Abstract
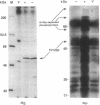
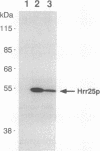
The Saccharomyces cerevisiae HRR25 gene was identified as a regulator of DNA strand-break repair. HRR25 encodes a protein kinase that is closely related to bovine casein kinase I (CKI). CKI is a ubiquitous multipotential protein kinase. Rabbit polyclonal antibodies that recognize and immunoprecipitate Hrr25p have been generated and an immune complex protein kinase assay has been developed. The reaction depends upon HRR25 and shows that Hrr25p uses casein as a substrate. The identity between Hrr25p and bovine CKI suggests that Hrr25p is a yeast isoform of the CKI family and that CKIs may play a role in regulating DNA metabolism.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen-Wu J. L., Padmanabha R., Glover C. V. Isolation, sequencing, and disruption of the CKA1 gene encoding the alpha subunit of yeast casein kinase II. Mol Cell Biol. 1988 Nov;8(11):4981–4990. doi: 10.1128/mcb.8.11.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chijiwa T., Hagiwara M., Hidaka H. A newly synthesized selective casein kinase I inhibitor, N-(2-aminoethyl)-5-chloroisoquinoline-8-sulfonamide, and affinity purification of casein kinase I from bovine testis. J Biol Chem. 1989 Mar 25;264(9):4924–4927. [PubMed] [Google Scholar]
- Dailey D., Schieven G. L., Lim M. Y., Marquardt H., Gilmore T., Thorner J., Martin G. S. Novel yeast protein kinase (YPK1 gene product) is a 40-kilodalton phosphotyrosyl protein associated with protein-tyrosine kinase activity. Mol Cell Biol. 1990 Dec;10(12):6244–6256. doi: 10.1128/mcb.10.12.6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould K. L., Hunter T. Platelet-derived growth factor induces multisite phosphorylation of pp60c-src and increases its protein-tyrosine kinase activity. Mol Cell Biol. 1988 Aug;8(8):3345–3356. doi: 10.1128/mcb.8.8.3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L., Ptashne M. Fusion of Escherichia coli lacZ to the cytochrome c gene of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2199–2203. doi: 10.1073/pnas.78.4.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M. Protein kinase catalytic domain sequence database: identification of conserved features of primary structure and classification of family members. Methods Enzymol. 1991;200:38–62. doi: 10.1016/0076-6879(91)00126-h. [DOI] [PubMed] [Google Scholar]
- Herman P. K., Stack J. H., DeModena J. A., Emr S. D. A novel protein kinase homolog essential for protein sorting to the yeast lysosome-like vacuole. Cell. 1991 Jan 25;64(2):425–437. doi: 10.1016/0092-8674(91)90650-n. [DOI] [PubMed] [Google Scholar]
- Hoekstra M. F., DeMaggio A. J., Dhillon N. Genetically identified protein kinases in yeast. II: DNA metabolism and meiosis. Trends Genet. 1991 Sep;7(9):293–297. doi: 10.1016/0168-9525(91)90311-D. [DOI] [PubMed] [Google Scholar]
- Hoekstra M. F., Demaggio A. J., Dhillon N. Genetically identified protein kinases in yeast. I: Transcription, translation, transport and mating. Trends Genet. 1991 Aug;7(8):256–261. doi: 10.1016/0168-9525(91)90325-K. [DOI] [PubMed] [Google Scholar]
- Hoekstra M. F., Liskay R. M., Ou A. C., DeMaggio A. J., Burbee D. G., Heffron F. HRR25, a putative protein kinase from budding yeast: association with repair of damaged DNA. Science. 1991 Aug 30;253(5023):1031–1034. doi: 10.1126/science.1887218. [DOI] [PubMed] [Google Scholar]
- Hoekstra M. F., Seifert H. S., Nickoloff J., Heffron F. Shuttle mutagenesis: bacterial transposons for genetic manipulations in yeast. Methods Enzymol. 1991;194:329–342. doi: 10.1016/0076-6879(91)94025-8. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazwinski S. M. CDC7-dependent protein kinase activity in yeast replicative-complex preparations. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2101–2105. doi: 10.1073/pnas.85.7.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazwinski S. M. Preparation of extracts from yeast. Methods Enzymol. 1990;182:154–174. doi: 10.1016/0076-6879(90)82015-t. [DOI] [PubMed] [Google Scholar]
- Johnston L. H., Eberly S. L., Chapman J. W., Araki H., Sugino A. The product of the Saccharomyces cerevisiae cell cycle gene DBF2 has homology with protein kinases and is periodically expressed in the cell cycle. Mol Cell Biol. 1990 Apr;10(4):1358–1366. doi: 10.1128/mcb.10.4.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerner T. J., Hill J. E., Myers A. M., Tzagoloff A. High-expression vectors with multiple cloning sites for construction of trpE fusion genes: pATH vectors. Methods Enzymol. 1991;194:477–490. doi: 10.1016/0076-6879(91)94036-c. [DOI] [PubMed] [Google Scholar]
