Abstract
Background
Green tea intake has been shown to improve endurance capacity in animal studies, but whether it has a similar effect on humans remains unclear. A randomized, double-blinded, parallel-controlled study was conducted to evaluate the short-term effect of STA-2, a pharmaceutical preparation of green tea polyphenols, in patients with effort-induced angina and documented positive exercise tolerance test.
Methods
A total of 79 patients recruited from three medical centers were randomly assigned to receive 2 STA-2 250 mg capsules, each containing 100 mg green tea polyphenols, three times daily, or placebo for six weeks after two consecutive symptom-limited treadmill exercise tests to ascertain the reproducibility of exercise tolerance.
Results
There was no difference in total exercise tolerance time from baseline to Week 6 between two groups (p = 0.639). There were also no observed improvements in subgroup analyses stratified by age, gender, and BMI categories. However, a significant reduction in low-density lipoprotein levels was shown in patients in the STA-2 group (-8.99 ± 19.18 mg/dL) versus the placebo group (0.57 ± 19.77 mg/dL), p = 0.037, with greater benefits in patients not taking antihyperlipidemic drugs (STA-2: -9.10 ± 19.96 mg/dL vs. placebo: 4.42 ± 15.08 mg/dL, p = 0.037).
Conclusions
STA-2 treatment for 6 weeks did not increase exercise time as measured on a treadmill. However, this study also indicated that STA-2 treatment could have potential beneficial effects on LDL-cholesterol concentrations.
Keywords: Chronic stable angina, Coronary disease, Green tea polyphenols, Ischemia, Low density lipoprotein (LDL)
INTRODUCTION
Clinical and epidemiological studies have shown that green tea consumption may be associated with a reduced risk of cardiovascular disease.1-4 There has been increased interest in the beneficial effects of green tea catechins on cardiovascular and metabolic health. These benefits are mainly attributed to the high levels of polyphenols, particularly flavonoids, found in tea.5 Reduced plasma oxidized low-density lipoprotein (LDL) levels were reported following daily consumption of 500 mg of catechins (equivalent to 6-7 cups of green tea) by healthy adults.6 This evidence sheds light on the treatment of patients with coronary artery disease.
Several studies have demonstrated the antioxidant effects of green tea, as evidenced by similar reductions in LDL levels.7-9 However, dietary habits and brewing practices vary among regions, so the benefits of drinking green tea may not be consistent. These different results can be minimized while the common advantages are magnified if green tea is used in the form of a pharmaceutical preparation of the extract(s). A genotoxicity study has provided green tea catechins with no evidence of mutagenic or clastogenic activity up to 2000 mg/kg.10 For all of the above-stated reasons, the Sinphar Pharmaceutical Co., Ltd. was prompted to develop the STA-2 capsule, a botanical drug preparation of green tea polyphenols.
Green tea intake has been shown to improve endurance capacity in mice. Murase et al.11,12 have shown improvement in swimming and running times to exhaustion of approximately 24% and 30%, respectively, after administering green tea extracts in mice. However, whether it may provide comparable benefits for humans has remained unclear. Exercise-induced oxidative stress may be associated with muscle fatigue, muscle damage, and a decrease in physical performance.13 One study conducted in 2008 revealed that green tea consumption may offer protection against the oxidative damage caused by exercise in young men undergoing resistance exercise.14 Previous studies were aimed at investigating the extract of green tea polyphenols in muscle fatigue and exercise tolerance in athletes or healthy individuals. However, the effect of green tea polyphenols on muscle fatigue and exercise tolerance in athletes remains very controversial. Therefore, we conducted this pilot study to investigate the efficacy and safety of STA-2 on selected blood markers of oxidative stress and exercise tolerance in patients with chronic stable angina. We hypothesized that STA-2 supplementation would elevate antioxidant potential and improve exercise duration.
MATERIALS AND METHODS
Investigational product
EpigallinTM, as a STA-2 active ingredient, is a green tea Camellia sinensis (L.) Kuntze (C. sinensis) extract standardized to contain 98% tea polyphenols and 1% caffeine. Teapol, Sinphar’s commercial product name corresponding to the generic product name of Xinnaojian Capsule using EpigallinTM as the active ingredient, has been approved and registered as a drug in China. Teapol has been marketed in China since 1996. To date, Teapol has been prescribed in over 500,000 prescriptions in China, and no apparent adverse reactions were observed or reported.
The ingredients of STA-2 250 mg capsules used in this study were manufactured according to the standard operation procedure of Jiangxi Lukang, a local material company in China. Each capsule contained 100 mg of green tea polyphenols extracts as the drug substance. The green tea polyphenols powder was manufactured through a series of processes including repeated extraction, filtration, concentration, decaffeination, and drying. The finished powder contained tea polyphenols at more than 98% w/w and less than 1% caffeine. The green tea polyphenols contents [mainly catechins including epigallocatechin 3-gallate [epigallocatechin gallate (EGCG), the most abundant catechin in tea), (-)-epigallocatechin, (±)-catechin, (-)-epicatechin, (±)-gallocatechin gallate, (-)-epicatechin-3-gallate (ECG), etc.] were first detected by the color reaction with ferrous tartrate and preliminarily quantitated by spectrophotometry. The formal identification of the active pharmaceutical ingredients was accomplished by the comparison of Thin-Layer chromatography (TLC) spots and high-performance liquid chromatography (HPLC) retention times of the assay samples with those of the standards, while the formal quantitation also achieved in the same run of HPLC. Active substance of STA-2 was identified by comparison of TLC spots of ECG and EGCG and HPLC retention times of catechins with those of standard preparation. Also, EGCG and total catechins were confirmed as marker ingredients.
EGCG and total catechins were selected as the marker ingredients for both routine qualitative and quantitative assays. The lowest acceptance levels for EGCG and catechins were set as 60% and 85%, respectively, while the highest counterparts were 85% and 102%, respectively. The upper acceptance limits for water content, total ash, and acid insoluble ash were all in accordance with the supplier’s specification.
