Abstract
Introduction
Heart failure (HF) is a medical condition with a rapidly increasing incidence both in Taiwan and worldwide. The objective of the TSOC-HFrEF registry was to assess epidemiology, etiology, clinical management, and outcomes in a large sample of hospitalized patients presenting with acute decompensated systolic HF.
Methods
The TSOC-HFrEF registry was a prospective, multicenter, observational survey of patients presenting to 21 medical centers or teaching hospitals in Taiwan. Hospitalized patients with either acute new-onset HF or acute decompensation of chronic HFrEF were enrolled. Data including demographic characteristics, medical history, primary etiology of HF, precipitating factors for HF hospitalization, presenting symptoms and signs, diagnostic and treatment procedures, in-hospital mortality, length of stay, and discharge medications, were collected and analyzed.
Results
A total of 1509 patients were enrolled into the registry by the end of October 2014, with a mean age of 64 years (72% were male). Ischemic cardiomyopathy and dilated cardiomyopathy were diagnosed in 44% and 33% of patients, respectively. Coronary artery disease, hypertension, diabetes, and chronic renal insufficiency were the common comorbid conditions. Acute coronary syndrome, non-compliant to treatment, and concurrent infection were the major precipitating factors for acute decompensation. The median length of hospital stay was 8 days, and the in-hospital mortality rate was 2.4%. At discharge, 62% of patients were prescribed either angiotensin-converting enzyme-inhibitors or angiotensin receptor blockers, 60% were prescribed beta-blockers, and 49% were prescribed mineralocorticoid receptor antagonists.
Conclusions
The TSOC-HFrEF registry provided important insights into the current clinical characteristics and management of hospitalized decompensated systolic HF patients in Taiwan. One important observation was that adherence to guideline-directed medical therapy was suboptimal.
Keywords: Beta-blocker, Heart failure, Guideline, Renin-angiotensin system blocker, Taiwan, Treatment
INTRODUCTION
The high prevalence of acute decompensated heart failure (HF) is a major public health concern. Owing to the rapidly aging population and improved survival of patients who suffered from acute myocardial infarction and various heart diseases, the HF population is growing rapidly in Taiwan and around the world.1-3 In fact, it is believed that HF is one of the most common causes of hospitalization for elderly patients.4
Many HF patients have multiple comorbidities and present with acute exacerbation of chronic HF. Acute HF is characterized by rapid onset of signs and symptoms of HF secondary to cardiac decompensation, and requires urgent intervention. Acute decompensated HF can lead to further cardiac and renal injuries, which therefore contribute to deterioration of HF and increased patient mortality.
Evidence-based medical therapy is the most effective way to treat HF that reduces mortality and morbidity. In Europe and the United States, guidelines for the diagnosis and management of HF were first published in 1995. Later on, further guidelines were published by the European Society of Cardiology and the American Heart Association based on evidence-based medicine and clinical trials.5,6 In 2012, the Heart Failure Committee of the Taiwan Society of Cardiology (TSOC) published its own Guideline for the Diagnosis and Treatment of Heart Failure.7 However, there remains a wide gap between guideline-directed treatment and real world practice in HF management.7,8
Guideline-driven HF treatment and organization of HF care via multi-disciplinary approach have not received much attention and recognition in Taiwan. Currently, clinicians establish a HF clinical pathway in the newly developed Diagnosis Related Groups System to further monitor the quality of HF care. Continuous medical education sessions had been organized for physicians to familiarize them with TSOC’s updated clinical practice guideline. However, a nationwide registration program is required to further improve the awareness of HF management status in Taiwan. A registry collecting the clinical information of all HF patients will allow improved evaluation of the epidemiology and outcomes of real-world HF management.
The aim of the Taiwan Society of Cardiology – Heart Failure with reduced Ejection Fraction (TSOC-HFrEF) registry was to describe the epidemiology of patients admitted to hospital with systolic HF, and the diagnostic and therapeutic procedures used to treat HF patients in Taiwan.
METHODS
Study designs and study population
The TSOC-HFrEF registry was a prospective, multicenter, observational survey of patients presenting to 21 medical centers or teaching hospitals in Taiwan. The institutional review board of each hospital agreed to participate in the registry, and all participating hospitals were listed in the Supplement Material.
Patients being enrolled in this study were those presenting with either acute new-onset HF or acute decompensation of chronic HFrEF. The symptoms of these patients had to be deemed severe enough to warrant hospitalization, with typical HF signs presented. The patients’ left ventricular ejection fraction (LVEF) had to be documented as less than 40% before enrollment; the ejection fraction was obtained by either echocardiography or left ventriculography during the index hospitalization. There were no specific exclusion criteria, except all patients should to be over 18 years of age. Participating sites were encouraged to enroll patients as consecutively as possible. In hospital HF management including administration of drugs, diagnostic or therapeutic procedures were left to the discretion of the attending cardiologists. There were no specific protocols or recommendation for evaluation and management of HF during this observation study. The timeframe of TSOC-HFrEF registration was shown in Figure 1.
Figure 1.
Time frame of the TSOC-HFrEF Registry.
Data collection
Data were collected according to the typical case report form and were entered using a web-based electronic system by the hospital’s investigator or research coordinator after patients read the study information and signed informed consent. Data including demographic characteristics, medical history, primary etiology of HF, precipitating factors for HF hospitalization, presenting symptoms and signs at the hospital, diagnostic and treatment procedures, in-hospital mortality, length of stay, and discharge medications, were gathered from medical records.
