Abstract
Background
It has been observed that acetaminophen shows cardioprotective efficacy in mammals. In this study, we investigated the electromechanical effects of acetaminophen on the left atrium (LA).
Methods
Conventional microelectrodes were used to record the action potentials (AP) in rabbit LA preparations. The action potential duration (APD) at repolarization levels of 90%, 50% and 20% of the AP amplitude (APD90, APD50, and APD20, respectively), resting membrane potential, and contractile force were measured during 2 Hz electrical stimulation before and after sequential acetaminophen administration to the LA.
Results
Acetaminophen (0.1, 0.3, 1, and 3 mM) reduced APD20 from 9.4 ± 1.2 to 8.0 ± 1.1 (p < 0.05), 7.1 ± 0.8 (p < 0.05), 7.8 ± 1.1, and 6.8 ± 1.2 ms (p < 0.05), respectively, and APD50 from 20.2 ± 1.9 to 17.4 ± 2.0, 15.6 ± 1.8 (p < 0.05), 15.8 ± 2.2 (p < 0.05), and 14.1 ± 2.4 ms (p < 0.05), respectively, in a concentration-dependent manner. APD90 was reduced from 72.0 ± 3.6 to 64.7 ± 4.2, 61.9 ± 4.3, 60.5 ± 3.7, and 53.4 ± 4.4 ms (p < 0.05), respectively. Acetaminophen increased LA contractility from 45 ± 9 to 52 ± 10 (p < 0.05), 55 ± 9 (p < 0.01), 58 ± 9 (p < 0.01), and 60 ± 9 mg (p < 0.01), respectively, in a concentration-dependent manner. In the presence of the NOS inhibitor L-NAME or PKG-I inhibitor DT-2, additional acetaminophen treatment did not significantly increase LA contractility.
Conclusions
Acetaminophen modulated the electromechanical characteristics of LA by inhibiting the NOS and PKG I pathway, and then contributed to the positive inotropic effect.
Keywords: Acetaminophen, Contractility, Left atrium
INTRODUCTION
Acetaminophen has been clinically proven to be effective for the temporary relief of minor aches and pains associated with the common cold, headache, toothache, muscular aches, and backache. It can also be effective against the minor pain of arthritis, for menstrual cramp pain and fever reduction.1 Acetaminophen has been used as an efficacious antipyretic and analgesic for more than a century. In recent years, acetaminophen has been demonstrated to possess cardioprotective benefits in mammals.2 For example, Merrill reported anti-arrhythmic properties of acetaminophen3 and that acetaminophen may be effective for myocardial infarction in dogs.4 During ischemia and reperfusion, acetaminophen attenuates the release of hydroxyl radicals and peroxynitrite, indicating a cardioprotective, antioxidant behavior.5 Acetaminophen is cardioprotective against H2O2-induced injury in vivo.4 Additionally, it has been observed to protect against iron-induced cardiac damage in gerbils.6
Atrial fibrillation (AF) is the most common cardiac arrhythmia encountered in clinical practice that induces cardiac dysfunction and stroke.7,8 Previous studies have shown that the left atrium (LA) plays an important role in the pathophysiology of AF.9,10 However, the electromechanical effects of acetaminophen on the LA are not clear. A previous study reported that acetaminophen inhibits nitric oxide synthesis.11 Nitric oxide (NO) plays an important role in the cardiovascular system. In the basal state, the effect of NO is bimodal, with a positive inotropic effect at low amounts of NO exposure but a negative one at higher amounts. The effect of NO donors and endogenous NO on the contractile force was enhanced in hearts.12 The purpose of this study was to investigate the electromechanical effects of acetaminophen on the LA and to determine whether acetaminophen can affect cardiac physiology through nitric oxide.
