mansoori et al., 2003 | 1996–2000 | Agar proportion | 273 | 76 | 28 | 50 | 18.5 | 50 | 18.5 | 28 | 10 | |
| Bahrmand et al., 2000 | 1998–1999 | Agar proportion | 563 | 35 | 6 | 25 | 4 | 55 | 10 | 17 | 3 | |
| Mohammadi et al., 2002 | 1999–2000 | MGIT Direct MGIT in Direct Agar proportion | 15 | 10 10 7 | 67 67 47 | 11 11 8 | 73 73 53 | 5 6 7 | 33 40 47 | 5 5 5 | 33 33 33 | |
| Dinmohammadi et al., 2010 | 1999–2008 | Agar proportion | 90 | 52 | 58 | … | … | … | … | … | … | |
| Shamaei et al., 2009 | 2000–2003 | Agar proportion | 548 | 152 | 28 | 119 | 22 | 184 | 34 | 75 | 14 | |
| Merza et al., 2011 | 2000–2005 | Agar proportion | 1742 | 414 | 24 | 307 | 18 | 478 | 27 | 207 | 12 | |
| Mirsaeidi et al., 2007 | 2003–2004 | Agar proportion | 264 | 93 | 35 | 52 | 20 | 96 | 36 | 35 | 13 | |
| Marjani et al., 2012 | 2003–2008 | Agar proportion | 554 | 81 | 15 | 27 | 5 | 116 | 21 | 22 | 4 | |
| Varahram et al., 2014 | 2003–2011 | Agar proportion and Allele specific PCR | 4825 | 296 | 6 | … | … | … | … | … | … | |
| Farnia et al., 2008b | 2006–2007 | Agar proportion | 258 | 9 | 3 | 3 | 1 | 7 | 3 | 1 | 0.4 | |
| Mohammadi, 2012 | 2006–2008 | Agar proportion MAS-PCR(h) | 90 | … | … | 37 29 | 41 32 | … | … | … | … | |
| Tasbiti et al., 2011 | 2006–2009 | Agar proportion | 1027 | 116 | 11 | 110 | 11 | 232 | 23 | 104 | 10 | |
| Taghavi et al., 2011 | 2008–2009 | Agar proportion MAS-PCR(i) | 96 | 56 43 | 58 45 | … | … | … | … | |||
| Ali et al., 2014 | 2009–2011 | Agar proportion PCR-SSCP | 103 | 12 5 | 12 5 | 9 4 | 9 4 | … | … | … | … | |
| Velayati et al., 2014 | 2010–2011 | PCR(a) | 324 | 20 | 6 | 26 | 8 | |||||
| Derakhshani Nezhad et al., 2012 | 2010–2011 | Agar proportion Allele-specific PCR | 106 | … | … | … | … | … | … | 36 13 | 34 28 | |
| Tahmasebi et al., 2012 | 2010–2011 | Agar proportion | 97 | 68 | 70 | 63 | 65 | 28 | 29 | 47 | 48 | |
| Bahrami et al., 2013 | 2010–2012 | Agar proportion | 176 | … | … | … | … | … | … | 48 | 27 | |
| Nasiri et al., 2014 | 2010–2012 | Agar proportion | 85 | 6 | 7 | 7 | 8 | 14 | 16 | 6 | 7 | |
| Sheikh Ghomi et al., 2014 | 2012–2013 | Agar proportion and Multiplex PCR | 83 | 35 | 42 | 47 | 56 | … | … | … | … | |
| Tabriz | Zamanlou et al., 2009 | 2005–2007 | Agar proportion | 50 | 25 | 50 | … | … | … | … | … | … |
| Rafi et al., 2009 | … | Agar proportion | 90 | 6 | 7 | 3 | 3 | 17 | 19 | … | … | |
| Moadab and Rafi, 2006 | 1999–2003 | Agar proportion | 90 | 7 | 8 | 2 | 2 | 17 | 19 | … | … | |
| Asgharzadeh et al., 2007 | … | Agar proportion MAS-PCR(j) | 120 | 13 | 11 | 12 | 10 | 27 | 22.5 | 4 10 | 3 8 | |
| Roshdi and Moadab, 2009 | … | Agar proportion | 103 | 2 | 2 | 0 | 0 | 8 | 8 | 0 | 0 | |
| Varshochi et al., 2006 | 2003–2004 | Agar proportion | 90 | 20 | 22 | 9 | 10 | 28 | 31 | 5 | 5.5 | |
| Hassan Heidarnejad and Nagili, 2001 | … | Agar proportion | 155 | 12 | 8 | 1 | 1 | 20 | 13 | 0 | 0 | |
| Asgharzadeh et al., 2014 | … | Agar proportion | 120 | 13 | 11 | 12 | 10 | 27 | 22.5 | 4 | 3 | |
| Yazd | Velayati et al., 2014 | 2010–2011 | PCR(a) | 12 | 0 | 0 | 1 | 8 | … | … | … | … |
| Tehran-Arak | Taheri et al., 2013 | … | Agar proportion | 40 | … | … | 20 | 50 | … | … | … | … |
| Tehran–Alborz-Sistan va Blochestan-Hormozgan-Kermanshah | Haeili et al., 2013 | 2010–2012 | Agar proportion | 291 | 4 | 1 | 2 | 1 | 21 | 7 | 2 | 1 |
| Tehran-Zabol-Kermanshah-Mashad-Tabriz | Bostanabad et al., 2011 | 2007–2008 | Agar proportion | 163 | 42 | 26 | 38 | 23 | 38 | 23 | 12 | 7 |
| Unknown | Moaddab et al., 2011 | … | Agar proportion and MIC | 50 | 25 | 50 | … | … | … | … | … | … |
(a): Multiplex-PCR assay for detection of mutations in RRDR(Rifampin resistance determinant region), and PCR assay for detection of mutations in IRDR (Isoniazid resistance determinant region) of katG, inhA.