Several good laboratory practice (GLP) compliant toxicity studies have been conducted to support the safe use of STA-2 for clinical investigation. The 13-week repeated dose oral toxicity study NOAEL (No Observed Adverse Effect Level) dose for STA-2 (containing EpigallinTM alone without excipients) is determined to be 400 mg/kg/day. The dose provides a safety margin which is 6.7-fold higher than the maximal human recommended daily dose on a body surface basis. The results of genotoxicity studies reveal no evidence of mutagenic or clastogenic activity of STA-2.
Study design
This was a double-blind, randomized, parallel-group, and placebo-controlled study conducted from May 2007 to June 2008 at the National Taiwan University Hospital, Taipei Veterans General Hospital, and the Chi Mei Medical Center to assess the efficacy and safety of STA-2 in comparison with that of placebo for the management in patients with chronic stable angina. Neither the investigator nor the patient knew what treatment the patient had received. The disposition of patients is summarized in Figure 1, and a disposition of patients by sites is provided in the supplementary material (Supplement Table 1). The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The trial protocol was reviewed and approved by the regulatory authorities in both the US and Taiwan, and also respective Institutional Review Boards of the medical centers involved. All patients provided written informed consent before enrollment. This study is registered as a Clinical Trial, number NCT01484912 (http://clinicaltrials.gov/ct2/show/NCT01484912).
Figure 1.
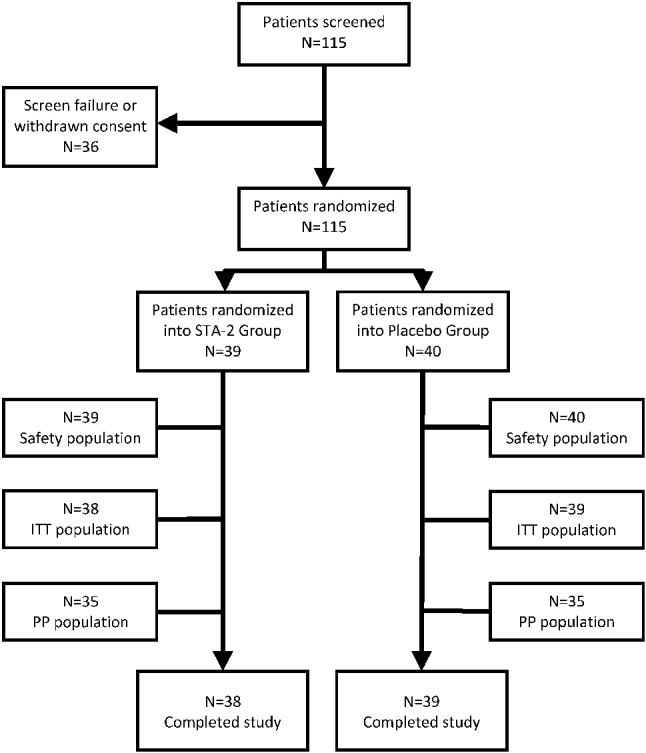
Disposition of patients. STA-2: STA-2 capsule, 250 mg/capsule, oral, take 2 capsules three times daily. Placebo: Placebo capsule, 250 mg/capsule, oral, take 2 capsules three times daily.
Supplement Table 1. Deposition of patients by sites.
| NTUH | TVGH | CMMC | All Sites | |
| Screened | 18 (16%) | 44 (38%) | 53 (46%) | 115 (100%) |
| Randomized | 8 (10%) | 36 (46%) | 35 (44%) | 79 (100%) |
| Completed | 8 (10%) | 34 (44%) | 35 (45%) | 77 (100%) |
CMMC, Chi Mei Medical Center; NTUH, National Taiwan University Hospital; TVGH, Taipei Veterans General Hospital.
Inclusion and exclusion criteria
Patients were enrolled in this study only if they met all of the following criteria: (1) male or female aged ≥ 20; (2) with effort-induced angina that was readily relieved by rest or nitroglycerin, or with catheterization-documented coronary artery disease, or with ≥ 3 months of myocardial infarction before screening; (3) manifesting positive exercise treadmill test (ETT, defined as giving ST-segment depression at least 1 mm greater than the resting one, with or without limiting angina) on both the days of screening (Day -7) and enrollment (Day 0), with the difference in total exercise tolerance time (TETT) not exceeding 20% of Day 0’s TETT; and (4) women of childbearing potential were included only if they implemented adequate contraception, or were non-lactating, or had a negative pregnancy test within 14 days prior to the study.
Patients were excluded from the study for any of the following reasons: (1) conduction abnormalities, unstable angina, or myocardial infarction within the preceding 3 months; (2) with heart failure (New York Heart Association class III or IV), uncorrected valvular, or congenital heart disease; (3) with electrocardiogram abnormalities preventing the interpretation of ischemia; and (4) with hepatic or gastrointestinal abnormalities, or conditions that could interfere with ETT.
Treatment
Each patient was asked to make a total of 5 study visits on Days -7, 0, 14 (±2), 28 (±2), and 42 (±2). At the screening visit (Day -7), demographic data, medical history, and concomitant medications were recorded, and the patients underwent physical examination, including assessment of vital signs and laboratory tests. A resting electrocardiogram was recorded, and an ETT was undertaken according to a standard Bruce multistage exercise test protocol, followed by a washout period of 1 week during which patients received placebo treatment. At baseline (Day 0), the patients again underwent an ETT before being randomized to receive either 2 capsules of 250 mg STA-2 or placebo in a fasting state, three times daily, for 6 weeks. The block randomization list was provided by the study sponsor, and individual treatment codes were kept in sealed opaque envelopes. The identical-looking study drug and placebo ensured that both patients and investigators were blinded to treatment. At each study visit, vital signs were assessed, and adverse events and concomitant medications recorded. At the final visit, all assessments including physical examination, resting electrocardiogram, laboratory tests, and ETT, were repeated.
The patients were allowed to take routine medications for other indications, provided they did not affect the study assessments. Medications for angina pectoris were also allowed; however, doses of such drugs as isosorbide dinitrate, nicorandil, beta-blockers, and calcium channel blockers known to influence ETT results were kept constant, starting 2 weeks prior to visit 1 until the end of the study. Beta-blockers were discontinued the day before ETT, and isosorbide dinitrate and nicorandil were given after assessments on the day of ETT. Drugs interfering with ST-segment changes (e.g., class I antiarrhythmic agents, digitalis, and monoamine oxidase inhibitors), theophylline, isosorbide mononitrate, and nitroglycerin sustained-release preparations were prohibited. Short-acting nitrates were prohibited 1 hour before the ETT procedure. A complete summary of concomitant medications used by study subjects during study period was listed in supplement Table 2.