MATERIALS AND METHODS
Electromechanical and pharmacological studies of LA preparations
The experiments in this study conformed to requirements of the institutional Guide for the Care and Use of Laboratory Animals. Male rabbits (weight, 1.5-2.0 kg) were anesthetized with an intraperitoneal injection of sodium pentobarbital (100 mg/kg). A midline thoracotomy was performed, and the heart and lungs were then removed.10 To dissect the LA, the LA was opened by an incision along the mitral valve annulus, extending from the coronary sinus to the septum, and suspended in Tyrode’s solution composed of 137 mM NaCl, 4 mM KCl, 15 mM NaHCO3, 0.5 mM NaH2PO4, 0.5 mM MgCl2, 2.7 mM CaCl2, and 11 mM dextrose. Preparations were connected to a WPI Duo 773 electrometer under a diastolic tension of 150 mg as previously described.10 One end of the preparations was pinned with needles to the bottom of a tissue bath. The other end was connected to a Grass FT03C force transducer with a silk thread. The adventitial or epicardial side of the preparations faced upward. The LA tissue strips were superfused at a constant rate (3 mL/min) with Tyrode’s solution saturated with a 97% O2-3% CO2 gas mixture. The temperature was maintained at 37 °C, and the preparations were allowed to equilibrate for 1 h before electrophysiological assessment. Transmembrane action potentials (AP) were recorded by using machine-pulled glass capillary microelectrodes filled with 3 mol/L KCl. Electromechanical events (contractile force and diastolic tension) were simultaneously displayed on a Gould 4072 oscilloscope and a Gould TA11 recorder. By using a data acquisition system, signals were recorded with direct current coupling and a 10-KHz low-pass filter cut-off frequency. Signals were recorded digitally with a 16-bit accuracy at a rate of 125 KHz. Electrical stimulation was provided by using a Grass S88 stimulator through a Grass SIU5B stimulus isolation unit. The AP amplitude (APA) was calculated by measuring the difference between the resting membrane potential (RMP) and the peak of AP depolarization. AP durations (APD) at repolarization of 90%, 50%, and 20% of the APA were respectively measured as APD90, APD50, and APD20. The RMP, APA, APD90, APD50, APD20, and contractile forces were measured under 2-Hz pacing of the LA before and after the sequential administration of acetaminophen (0.1, 0.3, 1, and 3 mM). In order to study the mechanical effects of acetaminophen, an NG-nitro-L-arginine methyl ester (L-NAME) solution (a NOS inhibitor, 100 μM), DT-2 trifluoroacetate salt [cell permeable cGMP protein kinase (PKG) inhibitor, 1 μM]13 and acetaminophen solution (1 mM) were sequentially added to the LA preparations.
Statistical analysis
All continuous variables are expressed as mean ± standard error of the mean (SEM). A one-way repeated-measures analysis of variance (ANOVA) was used to compare the difference before and after drug administration on the LA. The electrophysiological and mechanical characteristics between the different groups were compared by using a Wilcoxon rank-sum test or unpaired t-test, depending on the outcome of the normality test. A p < 0.05 was considered statistically significant.
RESULTS
Acetaminophen affects the electromechanical characteristics of the LA
As shown in Figure 1, acetaminophen (0.1, 0.3, 1, and 3 mM) reduced the LA (n = 8) APA from 97.3 ± 3.3 to 96.9 ± 3.5, 90.7 ± 2.8, 91.2 ± 3.2 (p < 0.05, vs. the control), and 85.4 ± 3.0 mV (p < 0.01, vs. the control), respectively, in a concentration-dependent manner. At concentrations of 0.1, 0.3, 1, and 3 mM, acetaminophen reduced the LA (n = 8) APD20 from 9.4 ± 1.2 to 8.0 ± 1.1 (p < 0.05, vs. the control), 7.1 ± 0.8 (p < 0.05, vs. the control), 7.8 ± 1.1, and 6.8 ± 1.2 ms (p < 0.05, vs. the control), respectively, in a concentration-dependent manner. Similarly, the LA (n = 8) APD50 was reduced from 20.2 ± 1.9 to 17.4 ± 2.0, 15.6 ± 1.8 (p < 0.05, vs. the control), 15.8 ± 2.2 (p < 0.05, vs. the control), and 14.1 ± 2.4 ms (p < 0.05, vs. the control), respectively, and the LA APD90 was reduced from 72.0 ± 3.6 to 64.7 ± 4.2, 61.9 ± 4.3, 60.5 ± 3.7, and 53.4 ± 4.4 ms (p < 0.05, vs. the control), respectively. However, acetaminophen did not significantly affect the RMP. At concentrations of 0.1, 0.3, 1, and 3 mM acetaminophen increased LA (n = 8) contractility from 45 ± 9 to 52 ± 10 (p < 0.05, vs. the control), 55 ± 9 (p < 0.01, vs. the control), 58 ± 9 (p < 0.01, vs. the control), and 60 ± 9 mg (p < 0.01, vs. the control), respectively, in a concentration-dependent manner.
Figure 1.