The primary etiology of HF (single choice only) included ischemic heart disease, dilated cardiomyopathy, hypertensive cardiovascular disease, valvular heart disease, and tachycardia-related cardiomyopathy. The precipitating factors for HF hospitalization (multiple answers allowed) included: (1) myocardial ischemia or acute coronary syndrome (ACS); (2) non-compliant behavior; (3) use of non-steroid anti-inflammatory drug; (4) atrial fibrillation with rapid ventricular response; (5) ventricular arrhythmia; (6) bradyarrhythmia; (7) uncontrolled hypertension; (8) infection; (9) acute renal injury or dysfunction; (10) anemia; (11) chronic obstructive pulmonary disease or asthma with acute exacerbation. Regarding alcohol consumption, the patients were classified into: (1) patients who never consumed alcohol; (2) former drinkers; (3) current social drinkers; and (4) current heavy drinkers (defined as average daily consumption either more than 300 ml of wine or more than 60 ml of liquor per day).
Data were collected from the point of initial presentation to hospital until the patient was either discharged or died in-hospital. Patient confidentiality was preserved because direct patient identifiers were not collected. A longitudinal unique identifier code was created for each individual patient in the web-based electronic system.
Statistical analysis
All patients enrolled were included in the analysis. Descriptive summaries were presented for all patients, as well as for subgroups of patients. The quantitative data were expressed as the mean value ± standard deviation or as median and inter-quartile range (IQR); categorical variables were reported as percentages. The Student’s t-test or the Mann-Whitney U-test was used for comparisons between the continuous data, and a Chi-square test was used for comparisons between the categorical data. A Cox regression was performed to evaluate patient baseline characteristics, underlying diseases, precipitating factors and other clinical parameters for possible association with in-hospital mortality. Variables with a p value < 0.1 from the univariate model were selected for multivariate analysis using forward stepwise Cox regression to determine the independent predictors, presenting as hazard ratios (HR) and 95% confidence intervals (CI). A p value of < 0.05 was considered statistically significant. The statistical analyses were performed using SPSS Statistics 17.0 software (Chicago, IL, USA).
RESULTS
Baseline characteristics
From May 2013 to October 2014, 1509 patients (age 64.0 ± 15.8 years, 72.4% male) were included in the TSOC-HFrEF registry in 21 medical centers or teaching hospitals. The most common etiology of HF was ischemic cardiomyopathy (44.1%), followed by dilated cardiomyopathy (32.9%), and valvular heart disease (7.9%).
Co-morbidities were common in the study population. Among these patients, 43.6% had diabetes mellitus, 41.8% had coronary artery disease, 34.5% had hypertension, 31.6% had chronic kidney disease, and 11% had chronic obstructive pulmonary disease (COPD) or asthma. Detailed baseline characteristics are shown in Table 1.
Table 1. Baseline characteristics of TSOC-HFrEF registry patients.
| N = 1,509 | |
| Age (years) | 64.0 ± 15.8 |
| Male | 1093 (72.4%) |
| Smoking (ex/current) | 412 (27.3%)/350 (23.2%) |
| Alcohol consumption (former/current social/current heavy) | 191 (12.7%)/275 (18.2%)/48 (3.2%) |
| Hypertensive heart disease | 520 (34.5%) |
| Diabetes mellitus | 658 (43.6%) |
| Hypercholesterolemia | 336 (22.2%) |
| Atherogenic dyslipidemia | 308 (20.4%) |
| Previous hospitalization for heart failure | 609 (40.4%) |
| Coronary artery disease | 631 (41.8%) |
| Old myocardial infarction | 376 (24.9%) |
| Previous valvular surgery | 70 (4.6%) |
| Peripheral vascular disease | 100 (6.6%) |
| Stroke/transient ischemic attack | 138 (9.1%) |
| Atrial fibrillation | 393 (26.0%) |
| Permanent pacemaker | 48 (3.2%) |
| Implantable cardioverter-defibrillator | 25 (1.7%) |
| Cardiac resynchronization therapy pacemaker/defibrillator | 15 (1.0%)/14 (0.9%) |
| Chronic kidney disease | 476 (31.5%) |
| Chronic obstructive pulmonary disease/asthma | 166 (11.0%) |
| Cancer, receiving chemotherapy | 41 (2.7%) |
| Hyperthyroidism | 39 (2.6%) |
| Hypothyroidism | 31 (2.1%) |
| Depression | 23 (1.5%) |
| Sleep apnea | 40 (2.7%) |
Clinical presentations at hospital entry
At hospital entry, clinically “wet” presentations were common. Pulmonary congestion and pulmonary rales were detected in 63.5% of the patients, peripheral edema in 49.3%, pleural effusion in 28.8% and engorged jugular vein in 23.9% of the cases. On the contrary, “cold” presentations, such as peripheral hypoperfusion, were detected in 14.1%, and hypotension was detected in 9.9%; additionally, 5.1% of admitted patients were found to be confused or somnolent.
Vital signs, body weight and severity of HF at presentation were recorded at the emergency room and in the ward during hospitalization. Mean systolic blood pressure was 130.9 ± 27.6 mmHg, mean diastolic blood pressure was 80.7 ± 19.5 mmHg, and mean heart rate was 92.7 ± 22.2 mmHg. In total, 31.9% of registry patients had a systolic blood pressure of more than 140 mmHg, and only 2.7% had a systolic blood pressure of less than 90 mmHg at hospital admission. Mean admission body weight was 67.4 ± 17.0 kg and BMI was 25.4 ± 6.5 kg/m2. The percentages of moderate (NYHA Fc II) and severe HF (NYHA Fc III and IV) were 11.8% and 88.2%, respectively.