Supplement Table 2. Summary of concomitant medications.
| Medications | STA-2 group (n = 38) | Placebo group (n = 39) |
| N (%) | N (%) | |
| Antianginal drugs | 32 | 33 |
| Nitrates | 9 | 12 |
| Beta blockers | 24 | 32 |
| Calcium channel blockers | 25 | 25 |
| Antiplatelet | 32 | 33 |
| Others | 7 | 7 |
| Hypertension* | ||
| Angiotensin II receptor blocker | 14 | 16 |
| ARB+diuretics | 4 | 0 |
| Angiotensin-converting enzyme inhibitor | 5 | 3 |
| Diuretics | 5 | 5 |
| Alpha-1 blockers | 5 | 6 |
| Diabetes mellitus | ||
| Biguanides | 3 | 5 |
| Glucosidase inhibitors | 0 | 2 |
| Insulin secreagogues | 1 | 5 |
| Thiozonidines | 1 | 1 |
| Hyperlipidemia | ||
| HMG-CoA reductase | 10 | 10 |
| ST + Cholesterol absorption inhibitor | 0 | 3 |
| Cholesterol absorption inhibitor | 3 | 2 |
| Folic acid derivatives | 1 | 2 |
| Antiarrythmia | ||
| Class III | 1 | 0 |
| Class IB | 1 | 0 |
* Excluding beta blockers and calcium channel blockers.
The primary endpoint was the change from baseline in TETT. A 12-lead electrocardiogram was used to continuously monitor vital signs. Patients were asked to complete 9-12 min of exercise or to exercise until 85% of the maximum predicted heart rate was reached. Exercise was terminated when limiting angina developed or when ST-segment depression measured 80 ms from the J point for 3 mm, whichever occurred first. Other limiting endpoints leading to exercise termination included: (1) a significant decrease (i.e., down to below 80 mmHg) or increase (i.e., up to above 240 mmHg) in systolic blood pressure; (2) ataxia, vertigo, dizziness, cyanosis, pallor, or any other symptoms suggestive of distress; (3) clinical evidence of acute cardiac decompensation; (4) clinically serious atrial or ventricular arrhythmias; and (5) heart block. The primary efficacy endpoint was further analyzed to determine the influence of prognostic factors such as gender (male or female), age (≥ 65 or < 65), and body mass index (BMI ≥ 25 or < 25 kg/m2). Change in time to 1-mm ST-segment depression (measured 80 ms from the J point) during ETT was assessed as a secondary endpoint and further analyzed depending on whether the patients received isosorbide dinitrate. Certain parameters were set to precisely delineate the 1-mm ST-segment depression. The assessments of ST-segment and ETT were implemented at the same time and in the same manner for all patients to ensure proper comparison with the baseline. Moreover, in order to avoid any possible confounds, the assessments were done by a person blind to the study groups. Safety was assessed based on the incidence of adverse events and serious adverse events, their relationship to the study treatment, vital signs, and laboratory assessments. Fasting plasma was analyzed for blood glucose, cholesterol, triglyceride, high density lipoprotein (HDL) and low-density lipoproteins (LDL) using Hitachi 7060 Autoanalyzer (Hitachi, Tokyo, Japan). Pharmacological parameters including oxidative stress parameters (isoprostane, homocysteine), homeostasis parameters (plasma plasminogen activator inhibitor-1, PAI-1 activity), inflammatory markers [fibrinogen, high sensitive C-reactive protein (hsCRP), soluble CD40 ligand] and cardiac enzymes [creatine phosphokinase-MB (CPK-MB) and lactic dehydrogenase (LDH)] were also measured.
Statistical analysis
Results were presented as mean ± standard deviation (SD). The calculation of sample size was based on exploration of efficacy and collected important information for future studies. Approximately 60 patients were planned to be enrolled, with 30 patients in each group. The intent-to-treat (ITT) population comprised all the randomized patients that received at least 1 dose of the study medication and had at least 1 follow-up efficacy evaluation. The per protocol (PP) population comprised all the randomized patients that completed the study with complete ETT data at baseline and at the end of the study without major protocol violations. The safety population comprised all the patients that had taken at least 1 dose of the study medication. All efficacy evaluations were performed on the ITT and PP populations, while the safety population was used for safety evaluation.
The demographic characteristics of the two groups were compared at baseline using chi-square or Fisher’s exact tests for categorical variables; t-tests or Wilcoxon Rank Sum test were used for continuous variables. If the hypothesis of normality was violated, the results from Wilcoxon Rank Sum test will be adopted. For the primary endpoint, the change in TETT was compared between the two groups in the ITT population; the test of superiority of STA-2 over placebo was conducted at a one-sided significance level of 0.025. A sensitivity analysis was also conducted in the PP population to validate the estimation of efficacy in the primary endpoint. For the secondary endpoint, hypothesis testing was conducted at a two-sided significance level of 0.05. Safety assessment was performed for safety population. Analyses were conducted by SAS software, version 9.3 (SAS Institutes Inc., Cary, NC, USA).
RESULTS
Of the 115 patients screened, 79 were enrolled and 77 completed the study (STA-2 group, 38; placebo group, 39) (Figure 1). One patient each from STA-2 and placebo groups discontinued due to withdrawal of consent and experience of abnormal liver function, respectively, before the primary endpoint analysis. The two groups had comparable demographics and baseline characteristics (Table 1).
Table 1. Demographic and baseline characteristics of the patients.
| Variable | STA-2 (n = 38) | Placebo (n = 39) |
| Demographics (Mean ± SD) | ||
| Age (year) | 62.6 ± 11.0 | 61.5 ± 12.4 |
| Height (cm) | 164.0 ± 7.1 | 164.1 ± 7.7 |
| Weight (kg) | 68.3 ± 11.9 | 70.1 ± 10.1 |
| Body mass index (kg/m2) | 25.3 ± 3.8 | 25.9 ± 2.6 |
| Baseline characteristics (Mean ± SD) | ||
| Angina frequency (no. of events per day) | 0.1 ± 0.3 | 0.1 ± 0.2 |
| Laboratory measurements (Mean ± SD) | ||
| Triglyceride (mg/dL) | 141.2 ± 67.5 | 153.6 ± 71.1 |
| Low-density lipoprotein (mg/dL) | 125.3 ± 37.8 | 124.5 ± 33.0 |
| High-density lipoprotein (mg/dL) | 44.2 ± 12.5 | 47.1 ± 12.6 |
SD, standard deviation.