(b): PCR assay for detection of mutations in RRDR of rpoB and IRDRof katG and inhA.
(c): PCR assay for detection of mutations in RRDR of rpoB and IRDR of katG.
(d): PCR assay for detection of mutations in RRDR of rpoB and IRDR of katG and inhA.
(e): Line Probe Assay.
(f): PCR-RFLP assay for detection of mutations in ERDR (Ethambotol resistance determinant region) of embB.
(g): PCR-SSCP assay for detection of mutations in RRDR of rpoB, ahpC and IRDR of katG, inhA.
(h): Mass-PCR assay for detection of mutations in RRDR of rpoB.
(i): Mass-PCR assay for detection of mutations in IRDR of katG.
(j): MAS-PCR assay for detection of mutations in ERDR of embB.
MGIT, Mycobacterium growth indicator tube; MIC, minimum inhibitory concentration; MGMT, malachite green microtube.
Table 2.
Summary multiple drug resistance of included studies.
| Author | location | Method | INH,RIF | INH,EMB | INH,SM | RIF,EMB | EMB,SM | RIF,SM | RIF,EMB,SM | INH,EMB,SM | INH,RIF,EMB | INH,RIF,SM | INH,RIF,SM,EMB | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | |||
| Velayati et al., 2014 | Ardebil | PCR | 4 | 6 | ||||||||||||||||||||
| Velayati et al., 2014 | Fars | PCR | 5 | 12.5 | ||||||||||||||||||||
| Velayati et al., 2014 | Gilan | PCR | 3 | 8 | ||||||||||||||||||||
| Velayati et al., 2014 | Golestan | PCR | 2 | 4 | ||||||||||||||||||||
| Javid et al., 2009 | Golestan | ProportionPCR | 4 2 | 9 2 | ||||||||||||||||||||
| Livani et al., 2011 | Golestan | MGIT | 5 | 3 | ||||||||||||||||||||
| Velayati et al., 2014 | Ghom | PCR | 4 | 6.5 | ||||||||||||||||||||
| Velayati et al., 2014 | Hormozgan | PCR | 3 | 8 | ||||||||||||||||||||
| Nasiri et al., 2014 | Hormozgan | Proportion | 2 | 4 | ||||||||||||||||||||
| Velayati et al., 2014 | Hamedan | PCR | 0 | 0 | ||||||||||||||||||||
| Velayati et al., 2014 | Isfahan | PCR | 2 | 5 | ||||||||||||||||||||
| Moniri, 2001 | Isfahan | Proportion | 16 | 17 | 9 | 10 | 2 | 2 | 12 | 13 | 8 | 8.5 | 1 | 1 | ||||||||||
| Nasiri et al., 2014 | Isfahan | Proportion | 2 | 4 | ||||||||||||||||||||
| Velayati et al., 2014 | Khorasan | PCR | 2 | 2 | ||||||||||||||||||||
| Namaei et al., 2006 | Khorasan | Proportion | 1 | 1 | 1 | 1 | ||||||||||||||||||
| Sani et al., 2015 | Khorasan | Proportion | 4 | 4 | 3 | 3 | 4 | 4 | 2 | 2 | ||||||||||||||
| Velayati et al., 2014 | Kermanshah | PCR | 1 | 6 | ||||||||||||||||||||
| Izadi et al., 2011 | Kermanshah | Proportion | 5 | 36 | ||||||||||||||||||||
| Nasiri et al., 2014 | Kermanshah | Proportion | 3 | 20 | ||||||||||||||||||||
| Mohajeri et al., 2014 | Kermanshah | Proportion | 16 | 14 | ||||||||||||||||||||
| Velayati et al., 2014 | Khozestan | PCR | 6 | 5 | ||||||||||||||||||||
| Khosravi et al., 2006 | Khozestan | Proportion | 7 | 9 | ||||||||||||||||||||
| Velayati et al., 2014 | Kerman | PCR | 3 | 12.5 | ||||||||||||||||||||
| Velayati et al., 2014 | Kordestan | PCR | 0 | 0 | ||||||||||||||||||||
| Velayati et al., 2014 | Lorestan | PCR | 0 | 0 | ||||||||||||||||||||
| Velayati et al., 2014 | Mazandaran | PCR | 1 | 4 | ||||||||||||||||||||
| Babamahmoodi et al., 2014 | Mazandaran | LPA | 0 | 0 | ||||||||||||||||||||