The precipitating factors for HF decompensation were identified, and myocardial ischemia or ACS was the most commonly identified cause for 31.3% of the patients. Other factors noted included noncompliance with diet or medication (24.6%), infection (17.0%), rapid atrial fibrillation (16.4%), renal dysfunction (14.5%), ventricular arrhythmia (5.2%) and uncontrolled hypertension (4.8%).
Specific examinations and laboratory studies
From the electrocardiogram obtained during hospital admission, sinus rhythm was noted in 66.7% of the cases, and atrial fibrillation was diagnosed in 26.7% of the patients. Prolonged QRS duration exceeding 120 ms was detected in 28.9% of the patients, including 8.8% with left bundle branch block pattern. Left ventricular hypertrophy by voltage criteria was noted in 19.0% of the patients. Echocardiogram was performed in 94.5% patients, the mean LVEF was 28.2 ± 8.2%, mean left ventricular end diastolic diameter was 60.8 ± 10.0 mm and mean left atrial diameter was 46.3 ± 8.7 mm. Moderate and severe mitral regurgitation were diagnosed in 39.6% and 12.7% of the patients, respectively. Moderate and severe tricuspid regurgitation were noted in 30.1% and 9.4% of the patients, respectively. Additionally, severe aortic regurgitation and aortic stenosis were diagnosed in 1.7% and 0.8% of the patients, respectively.
According to the estimated glomerular filtration rate (eGFR), 36.6% of the patients had stage III chronic kidney disease (eGFR 30-60 mL/min/m2), and 27.4% of the patients had stage IV/V chronic kidney disease (eGFR < 30 ml/min/m2). Anemia (hemoglobin level < 12 g/dL) was noted in 34.9% of the patients, and hyponatremia (serum sodium < 135 mEq/L) was noted in 19.9% of the patients. N-terminal pro-brain natriuretic peptide (NT-proBNP) and brain natriuretic peptide (BNP) were measured during hospitalization in 22.7% and 32.4% of the patients, respectively. The median value of NT-proBNP was 3534 pg/mL (IQR 1896~6338), and the median value of BNP was 1250 pg/mL (IQR 554~2487). Troponin-I was measured in 64.4% of the cases, with the median value of 0.09 ng/mL (IQR 0.04~0.32). Detailed laboratory findings are shown in Table 2.
Table 2. Laboratory findings of TSOC-HFrEF registry patients.
| N = 1,509 | Mean/Percentage | Median | IQR |
| Blood urine nitrogen (mg/dL) | 32.2 ± 23.3 | 24.7 | 17.3-38.0 |
| Creatinine (mg/dL) | 1.9 ± 1.8 | 1.3 | 1.0-1.9 |
| Estimated glomerular filtration rate (mL/min/m2) | 55.2 ± 39.6 | 48 | 27.9-73.7 |
| Stage III chronic kidney disease | 0.366 | ||
| Sage IV or V chronic kidney disease | 0.274 | ||
| Sodium (mEq/L) | 137.7 ± 4.6 | 138 | 135-140 |
| Potassium (mEq/L) | 4.0 ± 0.6 | 4 | 3.6-4.4 |
| Hemoglobin (g/dL) | 12.9 ± 2.4 | 13 | 11.2-14.7 |
| Anemia (hemoglobin < 12 g/dL) | 0.349 | ||
| Blood glucose (mg/dL) | 149.5 ± 81.7 | 125 | 102.5-168.5 |
| HbA1c (%) | 7.0 ± 1.7 | 6.5 | 5.9-7.6 |
| Total bilirubin (mg/dL) | 1.4 ± 2.0 | 1 | 42371 |
| Aspartate aminotransferase (U/L) | 87.3 ± 364.0 | 30 | 23-47 |
| Alanine aminotransferase (U/L) | 69.5 ± 224.4 | 26 | 17-45 |
| Free T4 (ng/mL) | 1.86 ± 3.11 | 1.27 | 1.08-1.51 |
| Thyroid stimulating hormone (μIU/mL) | 2.7 ± 4.3 | 1.7 | 0.9-3.0 |
| Brain natriuretic peptide (pg/mL) | 1749 ± 1589 | 1250 | 554-2487 |
| N-terminal pro-brain natriuretic peptide (pg/mL) | 4887 ± 5066 | 3534 | 1896-6338 |
| Troponin-I (μg/L) | 2.8 ± 21.9 | 0.09 | 0.04-0.32 |
| Uric acid (mg/dL) | 8.6 ± 2.9 | 8.5 | 6.6-10.3 |
| Ferritin (ng/mL) | 285 ± 367 | 152 | 84-365 |
| Iron (μg/dL) | 67.9 ± 52.4 | 54 | 36-85 |
| Total iron-binding capacity (μg/dL) | 307.3 ± 85.2 | 298 | 248-366 |
Specific management and hospital course
Intravenous diuretics were used in 62.6% of the patients. The median duration of intravenous diuretics was 4 days (IQR 2~7), and patients saw a median body weight change of -2.1 kg (IQR -4.7~-0.6). Intravenous nitroglycerin and inotropes were given in 27.1% and 36.5% of the patients, respectively. The most commonly used inotropic agents were dobutamine (20.4% of the patients) and dopamine (18.6% of the patients).