Table 2 summarized the changes in TETT from baseline among study subjects. At baseline, the mean (SD) TETT of the STA-2 and placebo groups was 437.2 (106.7) and 405.1 (120.7) s, respectively, with no significant difference. At Week 6, these values were 450.4 (106.7) and 411.2 (118.1) s, respectively. There was no observed difference in changes in TETT from baseline to Week 6 between STA-2 group: 13.2 (49.9) and placebo group: 6.1 (76.3). Besides, the changes in TETT were also analyzed for subgroups of patients based on body mass index (BMI ≥ 25 or < 25 kg/m2), gender (male or female), and age (≥ 65 or < 65). However, no statistical significance was reached for any of the subgroups analyses. Furthermore, sensitivity analysis showed similar results conducted in the PP population, the improvement from baseline in TETT was approximately 16 s greater in the STA-2 group than in the placebo group, changes in TETT from baseline to Week 6 in STA-2 group: 14.4 (49.8) and placebo group: -2.1 (74.4), with no statistical significance (Figure 2).
Table 2. Changes in total exercise tolerance time from baseline among study subjects.
| Study subjects | STA-2 | Placebo | p-value* | ||||||
| Baseline | Week 6 | Changes | Baseline | Week 6 | Changes | ||||
| n | Mean ± SD | Mean ± SD | Mean ± SD | n | Mean ± SD | Mean ± SD | Mean ± SD | ||
| All subjects | 38 | 437.2 ± 106.7 | 450.4 ± 106.7 | 13.2 ± 49.9 | 39 | 405.1 ± 120.7 | 411.2 ± 118.1 | 6.1 ± 76.3 | 0.639 |
| BMI (kg/m2) | |||||||||
| ≥ 25 | 20 | 445.5 ± 133.7 | 459.8 ± 130.4 | 14.4 ± 48.7 | 23 | 415.2 ± 126.4 | 415.6 ± 126.1 | 0.39 ± 80.9 | 0.715 |
| < 25 | 18 | 427.9 ± 68.1 | 439.9 ± 74.6 | 12.0 ± 52.5 | 16 | 390.5 ± 114.5 | 404.8 ± 109.3 | 14.3 ± 70.9 | 0.916 |
| Gender | |||||||||
| Male | 34 | 439.1 ± 111.4 | 455.1 ± 111.6 | 15.9 ± 51.1 | 32 | 409.8 ± 124.5 | 415.6 ± 111.3 | 5.8 ± 76.0 | 0.59 |
| Female | 4 | 421.0 ± 65.5 | 411.5 ± 34.4 | -9.5 ± 34.3 | 7 | 383.6 ± 107.5 | 390.7 ± 154.1 | 7.14 ± 83.7 | 0.718 |
| Age (years) | |||||||||
| ≥ 65 | 15 | 384.5 ± 110.6 | 399.4 ± 103.6 | 14.9 ± 55.8 | 15 | 360.9 ± 132.5 | 342.0 ± 96.0 | -18.9 ± 82.0 | 0.254 |
| < 65 | 23 | 471.5 ± 90.8 | 483.7 ± 96.9 | 12.1 ± 46.9 | 24 | 432.7 ± 106.4 | 454.4 ± 111.2 | 21.7 ± 69.6 | 0.585 |
SD, standard deviation.
* T-test of Wilcoxon Rand Sum test was used.
Figure 2.
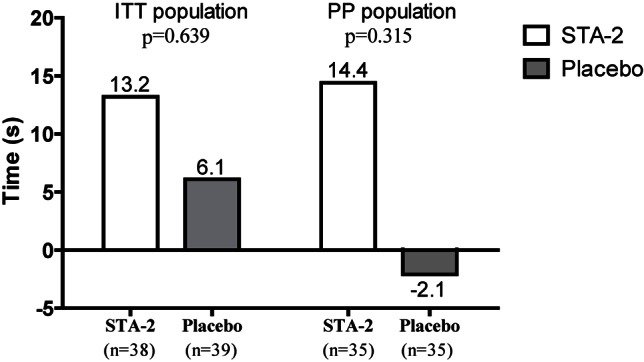
Change in total exercise tolerance time from baseline to Week 6 in the ITT and PP populations.
Furthermore, analyses of secondary endpoint also showed no difference between two groups (Table 3). At baseline, the mean time to 1-mm ST-segment depression during ETT for the STA-2 and placebo groups was 349.5 (104.8) and 321.5 (132.3) s, respectively. At Week 6, these values became 396.8 (109.0) and 341.7 (138.5) s, respectively. There was no observed difference in changes from baseline to Week 6 between the STA-2 group [37.4 (61.3)] and the placebo group [17.4 (103.4), p = 0.943]. There were no significant changes of urinary isoprostanes in either STA-2 or placebo group [from 119 (69) to 111 (77) pg/mg creatinine in the STA-2 group and from 117 (59) to 113 (62) pg/mg creatinine in the placebo group, p = 0.40]. No differences were observed in other pharmacological parameters including homocysteine, PAI-1, fibrinogen, hsCRP, soluble CD 40 ligand, CPK-MB and LDH.