| Velayati et al., 2014 | Markazi | PCR | 2 | 13 | ||||||||||||||||||||
| Farazi et al., 2013 | Markazi | Proportion | 9 | 8 | 2 | 2 | ||||||||||||||||||
| Velayati et al., 2014 | Qazvin | PCR | 2 | 20 | ||||||||||||||||||||
| Velayati et al., 2014 | Semnan | PCR | 0 | 0 | ||||||||||||||||||||
| Velayati et al., 2014 | Sistan va Blochestan | PCR | 1 | 1 | ||||||||||||||||||||
| Nasiri et al., 2014 | Sistan va Blochestan | Proportion | 3 | 5 | ||||||||||||||||||||
| Bahrmand et al., 2009 | Sistan va Blochestan | Proportion | 37 | 13 | ||||||||||||||||||||
| Velayati et al., 2014 | Tehran | PCR | 32 | 10 | ||||||||||||||||||||
| Tahmasebi et al., 2012 | Tehran | Proportion | 63 | 65 | ||||||||||||||||||||
| Mohammadzadeh et al., 2007 | Tehran | Proportion | 11 | 48 | ||||||||||||||||||||
| Ostadzadeh et al., 2011 | Tehran | Proportion | 13 | 26 | ||||||||||||||||||||
| Taghavi et al., 2011 | Tehran | Proportion MAS-PCR | 36 26 | 38 27 | ||||||||||||||||||||
| Masjedi et al., 2006 | Tehran | Proportion | 150 | 12 | ||||||||||||||||||||
| Bahrami et al., 2013 | Tehran | Proportion | 10 | 6 | 12 | 7 | 19 | 11 | 8 | 4.5 | ||||||||||||||
| Shamaei et al., 2009 | Tehran | Proportion | 106 | 19 | ||||||||||||||||||||
| Mirsaeidi et al., 2007 | Tehran | Proportion | 43 | 16 | 0 | 0 | 23 | 9 | 0 | 0 | 2 | 1 | 4 | 1.5 | 0 | 0 | 2 | 1 | 0 | 0 | 9 | 3 | 26 | 10 |
| Seyed-Davood Mansoori et al., 2003 | Tehran | Proportion | 42 | 15.5 | 26 | 9.5 | 40 | 14.5 | 21 | 7.5 | 22 | 8 | 23 | 8.5 | 17 | 6 | 21 | 7.5 | 21 | 7.5 | 22 | 8 | 17 | 6 |
| Bahrmand et al., 2000 | Tehran | Proportion | 3 | 0.5 | 4 | 1 | 1 | 0.1 | 1 | 0.1 | 7 | 1 | ||||||||||||
| Farnia et al., 2008a | Tehran | MGMT | 8 | 19 | ||||||||||||||||||||
| Nasiri et al., 2014 | Tehran | Proportion | 6 | 7 | ||||||||||||||||||||
| Sheikholslami et al., 2011 | Tehran | Proportion PCR-SSCP | 16 4 | 22 5 | ||||||||||||||||||||
| Merza et al., 2011 | Tehran | Proportion | 263 | 15 | ||||||||||||||||||||
| Marjani et al., 2012 | Tehran | Proportion | 12 | 2 | ||||||||||||||||||||
| Sheikh Ghomi et al., 2014 | Tehran | Proportion and PCR | 30 | 36 | ||||||||||||||||||||
| Imani Fooladi et al., Ali et al., 2014 | Tehran | Proportion PCR-SSCP | 9 3 | 9 3 | ||||||||||||||||||||
| Rafi et al., 2009 | Tabriz | Proportion | 2 | 2 | 1 | 1 | 3 | 3 | 2 | 2 | ||||||||||||||
| Moadab and Rafi, 2006 | Tabriz | Proportion | 1 | 1 | 6 | 7 | 1 | 1 | 1 | 1 | 3 | 3 | 2 | 2 | ||||||||||
| Asgharzadeh et al., 2007 | Tabriz | Proportion | 1 | 1 | 5 | 4 | 1 | 1 | 2 | 2 | 2 | 2 | ||||||||||||
| Roshdi and Moadab, 2009 | Tabriz | Proportion | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | ||||||||||||||
| Varshochi et al., 2006 | Tabriz | Proportion | 1 | 1 | ||||||||||||||||||||
| Hassan Heidarnejad and Nagili, 2001 | Tabriz | Proportion | 5 | 3 | ||||||||||||||||||||
| Asgharzadeh et al., 2014 | Tabriz | Proportion | 6 | 5 | ||||||||||||||||||||
| Velayati et al., 2014 | Yazd | PCR | 0 | 0 | ||||||||||||||||||||
| Haeili et al., 2013 | Tehran-Alborz Sistan va Blochestan Hormozgan Kermanshah | Proportion | 15 | 5 | ||||||||||||||||||||
Table 3.