Median length of hospital stay was 8 days (IQR 5~15). During hospitalization, 33% of the patients admitted to the intensive care unit had median stay of 4 days (IQR 3~7). Mechanical ventilator support for respiratory failure was used in 12.9% of the cases, and intra-aortic balloon pump and extracorporeal membrane oxygenation were applied in 2.7% and 0.5% of the patients, respectively. A total of 55 patients (3.6%) received cardiac implantable electronic device implantation during hospitalization: 22 (1.4%) of them received permanent pacemaker implantation, 15 (1.0%) received implantable cardioverter defibrillator (ICD), 12 (0.8%) received cardiac resynchronization therapy pacemaker (CRT-P) and 6 received (0.4%) cardiac resynchronization therapy defibrillator (CRT-D). One patient received left ventricular assisted device during hospitalization, but this patient died 4 days after the device was implanted.
In-hospital mortality: characteristics and predictors
Thirty-six (2.4%) patients died during the index hospitalization, and 63.9% of all deaths were of cardiovascular origin. Compared with the surviving patients, deceased patients were significantly older (70.3 ± 15.4 vs. 63.9 ± 15.8 years, p = 0.017), and more likely to present with lower blood pressure (systolic blood pressure 116.2 ± 28.1 vs. 131.2 ± 27.3 mmHg, p = 0.005; diastolic blood pressure 72.0 ± 17.5 vs. 80.8 ± 19.2 mmHg, p = 0.035). Moreover, 17.1% of deceased patients received ICD or cardiac resynchronization therapy (CRT) implantation, which was significantly higher than that in survived patients (3.3%, p < 0.001). Concomitant acute kidney injury (37.1% vs. 14.0%, p = 0.049), and COPD/asthma exacerbation (14.3% vs. 3.1%, p = 0.003) were also more common in deceased patients. Laboratory findings showed that deceased patients had significantly worse renal function (eGFR 33.7 ± 25.3 vs. 55.7 ± 39.8 mL/min/m2, p = 0.03) than surviving patients.
Multivariate Cox-regression analysis identified four independent predictors for in-hospital mortality (Table 3): (1) low systolic blood pressure during hospital admission (hazard ratio 0.98, 95% CI 0.96~0.99, p = 0.008); (2) history of chronic kidney disease (hazard ratio 2.65, 95% CI 1.19~5.88, p = 0.017); (3) history of ICD or CRT implantation (hazard ratio 7.45, 95% CI 2.81~19.73, p < 0.001); and (4) concomitant COPD/asthma exacerbation (hazard ratio 4.90, 95% CI 1.27~18.95, p = 0.021).
Table 3. Predictors of in-hospital mortality in TSOC-HFrEF registry.
| Univariate analysis | Multivariate analysis | ||||
| Mortality | Alive | p value | HR (95% CI) | p value | |
| Baseline and hospitalization characteristics | |||||
| Age (y/o) | 70.3 ± 15.4 | 63.9 ± 15.8 | 0.017 | ||
| Systolic blood pressure (mmHg) | 116.2 ± 28.1 | 131.2 ± 27.3 | 0.005 | 0.98 (0.96-0.99) | 0.008 |
| Diastolic blood pressure (mmHg) | 72.0 ± 17.5 | 80.8 ± 19.2 | 0.035 | ||
| Past and personal history | |||||
| Chronic kidney disease | 62.9% | 30.8% | 0.009 | 2.65 (1.19-5.88)0 | 0.017 |
| COPD/asthma | 20.0% | 10.8% | 0.008 | ||
| Hypothyroidism | 5.7% | 2.0% | 0.067 | ||
| Previous ICD/CRT implantation | 17.1% | 3.3% | < 0.001 | 7.45 (2.81-19.73) | < 0.001 |
| Coexisting problems during index hospitalization | |||||
| Non-compliance behavior | 97.1% | 74.9% | 0.056 | ||
| Acute kidney injury | 37.1% | 14.0% | 0.049 | ||
| COPD/asthma with acute exacerbation | 14.3% | 3.1% | 0.003 | 4.90 (1.27-18.95) | 0.021 |
| Electrocardiography | |||||
| QTc interval (msec) | 483 ± 55 | 470 ± 48 | 0.069 | ||
| Laboratory studies | |||||
| eGFR (mL/min/m2) | 33.7 ± 25.3 | 55.7 ± 39.8 | 0.03 | ||
| Specific managements | |||||
| Mechanical ventilator | 63.6% | 12.1% | 0.03 | ||
| Extracorporeal membrane oxygenation | 9.1% | 0.3% | 0.081 |
COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; eGFR, estimated glomerular filtration rate; ICD, implantable cardioverter defibrillator.
Pharmacological treatment at discharge
The pharmacological treatments at discharge are shown in Table 4. Diuretics were the most commonly prescribed medication and were used in 82.2% of the patients. Renin-angiotensin system blockers were prescribed in 62.1% of the patients, and beta-blockers and mineralocorticoid receptor antagonists were prescribed in 59.6% and 49.0% of the patients, respectively.
Table 4. Prescribed pharmacological treatments at discharge.
| N = 1,462 | Total | Rate of use |
| Angiotensin-converting enzyme inhibitors | 402 (27.5%) | |
| Ramipril | 136 (33.8%) | |
| Captopril | 122 (30.3%) | |
| Enalapril | 95 (23.6%) | |
| Others | 49 (12.2%) | |
| Angiotensin receptor blockers | 506 (34.6%) | |
| Candesartan | 201 (39.7%) | |
| Valsartan | 177 (35.0%) | |
| Losartan | 83 (16.4%) | |
| Others | 45 (8.9%) | |
| Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers | 908 (62.1%) | |
| Beta-blockers | 872 (59.6%) | |
| Bisoprolol | 505 (57.9%) | |
| Carvedilol | 327 (37.5%) | |
| Metoprolol | 11 (1.3%) | |
| Mineralocorticoid receptor antagonist | 716 (49.0%) | |
| Spironolactone | 707 (98.7%) | |
| Eplerenone | 9 (1.3%) | |
| Diuretics | 1202 (82.2%) | |
| Digitalis | 379 (25.9%) | |
| Antiplatelet agents | 869 (59.4%) | |
| Anticoagulants | 312 (21.3%) | |
| Nitrates | 532 (36.4%) | |
| Hydralazine | 71 (4.9%) | |
| Antiarrhythmic drugs | 229 (15.7%) |
DISCUSSION
Previously, the characteristics and clinical outcome of acutely decompensated HF patients in Taiwan were poorly defined despite its high prevalence and public health concern.9 The TSOC-HFrEF registry is the first large-scale, prospective multicenter database involving patients hospitalized for HF in Taiwan.