Table 3. Changes in secondary endpoints and pharmacological parameters from baseline among study subjects.
| Variables | STA-2 | Placebo | p-value* | ||||||
| Baseline | Week 6 | Changes | Baseline | Week 6 | Changes | ||||
| n | Mean ± SD | Mean ± SD | Mean ± SD | n | Mean ± SD | Mean ± SD | Mean ± SD | ||
| Time to 1 mm ST-segment | 29 | 349.5 ± 104.8 | 396.8 ± 109.0 | 37.4 ± 61.3 | 29 | 321.5 ± 132.3 | 341.7 ± 138.5 | 17.4 ± 103.4 | 0.943 |
| Pharmacological parameters | |||||||||
| Isoprostane (pg/mg creatinine) | 38 | 118.9 ± 68.8 | 111.2 ± 77.4 | -6.4 ± 72.9 | 39 | 116.9 ± 59.3 | 112.5 ± 62.3 | -4.4 ± 70.9 | 0.553 |
| Homocysteine (μmol/L) | 37 | 14.6 ± 7.5 | 14.3 ± 5.6 | 0.7 ± 2.6 | 39 | 13.1 ± 6.5 | 13.3 ± 6.8 | 0.2 ± 2.8 | 0.082 |
| PAI-1 (ng/mL) | 36 | 26.8 ± 34.4 | 31.7 ± 30.1 | 5.6 ± 29.0 | 38 | 26.5 ± 24.6 | 40.0 ± 58.6 | 14.4 ± 66.0 | 0.18 |
| Fibrinogen (mg/dL) | 38 | 310.2 ± 53.0 | 314.3 ± 49.4 | 6.2 ± 51.3 | 39 | 303.2 ± 44.7 | 307.7 ± 57.7 | -1.0 ± 45.5 | 0.538 |
| hsCRP (mg/L) | 35 | 1.5 ± 1.5 | 2.1 ± 2.7 | 0.7 ± 3.1 | 36 | 2.1 ± 2.7 | 2.4 ± 4.1 | -0.9 ± 8.8 | 0.223 |
| Soluble CD40 ligand (ng/mL) | 36 | 0.7 ± 0.6 | 0.6 ± 0.5 | -0.1 ± 0.8 | 38 | 0.8 ± 0.8 | 0.9 ± 0.9 | 0.2 ± 0.9 | 0.251 |
| CPK-MB (ng/mL) | 38 | 2.5 ± 1.4 | 2.8 ± 1.5 | 0.2 ± 1.2 | 39 | 2.5 ± 1.3 | 2.8 ± 1.5 | 0.3 ± 0.9 | 0.58 |
| LDH (U/L) | 38 | 172.5 ± 38.8 | 170.42 ± 32.3 | -1.7 ± 31.1 | 38 | 170.4 ± 32.3 | 177.1 ± 31.8 | -4.6 ± 18.4 | 0.953 |
CPK-MB, creatine phosphokinase-MB; hsCRP, high sensitive C-reactive protein; LDH, lactic dehydrogenase; PAI-1, plasminogen activator inhibitor-1; SD, standard deviation.
* T-test of Wilcoxon Rand Sum test was used.
A greater reduction in LDL levels from baseline to Week 6 was observed in the STA-2 group [-9.0 (19.2) mg/dL] than in the placebo group [0.6 (19.8) mg/dL) (Figure 3), with the difference being statistically significant (p = 0.035). Further analysis of the LDL profile showed a significant benefit of STA-2 treatment in patients not receiving antihyperlipidemic drugs [change from baseline to Week 6: STA-2, -9.1 (20.0) mg/dL; placebo, 4.4 (15.1) mg/dL; difference > 13 mg/dL; p = 0.037] as opposed to those receiving antihyperlipidemic drugs [STA-2, -8.9 (19.2) mg/dL; placebo, -2.7 (22.9) mg/dL; p = 0.328)].
Figure 3.
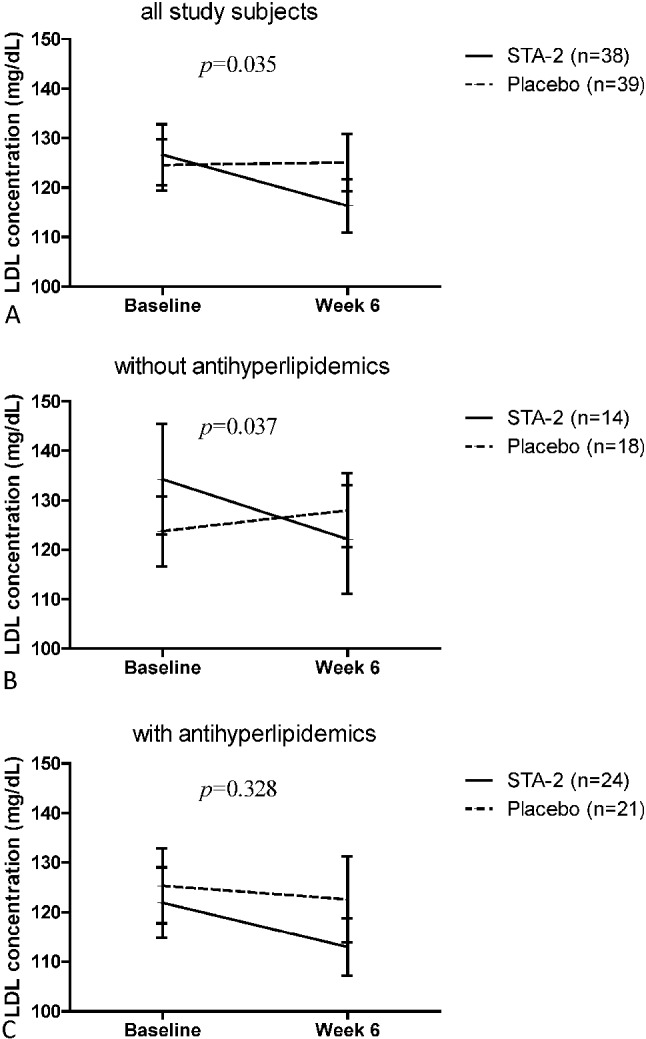
LDL concentration at baseline and Week 6 in: (A) the all study subjects; (B) subjects not receiving antihyperlipidemics; and (C) subjects receiving antihyperlipidemics.
The incidence of adverse events was comparable between the two groups; most adverse events were of mild to moderate severity and none led to study discontinuation. Treatment-related adverse events included palpitations in 2 patients, chest pain, chest discomfort, upper abdominal pain, and dry mouth in 1 patient each in the STA-2 group, abdominal distension in 1 patient in the placebo group. The adverse events are summarized in Table 4.