Different geographical regions of Iran.
| Region | Provinces |
|---|---|
| North | Golestan, Gilan, Mazandaran |
| South | Fars, Hormozgan |
| West | Kordestan, Kermanshah, Lorestan, Hamedan |
| Center | Isfahan, Qom, Markazi, Yazd |
| Northeast | Khorasan, Semnan |
| Northwest | Ardebil, Ghazvin |
| Southeast | Sistan-Blochestan, Kerman |
| Southwest | Khozestan |
Discussion
This review addressed the prevalence of first-line anti-tubercular drug resistance of M. tuberculosis in Iran. Various types of methods were used for determination of the susceptibility of M. tuberclusis: agar proportion (reference method), different types of PCR (PCR-RFLP, Real time PCR, PCR-SSCP, MAS-PCR, and Allele specific PCR), MGMT, and MGIT (direct and indirect). But most of them that used were agar proportion or PCR. In all the studies that use both of them, the results of reference method (agar proportion) had the highest of sensitivity and specificity (Javid et al., 2009; Sheikholslami et al., 2011; Derakhshani Nezhad et al., 2012; Mohammadi, 2012; Taherahmadi et al., 2013). In this study, evaluation of first-line anti-tubercular drug resistance in various provinces of Iran was based on PCR method that is not very accurate. It seems that the prevalence of drug resistance is higher than the results of studies that use mentioned method (PCR). As can be seen in the Table 1, the highest resistance of M. tuberculosis to first line drugs was observed in Tehran, INH:26%, RIF:23%, SM:22.5%, and EMB:16%. This could be due to transferring of patients with treatment failure to referral Hospitals in Tehran. The other reason could be presence of different nations such as Afghan, Iraq and Pakistan in Tehran. Between 1996 to 2000, three studies have been conducted in Tehran, Mohammadi et al. (2002), Bahrmand et al. (2000), and Seyed-Davood Mansoori et al. (2003) reported the resistance prevalence of 46.6, 6.2, and 28% to isoniazid, respectively. The reason of this difference could be due to small sample size in first study (Mohammadi et al., 2002). In Mohamadi et al. study, M. tuberculosis was isolated from referral patients that can be the reason of high resistance to isoniazid in this study. Two studies have been conducted in 2010–2011, in Velayati et al. (2014) reports, the prevalence of isoniazid resistance was 6% and in Tahmasebi et al. (2012) this level was 70.1%, that the reason of this difference could be used to strains that isolate from patients with treatment failure in second study. Over the years the increasing level of resistance to isoniazid might be due to incomplete treatment. The failure treatment can be for two reason, inappropriate drug prescribing and drug usage regularly and on time. This process has been seen about rifampin resistance. During 2000 and 2008, Shamaei et al. (2009) and Merza et al. (2011) reported the highest prevalence of rifampin resistance. These studies have been done in Masih daneshvari Hospital that is a referral hospital in Iran and most of the patients refer to this hospital due to treatment failure. As mentioned in results, high prevalence of resistance to INH (20%) and RIF (12%) was seen in Sistan va Blochestan due to vicinity of this province to Afghanistan and Pakistan. Rifampin and isoniazid resistance is a surrogate marker for MDR- M. tuberculosis. Most of studies reporting isoniazid and rifampin resistance were conducted in Tehran. These studies report the highest prevalence of resistance to INH and RIF (22%). In Masjedi et al. (2008) study, among 77% Iranian and 23% afghan cases, 131 Iranian (65%), and 13 afghan cases (22%) were susceptible to all 4 drugs tested and 72 patients (28%) were MDR-TB case. Notably, 38 MDR-TB cases (52.7%) were isolated from afghan immigrants. Twenty patients (47%) had mono drug resistant strains (nine were INH, seven SM, three RF, and one EMB mono resistant) and 22 (52%) had combined resistance.
In Al-Akhali et al. (2007) study that was performed in Yemen, the prevalence of resistance to any one of the four drugs was 9.8% in the new cases and 17.4% in the previously treated cases. The prevalence of MDR-TB, defined as TB cases excreting M. tuberculosis resistant at least to INH and RIF, was 3%. In Ayaz et al. (2012) study that conducted in Pakistan, resistance to one or more of the first-line anti-TB drugs was noted in 23% of patients. The INH resistance was 9% in untreated and 28.5% in treated patients. Resistance to other first-line drugs was as follow: SM 17%, EMB 5%, and RIF 5%.
Some limitations of this systematic review should be considered for results interpretation. First, few studies have been conducted in our country about resistance of TB to first and second line-drugs. Second, the probable influence of age, sex, ethnicity, economic level, and life styles could not be analyzed due to the limited information obtained from the original articles. Third, most included studies were hospital-based rather than population based which makes the results more prone to potential selection bias. Because of the small number of studies particularly in other cities except Tehran, we cannot judge about the prevalence of resistance against first-line anti-tuberculosis drugs properly. However, in recent years, emergence and spread of MDR-TB threaten the TB control strategy. In many law-and middle-income countries, due to inadequate laboratory capacity, most of the patients with MDR-TB are not diagnosed. Treatment of these cases mostly failed and significant expenditure of health care resources is needed.
In conclusion, this systematic review summarized the prevalence and distribution of first-line anti-tubercular drug resistance of M. tuberculosis in Iran. Our results suggest effective strategies to minimize the acquired drug resistance, to control the transmission of resistance and improve the diagnosis measures for TB control in our country.
An important element in gaining control of this epidemic is developing an understanding of the molecular basis of resistance to the most important anti-tuberculosis drugs. Since the mechanism of action of rifampin is to inhibit mycobacterial transcription by targeting DNA-dependent RNA polymerase (Somoskovi et al., 2001), routine application of rapid molecular tests in the clinical management of drug-resistant tuberculosis is highly recommended.