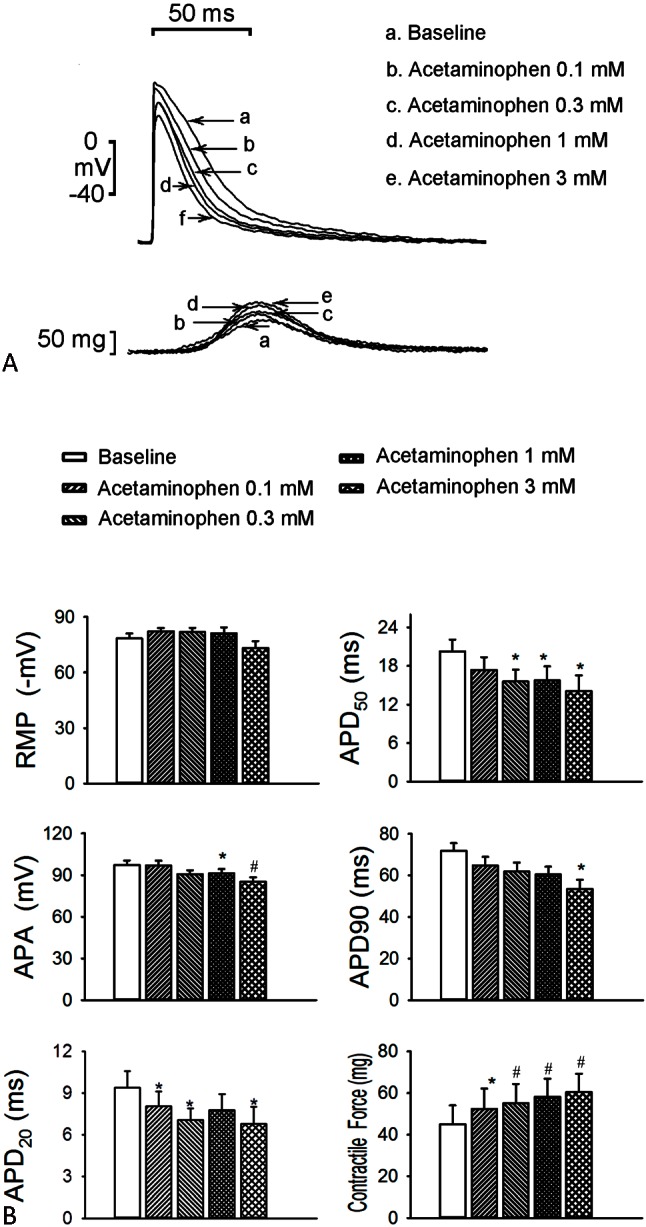
Effects of acetaminophen on the action potential (AP) of the left atrium (LA) without spontaneous activity. (A) Superimposed tracings and (B) average data of the AP and contractility in the LA (n = 8) before and after administration of the different concentrations (0.1, 0.3, 1, and 3 mmol/L) of acetaminophen. * p < 0.05, # p < 0.01, † p < 0.005 versus the baseline. APA, AP amplitude; APD20, APD50, and APD90, AP duration at a repolarization of 20%, 50%, and 90% of the APA, respectively; RMP, resting membrane potential.
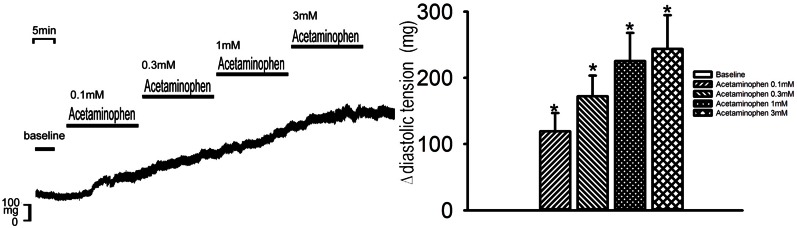
As shown in Figure 2, acetaminophen at 0.1, 0.3, 1, and 3 mM increased the diastolic tension of the LA (n = 8) to 151 ± 51 (p < 0.05, vs. the control), 229 ± 82 (p < 0.05, vs. the control), 274 ± 83 (p < 0.05, vs. the control), and 305 ± 87 mg (p < 0.05, vs. the control), respectively, in a concentration-dependent manner.
Figure 2.
Effect of acetaminophen on the diastolic tension of the left atrium (LA). An example and average data (n = 6) show that acetaminophen increased the diastolic tension of the LA in a concentration-dependent manner. (Δ, change in diastolic tension). * p < 0.05, # p < 0.01, † p < 0.005 versus the control.