Table 4. Summary of adverse events.
| STA-2 group (n = 39) | Placebo group (n = 40) | |
| N (%) | N (%) | |
| Total AEs | 32 | 33 |
| Patients with AEs | 17 (44%) | 18 (45%) |
| Patients with SAEs | 0 | 1 (3%) |
| Patients with treatment-related AEs | 6 (15%) | 1 (3%) |
| Most commonly reported AEs | ||
| Palpitations | 2 (5%) | 0 |
| Abdominal distension | 0 | 3 (8%) |
| Upper abdominal pain | 2 (5%) | 0 |
| Chest discomfort | 1 (3%) | 2 (5%) |
| Chest pain | 2 (5%) | 0 |
| Upper respiratory tract infection | 2 (5%) | 1 (3%) |
| Hypertriglyceridemia | 0 | 2 (5%) |
| Spinal osteoarthritis | 0 | 2 (5%) |
| Insomnia | 2 (5%) | 0 |
| Renal impairment | 0 | 2 (5%) |
AE, adverse event; SAE, serious adverse event.
DISCUSSION
To the best of our knowledge, this is the first report of the therapeutic benefits of STA-2, a pharmaceutical preparation of green tea extracts, in patients with chronic stable angina. Although there was no observed improvement in exercise capacity, this study demonstrated a substantial tendency of STA-2 in enhancing exercise tolerance, especially in patients with BMI ≥ 25 kg/m2, of male gender, or ≥ 65 years of age. This finding deserves further exploration.
Secondary, our results suggested that intake of STA-2 was associated with a significant decrease in LDL levels. Recent meta-analyses have reported significantly reduced concentrations of LDL in association with green tea intake.15 Risk of heart attack is 3-fold higher in subjects with hyperlipidemia than in subjects with normal lipid status,16 whereas a 1% decrease in serum cholesterol has been shown to reduce risk of cardiovascular disease by 3%.17 Furthermore, the reduction in LDL was noted in management of CVD risks.17 Lipid level modification remains an important target for cardiovascular disease prevention and treatment. Animal studies have suggested that green tea catechins reduce lipid absorption in the intestines,18 promote fecal excretion of cholesterols,19 and inhibit enzymes involved in hepatic cholesterol synthesis.20 EGCG, the most abundant green tea catechin, has been found to strongly inhibit hydroxyl-3-methyl-glutaryl-CoA reductase, the rate-controlling enzyme of cholesterol synthesis.21 The significance of our finding of the beneficial effects of green tea on lipid in relation to adjunctive treatment of coronary artery disease remains to be established. However, with the strong link between hyperlipidemia and coronary artery disease, beneficial effects of green tea on lipids may have clinical importance.
Green tea catechins have been reported to be capable of regulating the biomarkers of oxidative stress, lipid peroxidation, and plasma antioxidant capacity.7,22 One previous article further demonstrated that 12 weeks of green tea extracts (136 mg EGCG; daily) intake was associated with decreased malondialdehyde-modified LDL in human.9 Examining both plasma and urinary isoprostane markers of in vivo lipid peroxidation, this study applied urinary isoprostanes as measurements of antioxidation effect which are nonesterified and may represent both system and renal production and renal excretion. However, our results did not reveal the antioxidation effect of STA-2 assessed by urinary 8-isoprostane. This discrepancy could be explained by the following: first, compared to our study, a relatively lower dose was used in Nagao’s study. Second, small sample sizes, a different study population and the use of different green tea agents in this current study may reduce its significance in the findings related to LDL-cholesterol. However, our results were consistent with the findings of Hodgson et al.,23 showing that antioxidants derived from tea cannot inhibit in vivo lipid peroxidation assessed by urinary F2-isoprostane levels. Therefore, the benefits of green tea catechins still await further clarification.
Non-clinical studies (unpublished) were summarized in the investigative brochure. In short, catechins can effectively reduce the cell death of myocardial ischemia. Besides, there is a significant superiority of inhibitive and protective effects against myocardial infarction when STA-2 and the nitrates-based antianginal drugs are used in combination as compared to only using the nitrates-based drugs. Furthermore, a combination of catechins and the nitrates-based drugs can be used preventively against angina or myocardial infarction caused by myocardial ischemia. In general, the death of cardiac muscles involved in three stages: (1) ATP insufficiency due to a shortage of blood supply; (2) elevation in intracellular calcium concentration; and (3) lysosomes are released as a result. The possible mechanisms that link catechins to the reduction of myocardial ischemia may be explained by the catechol structure found in a part of the chemical structure of catechins, which can chelate calcium ions. The 2-hydroxy structure has been scientifically proven to be capable of chelating calcium, or the mildly acidic catechol found in tea polyphenols is able to combine with and break down enzymes, which inhibits the enzymes’ catalytic activities. In addition, the chelating effect of catechol may allow it to act like a calcium antagonist and lowers the calcium concentration within blood vessel cells, which in turn widens the blood vessels and lowers the afterload for the heart, subsequently reducing the cardiac work and preserving ATP for the heart, thereby alleviating angina.24-28
The strength of our study was in its design as a randomized and placebo-controlled clinical trial which was less sensitive to bias through the balanced study design and data acquisition. Second, studies have shown that caffeine affects muscle, adipose, and central nervous tissue indirectly by enhancing the release of catecholamines and the activity of adenylate cyclase, which catalyzes the formation of cAMP from ATP, thus enhancing lipolysis, fatty acid oxidation, and exercise performance.29 The independent effect of caffeine might have been a confounding factor that influenced the results of this antioxidation effect. The STA-2 used in this study contained less caffeine than the preparations used in previous studies. Besides, the investigational product of STA-2 (600 mg/day) contained only 6 mg caffeine, which is less than general commercial green tea (10-20 mg/100 ml) and also below the recommended caffeine daily intake (300 mg/day) in western countries including the US and Europe. Furthermore, STA-2 treatment was well-tolerated, and no new safety concerns were identified. The most commonly reported adverse events included palpitations, upper abdominal pain, insomnia, upper respiratory tract infection, chest discomfort, and chest pain, similar to those previously reported, i.e., excess gas, upset stomach, nausea, heartburn, stomach ache, abdominal pain, dizziness, headache, and muscle pain.22