- Krek W., Maridor G., Nigg E. A. Casein kinase II is a predominantly nuclear enzyme. J Cell Biol. 1992 Jan;116(1):43–55. doi: 10.1083/jcb.116.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lee J. M., Greenleaf A. L. CTD kinase large subunit is encoded by CTK1, a gene required for normal growth of Saccharomyces cerevisiae. Gene Expr. 1991 May;1(2):149–167. [PMC free article] [PubMed] [Google Scholar]
- Lemontt J. F. Pathways of ultraviolet mutability in Saccharomyces cerevisiae. III. Genetic analysis and properties of mutants resitant to ultraviolet-induced forward mutation. Mutat Res. 1977 May;43(2):179–204. doi: 10.1016/0027-5107(77)90003-3. [DOI] [PubMed] [Google Scholar]
- Lerch K., Muir L. W., Fischer E. H. Purification and properties of a yeast protein kinase. Biochemistry. 1975 May 6;14(9):2015–2023. doi: 10.1021/bi00680a032. [DOI] [PubMed] [Google Scholar]
- Lindberg R. A., Hunter T. cDNA cloning and characterization of eck, an epithelial cell receptor protein-tyrosine kinase in the eph/elk family of protein kinases. Mol Cell Biol. 1990 Dec;10(12):6316–6324. doi: 10.1128/mcb.10.12.6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lörincz A. T., Reed S. I. Primary structure homology between the product of yeast cell division control gene CDC28 and vertebrate oncogenes. Nature. 1984 Jan 12;307(5947):183–185. doi: 10.1038/307183a0. [DOI] [PubMed] [Google Scholar]
- Padmanabha R., Chen-Wu J. L., Hanna D. E., Glover C. V. Isolation, sequencing, and disruption of the yeast CKA2 gene: casein kinase II is essential for viability in Saccharomyces cerevisiae. Mol Cell Biol. 1990 Aug;10(8):4089–4099. doi: 10.1128/mcb.10.8.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pausch M. H., Kaim D., Kunisawa R., Admon A., Thorner J. Multiple Ca2+/calmodulin-dependent protein kinase genes in a unicellular eukaryote. EMBO J. 1991 Jun;10(6):1511–1522. doi: 10.1002/j.1460-2075.1991.tb07671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson L. C., Hubbard E. J., Graves P. R., DePaoli-Roach A. A., Roach P. J., Kung C., Haas D. W., Hagedorn C. H., Goebl M., Culbertson M. R. Yeast casein kinase I homologues: an essential gene pair. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):28–32. doi: 10.1073/pnas.89.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowles J., Slaughter C., Moomaw C., Hsu J., Cobb M. H. Purification of casein kinase I and isolation of cDNAs encoding multiple casein kinase I-like enzymes. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9548–9552. doi: 10.1073/pnas.88.21.9548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siede W., Robinson G. W., Kalainov D., Malley T., Friedberg E. C. Regulation of the RAD2 gene of Saccharomyces cerevisiae. Mol Microbiol. 1989 Dec;3(12):1697–1707. doi: 10.1111/j.1365-2958.1989.tb00155.x. [DOI] [PubMed] [Google Scholar]
- Stern D. F., Zheng P., Beidler D. R., Zerillo C. Spk1, a new kinase from Saccharomyces cerevisiae, phosphorylates proteins on serine, threonine, and tyrosine. Mol Cell Biol. 1991 Feb;11(2):987–1001. doi: 10.1128/mcb.11.2.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitz T., Eli D., Penner M., Bakhanashvili M., Naiman T., Timme T. L., Wood C. M., Moses R. E., Canaani D. Expression of the cDNA for the beta subunit of human casein kinase II confers partial UV resistance on xeroderma pigmentosum cells. Mutat Res. 1990 Jul;236(1):85–97. doi: 10.1016/0921-8777(90)90036-5. [DOI] [PubMed] [Google Scholar]
- Timmons T. M., Dunbar B. S. Protein blotting and immunodetection. Methods Enzymol. 1990;182:679–688. doi: 10.1016/0076-6879(90)82053-5. [DOI] [PubMed] [Google Scholar]
- Toda T., Cameron S., Sass P., Zoller M., Wigler M. Three different genes in S. cerevisiae encode the catalytic subunits of the cAMP-dependent protein kinase. Cell. 1987 Jul 17;50(2):277–287. doi: 10.1016/0092-8674(87)90223-6. [DOI] [PubMed] [Google Scholar]
- Toyn J. H., Araki H., Sugino A., Johnston L. H. The cell-cycle-regulated budding yeast gene DBF2, encoding a putative protein kinase, has a homologue that is not under cell-cycle control. Gene. 1991 Jul 31;104(1):63–70. doi: 10.1016/0378-1119(91)90465-n. [DOI] [PubMed] [Google Scholar]
- Tuazon P. T., Traugh J. A. Casein kinase I and II--multipotential serine protein kinases: structure, function, and regulation. Adv Second Messenger Phosphoprotein Res. 1991;23:123–164. [PubMed] [Google Scholar]
- Wang P. C., Vancura A., Mitcheson T. G., Kuret J. Two genes in Saccharomyces cerevisiae encode a membrane-bound form of casein kinase-1. Mol Biol Cell. 1992 Mar;3(3):275–286. doi: 10.1091/mbc.3.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]