On the other hand, INH is activated by the mycobacterial enzyme KatG, a multifunctional catalase-peroxidase that has other activities including peroxynitritase and NADH oxidase. Therefore, inhibition of both cell wall lipid, and nucleic acid synthesis by INH-NAD and INH-NAPD adducts together with respiratory inhibition by INH-derived NO can provides a potent antituberculosis cocktail. Some strategies such as developing agents that produce the isonicotinoyl radical, screening for molecules which increase mycobacterial levels of NAD+ or NADP+ for in co-administration use with INH, to designing of more drug-like molecules using the structure of INH-NAD adducts to inhibit specifically mycobacterial enzymes; and developing of mycobacterial enzyme inhibitors which can inactivate INH might be useful to control INH-TB resistance propagation (Timmins and Deretic, 2006).
Author contributions
All authors listed, have made substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Al-Akhali A., Ohkado A., Fujiki A., Mitarai S., Yamada N., Masui T., et al. (2007). Nationwide survey on the prevalence of anti-tuberculosis drug resistance in the Republic of Yemen, 2004. Int. J. Tuberc. Lung Dis. 11, 1328–1333. [PubMed] [Google Scholar]
- Ali I. F. A., Babak F., Fazlollah M. S., Nematollah J. J. (2014). Rapid detection of MDR-Mycobacterium tuberculosis using modified PCR-SSCP from clinical Specimens. Asian Pac. J. Trop. Biomed. 4(Suppl. 1), S165–S170. 10.12980/APJTB.4.2014C1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asgharzadeh M., Jahantabi A. R., Shahbabian K., Nahaei M. R., Rafi A. N. (2007). Detection of Ethambutol - resistant Mycobacterium tuberculosis strains by MAS-PCR method and comparison with Proportion. J. Mazandaran Univ. Med. Sci. E. 17, 50–56. [Google Scholar]
- Asgharzadeh M., Kafil H. S., Pourostadi M. (2014). Limited transmission of multidrug-resistant tuberculosis in East Azarbaijan, Iran. Beni-Suef Univ. J. Basic Appl. Sci. 3, 254–259. 10.1016/j.bjbas.2014.11.004 [DOI] [Google Scholar]
- Ayaz A., Hasan Z., Jafri S., Inayat R., Mangi R., Channa A. A., et al. (2012). Characterizing Mycobacterium tuberculosis isolates from Karachi, Pakistan: drug resistance and genotypes. Int. J. Infect. Dis. 16, e303–e309. 10.1016/j.ijid.2011.12.015 [DOI] [PubMed] [Google Scholar]
- Babamahmoodi F., Mahdavi M. R., Jalali H., Talebi B., Roshan P., Mahdavi M. (2014). Evaluation of gene mutations involved in drug resistance in Mycobacterium tuberculosis strains derived from tuberculosis patients in Mazandaran, Iran, 2013. Int. J. Mol. Cell. Med. 3, 190. [PMC free article] [PubMed] [Google Scholar]
- Bahrami S., Bahrmand A. R., Safarpour E., Masoumi M., Saifi M. (2013). Detection of ethambutol-resistant associated mutations in Myco-bacterium tuberculosis Isolates from Iran using multiplex allele-spe-cific PCR. J. Med. Microbiol. 1, 42. [Google Scholar]
- Bahrmand A. R., Titov L. P., Tasbiti A. H., Yari S., Graviss E. A. (2009). High-level rifampin resistance correlates with multiple mutations in the rpoB gene of pulmonary tuberculosis isolates from the Afghanistan border of Iran. J. Clin. Microbiol. 47, 2744–2750. 10.1128/JCM.r00548-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrmand A. R., Velayati A. A., Bakayev V. V. (2000). Treatment monitoring and prevalence of drug resistance in tuberculosis patients in Tehran. Int. J. Tuberc. Lung Dis. 4, 544–549. [PubMed] [Google Scholar]
- Bostanabad S. Z., Bahrmand A., Titov L. P., Taghikhani M. (2007). Identification of mutations in the rpoB encoding the RNA polymerase beta subunit in rifampicine-resistant Mycobacterium tuberculosis strains from Iran. Tuberk Toraks. 55, 370–377. [PubMed] [Google Scholar]
- Bostanabad S. Z., Nojoumi S. A., Jabbarzadeh E., Shekarabei M., Hoseinaei H., Rahimi M. K., et al. (2011). High level isoniazid resistance correlates with multiple mutation in the katG encoding catalase proxidase of pulmonary tuberculosis isolates from the frontier localities of Iran. Tuberkuloz ve Toraks. 59, 27. 10.5578/tt.761 [DOI] [PubMed] [Google Scholar]
- Derakhshani Nezhad Z. S. F., Farnia P., Deilami Khiabani Z., Ramazanzadeh R., Kazempoor M., Masjedi M. R., et al. (2012). Identification and genetic diversity of Ethambutol resistant strains of Mycobacterium tuberclusis by Allelic-specific PCR and spologiotyping. J. Ardabil. Univ. Med. Sci. 12, 248–255. [Google Scholar]