There are several potential limitations that existed in this study, which sought to establish a link between STA-2 and improvement in exercise tolerance. First, the current dose of STA-2 may still be below the optimal dosage to best observe the beneficial effects. However, the current dosage of STA-2 in this study was identical to that of Xin-Nao-Jian (literally heart-brain-healthy), the precursor of STA-2, approved by the China State Food and Drug Administration in 1997 for treatment of cardiovascular diseases associated with atherosclerosis and hyperfibrinogenemia. The dosage of 6 STA-2 capsules a day was equivalent to the polyphenols ingested by drinking 6 cups of green tea per day. Since the exact amount of polyphenols consumed by drinking green tea can vary depending on dietary practices and brewing habits, the use of a pharmaceutical preparation of green tea extracts is preferable in clinical practice. Kuriyama et al.1 demonstrated a dose-specific reduction in the hazard ratio of cardiovascular to all-cause mortality, with the greatest benefits obtained upon consumption of ≥ 5 cups of green tea per day. Second, this is the first exploratory study of the association of extract of green tea polyphenols on coronary artery disease (CAD) patients. For ethical consideration in conducting ETT on patients with severe myocardial infarction, the patients included in this study were selected according to a particular scenario at baseline (for example, subjects who are well-controlled or receiving optimal treatment), results from this study which may not be represented to general patients of CAD and only six weeks of treatment may be insufficient to reach functional improvement in this particular population. Furthermore, the finding that most patients did not experience angina during the exercise test may be explained by the fact that patients stop before they experience angina pectoris. Third, a significant malabsorption of carbohydrates after concomitant consumption of a mixture of polyphenols, including green tea catechins, has been reported.30 Tea components have been shown to inhibit the sodium-dependent glucose transporter I,31 which could be against high green tea extracts amounts in sport. Thus, it is possible that the STA-2 might have impaired energy metabolism, and subsequent exercise performance lead to insignificant findings of this study. Forth, the intervention of study duration was only for 6 weeks, and thus the longer-term effects of green tea on exercise tolerance are not addressed. Previous studies have shown that short-term studies with angiotensin-converting enzyme inhibitors in patients with stable angina pectoris have shown disappointing results.32,33 However, the long-term treatment with angiotensin-converting enzyme inhibition reduces exercise-induced myocardial ischemia.34 Thus, we cannot exclude the possibility of positive results after prolonged administration of STA-2. Finally, the limited sample size of the current exploratory study was not sufficient to support the association. However, future well-designed, long-term intervention studies with standardized green tea products would be warranted, to greatly facilitate our understanding of the benefits of green tea catechins.
CONCLUSIONS
The results of this pilot study showed that an amount of green tea extract which corresponds to 6 cups of tea per day for 6 weeks does not have significant beneficial effects on exercise performance. However, STA-2 treatment suggested beneficial effects on LDL-cholesterol concentrations. Further studies with a larger sample size, different dosage, and longer follow-up periods are needed to address the question of whether STA-2 may influence exercise performance.
Acknowledgments
This study was financially supported by the Department of Industrial Technology, Ministry of Economic Affairs (96-EC-17-A-20-I1-0003). We thank Dr. Muh-Hwan Su, Dr. JingJing Tang, and Mr. Young-Ming Huang for their assistance and advice in the study concept and design. We also thank Protech Pharmaservices Corporation for monitoring this study, and Drs. Yuan Lin and Yi-Hung Chen for their professional comments and suggestions on this study. The authors have not received external funding for the preparation and submission of the manuscript.
CONFLICT OF INTEREST
The authors have declared that no conflicting interests exist.
REFERENCES
- 1.Kuriyama S, Shimazu T, Ohmori K, et al. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: the Ohsaki study. JAMA. 2006;296:1255–1265. doi: 10.1001/jama.296.10.1255. [DOI] [PubMed] [Google Scholar]
- 2.Mineharu Y, Koizumi A, Wada Y, et al. Coffee, green tea, black tea and oolong tea consumption and risk of mortality from cardiovascular disease in Japanese men and women. J Epidemiol Community Health. 2011;65:230–240. doi: 10.1136/jech.2009.097311. [DOI] [PubMed] [Google Scholar]
- 3.Wen W, Xiang YB, Zheng W, et al. The association of alcohol, tea, and other modifiable lifestyle factors with myocardial infarction and stroke in Chinese men. CVD Prev Control. 2008;3:133–140. doi: 10.1016/j.cvdpc.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartley L, Flowers N, Holmes J, et al. Green and black tea for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;18:CD009934.. doi: 10.1002/14651858.CD009934.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hodgson JM. Tea flavonoids and cardiovascular disease. Asia Pac J Clin Nutr. 2008;17(Suppl 1):288–290. [PubMed] [Google Scholar]
- 6.Inami S, Takano M, Yamamoto M, et al. Tea catechin consumption reduces circulating oxidized low-density lipoprotein. Int Heart J. 2007;48:725–732. doi: 10.1536/ihj.48.725. [DOI] [PubMed] [Google Scholar]