Baseline characteristics: comparison with other HF registries
Many hospital-based registries have been published in recent years. Selected clinical patient characteristics of the TSOC-HFrEF registry compare with patients enrolled in 3 other major acute HF registries are shown in Table 5.
Table 5. Comparison of baseline clinical characteristics between TSOC HFrEF Registry and representative HF registries.
| ADHERE | EHFS II | ATTEND | TSOC-HF | |
| Patient numbers | 105388 | 3580 | 4842 | 1509 |
| Timeframe | 2001-2004 | 2004-2005 | 2007-2011 | 2013-2014 |
| Age (yrs) | 72 ± 14 | 70 ± 13 | 73 ± 14 | 64 ± 16 |
| Male | 48 | 54 | 58 | 72 |
| Ischemic etiology (%) | - | 54 | 31 | 44 |
| Left ventricular systolic dysfunction | 63 | 66 | 53 | 100 |
| Left ventricular ejection fraction (%) | 34 ± 16 | 38 ± 15 | - | 28 ± 8 |
| Hypertension | 73 | 63 | 69 | 35 |
| Hyperlipidemia | 37 | - | 37 | 34 |
| Coronary artery disease | 57 | 54 | - | 42 |
| Prior myocardial infarction | 31 | - | - | 25 |
| Atrial fibrillation | 31 | 39 | 40 | 26 |
| Diabetes mellitus | 44 | 33 | 34 | 44 |
| Chronic kidney disease | 30 | 17 | - | 32 |
| Chronic obstructive pulmonary disease | 31 | 19 | 10 | 11 |
| Heart rate (bpm) | - | 95 (77-114) | 99 ± 29 | 93 ± 22 |
| Systolic blood pressure (mmHg) | 144 ± 33 | 135 (110-160) | 145 ± 37 | 131 ± 28 |
| Sodium (mEq/L) | - | - | 139 ± 4 | 138 ± 5 |
| Creatinine (mg/dL) | 1.8 ± 1.6 | - | 1.4 ± 1.6 | 1.9 ± 1.8 |
| BNP (pg/mL) | 840 (430-1,730) | - | 707 (362-1,284) | 1250 (554-2,487) |
| Hemoglobin (g/dL) | - | - | 12.0 ± 2.6 | 12.9 ± 2.4 |
ADHERE, Acute Decompensated Heart Failure National Registry; ATTEND, Acute Decompensated Heart Failure Syndromes; BNP, B-type natriuretic peptide; EHFS II, European Heart Failure Survey II; TSOC HfrEF, Taiwan Society of Cardiology Heart Failure with reduced Ejection Fraction Registry.
The mean age of patients with primary diagnosis of HF ranged from 70 to 75 years, and approximately 40-50% of hospitalized HF patients were female in North American,10 European11 and Japanese12 registries. On the contrary, the mean age of patients in the TSOC-HFrEF registry was 64 years, and only 28% of those patients were female. These differences could be explained by the difference in the inclusion criteria. The other 3 registries generally enrolled hospitalized patients with HF, and the proportion of HF with preserved ejection fraction (HFpEF) patients ranged from 34-47%. Since female patients tend to be older at the time of initial diagnosis and are more likely to have HFpEF, it is reasonable to observe a younger, predominantly male patient population in the TSOC-HFrEF registry, which enrolled only HFrEF patients.
Ischemia was universally the most common cause of HF, ranging from 31-54% among the registries. Cardiac and non-cardiac comorbidities were also prevalent among hospitalized HF patients. About 42-57% of patients had known CAD, with 25-31% of cases diagnosed with myocardial infarction. Diabetes mellitus was found in 33-44% of patients, and chronic kidney disease was found in 17-32% of patients. Interestingly, the prevalence of chronic kidney disease was the highest in TSOC-HFrEF registry patients, with mean creatinine level of 1.9 ± 1.8 mg/dL. This may affect the initiation and titration of guideline-recommended medical therapy, especially renin-angiotensin system blockers.
Another notable finding was the lower prevalence of hypertension and atrial fibrillation in the TSOC-HFrEF registry. The prevalence of both hypertension and atrial fibrillation were higher in patients with HFpEF; both conditions cause diastolic dysfunction and impaired ventricular filling. Systolic blood pressure during admission was also lower in the TSOC-HFrEF patients.
Laboratory, electrocardiography and echocardiography findings: comparison with other HF registries
Data from the OPTIMIZE-HF registry showed that 50% of the hospitalized patients had low hemoglobin < 12.1 g/dL.13 In our registry, 34.9% of the patients had hemoglobin < 12 g/dL, thus demonstrating the high prevalence of anemia in these patient populations. The OPTIMIZE-HF registry also demonstrated that approximately 20% of patients were hyponatremic (serum sodium < 135 mEq/l) during admission. The prevalence and degree of hyponatremia were higher and more severe in patients admitted to the intensive care unit.14 Similar findings were also noted in the TSOC-HFrEF registry: hyponatremia was observed in 19.9% of the overall population, and 23.4% the patients admitted to intensive care unit.