Effect of L-NAME
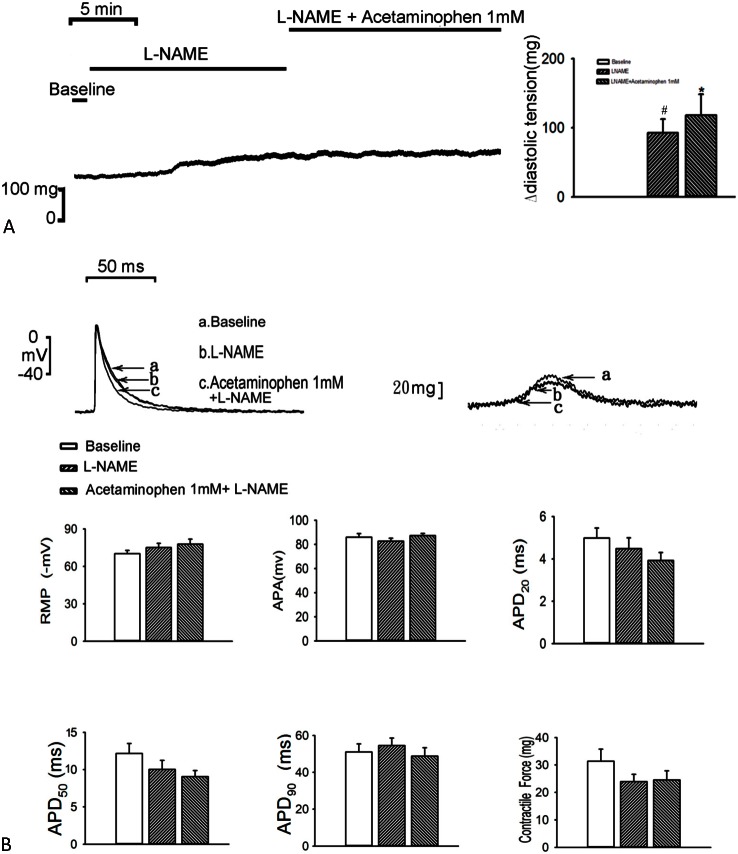
As compared with the control, treatment with L-NAME (100 μM) increased the diastolic tension of the LA (n = 7; 92 ± 20 mg; p < 0.01, vs. the control), and additional acetaminophen (1 mM) further increased the diastolic tension of the LA (n = 7; 118 ± 31 mg; p < 0.05, vs. the control; Figure 3A), but did not affect the contractility of the LA. In the presence of L-NAME (100 μM), additional acetaminophen (1 mM) treatment did not significantly further increase LA contractility (n = 7; Figure 3B).
Figure 3.
Effects of acetaminophen and L-NAME on the diastolic tension and action potential (AP) of the left atrium (LA). (A) Tracing and average data of the diastolic tension of the LA (n = 7) before and after administration of L-NAME and acetaminophen. (B) Superimposed tracings and average data of the AP and contractility in the LA (n = 7) before and after administration of L-NAME and acetaminophen. * p < 0.05, # p < 0.01, † p < 0.005 versus the baseline. APA, AP amplitude; APD20, APD50, and APD90, AP duration at a repolarization of 20%, 50%, and 90% of the APA, respectively; RMP, resting membrane potential.