- Dinmohammadi F., Farnia P., Biglari A., Kazempoor M., Ramazanzadeh R., Masjedi M. R., et al. (2010). Identification of the mutations related to resistance of Mycobacterium tuberculosis to isoniazid by use of PCR-RFLP in TB patients. Sci. J. Kurdistan Univ. Med. Sci. 14, 1–9. [Google Scholar]
- Farazi A., Sofian M., Zarrinfar N., Katebi F., Hoseini S. D., Keshavarz R. (2013). Drug resistance pattern and associated risk factors of tuberculosis patients in the central province of Iran. Caspian J. Intern. Med. Fall 4, 785–789. [PMC free article] [PubMed] [Google Scholar]
- Farnia P., Masjedi M. R., Mohammadi F., Tabarsei P., Mohammadzadeh A. R., Baghei P., et al. (2008a). Colorimetric detection of multidrug-resistant or extensively drug-resistant tuberculosis by use of malachite green indicator dye. J. Clin. Microbiol. 46, 796–799. 10.1128/JCM.01435-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnia P., Masjedi M. R., Varahram M., Mirsaeidi M., Ahmadi M., Khazampour M., et al. (2008b). The recent-transmission of Mycobacterium tuberculosis strains among Iranian and Afghan relapse cases: a DNA-fingerprinting using RFLP and spoligotyping. BMC Infect. Dis. 8:109. 10.1186/1471-2334-8-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeili M., Darban−Sarokhalil D., Fooladi A. A. I., Javadpour S., Hashemi A., Siavoshi F., et al. (2013). Spoligotyping and drug resistance patterns of Mycobacterium tuberculosis isolates from five provinces of Iran. Microbiologyopen 2, 988–996. 10.1002/mbo3.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan Heidarnejad M., Nagili B. (2001). Primary resistance of Mycobacterium tuberculosis to isoniazid, streptomycin, rifampin, and ethambutol in pulmonary tuberculosis. Arch. Irn. Med. 4, 1–4. [Google Scholar]
- Izadi B., Kanani M., Khazaei S., Madani S. H. (2011). Determination of Rifampin and Isoniazid resistant Mycobacterium tuberculosis in Kermanshah (2006-2008). J. Kermanshah Univ. Med. Sci. 15, 145–147. [Google Scholar]
- Javid S. N., Ghaemi A., Amirmozaffari N., Rafiee S., Moradi A., Dadgar T. (2009). Detection of Isoniazid and Rifampin Resistant Strain of Mycobacterium Tuberculosis Isolated from patients in Golestan province (North of Iran). Med. Lab. J. 3, 1–8. [Google Scholar]
- Khosravi A. D., Dezfulian A., Alavi S. M. (2006). Detection of Isoniazid and Rifampin resistant Mycobacterium tuberculosis isolated from tuberculosis patients using conventional method and PCR. Pak. J. Med. Sci. 22, 47. [Google Scholar]
- Livani S., Mirinargesi M., Nemati-Shoja E., Rafiei S., Taziki M., Tabarraei A. (2011). Prevalence of multidrug resistant Mycobacterium tuberculosis by Mycobacteria growth indicator tube in Golestan province, North of Iran. Med. Lab. J. 5, 7–14. [Google Scholar]
- Marjani M., Baghaei P., Tabarsi P., Shamaei M., Mansouri D., Masjedi M., et al. (2012). Drug resistance pattern and outcome of treatment in recurrent episodes of tuberculosis. East. Mediterr. Health J. 18, 957–961. [DOI] [PubMed] [Google Scholar]
- Masjedi M. R., Farnia P., Sorooch S., Pooramiri M. V., Mansoori S. D., Zarifi A. Z., et al. (2006). Extensively drug-resistant tuberculosis: 2 years of surveillance in Iran. Clin. Infect. Dis. 43, 841–847. 10.1086/507542 [DOI] [PubMed] [Google Scholar]
- Masjedi M. R., Varahram M., Mirsaeidi M., Ahmadi M., Khazampour M., Tabarsi P., et al. (2008). The recent-transmission of Mycobacterium tuberculosis strains among Iranian and Afghan Relapse Cases: a DNA-fingerprinting using RFLP and spoligotyping. BMC Infect. Dis. 8:09. 10.1186/1471-2334-8-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merza M. A., Farnia P., Tabarsi P., Khazampour M., Masjedi M. R., Velayati A. A. (2011). Anti-tuberculosis drug resistance and associated risk factors in a tertiary level TB center in Iran: a retrospective analysis. J. Infect. Dev. Ctries. 5, 511–519. 10.3855/jidc.1259 [DOI] [PubMed] [Google Scholar]
- Mirsaeidi M. S., Tabarsi P., Farnia P., Ebrahimi G., Morris M. W., Masjedi M. R., et al. (2007). Trends of drug resistant Mycobacterium tuberculosis in a tertiary tuberculosis center in Iran. Saudi Med. J. 28, 544–550. [PubMed] [Google Scholar]
- Moadab S. R., Rafi A. (2006). Susceptibility of Mycobacterium tuberculosis and nontuberculous mycobacteria to kanamaycin and amikacin. Pharm. Sci. 4, 5. [Google Scholar]