The ADHERE registry showed that 20% of the patients had severe renal impairment with eGFR < 30 ml/min/1.73 m2.15 In the TSOC-HFrEF registry, the prevalence of renal insufficiency was even higher, with 27.4% of patients having eGFR < 30 ml/min/1.73 m2. As mentioned above, the initiation and titration of renin-angiotensin system blockers and mineralocorticoid receptor antagonist was more difficult in TSOC-HFrEF patients due to the high prevalence of impaired renal function.
Natriuretic peptides, troponin, thyroid profiles, ferritin and iron profiles have been used commonly as diagnostic and prognostic predictors as well as precipitating risk factors in HF patients,5,16,17 although these laboratory data were only available in 55.1%, 76.9%, 33.7% and 13.1% of the patients in our registry, respectively. The timely collection of laboratory parameters will help clinicians to understand the impact of these comorbidities in-depth and guide clinical management of HF.
In the TSOC-HFrEF registry, atrial fibrillation was diagnosed in 26.7% of the patients. As mentioned above, the prevalence of atrial fibrillation was lower in our registry compared to other HF registries. In the RO-AHFS registry, atrial fibrillation was noted in 44.3% of patients, including 18.1% of new-onset atrial fibrillation.17 Prolonged QRS duration longer than 120 ms was detected in 28.9% of the TSOC-HFrEF registry patients, including 8.8% of left bundle branch block pattern. The reported incidences of prolonged QRS duration were higher in the RO-AHFS registry18 (31.6%) and the EVEREST trial19 (44.6%).
Nearly all TSOC-HFrEF registry patients underwent echocardiography during hospitalization. Since our study enrolled only HFrEF patients, it was expected that our results would demonstrate lower LVEF and larger left ventricular end-diastolic diameter than other registries.11,18
Initial management
The most commonly used intravenous medication was diuretics. However, the usage of vasodilators and inotropic agents exhibited substantial geographic variation (Table 6).8 A noteworthy finding is that TSOC-HFrEF registry patients less frequently received intravenous diuretic while more frequently receiving intravenous inotropes. The utilization of intravenous vasodilators was also less common in TSOC-HFrEF patients than in most other regions except North America. The TSOC-HFrEF registry only comprised patients with reduced ejection fraction, which may explain why we had more patients receiving inotropic agents. Although use of inotropic support has been associated with increased mortality,19 in-hospital mortality of TSOC-HFrEF registry patients was 2.4%, which was not higher than mortalities seen in other registries (4 to 12%).10-12,18,21
Table 6. Comparison of intravenous therapy and procedural interventions between TSOC HFrEF registry and representative HF registries.
| ADHERE | EHFS II | RO-AHFS | ATTEND | ALARM-HF | TSOC-HF | |
| Intravenous therapies | ||||||
| Diuretics (%) | 92 | 84.4 | 79.9 | 76.3 | 89.7 | 62.6 |
| Vasodilators (%) | 9 | 38 | 33.4 | 78.3 | 41.1 | 27.1 |
| Inotropes (%) | 15 | 30 | 17.7 | 18.5 | 39 | 36.5 |
| Procedural interventions | ||||||
| CAG (%) | 10 | - | 4.7 | - | - | 44.2 |
| PCI (%) | 8 | 8.4 | 2 | 8 | 12.8 | 23.5 |
| CABG (%) | - | 1.8 | 0.4 | 1.3 | 3 | 2.3 |
| IABP (%) | < 1 | 2.2 | 0.2 | 2.5 | 4.8 | 2.7 |
| MV (%) | 5 | 5.1 | 3.5 | 7.5 | 16.2 | 12.9 |
ADHERE, Acute Decompensated Heart Failure National Registry; ALARM-HF, Acute Heart Failure Global Registry of Standard Treatment; ATTEND, Acute Decompensated Heart Failure Syndromes; CABG, coronary artery bypass graft; CAG, coronary angiography; EHFS II, European Heart Failure Survey II; IABP, intra-aortic balloon pump; MV, mechanical ventilation; PCI, percutaneous coronary intervention; RO-AHFS, Romanian Acute Heart Failure Syndrome; TSOC HF, Taiwan Society of Cardiology Heart Failure with reduced Ejection Fraction Registry.
Although ischemic cardiomyopathy was the most common etiology of HF, and ACS was the most commonly identified cause for HF decompensation in the TSOC-HFrEF registry and other studies,22 only a minority of patients underwent coronary angiography and 2-12.8% of patients underwent percutaneous coronary intervention in other registries. The practice was significantly more aggressive in the TSOC-HFrEF registry, while 44.2% and 23.5% of the patients underwent coronary angiography and percutaneous coronary intervention, respectively. One possible explanation is that patients in our registry were younger, and the clinicians tend to attempt revascularization in order to improve heart function. Patients requiring coronary artery bypass surgery ranged from 0.4-3% in all registries. The HF was severe in both the ALARM-HF and TSOC-HFrEF registries, since more than 10% of the patients required mechanical ventilation, and 2.7-4.8% of patients required intra-aortic balloon pump support.