Effect of DT-2 trifluoroacetate salt
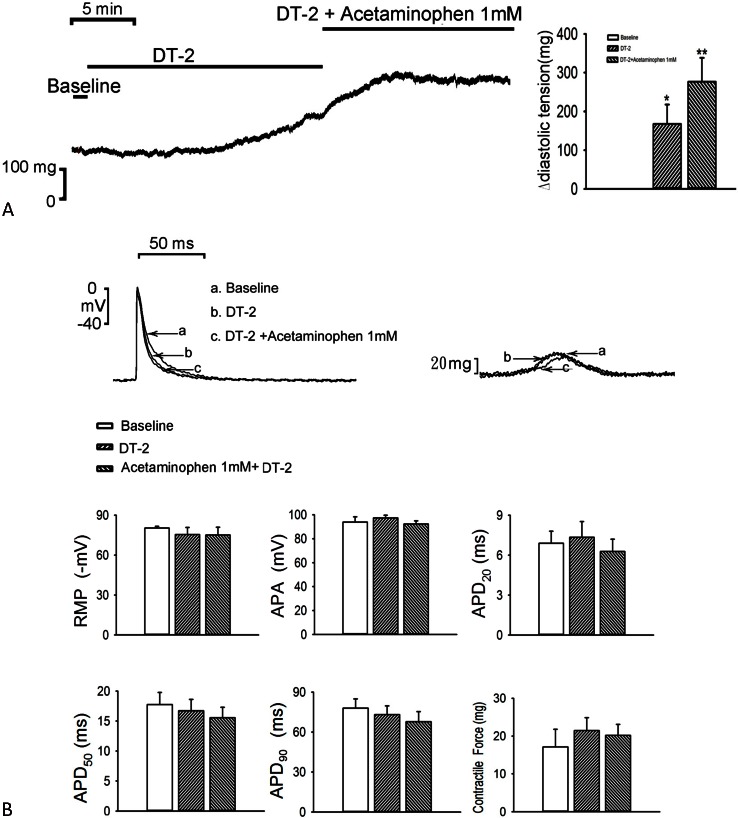
As compared with the control, treatment with DT-2 (1 μM) increased the diastolic tension of the LA (n = 7; 168 ± 50 mg; p < 0.05, vs. the control), and additional acetaminophen (1 mM) further increased the diastolic tension of LA (n = 7; 276 ± 62 mg; p < 0.01, vs. the control; Figure 4A) but did not affect the contractility of the LA. In the presence of DT-2 (1 μM), additional acetaminophen (1 mM) treatment did not significantly further increase LA contractility (n = 7; Figure 4B).
Figure 4.
Effects of acetaminophen and DT-2 on the diastolic tension and action potential (AP) of the left atrium (LA). (A) Tracing and average data of diastolic tension in the LA (n = 7) before and after administration of DT-2 and acetaminophen. (B) Superimposed tracings and average data of the AP and contractility in the LA (n = 7) before and after administration of DT-2 and acetaminophen. * p < 0.05, # p < 0.01, † p < 0.005 versus the baseline. APA, AP amplitude; APD20, APD50, and APD90, AP duration at a repolarization of 20%, 50%, and 90% of the APA, respectively; RMP, resting membrane potential.
DISCUSSION
In recent years, acetaminophen has been demonstrated to possess cardioprotective efficacy in mammals. A previous study reported both short- and long-term acetaminophen treatments (0.35 mM) to have cardioprotective effects.14 In the present study, we perfused the LA of rabbits with an acetaminophen solution in vitro. Acetaminophen reduced the APA, APD20, APD50, and APD90 in the LA but significantly enhanced the contractility of the LA in a concentration-dependent manner. However, acetaminophen did not significantly affect the RMP. Moreover, acetaminophen increased LA diastolic tension in a concentration-dependent manner, which could not be reversed after washout for 30 min. We found that acetaminophen significantly increased the contractility of the LA in a concentration-dependent manner. In addition, acetaminophen reduced the APD but enhanced the diastolic tension of the LA. The shortening of APD may make LA an excitable substrate to maintain AF.
Previous studies showed that cardiac myofilaments mediated an increase in diastolic fiber length via NO.12 Because of the increasing trend in diastolic tension, we predict that acetaminophen might inhibit NO release and then enhance the diastolic tension or contractility of the cardiac myofilaments. As pretreatment with L-NAME, an inhibitor of NOS,15 masks the inotropic properties of acetaminophen, it is suggested that acetaminophen either directly or indirectly inhibits the NO-generation pathway. Treatment with L-NAME and acetaminophen could increase diastolic tension more than L-NAME alone. The effects of acetaminophen may be attributable to not only the NO pathway.
Cyclic GMP is involved in the regulation of many cellular processes, including cardiac and smooth muscle contractility.16 The oligopeptide DT-2 is a specific PKG I inhibitor in vitro. We performed pretreatment with DT-2 to study whether acetaminophen affects cardiac physiology via the effect of PKG I. As pretreatment with DT-2 masks the inotropic properties of acetaminophen, it is suggested that acetaminophen either directly or indirectly inhibits the PKG I pathway. The treatment with DT-2 and acetaminophen could increase diastolic tension more than DT-2 alone. Accordingly, the effects of acetaminophen may be attributable to not only the PKG I pathway, but other causes as well.
CONCLUSIONS
Acetaminophen modulated LA electromechanical characteristics by inhibiting the NOS and PKG I pathways, and then contributed to the positive inotropic effect.
Acknowledgments
The current study was supported by grants from National Science Council of Taiwan (NSC102-2314-B-016-029-MY2), National Defense Medical Center (MAB-105-026) and Chi-Mei Medical Center (CMNDMC10410).