- 7.Basu A, Sanchez K, Leyva MJ, et al. Green tea supplementation affects body weight, lipids, and lipid peroxidation in obese subjects with metabolic syndrome. J Am Coll Nutr. 2010;29:31–40. doi: 10.1080/07315724.2010.10719814. [DOI] [PubMed] [Google Scholar]
- 8.Maron DJ, Lu GP, Cai NS, et al. Cholesterol-lowering effect of a theaflavin-enriched green tea extract: a randomized controlled trial. Arch Intern Med 23. 2003;163:1448–1453. doi: 10.1001/archinte.163.12.1448. [DOI] [PubMed] [Google Scholar]
- 9.Nagao T, Hase T, Tokimitsu I. A green tea extract high in catechins reduces body fat and cardiovascular risks in humans. Obesity (Silver Spring) 2007;15:1473–1483. doi: 10.1038/oby.2007.176. [DOI] [PubMed] [Google Scholar]
- 10.Ogura R, Ikeda N, Yuki K, et al. Genotoxicity studies on green tea catechin. Food Chem Toxicol. 2008;46:2190–2200. doi: 10.1016/j.fct.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 11.Murase T, Haramizu S, Shimotoyodome A, et al. Green tea extract improves running endurance in mice by stimulating lipid utilization during exercise. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1550–R1556. doi: 10.1152/ajpregu.00752.2005. [DOI] [PubMed] [Google Scholar]
- 12.Murase T, Haramizu S, Shimotoyodome A, et al. Green tea extract improves endurance capacity and increases muscle lipid oxidation in mice. Am J Physiol Regul Integr Comp Physiol. 2005;288:R708–R715. doi: 10.1152/ajpregu.00693.2004. [DOI] [PubMed] [Google Scholar]
- 13.Watson TA, MacDonald-Wicks LK, Garg ML. Oxidative stress and antioxidants in athletes undertaking regular exercise training. Int J Sport Nutr Exerc Metab. 2005;15:131–146. doi: 10.1123/ijsnem.15.2.131. [DOI] [PubMed] [Google Scholar]
- 14.Panza VS, Wazlawik E, Ricardo Schutz G, et al. Consumption of green tea favorably affects oxidative stress markers in weight-trained men. Nutrition. 2008;24:433–442. doi: 10.1016/j.nut.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Hooper L, Kroon PA, Rimm EB, et al. Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2008;88:38–50. doi: 10.1093/ajcn/88.1.38. [DOI] [PubMed] [Google Scholar]
- 16.Fletcher B, Berra K, Ades P, et al. Managing abnormal blood lipids:a collaborative approach. Circulation. 2005;112:3184–3209. doi: 10.1161/CIRCULATIONAHA.105.169180. [DOI] [PubMed] [Google Scholar]
- 17.Manson JE, Tosteson H, Ridker PM, et al. The primary prevention of myocardial infarction. N Engl J Med. 1992;326:1406–1416. doi: 10.1056/NEJM199205213262107. [DOI] [PubMed] [Google Scholar]
- 18.Ikeda I, Imasato Y, Sasaki E, et al. Tea catechins decrease micellar solubility and intestinal absorption of cholesterol in rats. Biochim Biophys Acta. 1992;1127:141–146. doi: 10.1016/0005-2760(92)90269-2. [DOI] [PubMed] [Google Scholar]
- 19.Yang TT, Koo MW. Chinese green tea lowers cholesterol level through an increase in fecal lipid excretion. Life Sci. 2000;66:411–423. doi: 10.1016/s0024-3205(99)00607-4. [DOI] [PubMed] [Google Scholar]
- 20.Muramatsu K, Fukuyo M, Hara Y. Effect of green tea catechins on plasma cholesterol level in cholesterol-fed rats. J Nutr Sci Vi-taminol (Tokyo) 1986;32:613–622. doi: 10.3177/jnsv.32.613. [DOI] [PubMed] [Google Scholar]
- 21.Cuccioloni M, Mozzicafreddo M, Spina M, et al. Epigallocatechin-3-gallate potently inhibits the in vitro activity of hydroxy-3-methyl-glutaryl-CoA reductase. J Lipid Res. 2011;52:897–907. doi: 10.1194/jlr.M011817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Babu PV, Liu D. Green tea catechins and cardiovascular health: an update. Curr Med Chem. 2008;15:1840–1850. doi: 10.2174/092986708785132979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hodgson JM, Croft KD, Mori TA, et al. Regular ingestion of tea does not inhibit in vivo lipid peroxidation in humans. J Nutr. 2002;132:55–58. doi: 10.1093/jn/132.1.55. [DOI] [PubMed] [Google Scholar]
- 24.Acton QA. Advances in Sorbitol Research and Application. 3rd ed. Atlanta GA: Cumberland; 2013. pp. 68–69. [Google Scholar]
- 25.Kang WS, Chung KH, Chung JH, et al. Antiplatelet activity of green tea catechins is mediated by inhibition of cytoplasmic calcium increase. J Cardiovasc Pharmacol. 2001;38:875–884. doi: 10.1097/00005344-200112000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Babu PV, Liu D. Green tea catechins and cardiovascular health: an update. Curr Med Chem. 2008;15:1840–1850. doi: 10.2174/092986708785132979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mattson MP, Chan SL. Calcium orchestrates apoptosis. Nat Cell Biol. 2003;5:1041–1043. doi: 10.1038/ncb1203-1041. [DOI] [PubMed] [Google Scholar]
- 28.Yuan XQ, Wang YF, Chen LJ. Tea Polyphenol Chemistry. 1st ed. Shanghai: Shanghai Science and Technology Press; 2003. pp. 412–422. [Google Scholar]
- 29.Graham TE, Spriet LL. Metabolic, catecholamine, and exercise performance responses to various doses of caffeine. J Appl Physiol (1985) 1995;78:867–874. doi: 10.1152/jappl.1995.78.3.867. [DOI] [PubMed] [Google Scholar]
- 30.Zhong L, Furne JK, Levitt MD. An extract of black, green, and mulberry teas causes malabsorption of carbohydrate but not of triacylglycerol in healthy volunteers. Am J Clin Nutr. 2006;84:551–555. doi: 10.1093/ajcn/84.3.551. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi Y, Suzuki M, Satsu H, et al. Green tea polyphenols inhibit the sodium-dependent glucose transporter of intestinal epithelial cells by a competitive mechanism. J Agric Food Chem. 2000;48:5618–5623. doi: 10.1021/jf0006832. [DOI] [PubMed] [Google Scholar]
- 32.van Gilst WH, Tio RA, van Wijngaarden J, et al. Effects of converting enzyme inhibitors on coronary flow and myocardial ischemia. J Cardiovasc Pharmacol. 1992;19(Suppl 5):S134–S139. [PubMed] [Google Scholar]
- 33.Klein WW, Khurmi NS, Eber B, Dusleag J. Effects of benazepril and metoprolol OROS alone and in combination on myocardial ischemia in patients with chronic stable angina. J Am Coll Cardiol. 1990;16:948–956. doi: 10.1016/s0735-1097(10)80347-x. [DOI] [PubMed] [Google Scholar]
- 34.van den Heuvel AF, Dunselman PH, Kingma T, et al. Reduction of exercise-induced myocardial ischemia during add-on treatment with the angiotensin-converting enzyme inhibitor enalapril in patients with normal left ventricular function and optimal beta blockade. J Am Coll Cardiol. 2001;37:470–474. doi: 10.1016/s0735-1097(00)01111-6. [DOI] [PubMed] [Google Scholar]