- Moaddab S. R., Farajnia S., Kardan D., Zamanlou S., Alikhani M. Y. (2011). Isoniazid MIC and KatG gene mutations among Mycobacterium tuberculosis Isolates in Northwest of Iran. Iran J. Basic Med. Sci. 14, 540–545. [PMC free article] [PubMed] [Google Scholar]
- Mohajeri P., Norozi B., Atashi S., Farahani A. (2014). Anti tuberculosis drug resistance in west of Iran. J. Global Infect. Dis. 6, 114. 10.4103/0974-777X.138506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohajeri P., Sadri H., Farahani A., Norozi B., Atashi S. (2015). Frequency of mutations associated with rifampicin resistance in Mycobacterium tuberculosis strains isolated from patients in West of Iran. Microb. Drug Resist. 21, 315–319. 10.1089/mdr.2014.0075 [DOI] [PubMed] [Google Scholar]
- Mohammadi F. D. (2012). Identifying Mycobacterium tuberculosis resistance to rifampin by multiplex specific PCR method. Qom Univ. Med. Sci. J. 5, 19–24. [Google Scholar]
- Mohammadi F., Farnia P., Mohammad-Taheri Z., Zia-Zarifi A. (2002). Recovery of mycobacteria from clinical specimens and assessing drug susceptibility test of Mycobacterium Tuberculosis specimens by mycobacteria growth indicator tube (MGIT). Tanaffos 1, 35–44. [Google Scholar]
- Mohammadzadeh A., Farnia P., Rashed T., Ghazvini K., Behdani M., Ghanaat J. (2007). Use of alamar blue assay as a colorimetric method for detection of multidrug-resistant Mycobacterium tuberculosis. Q. Horizon Med. Sci. 13, 47–51. [DOI] [PubMed] [Google Scholar]
- Moniri R. S. R. (2001). Mousavi sydghlambas. Evaluation of drug susceptibility of Mycobacterium tuberculosis isolated from clinical specimen in Kashan. J. Med. Sci. Health Servi. Yazd. 9, 67–70. [Google Scholar]
- Namaei M. H., Sadeghian A., Naderinasab M., Ziaee M. (2006). Prevalence of primary drug resistant Mycobacterium tuberculosis in Mashhad, Iran. Indian J. Med. Res. 124, 77–80. [PubMed] [Google Scholar]
- Nasiri M. J., Rezaei F., Zamani S., Darban-Sarokhalil D., Fooladi A. A., Shojaei H., et al. (2014). Drug resistance pattern of Mycobacterium tuberculosis isolates from patients of five provinces of Iran. Asian Pac. J. Trop. Med. 7, 193–196. 10.1016/S1995-7645(14)60019-5 [DOI] [PubMed] [Google Scholar]
- Organization W. H. (2000). Anti-Tuberculosis Drug Resistance in the World/the WHO/IUATLD Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Report 2, Prevalence and trends. [Google Scholar]
- Organization W. H. (2011). Global Health Observatory Data Repository. Available online at: http://appswhoint/ghodata
- Organization W. H. (2013). Global Tuberculosis Report 2013. World Health Organization. [Google Scholar]
- Ostadzadeh F., Hashemi M., Zakerbostanabad S., Rahimi M. K., Nouri S., Ghalami M. (2011). Identification of polymorphism in rpoB gene (a marker of gene resistance to rifampin drug) in Mycobacterium tuberculosis isolated from patients in Tehran. 2. Experimental 21, 24–31. [Google Scholar]
- Pourhajibagher M., Nasrollahi M., Musavi S. R., Esboei B., Ghorbani Pashakolaei A. (2012). Drug resistance in Mycobacterium tuberculosis isolates to isoniazid and rifampin. J. Babol Univ. Med. Sci. 14, 66–72. [Google Scholar]
- Rafi A. A. N., Moaddab S. R., Radmehr R. (2009). Drug resistance study of Mycobacterium tuberculosis strains and mycobacteria other than tubercle bacilli strains to ofloxacin and ciprofloxacin isolated from patients admitted to research center for TB and pulmonary diseases of Tabriz. Pharm. Sci. 15, 241–246. [Google Scholar]
- Rieder H. L., Chonde T. M., Myking H., Urbanczik R., Laszlo A., Kim S., et al. (1998). The Public Health Service National Tuberculosis Reference Laboratory and the National Laboratory Network; Minimum Requirements, Role and Operation in a Low-Income Country. International Union against Tuberculosis and Lung Disease; (Paris: IUATLD: ). [Google Scholar]
- Roshdi M. M., Moadab S. R. (2009). Drug susceptibility pattern of Mycobacterium tuberculosis strains to first and second line drugs in Tabriz, Iran. Iran. J. Med. Microbiol. 3, 18–24. [Google Scholar]
- Sani A. T., Shakiba A., Salehi M., Taghanaki H. R. B., Fard S. F. A., Ghazvini K. (2015). Epidemiological characterization of drug resistance among Mycobacterium tuberculosis Isolated from Patients in Northeast of Iran during (2012–2013). Biomed. Res. Int. 2015:747085. 10.1155/2015/747085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyed-Davood Mansoori M., Arami S., Zahra Mirabolhasani M., Velayati A.-A. (2003). The pattern of drug resistance among newly diagnosed and old cases of pulmonary tuberculosis in nritld. Arch. Iran. Med. 6, 255–260. [Google Scholar]