CONFLICT OF INTEREST
All the authors declare no conflict of interest.
REFERENCES
- 1.Leshnower BG, Sakamoto H, Zeeshan A, et al. Role of acetaminophen in acute myocardial infarction. Am J Physiol Heart Circ Physiol. 2006;290:H2424–H2431. doi: 10.1152/ajpheart.00962.2005. [DOI] [PubMed] [Google Scholar]
- 2.Spiler NM, Rork TH, Merrill GF. An old drug with a new purpose: cardiovascular actions of acetaminophen (paracetamol). Curr Drug Targets Cardiovasc Haematol Disord. 2005;5:419–429. doi: 10.2174/156800605774370335. [DOI] [PubMed] [Google Scholar]
- 3.Merrill GF, Merrill JH, Golfetti R, et al. Antiarrhythmic properties of acetaminophen in the dog. Exp Biol Med (Maywood) 2007;232:1245–1252. doi: 10.3181/0701-RM-19. [DOI] [PubMed] [Google Scholar]
- 4.Jaques-Robinson KM, Golfetti R, Baliga SS, et al. Acetaminophen is cardioprotective against H2O2-induced injury in vivo. Exp Biol Med (Maywood) 2008;233:1315–1322. doi: 10.3181/0802-RM-68. [DOI] [PubMed] [Google Scholar]
- 5.Merrill GF, Goldberg E. Antioxidant properties of acetaminophen and cardioprotection. Basic Res Cardiol. 2001;96:423–430. doi: 10.1007/s003950170023. [DOI] [PubMed] [Google Scholar]
- 6.Walker EM, Jr., Epling CP, Parris C, et al. Acetaminophen protects against iron-induced cardiac damage in gerbils. Ann Clin Lab Sci. 2007;37:22–33. [PubMed] [Google Scholar]
- 7.Krahn AD, Manfreda J, Tate RB, et al. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am J Med. 1995;98:476–484. doi: 10.1016/S0002-9343(99)80348-9. [DOI] [PubMed] [Google Scholar]
- 8.Psaty BM, Manolio TA, Kuller LH, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]
- 9.Oral H, Scharf C, Chugh A, et al. Catheter ablation for paroxysmal atrial fibrillation: segmental pulmonary vein ostial ablation versus left atrial ablation. Circulation. 2003;108:2355–2360. doi: 10.1161/01.CIR.0000095796.45180.88. [DOI] [PubMed] [Google Scholar]
- 10.Tsai CF, Chen YC, Lin YK, et al. Electromechanical effects of the direct renin inhibitor (aliskiren) on the pulmonary vein and atrium. Basic Res Cardiol. 2011;106:979–993. doi: 10.1007/s00395-011-0206-8. [DOI] [PubMed] [Google Scholar]
- 11.Godfrey L, Bailey I, Toms NJ, et al. Paracetamol inhibits nitric oxide synthesis in murine spinal cord slices. Eur J Pharmacol. 2007;562:68–71. doi: 10.1016/j.ejphar.2007.01.075. [DOI] [PubMed] [Google Scholar]
- 12.Massion PB, Feron O, Dessy C, Balligand JL. Nitric oxide and cardiac function: ten years after, and continuing. Circ Res. 2003;93:388–398. doi: 10.1161/01.RES.0000088351.58510.21. [DOI] [PubMed] [Google Scholar]
- 13.Dostmann WR, Taylor MS, Nickl CK, et al. Highly specific, membrane-permeant peptide blockers of cGMP-dependent protein kinase Ialpha inhibit NO-induced cerebral dilation. Proc Natl Acad Sci U S A. 2000;97:14772–14777. doi: 10.1073/pnas.97.26.14772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golfetti R, Rork T, Merrill G. Chronically administered acetaminophen and the ischemia/reperfused myocardium. Exp Biol Med (Maywood) 2003;228:674–682. doi: 10.1177/153537020322800605. [DOI] [PubMed] [Google Scholar]
- 15.Miller MJ, Thompson JH, Liu X, et al. Failure of L-NAME to cause inhibition of nitric oxide synthesis: role of inducible nitric oxide synthase. Inflamm Res. 1996;45:272–276. doi: 10.1007/BF02280990. [DOI] [PubMed] [Google Scholar]
- 16.Gambaryan S, Butt E, Kobsar A, et al. The oligopeptide DT-2 is a specific PKG I inhibitor only in vitro, not in living cells. Br J Pharmacol. 2012;167:826–838. doi: 10.1111/j.1476-5381.2012.02044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]