Guideline-directed medical therapy at discharge
The prescription of guideline-recommended therapies has increased over time, both in Northern America (ADHERE to OPTIMIZE-HF23) and Europe (EHFS-I24 to EHFS-II and ESC-HF Pilot25). The percentage of renin-angiotensin system blockers prescribed at discharge ranged from 74.7-88.5% in Northern America, Europe and Japan registries, but the percentage was lower in the TSOC-HFrEF registry. The percentage of beta-blockers prescription at discharge were higher in the OPTIMIZE-HF, ESC-HF Pilot and ATTEND registries, and the percentage of beta-blocker prescription in the TSOC-HFrEF registry was 8%-27% lower than that in the other three registries. Utilization of guideline-recommended medical therapy at discharge is shown in Figure 2.
Figure 2.
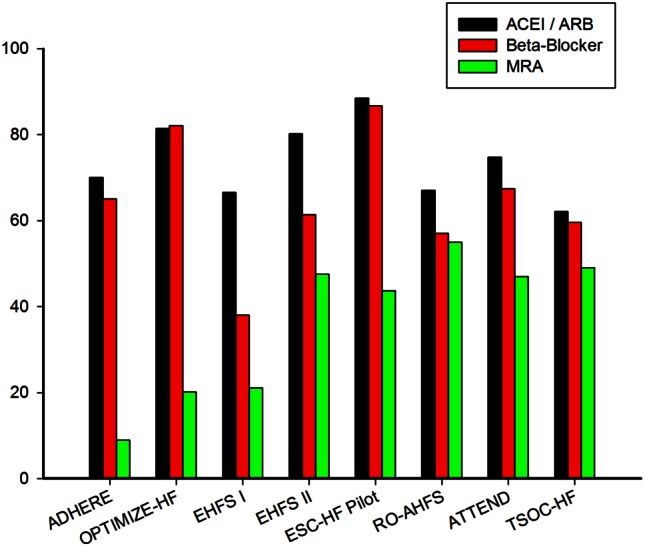
Guideline-directed medical therapy at discharge.
An important finding was that the rate of mineralocorticoid receptor antagonist prescription at discharge was markedly lower in North America. The prescription rate of mineralocorticoid receptor antagonist was less than 20% in the North America registries and was about 40-60% in other regions. The major concern involving the use of these medications was the risk of hyperkalemia in renal impaired patients. In our registry, the prevalence of chronic kidney disease was relatively high. The mean serum creatinine was 1.9 ± 1.8 mg/dL, and the mean eGFR was 55.5 ± 40.2 mL/min/m2. The percentage of mineralocorticoid receptor antagonist prescription was 49% in TSOC-HFrEF registry. This reflects the fact that our population was comprised of only those patients with reduced ejection fraction and was consistent with the population as described in the RALES trial.26
Hospitalization for acute HF is a critical event, which carries a high risk of morbidity and morality. The TSOC-HFrEF registry provides us the insight into current HF management and implementation of evidence-based medical treatment. The TSOC-HFrEF registry suggests that guideline-directed medical treatment was under-utilized. Physician managing HF needs to implement an evidence-based practice algorithm in order to improve quality of HF care.
Study limitations
This study had several limitations. First, although participating sites were encouraged to enroll patients as consecutively as possible, current registry design can still lead to selection bias and it may underestimate the severity of HF. Computer-based database should be designed for future HF registry in order to enroll all consecutive comers. Second, there is a need to standardize data collection. Data such as laboratory studies, biomarkers, route, dosage, and duration of HF medications should be standardized. Finally, the reasons for failure to initiate or up-titrate guideline-directed medical therapy should be documented. Otherwise, the percentage of “real under-utilization” of guide-directed therapy could not be calculated.
CONCLUSIONS
The TSOC-HFrEF registry which is the subject of this article is the largest national database to date involving acute decompensated HFrEF patients in Taiwan. It describes the epidemiology and diagnostic processes of acute HF patients. Our registry revealed the suboptimal guideline-directed medical care in Taiwan. Furthermore, one-year follow-up data will be collected, and our prospective registry will provide the foundation framework for further improving HF care in Taiwan.
SUPPLEMENTARY MATERIAL (LIST OF HOSPITALS IN ALPHABETICAL ORDER)
Chang Gung Memorial Hospital, Keelung, Taiwan; Chang Gung Memorial Hospital, Linkou, Taiwan; Cheng Hsin General Hospital, Taipei, Taiwan; Chimei Medical Center, Tainan, Taiwan; China Medical University Hospital, Taichung, Taiwan; Chung-Shan Medical University Hospital, Taichung, Taiwan; E-Da Hospital, Kaohsiung, Taiwan; Far Eastern memorial Hospital, New Taipei City, Taiwan; Hualien Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Hualien, Taiwan; Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan; Kaohsiung Medical University Chung-Ho Memorial Hospital, Kaohsiung, Taiwan; Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan; MacKay Memorial Hospital, Taipei, Taiwan; National Cheng Kung University Hospital, Tainan, Taiwan; National Taiwan University Hospital, Hsinchu Branch; National Taiwan University Hospital, Taipei, Taiwan; Shin Kong Wu Ho-Su Memorial Hospital, Taipei, Taiwan; Taichung Veterans General Hospital, Taichung, Taiwan; Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, New Taipei City, Taiwan; Taipei Veterans General Hospital, Taipei, Taiwan; Tri-Service General Hospital and National Defense Medical Center, Taipei, Taiwan.
CONFLICT OF INTEREST
All the authors declare no conflict of interest.