- Shamaei M., Marjani M., Chitsaz E., Kazempour M., Esmaeili M., Farnia P., et al. (2009). First-line anti-tuberculosis drug resistance patterns and trends at the national TB referral center in Iran–eight years of surveillance. Int. J. Infect. Dis. 13, e236–e240. 10.1016/j.ijid.2008.11.027 [DOI] [PubMed] [Google Scholar]
- Sheikh Ghomi S., Farnia P., Darbouy M. (2014). Detection of rpoB, inhA and katG genes mutations in clinical isolates of Mycobacterium tuberculosis by Real-Time PCR based on Taqman and HRM Assays. J. Ardabil. Univ. Med. Sci. 14, 147–157. [Google Scholar]
- Sheikholslami M. F., Farnia P., Tabarsi P., Merza M. A., Amiri M. V. P., Mohammadi F., et al. (2011). Comparison of polymerase chain reaction single-strand conformation polymorphism with DNA sequencing to detect drug resistance of Mycobacterium tuberculosis isolates. Arch. Clin. Infect. Diseases. 6, 66–70. [Google Scholar]
- Somoskovi A., Parsons L. M., Salfinger M. (2001). The molecular basis of resistance to isoniazid, rifampin, and pyrazinamide in Mycobacterium tuberculosis. Respir Res. 2, 164–168. 10.1186/rr54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghavi K., Farnia P., Varahram M., Sheikhoslami F. M., Ahmadi M., Kazempoor M., et al. (2011). Rapid detection of isoniazid resistance in Mycobacterium tuberculosis by a single multiplex allele-specific polymerase chain reaction assay. Cell J. Summer 13, 97–102. [PMC free article] [PubMed] [Google Scholar]
- Taherahmadi M., Ahmadi A., Shojapour M., Nazari R., Moadab S. R., Arjomanzadegan M. (2013). Rapid detection of susceptibility to ethambutol in clinical Mycobacterium tuberculosis isolated from tuberculosis patients by pcr- rflp. URMIA Med. J. 24, 566–576. [Google Scholar]
- Taheri B., Mirab S. S., Paryan M., Ghaznavi R. (2013). Rapid detection of rifampicin resistant Mycobacterium tuberculosis by using real time PCR. AMUJ 16, 50℃57. 27295919 [Google Scholar]
- Tahmasebi P., Farnia P., Sheikholslami F., Velayati A. (2012). Rapid identification of extensively and extremely drug resistant tuberculosis from multidrug resistant strains; using PCR-RFLP and PCR-SSCP. Iran. J. Microbiol. 4, 165–170. [PMC free article] [PubMed] [Google Scholar]
- Tasbiti A. R., Yari S., Karimi A., Fateh A., Saifi M., Jabarzadeh E., et al. (2011). Survey of extensively drug-resistant tuberculosis XDR-TB) in Iran-Tehran: A retrospective study. African J. Microbiol. Res. 5, 3795–3800. 10.5897/AJMR11.710 [DOI] [Google Scholar]
- Timmins G. S., Deretic V. (2006). Mechanisms of action of isoniazid. Mol. Microbiol. 62, 1220–1227. 10.1111/j.1365-2958.2006.05467.x [DOI] [PubMed] [Google Scholar]
- Varahram M., Nasiri M. J., Farnia P., Mozafari M., Velayati A. A. (2014). A retrospective analysis of isoniazid-monoresistant Tuberculosis: among Iranian pulmonary Tuberculosis patients. Open Microbiol. J. 8:1. 10.2174/1874285801408010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshochi M., Rastgar M. H., Raffi A., Nagili B. (2006). In vitro susceptibility of Mycobacterium tuberculosis to amoxicillin–clavulanate. Iran. J. Clin. Infect. Dis. 1, 121–125. [Google Scholar]
- Velayati A. A., Farnia P., Mozafari M., Sheikholeslami M. F., Karahrudi M. A., Tabarsi P., et al. (2014). High prevelance of rifampin-monoresistant tuberculosis: a retrospective analysis among Iranian pulmonary tuberculosis patients. Am. J. Trop. Med. Hyg. 90, 99–105. 10.4269/ajtmh.13-0057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Li X., Zhou F., Jin Q., Gao L. (2011). Prevalence of drug-resistant tuberculosis in mainland China: systematic review and meta-analysis. PLoS ONE 6:e20343. 10.1371/journal.pone.0020343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakerbostanabad S., Titov L. P., Bahrmand A. R. (2008). Frequency and molecular characterization of isoniazid resistance in katG region of MDR isolates from tuberculosis patients in southern endemic border of Iran. Infect. Genet. Evol. 8, 15–19. 10.1016/j.meegid.2007.09.002 [DOI] [PubMed] [Google Scholar]
- Zamanlou S., Farajnia S., Moadab R., Akhi M. (2009). Rapid diagnosis of Isoniazid resistant Mycobacterium Tuberculosis, isolated from East Azerbaijanian patients by PCR-RFLP method. Pharmaceutical Sci. 15, 263–268. [Google Scholar]