REFERENCES
- 1.Bonneux L, Barendregt JJ, Meeter K, et al. Estimating clinical morbidity due to ischemic heart disease and congestive heart failure: the future rise of heart failure. Am J Public Health. 1994;84:20–28. doi: 10.2105/ajph.84.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okura Y, Ramadan MM, Ohno Y, et al. Impending epidemic: future projection of heart failure in Japan to the year 2055. Circ J. 2008;72:489–491. doi: 10.1253/circj.72.489. [DOI] [PubMed] [Google Scholar]
- 3.Huang CH, Chien KL, Chen WJ, et al. Impact of heart failure and left ventricular function on long-term survival--report of a community-based cohort study in Taiwan. Eur J Heart Fail. 2007;9:587–593. doi: 10.1016/j.ejheart.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines: developed in collaboration with the international society for heart and lung transplantation. J Am Coll Cardiol. 2009;53:e1–e90. doi: 10.1016/j.jacc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 5.McMurray JJ, Adamopoulos S, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 6.WRITING COMMITTEE MEMBERS. Yancy CW, Jessup M, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 7.Wang CC, Chen JH, Yu WC, et al. 2012 Guidelines of the Taiwan Society of Cardiology (TSOC) for the diagnosis and treatment of heart failure. Acta Cardiol Sin. 2012;28:161–195. [Google Scholar]
- 8.Ambrosy AP, Fonarow GC, Butler J, et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63:1123–1133. doi: 10.1016/j.jacc.2013.11.053. [DOI] [PubMed] [Google Scholar]
- 9.Huang CH, Chien KL, Chen WJ, et al. Impact of heart failure and left ventricular function on long-term survival--report of a community-based cohort study in Taiwan. Eur J Heart Fail. 2007;9:587–593. doi: 10.1016/j.ejheart.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Adams KF, Jr., Fonarow GC, Emerman CL, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J. 2005;149:209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Nieminen MS, Brutsaert D, Dickstein K, et al. EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J. 2006;27:2725–2736. doi: 10.1093/eurheartj/ehl193. [DOI] [PubMed] [Google Scholar]
- 12.Sato N, Kajimoto K, Keida T, et al. Clinical features and outcome in hospitalized heart failure in Japan (from the ATTEND Registry). Circ J. 2013;77:944–951. doi: 10.1253/circj.cj-13-0187. [DOI] [PubMed] [Google Scholar]
- 13.Young JB, Abraham WT, Albert NM, et al. Relation of low hemoglobin and anemia to morbidity and mortality in patients hospitalized with heart failure (insight from the OPTIMIZE-HF registry). Am J Cardiol. 2008;101:223–230. doi: 10.1016/j.amjcard.2007.07.067. [DOI] [PubMed] [Google Scholar]
- 14.Gheorghiade M, Abraham WT, Albert NM, et al. Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: an analysis from the OPTIMIZE-HF registry. Eur Heart J. 2007;28:980–988. doi: 10.1093/eurheartj/ehl542. [DOI] [PubMed] [Google Scholar]
- 15.Heywood JT, Fonarow GC, Costanzo MR, et al. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail. 2007;13:422–430. doi: 10.1016/j.cardfail.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Ketchum ES, Levy WC. Establishing prognosis in heart failure: a multimarker approach. Prog Cardiovasc Dis. 2011;54:86–96. doi: 10.1016/j.pcad.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Masson S, Anand I, Favero C, et al. Serial measurement of cardiac troponin T using a highly sensitive assay in patients with chronic heart failure: data from 2 large randomized clinical trials. Circulation. 2012;125:280–288. doi: 10.1161/CIRCULATIONAHA.111.044149. [DOI] [PubMed] [Google Scholar]
- 18.Chioncel O, Vinereanu D, Datcu M, et al. The Romanian Acute Heart Failure Syndromes (RO-AHFS) registry. Am Heart J. 2011;162:142–153. doi: 10.1016/j.ahj.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 19.Wang NC, Maggioni AP, Konstam MA, et al. Clinical implications of QRS duration in patients hospitalized with worsening heart failure and reduced left ventricular ejection fraction. JAMA. 2008;299:2656–2666. doi: 10.1001/jama.299.22.2656. [DOI] [PubMed] [Google Scholar]
- 20.Abraham WT, Adams KF, Fonarow GC, et al. In-hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE). J Am Coll Cardiol. 2005;46:57–64. doi: 10.1016/j.jacc.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 21.Follath F, Yilmaz MB, Delgado JF, et al. Clinical presentation, management and outcomes in the Acute Heart Failure Global Survey of Standard Treatment (ALARM-HF). Intensive Care Med. 2011;37:619–626. doi: 10.1007/s00134-010-2113-0. [DOI] [PubMed] [Google Scholar]
- 22.Kociol RD, Pang PS, Gheorghiade M, et al. Troponin elevation in heart failure prevalence, mechanisms, and clinical implications. J Am Coll Cardiol. 2010;56:1071–1078. doi: 10.1016/j.jacc.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 23.Gheorghiade M, Abraham WT, Albert NM, et al. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA. 2006;296:2217–2226. doi: 10.1001/jama.296.18.2217. [DOI] [PubMed] [Google Scholar]
- 24.Cleland JG, Swedberg K, Follath F, et al. The EuroHeart Failure survey programme - a survey on the quality of care among patients with heart failure in Europe. Part 1: patient characteristics and diagnosis. Eur Heart J. 2003;24:442–463. doi: 10.1016/s0195-668x(02)00823-0. [DOI] [PubMed] [Google Scholar]
- 25.Maggioni AP, Dahlstrom U, Filippatos G, et al. EURObservational Research Programme: the Heart Failure Pilot Survey (ESC-HF Pilot). Eur J Heart Fail. 2010;12:1076–1084. doi: 10.1093/eurjhf/hfq154. [DOI] [PubMed] [Google Scholar]
- 26.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]


